Abstract
Post-translational modifications of proteins are a major determinant of biological function. Phosphorylation of proteins involved in signal transduction contributes to the induction and maintenance of several examples of cellular and synaptic plasticity. In this study we have identified phosphoproteins regulated by Pavlovian conditioning in lysates of Hermissenda nervous systems using 2DE in conjunction with 32P labeling, fluorescence based phosphoprotein in-gel staining, and mass spectrometry. Modification of protein phosphorylation regulated by conditioning was first assessed by densitometric analysis of 32P labeled proteins resolved by 2DE from lysates of conditioned and pseudorandom control nervous systems. An independent assessment of phosphorylation regulated by conditioning was obtained from an examination of 2D gels stained with Pro-Q Diamond phosphoprotein dye. Mass spectrometric analysis of protein digests from phosphoprotein stained analytical gels or Coomassie blue stained preparative gels provided for the identification of phosphoproteins that exhibited statistically significant increased phosphorylation in conditioned groups as compared to pseudorandom controls. A previously identified cytoskeletal related protein, Csp24, involved in intermediate-term memory exhibited significantly increased phosphorylation detected 24 hrs post-conditioning. Our results show that proteins involved in diverse cellular functions such as transcriptional regulation, cell signaling, cytoskeletal regulation, metabolic activity, and protein degradation contribute to long-term post-translational modifications associated with Pavlovian conditioning.
Keywords: β-thymosin repeat protein, Hermissenda, cytoskeletal proteins, Pavlovian conditioning, post-translational modification
Post-translational modifications that change the properties of proteins are of major importance for biological function. For example, a number of biological events such as signal transduction, transcriptional regulation, control of enzyme activity, and cell division are regulated by the phosphorylation of proteins (for reviews see Hunter and Karin, 1992; Johnson and Vaillancourt, 1994; Hunter, 1995; Karin and Hunter, 1995; Johnston and O’Reilly, 1996; Mann and Jensen, 2003). In addition, phosphorylation is now recognized as important in the induction and maintenance of cellular and synaptic plasticity (for example see Neary et al., 1981; Sweatt and Kandel, 1989; Crow et al., 1996; Kondo et al., 2005; Wang et al., 2005; Baratier et al., 2006; Moult et al., 2006; Sun et al., 2006; Sunyer et al., 2008). The onset and duration of protein phosphorylation varies over time following stimulation (Mooney and Anderson, 1989; Wahl et al., 1989; Ahn et al., 1990; Donaldson et al., 1991; Hagiwara et al., 1992; Moreland et al., 1992; Sato et al., 1999), and may be transient or long-term, depending upon the duration of the experimental treatment (Sweatt and Kandel, 1989; Homayouni et al., 1995, 1997). However, protein phosphorylation may also be detected within minutes-to-hours following a single experimental treatment(Crow et al., 1996).
In the marine mollusc Hermissenda the regulation of several phosphoproteins has been examined following multi-trial and one-trial Pavlovian conditioning (Neary et al., 1981; Alkon and Nelson, 1990; Crow et al., 1996; Nelson et al., 1996). Two phosphoproteins that have been identified and characterized in Hermissenda sensory neurons (photoreceptors) are calexcitin (CE), a GTP- and Ca2+ binding protein (Kuzirian et al., 2001), and Csp24, a protein containing repeated β-thymosin homology domains(Crow and Xue-Bian, 2000, 2003; Crow et al., 2003). The phosphorylation of Csp24 is regulated by one-trial in vitro conditioning. Previous studies of Hermissenda isolated nervous systems showed that one-trial in vitro conditioning resulted in a time-dependent increase in the phosphorylation of several proteins (Crow et al., 1996,1999; Crow and Xue-Bian, 2003). However, with the exception of Csp24, additional phosphoproteins regulated by one-trial conditioning have not been identified or characterized. In addition, it is not known if multi-trial Pavlovian conditioning regulates the same phosphoproteins that are modified following one-trial in vitro conditioning procedures. Here we report that multi-trial Pavlovian conditioning results in a significant increase in the phosphorylation of 12 of 20 proteins from the sample selected for analysis, as compared to control groups that received pseudorandom presentations of the conditioned stimulus (CS) and unconditioned stimulus (US). Tryptic digests of the selected spots from 2D gels of conditioned and control lysates followed by mass spectrometric sequencing resulted in peptide sequences that were assigned by homology to proteins with diverse cellular functions.
EXPERIMENTAL PROCEDURES
Pavlovian conditioning
A total of 170 animals were used in the experiments. Adult Hermissenda crassicornis obtained from Sea Life Supply, Sand City, CA were maintained in artificial sea water (ASW) aquaria at 14 ± 1°C on a 12 hr light/dark cycle. The details of the conditioning procedure have been described in previously published papers (Crow and Alkon, 1978; Crow and Offenbach, 1983; Crow, 1985). Briefly, adult Hermissenda were conditioned using light as a conditioned stimulus (CS) and orbital rotation as the unconditioned stimulus (US). Animals received 100 trials of the 10 sec CS paired with a 9 sec US. Controls received 100 trials of the CS and US programmed on independent random schedules with the restriction that the CS and US not overlap in time e.g. pseudorandom. Behavior was tested 24 hours following conditioning using automated procedures to determine start latencies in response to the CS, the measure of inhibition of light-evoked locomotion (Crow and Offenbach, 1983; Crow, 1985). Start latencies were examined before conditioning to establish a baseline for all animals. The animals were placed into glass tubes filled with ASW and confined to one end of the tube by a small foam plug used as a starting gate. Tubes were placed into spring clips on a modified turntable housed inside an incubator to maintain temperature at 15°C. Animals were dark-adapted for 12 minutes prior to baseline testing of behavior. A light spot (10−4 w/cm2, white light) provided by a fiber optic bundle was projected onto the center of the turntable, illuminating a circular area 15-16 cm in diameter. Following the period of dark adaptation the foam plugs were removed, and the elapsed time to initiate locomotion in the presence of the test light was recorded when an animal moved between an infrared emitter and a phototransistor at the starting end of each glass tube. When the infrared beam was interrupted, a free-running digital clock was automatically turned off and the time recorded for later data analysis. The conditioned groups were compared to pseudorandom controls that had received the same number of CS and US presentations as conditioned animals.
2D gel electrophoresis and protein phosphorylation
Following behavioral testing, modification of protein phosphorylation was examined in lysates of isolated circumesophageal nervous systems from conditioned and pseudorandom control preparations. Proteins in lysate samples were resolved by 2DE using a first-dimension isoelectric focusing (IEF) gel with an immobilized pH gradient (4-7) and a precast SDS polyacrylamide (8-18% linear gradient) second-dimension gel. In the first stage of the experiments protein phosphorylation in conditioned and control preparations were conducted with radiolabeling of proteins. The isolated nervous systems were incubated for 2 hr in 200 μl of oxygenated ASW containing 11 mM glucose and 0.125 mCi of 32PO4 (carrier-free; PerkinElmer Sciences, Waltham, MA). To minimize potential animal-to-animal variability in 32P uptake, nervous systems from five animals were used from conditioned and control procedures in five independent replications (total number of animals = 50). After the 2 hr incubation the samples were rinsed in an isotonic ice-cold wash solution (in mM: 460 NaCl, 10 KCl, 5 EDTA, and 100 Tris-HCl, pH 7.8) and lysed in a modified lysis solution containing 9.2 M urea, 2% Nonidet P-40, 5% β-mercaptoethanol, and 2% carrier ampholytes (1.6% pH 5-8, 0.4% pH 3.5-10), 100 mM NaF, 1 mM sodium orthovandate, 0.1 mM okadiac acid and stored frozen at −80°C. Proteins in the samples were resolved by 2DE as described above. Each precast gel provided the opportunity to run a side-by-side sample from a conditioned group and a pseudorandom control, thus providing paired comparisons of proteins resolved under the same conditions. Gels containing 32P labeled proteins were stained in SYPRO Ruby protein stain for 90 min, destained for 30 min and air dried overnight followed by exposure to storage phosphor screens for 24 hr. Phosphor screens were scanned and analyzed using ImageQuant software (GE Healthcare, Piscataway, NJ) for quantitative analysis of 32P labeling. Densitometric analysis of matched spots from SYPRO Ruby protein stained gels was conducted to determine total protein levels for conditioned groups and pseudorandom controls in order to control for potential differences in protein loading, and provided for normalization of 32P levels of phosphoproteins relative to total protein.
In the second stage of the experiments phosphorylation was examined in conditioned and control samples using a fluorescence-based technique for the detection of phosphoproteins (ProQ Diamond phosphoprotein gel stain, Molecular Probes, Eugene, OR). Proteins separated by 2DE from 10 circumesophageal nervous systems of conditioned and control animals in six independent replications (total number of animals =120) were stained using the phosphoprotein dye. The fluorescent dye staining was used in conjunction with SYPRO Ruby stain or Coomossie blue to provide for visualization from the same gel of phosphoproteins and total protein levels. Gels were fixed in 100 ml fixation solution (50% methanol, 10% acetic acid) for 30 min, followed by a change in fresh fixation solution for overnight fixation. The gels were washed with distilled H2O and incubated in 100 ml Pro-Q Diamond stain in the dark for 90 min, and destained (20% 1,2-propanedial, 50 mM sodium acetate, pH 4.0, three changes of 30 min and the 5 min wash period in distilled H2O). Images of the stained gels were acquired using a Molecular Dynamics Typhoon 9400 imager (GE Healthcare, Piscataway, NJ) with a 532 nm laser excitation and 580 nm bandpass emission filter. Following the scans of phosphoproteins the gels were stained in SYPRO Ruby protein stain for 90 min, destained in 10% methanol 17% acetic acid for 30 min, and 10% glycerol for 20 min. Gels were air dried overnight in the dark and scanned by a STORM 860 imager (GE Healthcare, Piscataway, NJ). For some experiments phosphoprotein stained gels were post-stained with Coomassie blue.
Spot selection from 2D gels
Only readily visible 32P labeled spots that could be easily and reliably identified from all 2D gels were included in the sample. With regards to the MS identification of proteins, protein abundance based upon the matching of the 32P labled spots to corresponding Coomassie blue stained preparative gels was a criterion for selection. Previously analyzed, but unidentified phosphoproteins from studies of one-trial in vitro conditioning were also included in the final sample. The inclusion of all 32P labeled phosphoproteins that could be detected from 2D gels was not attempted.
Following densitometric analysis, proteins of interest were excised by hand from Coomassie blue stained 2D gels or gels treated with the phosphoprotein stain and prepared for MS analysis. In the first experimental stage involving radiolabeling, 32P labeled proteins were matched to multiple Coomassie blue stained preparative gels. Spot matching from multiple gels was carried-out by measurements of the selected spots relative to several abundant evenly spaced proteins used as reference points. For some experiments the reliability of the procedure for localizing spots from multiple Coomassie stained gels was validated by an independent comparison of the results of de novo sequencing from a second group of samples. Overall validation was based upon identical MS results from the analysis of phosphoprotein stained analytical gels and preparative gels matched to 32P labeled samples.
Protein identification by Mass Spectrometry
Protein Digestion
Gel spots were dehydrated in 50% acetonitrile in 0.1M NH4HCO3, dried in a SpeedVac and dissolved in 0.05 M NH4HCO3 containing 10ng/μl modified trypsin (Promega) and digested for 20hr at 37° C. The supernatant was removed and fragments extracted with aqueous 50% acetonitrile, 1% formic acid for 30 min. Samples were then evaporated to ~10 μl, acidified with formic acid to ~pH 3 and desalted on a C18 ZipTip (Millipore). Peptides were eluted from the ZipTip with 3-5μl of an aqueous solution of 50% acetonitrile containing 2% formic acid for analysis by mass spectrometry.
MALDI-TOF and Nanospray MS/MS Analysis of Protein Digests
Protein digests (1μl) were spotted on a MALDI target plate, with matrix (HCCA, alpha-cyano-4-hydroxycinnamic acid), dried and analysis performed in reflector mode on an ABI/SCIEX 4700 Proteomics Analyzer TOF/TOF mass spectrometer with internal calibration using trypsin autodigestion peptides. Dectected monoisotopic peptide masses were analyzed using MS-Fit (Protein Prospector, University of California, San Francisco) or ProFound (PROWL, Rockefeller University) for protein database searches and protein identification by peptide mass fingerprinting (PMF). For most of the samples there were no good matches or identifications by PMF since the Hermissenda genome has not been sequenced. The remainder of the digests were then analyzed on an ABI 4000 Q TRAP, a hybrid triple quadrupole linear ion trap mass spectrometer. Samples were directly introduced into the mass spectrometer via a nano-electrospray capillary tube (Protana, Odense, Denmark). MS/MS fragmentation spectra were collected on selected peptide ions in the positive ion mode and sequences were determined by visual inspection. The NCBInr database was searched using the MS-Pattern search program from Protein Prospector, allowing for several mismatches in the sequence that was entered. A protein was identified as the target protein if it contained at least 2 peptide sequences that were homologous to those determined from the MS/MS peptide sequence that was entered.
RESULTS
Conditioning
The experiments were conducted in two stages. In the first, 25 animals were in the conditioned group and 25 animals were assigned to the pseudorandom control group. Protein phosphorylation after conditioning was assessed by 32P labeling of isolated nervous systems. In the second stage, 60 animals were conditioned and 60 animals were in the pseudorandom control group. Differences in protein phosphorylation between groups was assesses by phosphoprotein staining of 2D gels in the second stage. The conditioned groups received 100 conditioning trials and control animals received 100 pseudorandom presentations of the CS and US. Inhibition of light-evoked locomotion, the conditioned response, was tested 24 hr following conditioning by comparing the post-conditioning start latencies elicited in light to baseline start latencies elicited in light before conditioning. Assessment of the effectiveness of conditioning was determined by computing suppression ratios (A/A+B) that compared post-conditioned behavior (B) with pre-conditioned measurements (A). The mean suppression ratios for the conditioned group and pseudorandom controls were 0.35±0.04 and 0.52±0.03 respectively. The statistical analysis revealed that 100 conditioning trials produced significant inhibition of light-elicited locomotion as compared with the group that received 100 pseudorandom presentations of the CS and US (t168=2.7; p < .005).
Post-conditioning 32P labeling of proteins
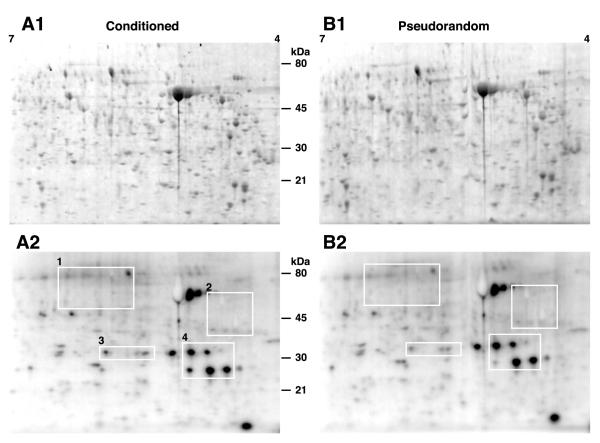
Changes in protein phosphorylation were examined by 2DE separation of proteins in lysates from the nervous systems of conditioned and control animals incubated in 32PO4 after behavioral testing. To minimize animal-to-animal variability in 32P uptake, the circumesophageal nervous systems from five conditioned and five pseudorandom controls were used for each experimental replication. To reduce the effects of gel-to-gel variability, each experimental replication (n=5) consisted of lysates from conditioned and pseudorandom controls run side-by-side on the same 2D gel. Therefore densitometric comparisons were between the conditioned group and the corresponding pseudorandom control on each 2D gel. Densitometric analyses of the 2D gels revealed that multi-trial Pavlovian conditioning resulted in an increase in 32P incorporation into proteins as compared to the lysate samples from pseudorandom controls. Representative images from scans of SYPRO Ruby stained proteins in conditioned and control samples are shown in Fig. 1A1-B1. A sample of twenty phosphoproteins were slelected for analysis from 32P labeled proteins and spots extracted from phophosprotein stained gels based upon the criteria listed in the Methods section. Densitometric analysis of the selected proteins on SYPRO stained 2D gels was conducted to verify approximately equal loading of proteins after the experimental and control procedures, and to provide for normalization of phosphorylation relative to total protein to compensate for potential differences in protein abundance between samples. Figure 1A2-B2 shows representative examples of images from a storage phosphor screen of 2D gels with 32P labeling of proteins from nervous systems of conditioned and pseudorandom control animals from the same gels stained with SYPRO Ruby shown in Fig. 1A1-B1. The phosphoproteins selected for statistical analysis are enclosed by white rectangles shown in Fig. 1A2-B2. Adjacent spot comparisons between examples of phosphoproteins from conditioned and pseudorandom controls are shown in Fig.1. The spots indicated by the arrows in Figure 2 correspond to the different regions of interest shown in Fig 1A2-2B2. The densitometric analysis of the phosphoimaging scans of 32P labeled proteins shown in Fig. 1A2-B2 and Fig. 2 revealed that conditioning resulted in an increase in 32P incorporation in proteins from conditioned samples relative to the samples from pseudorandom controls. The statistical analysis (ANOVA) of the densitometric measurements of sample phosoproteins indicated significant overall differences between the conditioned group and pseudorandom controls (F(1,72)=5.4; p < 0.02). Differences in 32P incorporation in phosphoproteins from both the conditioned group and controls also showed an overall significant difference (F(8,72)=5.5; p < .001). Since each side-by-side 2D gel contained proteins from conditioned preparations and controls separated under the same conditions, experimental/control ratios were computed for the statistical analysis of paired comparisons of individual proteins. For the statistical analysis of the densitometric measures, 32P incorporation into each protein was normalized to the corresponding densitometric analysis of protein levels generated from scans of the SYPRO stained 2D gels. Densitometric analyses also were conducted on the ratios of phosphoprotein dye stained spots from conditioned and pseudorandom control samples (see below). The results of the statistical analysis of the ratios of conditioned to pseudorandom controls for each of the proteins selected for analysis revealed significant differences in 32P incorporation or phosphoprotein staining for 12 of 20 phosphoproteins in the sample (see Table1).
Fig. 1.
Pavlovian conditioning results in an increase in 32P incorporation into phosphoproteins. (A1) Representative print of a scan of SYPRO Ruby labeled proteins separated by 2DE from lysates of nervous systems (n=5) from conditioned animals. (A2) Print from a representative phosphoimaging scan of the same gel showing 32P labeling of proteins from the conditioned group. (B1) Representative print of a scan of SYPRO Ruby labeled proteins separated by 2DE from lysates of nervous systems (n=5) from pseudorandom control animals. (B2) Print from a representative phosphoimaging scan of the same gel showing 32P labeling of proteins from the pseudorandom control groups. The white rectangles numbered 1-4 enclose the areas of interest containing phosphoproteins that were included in the sample subjected to densitometric analysis.
Fig. 2.
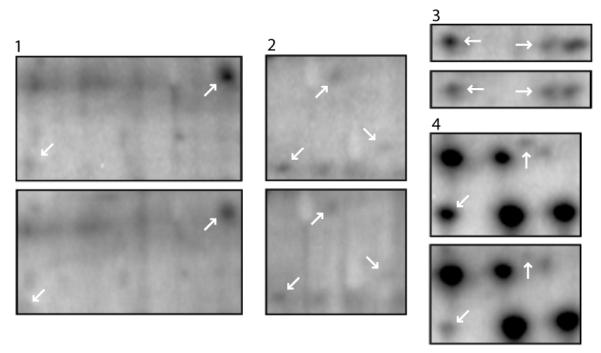
Enlargements of the four areas indicated by the white rectangles in Figure 1. For each pair of rectangles the upper enlargement is from the conditioned group and lower is from the corresponding pseudorandom controls. The white arrows in each upper rectangle indicate proteins exhibiting statistically significant increased 32P incorporation following Pavlovian conditioning as compared to the pseudorandom control groups.
Table1.
Sample of identified phosphoproteins regulated by conditioning
| Name | Mean ratio (Cond./Pseudo.) ± SEM | Computed statistic |
Significance Level |
|||
|---|---|---|---|---|---|---|
| (a) |
(b) |
(a) |
(b) |
(a) |
(b) |
|
| Intermediate Filament Protein A | 1.6 ± .2 | 2.8 ± .6 | t4=3.2 | t5=2.7 | p< .025 | p< .025 |
| Retrograde Protein 51 | 2.04 ± .4 | 2.23 ± .4 | t4=2.4 | t5=3.03 | p< .05 | p< .025 |
| Enolase I | 1.43 ±.16 | 1.31 ± .1 | t4=2.6 | t5=3.02 | p< .05 | p< .025 |
| Actin | ---- | 1.51 ± .2 | ---- | t5= 2.2 | ---- | p< .05 |
| Tubulin Folding Co-factor B | 1.74 ± .2 | 1.6 ± .2 | t4=3.09 | t5=2.6 | p< .025 | p< .025 |
| Tropomyosin (A) | 1.7 ± .3 | 1.34 ± .14 | t4=2.42 | t5=2.34 | p< .05 | p< .05 |
| Tropomyocin (B) | 2.09 ± .4 | 1.62 ± .22 | t4=2.51 | t5=2.75 | p< .05 | p< .025 |
| 20S Proteasome subunit | 1.82 ± .2 | 1.50 ± .21 | t4=3.43 | t5=2.35 | p< .025 | p< .05 |
| Pirin | ---- | 1.59 ± .2 | ---- | t5=2.64 | ---- | p< .025 |
| Ribosomal Protein PO | ---- | 1.33± .1 | ---- | t5=2.34 | ---- | p< .05 |
| Csp24 | 3.92 ± .5 | 5.43 ± .9 | t4=4.8 | t5=4.53 | p< .005 | p< .005 |
| Hypothetical Protein | 2.73 ± .7 | 1.49 ± .17 | t4=2.7 | t5=2.9 | p< .05 | p< .025 |
a) 32P labeling
b) phosphoprotein stain
Phosphoprotein staining
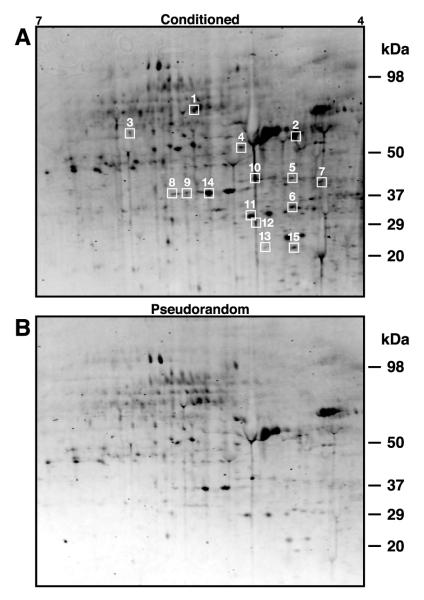
To provide for the identification of phosphoproteins independent of radiolabeling experiments, we examined proteins in lysate samples from conditioned and pseudorandom control circumesophageal nervous systems separated by 2DE, stained with Pro-Q Diamond phosphoprotein fluorescent dye and SYPRO Ruby or Coomassie blue stain, followed by MS analysis. The compatability of the phosprotein stain with MS provided for the opportunity to obtain sequence data from each analytical gel. This provided an independent replication of protein identification obtained initially from the Coomassie stained preparative gels. Six independent replications consisting of nervous systems from 10 conditioned and 10 pseudorandom controls were used in the analysis of phosphoprotein stained gels. Figure 3A shows a representative image from a scan of a 2D gel from conditioned preparations stained with Pro-Q Diamond phosphoprotein dye and Fig. 3B is an example of an image from a scan of a 2-D gel from the corresponding pseudorandom controls stained with the phosphoprotein dye. The phosphoproteins enclosed by the white boxes were selected for MS analysis. Consistent with the results of the radiolabeling experiments, statistically significant changes in the density of phosphoprotein staining of 2D gels from conditioned and control samples were detected following multi-trial Pavlovian conditioning. The statistical analysis (ANOVA) of the densitometric measurements of the spots revealed a significant overall difference between the conditioned group and pseudorandom controls (F(1,150)=8.77;p <.01).
Fig. 3.
Phosphoprotein stained proteins from lysates of conditioned and pseudorandom control circumesophageal nervous systems separated by 2DE. (A) Print of a 2D gel stained with Pro-Q Diamond phosoprotein dye from conditioned nervous systems (n=10). The 15 phosphoproteins enclosed by the white boxes were extracted from the analytical gels for identification by MS analysis. Identified proteins are indicated by the numbers corresponding to the assignments listed in Table 2. (B) Print of a 2D gel from pseudorandom control nervous systems (n=10) stained with phosphoprotein dye.
MS/MS identification of proteins
Proteins in lysate samples from nervous systems of conditioned animals were separated by 2DE, and stained with Coomassie blue or phosphoprotein stain. Spots were excised from preparative and analytical 2D gels followed by tryptic digests, the generation of fragmentation data and de novo peptide sequencing. Since no molluscan genome has been sequenced, protein assignment was based upon database searches for homologous proteins exhibiting conserved peptides. The database homology search engine using the amino acid sequences of peptides derived from MS/MS fragmentation spectra resulted in the assignment of 19 phosphoproteins in the sample not previously examined in conditioned Hermissenda. The amino acid sequences of peptides generated from the MS/MS analysis are shown in Table 2. The spot numbers of identified proteins in Table 2 correspond to the spots enclosed by the numbered white boxes in Fig.3. Five of the 20 MS analyzed spots were not identified, and are listed with their peptide sequences in Table 2 as unknown. Assignment based upon the MS/MS analysis resulted in the classification of proteins with diverse functions and interactions potentially contributing to the establishment of long-term memory. The functions of proteins in the sample involved cell signaling, cytoskeletal changes, transcriptional and translational control, protein degradation, and metabolic changes. In addition to previously identified Csp24, a number of proteins in the sample identified by conserved peptide sequences were cytoskeletal-related. Intermediate filament protein A, retrograde protein 51, actin, tublin folding co-factor B, tropomysin, and Csp24 exhibited significant regulation of phosphoryl in conditioned animals. Interestingly, actin did not exhibit changes in 32P incorporation with conditioning. However phosphorylation regulated by conditioning was detected from phosphoprotein stained gels. Proteins that were visible with the phosphoprotein stain but not observed on the phosphor image may represent phosphorylated proteins that have lower turnovers. Three additional phosphoproteins were identified from the phosphoprotein stained 2D gels that were not initially selected for analysis from Coomassie stained preparative gels. Neurocalcin, pirin, and ribosomal protein PO were identified from the MS/MS analysis of phosphoprotein stained analytical gels. Pirin (t5=2.64; p<.025) and ribosomal protein PO (t5=2.34; p<.05) exhibited statistically significant increased phosphorylation with conditioning. Neurocalcin showed an increase in phosphorylation in conditioned animals (mean=1.52±.4) that was not statistically significant (t5=1.29; NS). Phosphoprotein Csp24 that was originally identified from 32P labeling, also exhibited increased phosphorylation with conditioning using the Pro-Q Diamond stain. These results provide the first evidence for long-term post-translational modification of Csp24 by multi-trial Pavlovian conditioning.
Table2.
Peptide sequences used for protein assignments
| Spot(a) | Name | Accession Number | Peptide Sequences(b) | %ID(c) | % coverage(d) |
|---|---|---|---|---|---|
| 1 | Intermediate Filament Protein A |
CAA42839 | YENELAQAR LVGLQDEISQLR KIDSLGNQIGIOEYEGELHTLR |
84 | 9 |
| 2 | Retrograde Protein 51 | AAT01542 | LAGLQDEIGSLR IDSLGNQLGEF EGELQSLR |
78 | 7 |
| 3 | Enolase I | NP-O11770 | GNPTVEVELTTEK SIVPSGASTG VHEALEMER IGSEVYHNLK |
100 | 13 |
| 4 | Actin | AAS02073 | VAPEEHPVLLTEAPLNPK | 100 | 5 |
| 5 | Tubulin-Folding Cofactor B |
AAI06703 | FEISEDHYDKR TDFKPGYWVGVK |
78 | 9 |
| 6 | Tropomyosin (A) | AAA27817 | IQLLEEDLE TLSIQNDQASQ |
95 | 8 |
| 7 | Tropomyosin (B) | BABO1765 | TTENNFDVANEQLQEANVK LQLLEEDFE LAFTEVDLER |
76 | 14 |
| 8 | 2O S Proteasome α subunit | AAH55520 | HITIFSPSGR AINQGGLTSVAVR |
96 | 9 |
| 9 | Pirin | XP001380970 | NLDPFLVLDEFR TPTNYLDFK |
86 | 7 |
| 10 | Ribosomal Protein PO | ABY87386 | ITALFDEYPK TGVVAPLD VRVPAQ(I/L)TALGPEK |
75 | 12 |
| 11 | Hypothetical Protein | XP001204192 | KSGHI(I/L)NISSDSGR KFFVEGVTQVLR |
73 | 11 |
| 12 | Short-Chain Alcohol Dehydrogenase |
EAA11743 | VAVVTGASSGIGA VTSVSPGAVKT |
75 | 10 |
| 13 | Csp24 | AAN08024 | NPLPTAEISQER TVLPSIDDIGGEKK NSLPPQEAVETEK |
100 | 26 |
| 14 | Hypothetical Protein | EEA40671 | TNIALPAGGFEFR MFYFDVGSNR DI(I/L)GEYAEK |
75 | 14 |
| 15 | Neurocalcin | Q16982 | FAEHVFR LSIEEFIEGAK |
95 | 10 |
| 16 | Unknown | YES(FM)(I/L)NA(I/L)DDHK (FM) VATDVNPAC(FM)(I/L) E |
|||
| 17 | Unknown | (I/L)VE (I/L)DGR (R/K)ESPED(I/L) P(I/L)ESNP (R/K)A[F/oxM]SN[F/oxM](I/L)K (R/K)(I/L) T[F/oxM] DENK |
|||
| 18 | Unknown | VGELLWEK VVT[I/L]P(K/Q) VNAA(D/N) |
|||
| 19 | Unknown | NDVVHD(I/L)PH E(ND)GVNVYY(I/L)EE V(FM)SNAEMN(FM) |
|||
| 20 | Unknown | (R/K)(I/L)N[F/oxM]AE[F/oxN]MAD[F/oxM]YSR (R/K)(I/L)SDE(I/L)END (R/K)(I/L)S[F/oxM]DE[F/oxM]YSA[F/oxM]NK |
Spot# 1-15 correspond to those shown in Figure 3.
Ambiguous residues represented within parentheses. Modifications to specific residues designated by lower case abbreviation.
% ID refers to the degree of identity between published peptide sequences and peptide sequences from Hermissenda
% sequence coverage was calculated by dividing the number of sequenced amino acid residues by the total number of amino acid residues of the protein
DISCUSSION
Pavlovian conditioning in Hermissenda produces modifications in synaptic efficacy and intrinsic excitability in identified neurons in the CS pathway (for a recent review see Crow, 2004). Associated with the electrophysiological and biophysical modifications produced by conditioning are post-translational modifications of proteins in the circumesophageal nervous system and in sensory neurons of the CS pathway (Neary et al., 1981; Crow et al., 1996; Crow and Xue-Bian, 2000; Kuzirian et al., 2001). One identified protein, CE is a GTP- and Ca2+−binding protein that is activated by Ca2+ influx and binds to ryanodine receptors to increase cytosolic Ca2+ (Nelson et al., 1996; Ascoli et al., 1997). CE is phosphorylated by PKC which results in its translocation to membranes. Conditioning increases CE levels in the sensory neurons (type B photoreceptors) and contributes to enhanced excitability by K+-channel inactivation (Kuzirian et al., 2001). A second protein that has been characterized, and contributes to enhanced excitability produced by one-trial conditioning is Csp24. Csp24 is a β-thymosin-like protein that is homologous to members of the family of β-thymosin repeat proteins that contain multiple actin binding domains (Crow and Xue-Bian, 2000; Crow et al., 2003). Actin co-precipitates with Csp24, and is localized with Csp24 in the cytosol of type B photoreceptor cell bodies (Crow and Xue-Bian, 2003). Protein kinase C influenced co-precipitation of Csp24 with actin is regulated by one-trial in vitro conditioning and recombinant Csp24 sequesters G-actin in vitro (Redell et al., 2007). The phosphorylation of Ser-122, but not Ser-49 of Csp24 is produced by conditioning procedures that result in intermediate-term and long-term intrinsic enhanced excitability, but not after procedures that result in short-term changes in excitability of sensory neurons (Crow et al., 1999; Crow and Xue-Bian, 2000, 2007). Moreover, treatment with a Csp antisense oligonucleotide (ON) before one-trial conditioning blocks intermediate-term enhanced excitability, without affecting the induction of short-term enhanced excitability (Crow et al., 2003). One-trial conditioning in isolated type B-photoreceptors produces a depolarized shift in the steady-state activation curve of the A-type transient K+ current (IA) without altering the inactivation curve (Yamoah et al., 2005). The conditioning-dependent reduction in IA is blocked by pre-incubation of isolated B-photoreceptors with Csp antisense ON. We now show that Csp24 phosphorylation is also regulated by multi-trial Pavlovian conditioning, and the increased phosphorylation of Csp24 persists for at least 24 hrs following conditioning and behavioral testing. Taken collectively, studies of post-translational modification of Csp24 suggest that it may be an important link in the regulation of K+ conductances underlying conditioning-dependent intrinsic enhanced excitability.
In addition to a role supporting cellular plasticity, the interaction between the cytoskeleton and β-thymosin, including β-thymosin repeat proteins, has been implicated in structural remodeling underlying development, growth and regeneration (Bouquet et al., 2000; Hertzog et al., 2004; Van Troys et al., 2004; Colby et al., 2005; Hermann et al., 2005; Van Troys et al., 2007). The loss of the function of the β-thymosin repeat protein Cib results in axonal growth defects in the central nervous system of Drosophila (Bouquet et al., 2000). Following nerve axotomy in Aplysia, β-thymosin and β-thymosin mRNA were detected at growth cones and in regenerating axons in vivo (Colby et al., 2005). In Hydra, Pedin, a β-thymosin-like repeat protein contributes to bud outgrowth (Hermann et al., 2005). Tetrathymosinβ identified in Caenorhabditis elegans has four thymosinβ4-like repeats and contains both G- and F-actin interaction sites. The activity of tetrathymosinβ may be of critical importance at specific developmental stages requiring actin polymerization (Van Troys et al., 2004). Recently identified β-thymosinHis and β-thymosinGlu found in hemolymph and whole ganglia releasates of Aplysia support the anchoring of neurons in culture and increase neurite sprouting and total neurite outgrowth in culture (Romanova et al., 2006).
In addition to Csp24, other proteins identified in this study whose phosphorylation is regulated by Pavlovian conditioning have been implicated in growth, regeneration and plasticity. The ubiquitin-proteasome pathway plays a major role in the regulation of long-term synaptic plasticity (for reviews see DiAntonio and Hicke, 2004; Hegde, 2004). Proteasomes are continually assembled and disassembled and their subunits are subjected to a variety of post-translational modifications (Glickman and Raveh, 2005). Proteasome activity in the synaptic terminals is higher compared to activity in the nucleus (Wood et al., 2005; Upadhya et al., 2006) and ubiquitin c-terminal hydroxylase is involved in spatial learning and working memory (Wood et al., 2005). Intermediate filament protein A has been reported to contribute to 5-HT-dependent synaptic facilitation in Aplysia (Homayouni et al., 1997). In Lymnaea, inhibition of the intermediate filament protein RGP51 by RNA interference inhibits regenerative outgrowth of adult neurons in culture (Perlson et al., 2004). In addition, tropomyosin is a major regulator of F-actin functional specialization in migrating cells and is important for regionally defining the properties of the actin cytoskeleton in mediating changes in cell morphology (Gupton et al., 2005). Our results also showed that conditioning produces a significant increase in the phosphorylation of tubulin cofactor B. Previous work has shown that cytoskeletal reorganization is regulated by microtubule dynamics induced by p21-activated kinase 1 (Pak1) phosphorylation of tubulin cofactor B (Vadlamudi et al., 2005).
The post-translational modification of cytoskeletal-related proteins by conditioning may contribute to actin-filament dynamics underlying structural remodeling. The activity of proteins is modulated by intracellular signals, resulting in the recruitment of actin nucleatin and polymerization at specific cellular sites. Structural proteins characterized in this study could play a pivotal role in influencing actin dynamics supporting the development of long-term memory.
Acknowledgements
This research was supported by NIMH grant MH40860 to T.C. We thank Diana Parker and Donna Wood for assistance with the manuscript and L.-M. Tian for technical assistance.
Abbreviations
- ASW
artificial sea water
- CS
conditioned stimulus
- US
unconditioned stimulus
- 2DE
two-dimensional electrophoresis
- MS
mass spectrometry
- IEF
isoelectric focusing
- SDS
sodium dodecyl sulfate
- Csp24
24kDa conditioned stimulus pathway phosphoprotein (accession number AAN08024)
- CE
calexcitin
- IA
A-type transient K+ current
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn NG, Weiel JE, Chan CP, Krebs EG. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990;26:11487–11494. [PubMed] [Google Scholar]
- Alkon DL, Nelson TJ. Specificity of molecular changes in neurons involved in memory storage. FASEB J. 1990;4:156–1576. doi: 10.1096/fasebj.4.6.2108074. [DOI] [PubMed] [Google Scholar]
- Ascoli G, Liu KX, Olds JL, Nelson TJ, Gusov PA, Bertucci C, Bramanti E, Raffaelli A, Salvador P, Alkon DL. Secondary structure of Ca2+-induced conformational change of calexcitin, a learning associated protein. J Biol Chem. 1997;272:29771–29779. doi: 10.1074/jbc.272.40.24771. [DOI] [PubMed] [Google Scholar]
- Baratier J, Peris L, Brocard J, Gory-Fauré S, Dufour F, Bose C, Fourest-Lieuvin A, Blanchoin L, Salin P, Job D, Andrieux A. Phosphorylation of microtubule-associated protein STOP by calmodulin kinase II. J Biol Chem. 2006;281:19561–19569. doi: 10.1074/jbc.M509602200. [DOI] [PubMed] [Google Scholar]
- Bouquet I, Boujemaa R, Cartier M-F, Préat T. Ciboulot regulates actin assembly during Drosophila brain metamorphosis. Cell. 2000;102:797–808. doi: 10.1016/s0092-8674(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Colby GP, Sung Y-J, Ambron RT. mRNAs encoding the Aplysia homologues of Fasciclin-I and β-thymosin are expressed only in the second phase of nerve injury and are differentially segregated in axons regenerating in vitro and in vivo. J Neurosci Res. 2005;82:484–498. doi: 10.1002/jnr.20645. [DOI] [PubMed] [Google Scholar]
- Crow T. Pavlovian conditioning of Hermissenda: current cellular, molecular, and circuit perspectives. Learn Mem. 2004;11:229–238. doi: 10.1101/lm.70704. [DOI] [PubMed] [Google Scholar]
- Crow T. Conditioned modification of phototactic behavior in Hermissenda. I. Analysis of light intensity. J Neurosci. 1985;5:209–214. doi: 10.1523/JNEUROSCI.05-01-00209.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Alkon DL. Retention of an associative behavioral change in Hermissenda. Science. 1978;201:1239–1241. doi: 10.1126/science.694512. [DOI] [PubMed] [Google Scholar]
- Crow T, Offenbach N. Modification of the initiation of locomotion in Hermissenda. Brain Res. 1983;281:301–310. doi: 10.1016/0006-8993(83)90292-5. [DOI] [PubMed] [Google Scholar]
- Crow T, Siddiqi V, Zhu Q, Neary JT. Time-dependent increase in protein phosphorylation following one-trial enhancement in Hermissenda. J Neurochem. 1996;66:1736–1741. doi: 10.1046/j.1471-4159.1996.66041736.x. [DOI] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ, Siddiqi V. Protein synthesis-dependent and mRNA synthesis-independent intermediate phase of memory in Hermissenda. J Neurophysiol. 1999;82:495–500. doi: 10.1152/jn.1999.82.1.495. [DOI] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ. Identification of a 24 kDa phosphoprotein associated with an intermediate stage of memory in Hermissenda. J Neurosci. 2000;20:RC74, 1–5. doi: 10.1523/JNEUROSCI.20-10-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ. One-trial in vitro conditioning regulates a cytoskeletal-related protein (Csp24) in the conditioned stimulus pathway of Hermissenda. J Neurosci. 2003;22:10514–10518. doi: 10.1523/JNEUROSCI.22-24-10514.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Redell JB, Tian LM, Xue-Bian JJ, Dash PK. Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the development of intermediate-term memory in Hermissenda. J Neurosci. 2003;23:415–3422. doi: 10.1523/JNEUROSCI.23-08-03415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Xue-Bian JJ. One-trial in vitro conditioning of Hermissenda regulates phosphorylation of Ser-122 of Csp24. Ann NY Acad Sci. 2007;1112:189–200. doi: 10.1196/annals.1415.012. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Hicke L. Ubiquitin-dependent regulation of the synapse. Annu Rev Neurosci. 2004;27:223–246. doi: 10.1146/annurev.neuro.27.070203.144317. [DOI] [PubMed] [Google Scholar]
- Donaldson RW, Hagedorn CH, Cohen S. Epidermal growth factor or okadaic acid stimulates phosphorylation of eukaryotic initiation factor 4F. J Biol Chem. 1991;266:3162–3166. [PubMed] [Google Scholar]
- Glickman MH, Raveh D. Proteasome plasticity. FEBS Letts. 2005;579:3214–3223. doi: 10.1016/j.febslet.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Anderson KL, Kole TP, Fischer RS, Ponti A, Hitchcock-DeGregori SE, Danuser G, Fouler VN, Wirtz D, Hanein D, Waterman-Storer CM. Cell migration without a lamellipodium: translation of actin dynamics into cell movements mediated by tropomyosin. J Cell Biol. 2005;158:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Alberts A, Brindle P, Meinkoth J, Feramisco J, Deng T, Karin M, Shenohikar S, Montminy N. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992;70:105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Hegde AN. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Progr Neurobiol. 2004;73:311–357. doi: 10.1016/j.pneurobio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Hermann D, Hatta M, Hoffmeister SAH. Thypedin, the multi copy precursor for the hydra peptide pedin, is a β-thymosin repeat-like domain containing protein. Mech Develop. 2005;122:1183–1193. doi: 10.1016/j.mod.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Hertzog M, Heijenoort CV, Didtry D, Gaudier M, Coutant J, Gigant B, Didelot G, Preat T, Knossow M, Guittet E, Carlier M-F. The β-thymosin/WH2 domain: structural basis for the switch from inhibition to promotion of actin assembly. Cell. 2004;117:611–623. doi: 10.1016/s0092-8674(04)00403-9. [DOI] [PubMed] [Google Scholar]
- Homayouni R, Byrne JH, Eskin A. Dynamics of protein phosphorylation in sensory neurons in Aplysia. J Neurosci. 1995;15:429–438. doi: 10.1523/JNEUROSCI.15-01-00429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayouni R, Nunez-Regueiro M, Byrne JH, Eskin A. Identification of two phosphoproteins affected by serotonin in Aplysia sensory neurons. Brain Res. 1997;750:87–94. doi: 10.1016/s0006-8993(96)01335-2. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the Yin and Yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Vaillancourt RR. Sequential protein kinase reactions controlling cell growth and differentiation. Cell. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- Johnston LN, O’Reilly M. Control by phosphorylation. Curr Opin Struct Biol. 1996;6:762–769. doi: 10.1016/s0959-440x(96)80005-4. [DOI] [PubMed] [Google Scholar]
- Karin M, Hunter T. Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus. Curr Biol. 1995;5:747–757. doi: 10.1016/s0960-9822(95)00151-5. [DOI] [PubMed] [Google Scholar]
- Kondo T, Kakegawa W, Yuzaki M. Induction of long-term depression and phosphorylation of the delta2 glutamate receptor by protein kinase C in cerebellar slices. Eur J Neurosci. 2005;22:1817–1820. doi: 10.1111/j.1460-9568.2005.04319.x. [DOI] [PubMed] [Google Scholar]
- Kuzirian AM, Epstein HT, Buck D, Child FM, Nelson T, Alkon DL. Pavlovian conditioning-specific increases of the Ca2+- and GTP-binding protein, calexcitin in identified Hermissenda visual cells. Neurocytol. 2001;12:993–1008. doi: 10.1023/a:1021836723609. [DOI] [PubMed] [Google Scholar]
- Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nature Biotechnol. 2003;21:255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Anderson DL. Phosphorylation of the insulin receptor in permeabilized adipocytes is coupled to a rapid dephosphorylation reaction. J Biol Chem. 1989;264:6850–6857. [PubMed] [Google Scholar]
- Moreland S, Nishimura J, Breemen C, Ahn HY, Moreland RS. Transient myosin phosphorylation at constant Ca2+ during agonist activation of permeabilized arteries. Am J Physiol. 1992;263:C540–C544. doi: 10.1152/ajpcell.1992.263.2.C540. [DOI] [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molner E, Collinridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Crow T, Alkon DL. Change in a specific phosphoprotein band following associative learning in Hermissenda. Nature. 1981;293:658–660. doi: 10.1038/293658a0. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Cavallaro S, Yi CL, McPhie D, Schreurs BG, Gusev PA, Favit A, Zohar O, Jim J, Beushausen S. Calexcitin: a signaling protein that binds calcium and GTP, inhibits potassium channels, and enhances membrane excitability. Proc Natl Acad Sci USA. 1996;93:13808–13813. doi: 10.1073/pnas.93.24.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E, Modzihradszky KF, Darula Z, Munno DW, Syed NI, Burcingame AL, Fainzilber M. Differential proteomics revals multiple components in retrogradely transported axoplasm after nerve injury. Molec Cell Proteom. 2004;3:510–520. doi: 10.1074/mcp.M400004-MCP200. [DOI] [PubMed] [Google Scholar]
- Redell JB, Xue-Bian JJ, Bubb MR, Crow T. One-trial in vitro conditioning regulates an association between the β-thymosin repeat protein Csp24 and actin. Neurosci. 2007;148:413–420. doi: 10.1016/j.neuroscience.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Romanova EV, Roth MJ, Rubakhin SS, Jukubowski JA, Kelley WP, Kirk MD, Kelleher NL, Sweedler JV. Identification and characterization of homologues of vertebrate β-thymosin in the marine mollusk Aplysia californica. J Mass Spectrom. 2006;41:1030–1040. doi: 10.1002/jms.1060. [DOI] [PubMed] [Google Scholar]
- Sato C, Nishizawa K, Nakayama T, Ohtsuka K, Nakamura H, Kobayashi T, Inagaai M. Rapid phosphorylation of MAP-2-related cytoplasmic and nuclear Mr 300,000 protein by serine kinases after growth stimulation in quiescent cells. Exp Cell Res. 1999;175:136–147. doi: 10.1016/0014-4827(88)90261-3. [DOI] [PubMed] [Google Scholar]
- Sun J, Bronk P, Liu X, Han W, Sudhof TC. Synapsins regulate use-dependent synaptic plasticity in the calyx of Held by a Ca2+/calmodulin-dependent pathway. Proc Natl Acad Sci USA. 2006;103:2880–2885. doi: 10.1073/pnas.0511300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer B, Diao W, Lubec G. The role of post-translational modifications for learning and memory formation. Electrophoresis. 2008;29:2593–2602. doi: 10.1002/elps.200700791. [DOI] [PubMed] [Google Scholar]
- Sweatt JD, Kandel ER. Persistent and transcriptionally-dependent increase in protein phosphorylation in long-term facilitation of Aplysia sensory neurons. Nature. 1989;330:51–54. doi: 10.1038/339051a0. [DOI] [PubMed] [Google Scholar]
- Upadhya SC, Ding L, Smith TK, Hegde AN. Differential regulation of proteasome activity in the nucleus and the synaptic terminals. Neurochem Intl. 2006;48:296–305. doi: 10.1016/j.neuint.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RK, Barnes CJ, Rayala S, Li F, Balasenthil S, Marcus S, Goodson HV, Sahin AA, Kumar R. p21-activated kinase 1 regulates microtubule dynamics by phosphorylating tublin cofactor B. Molec Cell Biol. 2005;25:3726–3736. doi: 10.1128/MCB.25.9.3726-3736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Ono K, Dewitte D, Jonckheere V, De Ruyck N, Vandekerckhove J, Ono S, Ampe C. Tetrathymosinβ is required for actin dynamics in Caenorhabditis elegans and acts via functionally different actin-binding repeats. Molec Biol Cell. 2004;5:735–4748. doi: 10.1091/mbc.E04-03-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Dhaese S, Vanderkerckhove J, Ampe C. Multirepeat β-thymosins. In: Lappalainer P, editor. Actin-Monomer-Binding Proteins. Springer; 2007. [Google Scholar]
- Wahl MI, Olashaw NE, Nishibe S, Rhee SG, Pledger WJ, Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-γ in quiescent BALB/c 3T3 cells. Mol. Cell Biol. 1989;9:2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Arora A, Yang L, Parelkar NK, Zhang G, Liu X, Choe FS, Mao L. Phosphorylation of AMPA receptors: mechanisms and synaptic plasticity. Mol Neurobiol. 2005;32:237–249. doi: 10.1385/MN:32:3:237. [DOI] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Brensinger CM, Guo W, Abel T. Ubiquitin C-terminal hydroxylase L3 (Uchl3) is involved in working memory. Hippocampus. 2005;15:610–621. doi: 10.1002/hipo.20082. [DOI] [PubMed] [Google Scholar]
- Yamoah EN, Levic S, Redell JR, Crow T. Inhibition of conditioned stimulus pathway phosphoprotein 24 expression blocks the reduction in A-type transient K+ current produced by one-trial in vitro conditioning of Hermissenda. J Neurosci. 2005;25:4793–4800. doi: 10.1523/JNEUROSCI.5256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]