Summary
Trace metals such as iron, copper, zinc, manganese, and cobalt are essential cofactors for many cellular enzymes. Extensive research on iron, the most abundant transition metal in biology, has contributed to an increased understanding of the molecular machinery involved in maintaining its homeostasis in mammalian peripheral tissues. However, the cellular and intercellular iron transport mechanisms in the central nervous system (CNS) are still poorly understood. Accumulating evidence suggests that impaired iron metabolism is an initial cause of neurodegeneration, and several common genetic and sporadic neurodegenerative disorders have been proposed to be associated with dysregulated CNS iron homeostasis. This review aims to provide a summary of the molecular mechanisms of brain iron transport. Our discussion is focused on iron transport across endothelial cells of the blood-brain barrier and within the neuro- and glial-vascular units of the brain, with the aim of revealing novel therapeutic targets for neurodegenerative and CNS disorders.
Keywords: Blood-brain barrier (BBB); Reactive-Oxygen Species (ROS); ferritin (Ft); transient receptor potential mucolipin 1 (TRPML1); transferrin (Tf); non-transferrin-bound iron (NTBI); divalent metal transporter-1 (DMT1, Slc11a2); ferroportin (Fpn); early endosome (EE); brain vascular endothelial cell (BVEC)
The Axis of Brain Iron, Oxidative Stress, and Neurodegeneration
Iron is likely an integral part of metabolism because it can gain (ferric to ferrous, or Fe3+ to Fe2+) or lose (Fe2+ to Fe3+) electrons relatively easily. Interestingly, iron has a functional split personality in the nervous system where it is essential for life yet toxic if levels are perturbed. At the cellular level, iron is required for the cell growth, however, excessive iron (iron overload) causes oxidative stress and cell death. Perhaps not surprisingly, iron levels are tightly regulated in a process referred to as iron homeostasis. The principal protective strategy to avoid iron overload in the brain is the blood-brain barrier (BBB), which limits iron entry to the brain from the blood via highly regulated, selective transport systems [1–3]. Within the brain, multiple feedback loops form an elaborate control system for cellular iron levels to ensure that a precisely balanced iron level exists for normal function of the nervous system [4, 5].
Iron is critical for a host of basic cellular processes such as mitochondrial ATP generation and DNA replication [6]. Iron deficiency in the brain likely affects normal cell division of brain cells such as neuronal precursor cells, astrocytes, and oligodendrocytes [5, 7]. In addition, iron is required for several neuronal specific functions such as dopaminergic neurotransmitter synthesis [5, 8] and myelination of axons [6]. Not surprisingly, iron deficiency in early development leads to neurological abnormalities [9], contributing to the so-called “early anemia, later mental retardation” effect [10].
Iron accumulation in the adult brain is known to cause neurodegeneration [5, 7, 8]. Owing to iron’s ability to donate electrons to oxygen, increased iron levels can lead to the formation of hydroxyl radicals and hydroxyl anions via the Fenton Reaction (Fe2+ + H2O2 → Fe3+ + OH· + OH−) (Fig. 1). Increased iron levels can also generate peroxyl/alkoxyl radicals due to Fe2+-dependent lipid peroxidation [11]. These reactive-oxygen species (ROS) can damage cellular macromolecules including proteins, lipids, and DNA. Under normal conditions, several detoxification systems and antioxidant defense mechanisms exist to prevent this damage; for example, catalase and glutathione peroxidase quickly convert H2O2 to water, helping to ensure that ROS-induced damage is minimal in the brain [12]. However, when the formation of ROS exceeds the cells’ detoxification/antioxidant systems, the cell experiences oxidative stress. Iron-induced oxidative stress is particularly dangerous because it can cause further iron release from iron-containing proteins such as ferritin (Ft), heme proteins, and iron-sulfur (Fe-S) clusters, forming a destructive intracellular positive-feedback loop that exacerbates the toxic effects of brain iron overload [13].
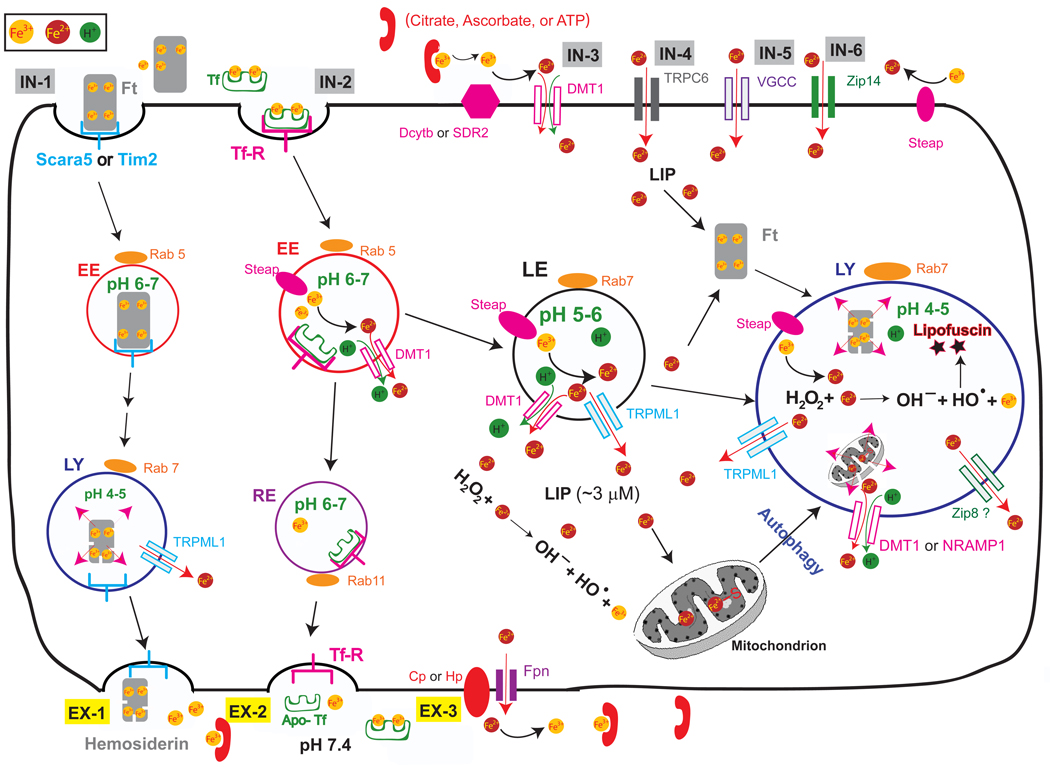
Figure 1. Molecular mechanisms of intracellular iron transport.
Most cellular uptake of ferric iron (Fe3+) occurs via receptor-mediated endocytosis of transferrin (Tf) (vesicular import pathway IN2). Fe3+ ions form complexes with the high affinity iron binding protein, Tf, which then binds to specific membrane-bound Tf receptor (TfR). After endocytosis, the acidic environment of the early endosomes (EE; compartment specificity defined by a small GTPase Rab5) triggers the release of Fe3+ from the Tf-TfR complex, which is recycled to the plasma membrane via Rab11-positive recycling endosomes (RE; vesicular export pathway EX2). Members of the Steap family of ferric reductases localize to the endosome and reduce Fe3+ (ferric) to its Fe2+ (ferrous) form before Fe2+ is released into the cytosol by the divalent metal transporter-1 (DMT1) in an H+-dependent manner. Fe3+ may also be sorted into Rab7-positive late endosomes (LE) and lysosomes (LY), where it is reduced into Fe2+ by Steap proteins in LE or LY. In LE and LY, Fe2+ can also be released by other endolysosomal iron release channels/transporters such as TRPML1, Nramp1 (in macrophages), or Zip8. Other sources of lysosomal Fe3+ include Fe3+-laden ferritin (Ft) complexes and autophagic ingestion of damaged organelles such as mitochondria. Other mechanisms for receptor-mediated iron uptake exist in oligodendrocytes and other cells, where Fe3+ ions bind the Ft receptors (Scara5 or Tim-2) and undergo receptor-mediated endocytosis (vesicular iron import pathway IN1). This pathway depends on lysosomal degradation of Ft-Fe complexes before iron is released into the cytosol. Lysosomal Fe3+ or Ft-Fe complexes may also be exported out of the cells via lysosomal exocytosis (vesicular export pathway EX1). In some cell types, uptake of non-Tf bound iron (NTBI) in its Fe2+ form can be mediated by plasma membrane localized iron importers such as DMT1 (non-vesicular import pathway IN3), TRPC6 (IN4), voltage-gated Ca2+ channels (VGCC; IN5), and Zip14 (Slc39a14; IN6). Free Fe2+ in the cytosol constitutes a “labile iron” pool (LIP; ~ 3 µM) for cellular utilization. If not immediately used, it can also be rapidly sequestered by cytosolic Ft into a non-reactive state. Finally, iron can be released from cells by the iron exporter ferroportin (Fpn). Cellular export of Fe2+ usually is coupled to a membrane bound or cytosolic ferroxidase such as Hephaestin (Hp) or Ceruloplasmin (Cp) that oxidizes reactive Fe2+ to its less reactive Fe3+ form before diffusing throughout the extracellular space. Fe3+ can be bound in the extracellular space by Tf, citrate, ascorbate, or ATP. Cytosolic or intralysosomal iron overload may catalyze the production of free radical oxides via the Fenton reaction. Radical oxides may cause cellular damage by oxidizing macromoleucles such as lipids, DNA, and proteins.
A cardinal pathology associated with several common sporadic neurodegenerative disorders, such as Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease, is iron accumulation in the brain coincident with sites of extensive neuronal cell death [5, 7, 8, 14–17]. Normally, iron-rich regions in the brain such as the globus pallidus, red nucleus, substantia nigra (SN), putamen and caudate nucleus seem to be most susceptible to neurodegenerative processes [14]. During the typical aging process, iron accumulation also occurs in multiple regions of brain_known to be more vulnerable to age-dependent neurodegeneration [8, 9]. For example, iron invasion with age predominates in motor areas of the brain such as the basal ganglia, which may explain the motor deterioration commonly exhibited in many neurodegenerative diseases.
The question remains whether iron invasion and subsequent radical formation is a primary or secondary event in neurodegenerative diseases. Recent evidence suggests that iron invasion is an initial cause of neuronal cell death and axonal degeneration [5, 8, 18]. Several inherited disorders of iron metabolism in humans are known to display neurodegeneration with brain iron accumulation (NBIA) [14]. This group of genetically heterogeneous progressive disorders includes NBIA type I (formerly Hallervorden-Spatz syndrome), neuroferritinopathy, infantile neuroaxonal dystrophy-1 (INAD1), and aceruloplasminemia (Table 1). Detailed studies of these disorders suggest that an iron gradient formed in brain tissues is causal for triggering neuronal toxicity [19–22]. Other CNS disorders that have been proposed to have an iron-dependent component include Friederich’s Ataxia [17], Restless Leg Syndrome [23], Ischemic/Hemorrhagic Stroke [24], and Multiple Sclerosis [16]. Dong et al. recently demonstrated that transient receptor potential mucolipin 1 (TRPML1), the gene that is mutated in mucolipidosis type IV (ML4) neurodegenerative disease [25], encodes an iron release channel found in the late endosome and lysosome (LEL) [26]. Furthermore, several studies of iron regulatory proteins using transgenic rodents [27, 28] suggest that iron may be a pathogenic factor for neurodegeneration. Finally, iron chelators [8, 29, 30] and genetic manipulation resulting in low iron levels [29, 31, 32] are found be to neuroprotective.
Table 1.
Iron-related genes involved in neurodegeneration
| Gene | Function (s) | Involvement in neurodegeneration | Human Disorder(s) |
|---|---|---|---|
| PANK2; Pantothenate kinase-2 [19] | Mitochondrial protein essential for Coenzyme A synthesis and fatty acid metabolism [19] | Pank2−/−mice show retinal degeneration [96]. | *NBIA Type I; PKAN, Hallervorden-Spatz Syndrome; MIM #243200 |
| Human patients show neurodegeneration with brain iron accumulation in the basal ganglia. | |||
| FLT1; L-Ferritin (ferritin light chain) [22] | Iron storage; lacks ferroxidase activity of H-ferritin [13] | Patients show low serum ferritin levels, but normal iron, hemoglobin, and transferrin levels. | *Neuroferritinopathy; hereditary ferritinopathy; MIM |
| Dense ferritin-Fe spheroid inclusions throughout forebrain and cerebellum; neurons, oligodendrocytes, and microglia. [22] | 606159 | ||
| PLA2G6; iPLA2; Ca2+-independent group VI phospholipase A2 [20] | Arachidonic acid release [97] | Phospolipase A2 is important for myelin breakdown and phagocytosis during axonal degeneration [98]. | *NBIA type II;MIM #610217 *Infantile Neuroaxonal Dystrophy-1; INAD1; MIM #256600 *Karak Syndrome; MIM 608395 |
| Cp; Ceruloplasmin [21] | Cu-dependent ferroxidase converting Fe2+ into Fe3+ [21] | Cp−/−mice exhibit brain iron accumulation in astrocytes. | Aceruloplasminemia (aCp); MIM #604290 |
| Slc11a2; DMT1 | H+-dependent Fe2+ transporter | Upregulated in models of Parkinson’s | None described |
| MCOLN1; mucolipin-1; TRPML1 | Ca2+/Fe2+ release channel in LEL | Loss-of-function mutations cause decreased cytosolic iron levels and intralysosomal iron accumulation; iron deficiency anemia; psychomotor retardation | Mucolipidosis type-IV (ML4); MIM #252650 |
| IRP2; Iron-regulatory protein-2; IRP2 [28] | Cytosolic iron sensor; Post-transcriptional regulator of iron-management protein expression | IRP2−/−mice exhibit ataxia, bradykinesia, and tremor; brain iron accumulation precedes neurodegeneration | None described |
Although we focus on iron toxicity in this review, it is worth mentioning that other transition metals have also been implicated in neurodegenerative disorders [33]. For example, copper accumulation has also been implicated in Alzheimer’s [33, 34]. As Cu+ is also able to catalyze the Fenton reaction, copper accumulation may also facilitate radical formation during oxidative stress [33]. Under different pathological conditions, iron and other transition metals accumulate in different brain regions at different levels [33, 34]. Therefore, it is difficult to isolate iron-specific toxicity. Furthermore, there is a crosstalk between iron and copper toxicity, as ceruloplasmin (Cp), the major ferroxidase, functions in a Cu-dependent manner [35]. Nevertheless, the abundance of iron suggests a major role of iron toxicity in neurodegeneration.
Cellular Iron Transport in the Peripheral Tissues
In normal human plasma, serum iron (~ 20 µM) exists primarily in the Fe3+ form and is complexed with the high affinity iron binding protein transferrin (Tf; ~ 40 µM) in a 2:1 ratio (Tf-Fe2; Fig. 1). Hence, Tf is only partially (~ 25%) saturated. Under iron overload conditions, however, Tf is saturated and non-transferrin-bound iron (NTBI) can reach 1–20 µM [36]. In the cerebrospinal fluid (CSF), in which the Tf concentration is low (< 0.5 µM), NTBI can reach a sub-micromolar concentration (<1 µM) [37, 38]. Depending on the microenvironment and availability of ferric reductases, NTBI can exist in both Fe3+ and Fe2+ forms. Inside the cell, free iron in its reduced form (Fe2+) constitutes the “labile iron” pool (~ 2–3 µM) [39], which supplies Fe2+ molecules as co-factors for many Fe2+-dependent enzymes in the cytosol, mitochondria, and nucleus. If cytosolic iron is not needed for immediate use, it is usually stored in Fe3+- Ft complexes [4].
Non-heme iron can enter mammalian cells via two distinct mechanisms: vesicular and non-vesicular iron import. Fe3+ forms a complex with Fe3+-binding proteins such as Tf to enter the cell through the receptor-mediated endocytic pathway (vesicular iron import pathways IN1 and IN2; Fig. 1). Fe2+, on the other hand, may influx into the cell through transmembrane channels or secondary transporters (non-vesicular iron import pathways IN3, IN4, and IN5; Fig. 1). Similarly, iron can leave the cell through mechanisms of vesicular and non-vesicular transport. Both Fe3+ and Fe2+ inside intracellular vesicles can exit the cell via exocytosis of recycling endosomes or lysosomes (vesicular iron export pathway EX2 and EX1; Fig. 1). Fe2+ may also efflux from the cell via transmembrane channels or transporters (non-vesicular iron export pathway EX3; Fig. 1). Most mammalian cells transport iron using a combination of these iron import and export pathways.
Iron transport in enterocytes
Systemic iron levels are principally regulated at the level of absorption by the small intestine [40, 41]. Intestinal iron absorption involves transcellular transport mediated by enterocytes—polarized epithelial cells that line the lumen of the intestines. Iron first crosses the apical brush border membrane, traverses the cell, and then exits across the basolateral membrane to enter the blood plasma [4, 42, 43]. Dietary iron, existing primarily in the insoluble Fe3+ form, is first reduced to Fe2+ by duodenal cytochrome B (DcytB, also called CYBRD1), a ferrireductase present on the apical membranes of enterocytes [44]. Fe2+ then enters the enterocytes by divalent metal transporter-1 (DMT1, Slc11a2), an H+-dependent Fe2+ transporter (non-vesicular iron import pathway IN3; Fig. 1) expressed on the apical membrane [45]. Once inside, Fe2+ can leave the enterocyte through ferroportin (Fpn, Ireg1, MTP1), an Fe2+ channel/transporter (non-vesicular iron export pathway EX3; Fig. 1) expressed on the basolateral membrane [46]. Released Fe2+ is quickly oxidized into the bio-unavailable Fe3+ form via a membrane bound or cytosolic ferroxidase such as Hephaestin (Hp) [47] or Ceruloplasmin (Cp) [21]. In order to be available for use, Fe3+ enters the plasma by forming complexes with high affinity iron-binding proteins such as Tf, or with low affinity iron binding molecules such as citrate, ascorbate, or ATP (Fig. 1). The inability to maintain sufficient iron levels in the plasma is defined as iron deficiency, often associated with anemia, while excessive iron absorption is termed iron overload.
Iron transport in erythroid precursor cells
In most mammalian cells, especially erythroid precursor cells, iron is sequestered in a non-reactive (Fe3+) state by Tf in the blood plasma, forming a Tf-Fe2 complex (Fig. 1). The Fe3+-binding affinity for Tf is extremely high (Kd ~ 10−23 M). Since Tf is abundant (~ 40 µM) and only partially (~ 25%) saturated in the plasma, Tf-Fe2 is the predominant form of iron present in the extracellular space surrounding erythroid precursor cells. In this classical iron uptake mechanism (see Fig. 1), the Tf-Fe2 complex is taken up by transferrin receptor 1 (TfR1 on erythroid precursor cells via receptor-mediated endocytosis into clathrin-coated vesicles (vesicular iron import pathway IN2; Fig.1) [40, 41]. The acidic environment of Rab5-positive early endosomes (EE) triggers the release of Fe3+ from the Tf-TfR complex, which is sorted into Rab11-positive recycling endosomes (RE) before trafficking to the cell membrane and subsequent exocytosis. In erythroid precursor cells, specifically, the endosomal ferric reductase six-transmembrane epithelial antigen of the prostate 3 (Steap3) reduces Fe3+ to Fe2+ [48], which is then released into the cytosol by DMT1 in a H+-dependent manner [45]. Ferrous iron in the cytosol comprises the “labile iron” pool and represents iron that is directly available for cellular utilization. The majority of cytosolic Fe2+, however, is transported into mitochondria by the iron importer Mfrn [49] for the synthesis of iron-sulfur (Fe-S) clusters and biogenesis of heme. If not immediately used, cytosolic Fe2+ can also be stored in ferritin-Fe3+ complexes, cytoplasmic iron storage proteins [13]. These complexes are eventually degraded in the lysosome, releasing reactive Fe2+ [50] via DMT1. In addition to utilization and storage, iron can also be released from the cell by the iron exporter Fpn (non-vesicular iron export pathway EX3; Fig. 1) [46].
Iron transport in most mammalian cells
Most other mammalian cell types recruit the cellular iron transport machinery that is used by enterocytes and erythroid precursor cells. This includes using the Tf-TfR system for iron import and Fpn for iron export. The intracellular iron transport machinery, however, may differ significantly. As DMT1 is specifically expressed on the endosomes of erythroid precursor cells, but not all cells [45], genetic evidence suggests that a DMT1-independent iron release mechanism must exist in the endolysosomes of most cells [51]. Dong et al. recently characterized a novel pathway for iron release from the LEL, mediated by the transient receptor potential cation channel mucolipin1 (TRPML1) [26]. Mutations in TRPML1 cause the neurodegenerative disease ML4 (Table 1), of which some patients exhibit iron-deficiency anemia. TRPML1 is ubiquitously expressed and functions as a conduit for endolysosomal iron release [26]. The electrophysiological properties of TRPML1 are somewhat similar to those of DMT1. Both iron-carrying currents are inwardly rectifying (cations flowing out of the endolysosome), which is consistent with a role in iron release from the vesicular lumen. Like DMT1, TRPML1 conducts Fe2+, but not Fe3+. Hence, a putative ferric reductase, presumably one of the Steap proteins [48, 52], is required for TRPML1-mediated Fe2+ release from LEL. Like DMT1, low pH (the environment of endolysosomes) potentiates Fe2+ permeation of TRPML1. Although the function of TRPML1 is similar to DMT1, its localization in specific endocytic compartments is distinct from that of DMT1. While DMT1 can be detected in lysosomes, the quasi steady-state location for DMT1 is early or late endosome [4, 53]. On the other hand, TRPML1 is primarily localized in LEL. Therefore, TRPML1 is localized in overlapping but later endocytic compartments compared to DMT1. Consistent with a role of TRPML1 in endolysosomal iron release, TRPML1-deficient fibroblast cells exhibit lysosomal iron overload as well as cytosolic iron deficiency [26], suggesting defective iron transport across LEL membranes (Fig. 2). However, as TRPML1 is also permeable to Ca2+, it is unlikely that TRPML1 is fully dedicated to intracellular iron metabolism. Other cell-type specific endolysosomal iron release proteins may exist. For example, Nramp1 (Scl11a1), a DMT1-related proton-dependent iron transporter, is expressed in the lysosomes of macrophages [54]. Similarly, TRPML2, a homologue of TRPML1 and also a potential iron release channel in LEL [26], is expressed in macrophages and B cells [55]. Additionally, ZIP8 (Slc39a8), previously known as a zinc transporter, has also recently been implicated in endolysosomal iron release [56].
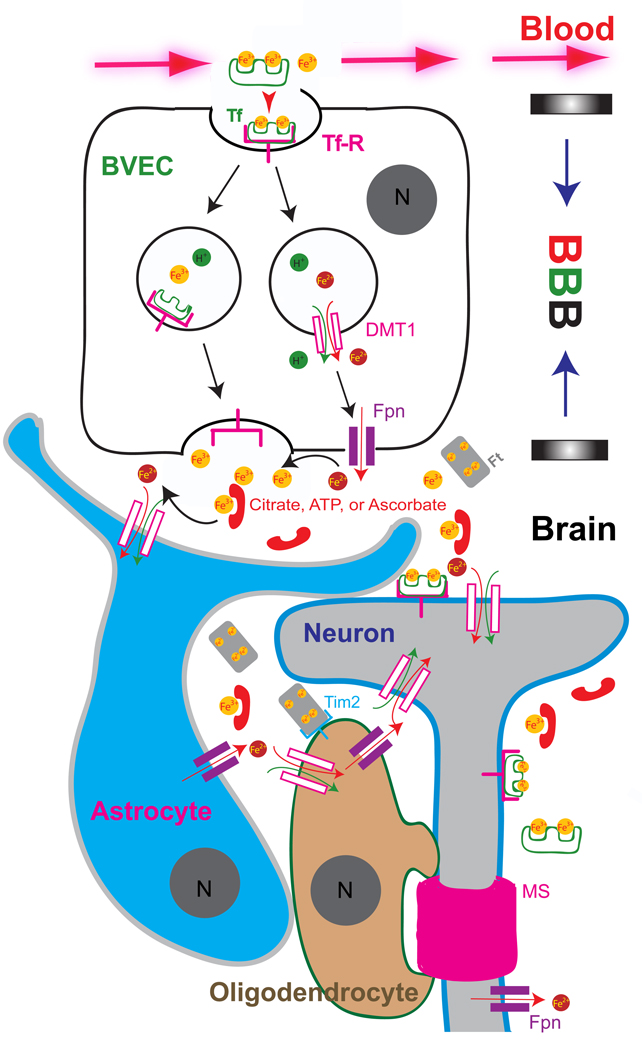
Figure 2. Iron transport across the blood-brain barrier (BBB).
Iron is sequestered in a non-reactive state in the blood plasma by transferrin (Tf; ~ 40 µM). Material transport from the blood plasma into the brain is limited by the blood-brain barrier, which is formed by tight junctions between the brain vascular endothelia cells (BVECs). Transferrin receptors (TfRs) line the lumen of the brain microvasculature and bind circulating Tf-Fe2 (~ 10 µM) and facilitate iron uptake into BVECs via receptor-mediated endocytosis. Two models for BVEC iron export are shown. The DMT1/ferroportin (Fpn)-independent pathway releases Fe3+ or Tf-Fe2 through the exocytosis of recycling endosomes (vesicular export pathway). Tf (mainly secreted from oligodendrocytes) concentration (<0.5 µM) is low in the CSF. Thus, the majority of Fe3+ forms complexes with ascorbate, citrate, and ATP as NTBI (~ 1 µM). Citrate and ATP are released from astrocytes. DMT1/Fpn-dependent (non-vesicular export) pathway releases Fe2+, which is rapidly converted into Fe3+ via ceruloplasmin (Cp) in the abluminal membranes. Alternatively, astrocytic foot-processes form close associations with BVECs, and polarized DMT1 expression in these astrocytic end-feet may facilitate rapid Fe2+ uptake after release (from BVECs) into the perivascular space of the brain. Neither astrocytes nor oligodendrocytes express detectable levels of TfR. Oligodendrocytes can take up iron via the ferritin (Ft) receptor Tim-2. In addition, oligodendrocytes may also uptake NTBI via DMT1 or other non-vesicular iron import mechanisms. The axons of neurons are wrapped with the myelin sheath (MS), which is made in oligodendrocytes in an iron-dependent manner.
The biologic reason for the existence of multiple iron release mechanisms remains unclear. For simplicity, we refer to DMT1 as an endosomal (early, recycling, and late) Fe2+ release pathway, and TRPML1/2 and Nramp1[54] as LEL iron release pathways. Importantly, the source of iron may determine the primary release mechanism. For example, iron acquired through the Tf-TfR cycle is likely to be released via DMT1, which is localized in the early endocytic compartments. Thus, endosomal iron release is mainly due to DMT1. Consistent with this notion, DMT1 is crucial for the iron uptake by erythroid precursor cells [51], in which the dominant uptake pathway is Tf-dependent. On the other hand, TRPML1/2 and Nramp1 pathways may be used for iron release following lysosomal degradation of Ft-Fe complexes and autophagic/phagosomal ingestion of iron-containing macromolecules [4]. In the scenario where macrophages recycle senescent red blood cells, Nramp1 may serve as the main release channel/transporter in the lysosome [4, 54]. Following lysosomal degradation of Ft-Fe complexes and iron-containing macromolecules, TRPML1/2 may be the primary release channel in LEL. Additionally, both DMT1- and TRPML1-dependent mechanisms may co-exist in some cell types, such as neurons [26, 45]. As endosomes undergo rapid Rab-dependent sorting, a certain amount of iron from the Tf cycle may not be released into the cytosol before sorted into LEL. Thus, DMT1 and TRPML1 may mediate iron release in a sequential manner. In cells with dual expression of DMT1 and TRPML1, impairment in DMT1-mediated endosomal iron release may be tolerated because iron entering the late endocytic pathways can still be released via TRPML1.
The regulation of iron release pathways is poorly understood. Regulators of intracellular traffic may play a role in iron metabolism by affecting both vesicular and non-vesicular iron transport pathways. For example, it was demonstrated that Mon1a, a protein involved in multiple steps of intracellular trafficking, affects cellular iron metabolism in an Fpn-dependent manner [57]. Rab proteins are a family of small GTPases that are known to control organelle identity and the specificity of intracellular membrane traffic [58]. For example, Rab5, Rab11, and Rab7 are primarily localized in the EE, recycling endosomes (RE), and LEL, respectively (see Fig. 1). Impaired Rab5 function [59] in zebrafish reportedly causes iron deficiency anemia [60]. Future work may reveal the role of vesicular trafficking in regulation of cellular iron transport.
Brain Iron Uptake across Blood-Brain Barrier
The brain, unlike most other organs in the body, rests behind a vascular barrier. Unlike peripheral endothelia, endothelial cells within the brain vasculature are bound together by tight junctions, resulting in a physical barrier to material exchange between blood and brain tissues and fluid. Therefore, the brain cannot take up iron directly from the circulatory system. The choroid plexus epithelium, comprised by ependymal cells, separates the blood from the ventricular cerebrospinal fluid (CSF). Although ependymal cells can mediate iron absorption into the brain from the blood, their contribution is thought to be rather small [61]. The major uptake pathway is mediated by the blood-brain barrier (BBB), which is formed by cerebrovascular endothelial cells (or brain vascular endothelial cells, BVECs) (see Fig. 2). Unlike small gas molecules such as O2 and CO2 that can diffuse freely across lipid membranes into the brain, iron crosses BVECs in a multi-step transcellular process similar to that of enterocytes in the duodenum.
Tf-dependent and Tf-independent iron import into BVECs
The initial observation that TfR is expressed on the luminal side of brain capillaries suggested a mechanism for iron transport across the BBB through TfR-mediated endocytosis into BVECs [62]. Now, accumulated evidence from functional and genetic studies reveals that, indeed, Tf-TfR plays a primary role in iron uptake across the BBB [5, 7, 37, 63, 64] (Fig. 2). The Tf-TfR-dependent iron uptake system in BVECs is similar to that used in most cell types (see Fig. 1).
Tf-independent mechanisms may also contribute to iron transport across the BBB [1–3, 61, 64–66]. Because hypotransferrinemic mice (<1% of normal circulating Tf) have reportedly normal amounts of iron in the brain, significant contributions from Tf-independent mechanism(s) of brain iron uptake must exist [67]. While iron may also be taken up by BVECs by Tf homologues such as lactoferrin or melanotransferrin [1], the extent of this activity is still unclear [2].
Non-transferrin-bound iron (NTBI) can enter BVECs through both vesicular and non-vesicular import mechanisms (Fig. 1). Fe3+ may form complexes with non-Tf iron-binding proteins, for example, secreted Ft in the serum [68]. Scara5 [69] and Tim-2 [70] have both been recently demonstrated to function as Ft receptors (Fig. 1). Because a molecule of Ft can bind as many as 4,500 iron atoms, these newly identified receptors may play an important role in providing iron for highly iron-dependent processes such as organogenesis [69] and myelination [6] during development. The existence and relative contribution of Ft receptor-dependent iron uptake in BVECs is still unclear. Non-vesicular import pathways may provide a direct mechanism to cross the BBB. Because the brains of DMT1-compromised rats containing the Belgrade hypomorphic mutation (G185R) show decreased iron levels in the brain, it was thought to play a direct role in brain iron uptake [61]. However, it is not clear whether DMT1 is actually expressed in BVECs [61, 71]. Thus, it is necessary to investigate whether other non-vesicular iron importers (IN4, IN5, and IN6; Fig. 1) are expressed on the luminal membranes of BVECs. Although DMT1 transports iron at the acidic pH of the endosome, it may also be able to function as an Fe2+ importer at the neutral pH of the lumen [72].
Intracellular iron transport in BVECs
While it is well established that iron enters brain endothelial cells through Tf-dependent receptor-mediated endocytosis, there is little agreement on the mechanisms of intracellular transport that ensue for iron to eventually traverse the abluminal membrane and enter the brain. The controversy of DMT1 expression in BVECs [61, 71] may determine how iron is transported intracellularly. Given its ubiquitous expression pattern, TRPML1 is likely to play an important role in endolysosomal iron release in BVECs [26]. Whether other iron release proteins such as ZIP8 and TRPML2 are expressed in BVEC cells is not yet known. Additionally, it is not known which ferric reductase(s) are expressed in the endosomes and lysosomes of BVECs, though they likely express one or more of the Steap proteins [48, 52].
Iron export across the abluminal membrane
Ferroportin is currently the only known iron exporter [46], making it a natural candidate to export iron across abluminal membranes. However, it is unclear whether it is expressed [73] [61, 74] in BVECs. It is also possible that the expression level of Fpn is low in these cells. Since knock-out of Fpn globally in mice results in embryonic lethality [75], an endothelium- or BVEC-specific inactivation of Fpn in mice may be necessary to provide a definite answer on whether Fpn plays a role in iron transport across BBB.
DMT1/Fpn-independent export mechanisms may also account for iron transport across the abluminal membranes of BVECs [74]. Moos et al. propose that exocytosis of the recycling endosomes containing Tf-Fe2 complexes to the abluminal surface of the BVEC, leading to the detachment of iron from Tf in the slightly lower pH of brain interstitial fluid (ISF) compared to blood plasma (vesicular iron export pathway EX2; Fig. 1). Compared to plasma, CSF/ISF solutions contain much lower concentrations of Tf (<0.5 µM), but several fold higher concentrations of citrate and ascorbate [37]. Thus, citrate and ascorbate in the ISF may serve as possible coordination partners for released Fe3+ (Fig. 1). Additionally, astrocytes form close associations with the brain’s microvasculature and are known to release ATP and other nucleotides which may play a role in this exocytosis-dependent abluminal membrane iron transport [76]. Indeed, significant sources of NTBI exist in the brain bound to citrate, ascorbate, and ATP [5]. Nevertheless, this model is lacking how abluminally exposed apo-Tf laden TfRs are recycled or degraded, since very little serum Tf crosses into the BBB. Another potential iron export mechanism in BVECs is lysosomal exocytosis (vesicular iron export pathway EX1; Fig. 1). Lysosomal exocytosis has recently been demonstrated to occur in all mammalian cell types studied thus far [77]. As Ft-receptor- (Scara5 or Tim-2) mediated endocytic iron uptake and reutilization of Ft-bound storage iron require lysosomes [50], this pathway may not only export iron but also provide secreted Ft or its degraded product hemosiderin to the CSF/ISF. Direct evidence for this lysosome-mediated iron export is currently still lacking. Nevertheless, if both exocytotic types of iron export mechanisms co-exist in BVECs, manipulations of compartment-specific Rab proteins may help determine the extent to which iron absorption by BVECs relies on the Rab7-depedent lysosomal pathway versus the Rab11-dependent endosomal pathway.
Iron Transport in Neurons and Glia
Iron transport in astrocytes
Astrocytic perivascular foot processes surround the vascular barrier formed by BVECs just outside the basal lamina, ensheathing the abluminal membranes of BVECs [63] (Fig. 2). These foot processes can also form direct connections to neurons. As such, astrocytes likely play a critical role in regulating brain iron absorption and metabolism at the junction of the BBB [78]. This unique arrangement allows astrocytes to act as gatekeepers in regulating the iron transport properties of the BBB. Astrocytes do not express TfR [78], although this is somewhat controversial [79]. They may, however, take up significant amounts of NTBI as a primary iron uptake pathway. Interestingly, DMT1 has been shown to have polarized expression in astrocytes, being only expressed in the end-foot processes that associate with the BBB [80]. Therefore, iron released by BVECs may quickly be taken up by nearby astrocytes through DMT1 (non-vesicular iron import pathway IN3; Fig. 1 and Fig. 2) and redistributed to the extracellular space in the brain parenchyma. Astrocytes have also been implicated in metabolizing heme, releasing iron degraded by heme oxygenase-1 (HO-1) [81].
Ferroportin (non-vesicular iron export pathway EX3; Fig. 1) forms the major iron efflux pathway in astrocytes [73]. Fpn is coupled to a GPI-anchored form of ceruloplasmin (Cp), the Cu-dependent ferroxidase that can rapidly oxidize released Fe2+ to its less reactive Fe3+ form [35]. Astrocytes cultured from Cp-deficient mice are unable to export iron, even though they have normal iron uptake [82]. Yet normal iron export is restored by simply supplying soluble Cp to the cell culture medium. Because astrocytes are closely associated to the brain’s vasculature and have a high capacity for iron accumulation, yet store little iron, they may be involved in brain iron distribution after uptake. Consistent with this notion, patients with aceruloplasminemia (Table 1) are observed to have primary iron accumulation in the astrocytic foot-processes along the BBB, forming inclusions known as “grumose foamy spheroid bodies” [83].
Iron transport in neurons
Iron is required for many neuronal functions and is a cofactor required for the synthesis of dopaminergic neurotransmitters [5, 8]. A substantial portion of oxygen consumption in the body (~20%) relies on mitochondrial respiration in the brain, of which iron is an essential trophic factor required for oxygen consumption and ATP generation [6]. As TfR is expressed in central neurons, the Tf-TfR system may be an important pathway for neurons to acquire iron. Furthermore, both DMT1 and TRPML1 are highly expressed in neurons [37, 55]. Since both proteins transport Fe2+ but not Fe3+, a ferric reductase(s) must exist in the endosomes and lysosomes of neurons. While Steap3 is shown to function in the endosomes of erythroid cells [48], both Steap1 and Steap2 are expressed in the brain [52]. In addition, the stromal cell-derived receptor-2 (SDR2) [84], a homologue of the duodenal ferrireductase DcytB [44], is expressed in the brain. It is unclear whether SDR2 is localized to the endolysosomal membranes or the plasma membrane.
Neurons are also able to utilize NTBI as an iron source, where it forms significant stores of brain iron[64]. Although citrate and ascorbate bind Fe3+ with affinities (Kd~ 10−11 M) more than 1010 times lower than Tf (Kd~ 10−23 M), they are much more abundant in the CSF/ISF than the plasma [37]. Hence citrate/ascorbate-bound NTBI can reach sub-micromolar concentrations (<1 µM ) in the CSF [37, 38]. It has been demonstrated that NTBI may enter the neurons through several non-vesicular import mechanisms (IN3, IN4, IN5, and IN6; Fig. 1). DMT1, Zip14 (Slc39a14) [85], voltage-gated Ca2+ channels (VGCC) [86], and TRPC6 [87] are all selective for Fe2+ over Fe3+. It remains an open question how Fe3+ can be dissociated from ascorbate/citrate and converted into Fe2+ by a membrane bound ferric reductase. Little iron is actually stored in neurons since Ft detection is relatively weak [5, 7, 70], suggesting that iron is taken up either for rapid utilization or secreted by the robustly detected iron exporter Fpn. ncreased neuronal Ft expression, as seen in Irp2−/− mice, may be toxic and has been reportedly associated with extrapyramidal neurodegeneration [27].
Abnormal intracellular iron transport in neurons may cause neurodegeneration. Cytosolic iron overload causes oxidative stress by increasing ROS levels via the Fenton reaction. Since neuronal iron is transported along axons and dendrites, iron overload in neurons may cause both neuronal cell death and axonal degeneration. Impaired endolysosomal iron release may result in intralysosomal iron overload, which in turn increases free radical oxides and facilitates the formation of “lipofuscin” (aka ‘aging pigment’) [26, 88]. Lipofuscin accumulation may affect the normal function of lysosomes, such as autophagy, resulting in neuronal cell death and axonal degeneration. Impaired intracellular iron transport may also cause other neuronal dysfunctions. For example, hippocampus-specific inactivation of DMT1 in mice results in iron deficiency in the hippocampal neurons and impaired learning and memory [89].
Iron transport of oligodendrocytes
Most of the histochemically detectable iron in the brain is found within oligodendrocytes, likely reflecting the high demand of iron required for myelination [6]. These cells are also the predominant producers of Tf in the brain [66], suggesting an indirect role of oligodendrocytes in neuronal iron transport. Ferritin, traditionally considered to be an intracellular iron storage protein, is also present in the serum [13] and known to bind the extracellular face of oligodendrocytes in vivo [90]. The recent identification of Ft receptors [69, 90] suggest that oligodendrocytes primarily take up NTBI iron from the ISF by a Ft-dependent mechanism (vesicular import pathway IN1; Fig. 1). Consistent with the stringent iron requirements for axon myelination (Fig. 2), the Ft receptor Tim-2 is highly expressed in oligodendrocytes, but not astrocytes [90].
CNS Iron Homeostasis, Dyshomeostasis, and Novel Therapies
Regulation of brain iron transport
Iron uptake across the BBB is dependent on the brain’s iron status. During development, and conditions of inadequate iron supply, the transport rate of iron into the brain is significantly increased [91]. However, this is not associated with increased TfR expression by BVECs, rather TfR expression is upregulated in neurons during periods of inadequate circulating iron [64]. Therefore, multiple regulatory mechanisms may exist to control iron levels in the brain, independent of peripheral iron status. Consistent with this notion, during peripheral iron-overload, such as in patients with hemochromatosis, brain iron levels are not elevated or disrupted [66].
Key proteins involved in iron metabolism are regulated post-transcriptionally by the IRE-IRP system in mammalian cells [4, 28]. Iron regulatory proteins (IRPs) are cytosolic iron-sensing proteins that, when cells are depleted on iron, interact with RNA transcripts containing a stem loop structure known as an iron-responsive element (IRE)[4]. IREs are present in many key proteins involved in iron acquisition, transport, and storage. Low cytosolic iron levels cause two homologous IRPs (IRP1 and IRP2) to inhibit Ft and Fpn translation and to stabilize mRNA transcripts of TfR1 and DMT1 [13]. In iron replete cells, neither IRP1 nor IRP2 binds IREs and Ft/Fpn expression increases while TfR/DMT1 expression decreases. The role of the IRE-IRP network in regulating systemic iron homeostasis has been excellently reviewed elsewhere [28].
Iron transport in the brain is also regulated by the IRE-IRP system. In brain tissues from Irp2−/− mice, Ft/Fpn expression is significantly increased. Conversely, TfR levels were found to be decreased, consistent with the notion that IRP2 ablation may constitute an inability to stabilize TfR transcripts [4, 28]. Although IRP double knockout mice (Irp1−/−/ Irp2−/−) do not survive, Irp2−/− mice exhibit microcytic anemia and progressive neurodegeneration [27]. Interestingly, brain iron accumulation precedes degeneration by several months in these mice [27]. Moreover, regions of brain iron accumulation in young mice accurately predict sites of CNS neurodegeneration. This work represents another example supporting the idea that defects in iron metabolism can act in a causal manner in CNS disorders.
Iron dyshomeostasis in animal models of CNS disorders
In Irp2−/− mice, immunolabeling of Ft revealed subunits along the length of axons, suggesting that Ft is transported from the site of synthesis (soma) to the axons prior to axonal degeneration. Lysosomal turnover of Ft-Fe complexes at the synaptic terminal may lead to increased reactive Fe2+ levels and oxidative stress [84]. Consistent with this notion, axonal iron staining of Irp2−/− mice is significantly greater than the wild-type control [27].
Increased DMT1 expression has also been shown to be associated with neurodegeneration. Salazar et al. report that the transporter contributes to neurodegeneration in animal models of Parkinson’s disease (PD) [32]. Using the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse intoxication model of PD, the authors demonstrate that DMT1 expression increases in the ventral mesencephalon leading to iron accumulation, increased oxidative stress, and dopaminergic cell death. A hypomorphic mutation that impairs iron transport (G185R) in DMT1 was sufficient to protect rodents against MPTP-induction of neurodegeneration, suggesting a critical role for DMT1-mediated neuronal iron uptake into regions of the brain commonly affected in animal models of PD. Consistent with a role for iron-mediated toxicity in the animal models of PD, Kaur et al. used transgenic expression of Ft (as a genetic iron chelator) to demonstrate its protective effect on dopaminergic neurons in the substantia nigra (SN) after injection of MPTP [31]. Similar results were obtained using oral administration of the iron chelator Clioquinol [31].
Mutations in TRPML1 cause the neurodegenerative disorder ML4. Dong et al. propose that the excessive accumulation of intralysosomal iron leads to the formation of iron-catalyzed ROS and eventually to formation and accumulations of lipofuscin in post-mitotic cells such as neurons [26]. Progressive damage to cellular macromolecules eventually leads to psychomotor retardation and retinal degeneration. It may be noteworthy that ML4 cells exhibit intralysosomal iron overload and concurrent cellular iron deficiency. Cellular iron deficiency observed in ML4 cells may lead to dysregulation of the IRE-IRP regulatory system, further enhancing iron mis-localization and neurodegeneration. Thus, it is the cellular iron mis-localization, but not the overall cellular iron content, that causes neuronal cell death and degeneration.
Therapeutic targets
Two principal iron-related therapies for neurodegenerative disorders are anti-oxidant or chelation therapies. While anti-oxidant therapy aims to attenuate oxidative stress by decreasing the amount of ROS, chelation therapy is geared toward suppressing radical formation by decreasing the level of the catalyst, i.e. iron. Desferrioxamine (DFO), a commonly known iron chelator, has been shown to slow cognitive decline in Alzheimer’s patients [29] and clioquinol has demonstrated neuroprotective success in animal models of PD [31]. Most therapeutic agents, however, are hampered by pitfalls. For example, clioquinol has notably neurotoxic side effects [30], and DFO crosses the BBB poorly [29].
Several new technologies may provide novel targeting methods for iron chelators. For example, DFO can be incorporated into drug-delivery systems with nanoparticles [92]. Iron chelator–nanoparticle systems allow therapeutic agents to cross the BBB by using low-density lipoprotein (LDL) transport machinery for uptake [93]. This approach minimizes toxicity by allowing the chelator–nanoparticle complex to be transported, together with iron, out of the brain. These carrier systems may be able to efficiently deliver any iron-related therapeutic compound or transgenic fusion protein to the CNS, equivalent to a “genetic iron chelator”. As iron accumulation in neurodegenerative diseases is often “regional”, research may eventually lead to targeting strategies specifically to regions of interest in the brain that are particularly vulnerable to iron accumulation and iron-mediated toxicity, such as the SN. Iron overload can also be a “subcellular” problem, as seen in the ML4 cells, which exhibit cytosolic iron deficiency and concurrent intralysosomal iron overload [26]. Therefore, iron chelators that target the lysosome such as alpha lipoic acid plus (LAP) [94] may specifically reduce intralysosomal iron overload and subsequent lipofuscin formation. Although LAP has the potential to cross the BBB, its conjugation with nanoparticles may greatly facilitate the transport into and out of the brain. The specificity and efficacy of iron chelators can be tested in the aforementioned animal models.
Finally, gene therapy may provide an alternative neuroprotection strategy. Using genetic engineering approaches to express chimeric TfR fused to a peptidomimetic monoclonal antibody, Boado et al. demonstrate that TfR can serve as a viable drug delivery mechanism to cross the BBB for therapeutic fusion proteins in mice [95]. Further, Chang et al. has shown that Tf-bound nanoparticles interact with cells in a specific manner and enter cells via the caveolae pathway [92]. As the mechanisms of brain iron transport begin to emerge, new pharmacological targets and drug-carrier systems are likely to develop.
Future Perspective
Several genetic disorders of iron metabolism clearly cause degenerative symptoms. These include inherited mutations in pantothenate kinase-2 (PANK2) leading to NBIA Type I, mutations in FLT1 (encoding L-Ft) leading to neuroferritinopathy, mutations in PLA2G6 leading to INAD1, and mutations in Cp leading to aceruloplasminemia. A number of non-genetic, sporadic neurodegenerative diseases have also been proposed to be related to changes in iron homeostasis, including Parkinson’s, Alzheimer’s, Huntington’s, Friedreich’s Ataxia, Restless Leg Syndrome, Ischemic/Hemorrhagic Stroke, and Multiple Sclerosis. As a clearer picture of brain iron metabolism begins to come into focus, future research will be able to help develop iron-centric therapies for these CNS disorders. Continuing efforts should be made to gain a mechanistic understanding of iron transport across the abluminal membrane of BVECs, the roles of glia cells in regulating brain iron uptake, and finally how neuronal iron accumulation may lead to neurodegeneration and CNS disease progression. Brain iron accumulation manifests at regional, cellular, and subcellular levels. Understanding the cellular and intercellular transport mechanism(s) may not only reveal the pathogenic cause of neurodegenerative diseases, but is also necessary for designing effective therapeutic approaches to take advantage of newly-developed drug-targeting methods.
Executive Summary.
Excessive iron levels lead to oxidative stress and, in the brain, cause neurodegeneration.
Non-heme iron can enter and exit mammalian cells via two distinct mechanisms: vesicular and non-vesicular.
Dietary ferric iron is transported across intestinal enterocytes in a coordinated effort between the apical ferrireductase DcytB and the iron importer DMT1, followed by its export via Fpn and a basolateral ferrioxidase, binding to Tf and entry into plasma.
Tf-bound iron is taken into erythroid precursor cells by the membrane-bound TfR via endocytosis, released in the early endosome, reduced by Steap3, and transported into the cytosol by DMT1 where it contributes to the labile iron pool, is transported to mitochondria, stored in ferritin, or transported out of the cell via Fpn.
Mutations in TRPML1, a protein involved in iron release from late endosome and lysosomes, cause the neurodegenerative disease ML4, of which some patients exhibit iron-deficiency anemia.
The brain cannot take up iron directly from the circulatory system; rather, iron crosses BVECs in a multi-step transcellular process similar to that of enterocytes in the duodenum.
The Tf-TfR system plays a primary role in iron uptake across the BBB, while the extent to which iron is taken up by Tf homologues such as lactoferrin or melanotransferrin is still unclear.
While there is controversy if DMT1 is expressed in BVECs, TRPML1 has a ubiquitous expression pattern and is likely to play an important role in endolysosomal iron release; however the role of other iron release proteins, such as ZIP8 and TRPML2, is not yet known.
The iron export mechanism across the abluminal membrane is undefined, however, both vesicular and non-vesicular exocytotic mechanisms may co-exist in BVECs.
Because astrocytes are closely associated to the brain’s vasculature and have a high capacity for iron accumulation, yet store little iron, they may be involved in brain iron distribution after uptake, acting as gatekeepers in regulating the iron transport properties of the BBB.
Little iron is typically stored in neurons, and since neuronal iron is transported along axons and dendrites, iron overload may cause both neuronal cell death and axonal degeneration.
Most detectable iron in the brain is found within oligodendrocytes, as is the high expression of the recently identified Ft receptor (Tim-2), likely reflecting the high demand of iron required for axon myelination.
Iron transport in the brain is governed by key proteins regulated post-transcriptionally by the IRE-IRP system.
Cellular iron mis-localization, but not the overall cellular iron content, causes neuronal cell death and degeneration.
New technologies for neuroprotection may provide novel targeting methods, including nanoparticle drug-delivery systems in conjunction with iron chelators as well as gene therapy strategies.
Key Terms.
Blood-brain barrier (BBB): A vascular barrier that prevents dissolved solutes in the blood from freely entering the central nervous system and is formed by tight junctions between adjacent brain vascular endothelial cells (BVECs) lining CNS vessels.
Reactive Oxygen Species (ROS): Highly reactive products of oxygen metabolism that form in high amounts when cells are under stress conditions. ROS includes oxygen ions, free radicals, and peroxides. Accumulation can lead to damage of several cellular structures including DNA, proteins, and lipids.
Ferritin (Ft): a globular intracellular iron storage protein consisting of 24 protein subunits. Ferritin maintains the solubility of iron by storing it in its ferric (Fe3+) form.
Transferrin (Tf): A blood plasma glycoprotein that transports Fe3+ through the body and delivers iron to tissues of the body which express transferrin receptors (TfRs), initiating the transferrin cycle of delivery.
Non-transferrin bound iron (NTBI): A population of iron in the blood plasma or CSF that is free or bound by molecules other than transferrin.
Brain vascular endothelial cells (BVECs): Specialized endothelial cells that form the lining of the brain’s circulatory system. Tight junctions between adjacent endothelial cells form the blood-brain barrier and restrict the passage of solutes from the blood into the brain parenchyma.
Acknowledgements
The work in the Xu laboratory is supported by startup funds to H.X. from the Department of MCDB and Biological Science Scholar Program, the University of Michigan, a NIH RO1 grant (NS062792 to H.X), pilot grants (to H.X.) from the UM Initiative on Rare Disease Research, Michigan Alzheimer’s Disease Research Center (NIH grant P50-AG08671 to Gilman), and National Multiple Sclerosis Society. W.F. is supported by Chinese Academy of Sciences One Hundred Talents Program (KSCX2-YW-R-151) and National Basic Research Program of China (973 Program; 2009CB941400). We appreciate the encouragement and helpful comments from other members of the Xu laboratory.
References
- 1.Fillebeen C, et al. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem. 1999;274(11):7011–7017. doi: 10.1074/jbc.274.11.7011. [DOI] [PubMed] [Google Scholar]
- 2.Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331(1):1–40. doi: 10.1016/s0304-4157(96)00014-7. [DOI] [PubMed] [Google Scholar]
- 3.Rothenberger S, et al. Coincident expression and distribution of melanotransferrin and transferrin receptor in human brain capillary endothelium. Brain Res. 1996;712(1):117–121. doi: 10.1016/0006-8993(96)88505-2. [DOI] [PubMed] [Google Scholar]
- 4.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 5.Moos T, et al. Iron trafficking inside the brain. J Neurochem. 2007;103(5):1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x. [DOI] [PubMed] [Google Scholar]
- 6.Todorich B, et al. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57(5):467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 7.Ke Y, Qian ZM. Brain iron metabolism: neurobiology and neurochemistry. Prog Neurobiol. 2007;83(3):149–173. doi: 10.1016/j.pneurobio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Zecca L, et al. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–73. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 9.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13(3):158–165. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Pollitt E. Early iron deficiency anemia and later mental retardation. Am J Clin Nutr. 1999;69(1):4–5. doi: 10.1093/ajcn/69.1.4. [DOI] [PubMed] [Google Scholar]
- 11.Schipper HM. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res Rev. 2004;3(3):265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Lillig CH, Berndt C, Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780(11):1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 13.MacKenzie EL, Iwasaki K, Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(6):997–1030. doi: 10.1089/ars.2007.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg D, Hochstrasser H. Iron metabolism in Parkinsonian syndromes. Mov Disord. 2006;21(9):1299–1310. doi: 10.1002/mds.21020. [DOI] [PubMed] [Google Scholar]
- 15.Lee DW, Andersen JK, Kaur D. Iron dysregulation and neurodegeneration: the molecular connection. Mol Interv. 2006;6(2):89–97. doi: 10.1124/mi.6.2.6. [DOI] [PubMed] [Google Scholar]
- 16.Abo-Krysha N, Rashed L. The role of iron dysregulation in the pathogenesis of multiple sclerosis: an Egyptian study. Mult Scler. 2008;14(5):602–608. doi: 10.1177/1352458507085550. [DOI] [PubMed] [Google Scholar]
- 17.Barnham KJ, Bush AI. Metals in Alzheimer's and Parkinson's diseases. Curr Opin Chem Biol. 2008;12(2):222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Moos T, Morgan EH. The metabolism of neuronal iron and its pathogenic role in neurological disease: review. Ann N Y Acad Sci. 2004;1012:14–26. doi: 10.1196/annals.1306.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhou B, et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001;28(4):345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
- 20.Morgan NV, et al. PLA2G6, encoding a phospholipase A2, is mutated in neurodegenerative disorders with high brain iron. Nat Genet. 2006;38(7):752–754. doi: 10.1038/ng1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris ZL, et al. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96(19):10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis AR, et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat Genet. 2001;28(4):350–354. doi: 10.1038/ng571. [DOI] [PubMed] [Google Scholar]
- 23.Clardy SL, et al. Is ferroportin-hepcidin signaling altered in restless legs syndrome? J Neurol Sci. 2006;247(2):173–179. doi: 10.1016/j.jns.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Hua Y, et al. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38(2 Suppl):759–762. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 25.Sun M, et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum Mol Genet. 2000;9(17):2471–2478. doi: 10.1093/hmg/9.17.2471. [DOI] [PubMed] [Google Scholar]
- 26.Dong XP, et al. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455(7215):992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LaVaute T, et al. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat Genet. 2001;27(2):209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- 28.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2(8):406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 29.Crapper McLachlan DR, et al. Intramuscular desferrioxamine in patients with Alzheimer's disease. Lancet. 1991;337(8753):1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- 30.Benvenisti-Zarom L, Chen J, Regan RF. The oxidative neurotoxicity of clioquinol. Neuropharmacology. 2005;49(5):687–694. doi: 10.1016/j.neuropharm.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Kaur D, et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37(6):899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 32.Salazar J, et al. Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105(47):18578–18583. doi: 10.1073/pnas.0804373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Curr Opin Chem Biol. 1999;3(2):220–225. doi: 10.1016/S1367-5931(99)80035-0. [DOI] [PubMed] [Google Scholar]
- 34.Smith DG, Cappai R, Barnham KJ. The redox chemistry of the Alzheimer's disease amyloid beta peptide. Biochim Biophys Acta. 2007;1768(8):1976–1990. doi: 10.1016/j.bbamem.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Patel BN, David S. A novel glycosylphosphatidylinositol-anchored form of ceruloplasmin is expressed by mammalian astrocytes. J Biol Chem. 1997;272(32):20185–20190. doi: 10.1074/jbc.272.32.20185. [DOI] [PubMed] [Google Scholar]
- 36.Tsushima RG, et al. Modulation of iron uptake in heart by L-type Ca2+ channel modifiers: possible implications in iron overload. Circ Res. 1999;84(11):1302–1309. doi: 10.1161/01.res.84.11.1302. [DOI] [PubMed] [Google Scholar]
- 37.Bradbury MW. Transport of iron in the blood-brain-cerebrospinal fluid system. J Neurochem. 1997;69(2):443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- 38.Savman K, et al. Non-protein-bound iron in brain interstitium of newborn pigs after hypoxia. Dev Neurosci. 2005;27(2–4):176–184. doi: 10.1159/000085990. [DOI] [PubMed] [Google Scholar]
- 39.Petrat F, de Groot H, Rauen U. Determination of the chelatable iron pool of single intact cells by laser scanning microscopy. Arch Biochem Biophys. 2000;376(1):74–81. doi: 10.1006/abbi.2000.1711. [DOI] [PubMed] [Google Scholar]
- 40.Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112(2):219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Domenico I, McVey Ward D, Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat Rev Mol Cell Biol. 2008;9(1):72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 42.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 43.Garrick MD, Garrick LM. Cellular iron transport. Biochim Biophys Acta. 2009;1790(5):309–325. doi: 10.1016/j.bbagen.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 44.McKie AT, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. 2001;291(5509):1755–1759. doi: 10.1126/science.1057206. [DOI] [PubMed] [Google Scholar]
- 45.Gunshin H, et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature. 1997;388(6641):482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 46.Donovan A, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 47.Vulpe CD, et al. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat Genet. 1999;21(2):195–199. doi: 10.1038/5979. [DOI] [PubMed] [Google Scholar]
- 48.Ohgami RS, et al. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet. 2005;37(11):1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw GC, et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440(7080):96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 50.Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am J Physiol Cell Physiol. 2006;291(3):C445–C455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- 51.Gunshin H, et al. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J Clin Invest. 2005;115(5):1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohgami RS, et al. The Steap proteins are metalloreductases. Blood. 2006;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam-Yuk-Tseung S, Gros P. Distinct targeting and recycling properties of two isoforms of the iron transporter DMT1 (NRAMP2, Slc11A2) Biochemistry. 2006;45(7):2294–2301. doi: 10.1021/bi052307m. [DOI] [PubMed] [Google Scholar]
- 54.Jabado N, et al. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med. 2000;192(9):1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puertollano R, Kiselyov K. TRPMLs: in sickness and in health. Am J Physiol Renal Physiol. 2009;296(6):F1245–F1254. doi: 10.1152/ajprenal.90522.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nguyen A, et al. B266 Mechanisms of iron release from lysosomes. Experimental Biology. 2009;921(11 I) [Google Scholar]
- 57.Wang F, et al. Genetic variation in Mon1a affects protein trafficking and modifies macrophage iron loading in mice. Nat Genet. 2007;39(8):1025–1032. doi: 10.1038/ng2059. [DOI] [PubMed] [Google Scholar]
- 58.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2(2):107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 59.Pal A, et al. Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease. J Cell Biol. 2006;172(4):605–618. doi: 10.1083/jcb.200509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lumsden AL, et al. Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet. 2007;16(16):1905–1920. doi: 10.1093/hmg/ddm138. [DOI] [PubMed] [Google Scholar]
- 61.Burdo JR, et al. Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res. 2001;66(6):1198–1207. doi: 10.1002/jnr.1256. [DOI] [PubMed] [Google Scholar]
- 62.Jefferies WA, et al. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312(5990):162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 63.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 64.Moos T, Morgan EH. Evidence for low molecular weight, non-transferrin-bound iron in rat brain and cerebrospinal fluid. J Neurosci Res. 1998;54(4):486–494. doi: 10.1002/(SICI)1097-4547(19981115)54:4<486::AID-JNR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 65.Burdo JR, et al. Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience. 2003;121(4):883–890. doi: 10.1016/s0306-4522(03)00590-6. [DOI] [PubMed] [Google Scholar]
- 66.Burdo JR, Connor JR. Brain iron uptake and homeostatic mechanisms: an overview. Biometals. 2003;16(1):63–75. doi: 10.1023/a:1020718718550. [DOI] [PubMed] [Google Scholar]
- 67.Beard JL, et al. Brain iron uptake in hypotransferrinemic mice: influence of systemic iron status. J Neurosci Res. 2005;79(1–2):254–261. doi: 10.1002/jnr.20324. [DOI] [PubMed] [Google Scholar]
- 68.Fisher J, et al. Ferritin: a novel mechanism for delivery of iron to the brain and other organs. Am J Physiol Cell Physiol. 2007;293(2):C641–C649. doi: 10.1152/ajpcell.00599.2006. [DOI] [PubMed] [Google Scholar]
- 69.Li JY, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. 2009;16(1):35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Todorich B, et al. Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem. 2008;107(6):1495–1505. doi: 10.1111/j.1471-4159.2008.05678.x. [DOI] [PubMed] [Google Scholar]
- 71.Siddappa AJ, et al. Developmental changes in the expression of iron regulatory proteins and iron transport proteins in the perinatal rat brain. J Neurosci Res. 2002;68(6):761–775. doi: 10.1002/jnr.10246. [DOI] [PubMed] [Google Scholar]
- 72.Mackenzie B, et al. Divalent metal-ion transporter DMT1 mediates both H+ -coupled Fe2+ transport and uncoupled fluxes. Pflugers Arch. 2006;451(4):544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- 73.Wu LJ, et al. Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res. 2004;1001(1–2):108–117. doi: 10.1016/j.brainres.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 74.Moos T, et al. Brain capillary endothelial cells mediate iron transport into the brain by segregating iron from transferrin without the involvement of divalent metal transporter 1. J Neurochem. 2006;98(6):1946–1958. doi: 10.1111/j.1471-4159.2006.04023.x. [DOI] [PubMed] [Google Scholar]
- 75.Donovan A, et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Montana V, et al. Vesicular transmitter release from astrocytes. Glia. 2006;54(7):700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 77.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106(2):157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 78.Dringen R, et al. The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res. 2007;32(11):1884–1890. doi: 10.1007/s11064-007-9375-0. [DOI] [PubMed] [Google Scholar]
- 79.Qian ZM, et al. Transferrin receptors on the plasma membrane of cultured rat astrocytes. Exp Brain Res. 1999;129(3):473–476. doi: 10.1007/s002210050916. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Ling EA. Studies of the ultrastructure and permeability of the blood-brain barrier in the developing corpus callosum in postnatal rat brain using electron dense tracers. J Anat. 1994;184(Pt 2):227–237. [PMC free article] [PubMed] [Google Scholar]
- 81.Ham D, Schipper HM. Heme oxygenase-1 induction and mitochondrial iron sequestration in astroglia exposed to amyloid peptides. Cell Mol Biol (Noisy-legrand) 2000;46(3):587–596. [PubMed] [Google Scholar]
- 82.Jeong SY, David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J Biol Chem. 2003;278(29):27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- 83.Kaneko K, et al. Astrocytic deformity and globular structures are characteristic of the brains of patients with aceruloplasminemia. J Neuropathol Exp Neurol. 2002;61(12):1069–1077. doi: 10.1093/jnen/61.12.1069. [DOI] [PubMed] [Google Scholar]
- 84.Vargas JD, et al. Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochim Biophys Acta. 2003;1651(1–2):116–123. doi: 10.1016/s1570-9639(03)00242-5. [DOI] [PubMed] [Google Scholar]
- 85.Liuzzi JP, et al. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci U S A. 2006;103(37):13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oudit GY, et al. L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med. 2003;9(9):1187–1194. doi: 10.1038/nm920. [DOI] [PubMed] [Google Scholar]
- 87.Mwanjewe J, Grover AK. Role of transient receptor potential canonical 6 (TRPC6) in non-transferrin-bound iron uptake in neuronal phenotype PC12 cells. Biochem J. 2004;378(Pt 3):975–982. doi: 10.1042/BJ20031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurz T, et al. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008;129(4):389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carlson ES, et al. Iron is essential for neuron development and memory function in mouse hippocampus. J Nutr. 2009;139(4):672–679. doi: 10.3945/jn.108.096354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hulet SW, et al. Characterization and distribution of ferritin binding sites in the adult mouse brain. J Neurochem. 1999;72(2):868–874. doi: 10.1046/j.1471-4159.1999.720868.x. [DOI] [PubMed] [Google Scholar]
- 91.Taylor EM, Crowe A, Morgan EH. Transferrin and iron uptake by the brain: effects of altered iron status. J Neurochem. 1991;57(5):1584–1592. doi: 10.1111/j.1471-4159.1991.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 92.Chang J, et al. Characterization of endocytosis of transferrin-coated PLGA nanoparticles by the blood-brain barrier. Int J Pharm. 2009 doi: 10.1016/j.ijpharm.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 93.Liu G, et al. Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer disease. Neurosci Lett. 2009;455(3):187–190. doi: 10.1016/j.neulet.2009.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Persson HL, et al. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radic Biol Med. 2003;34(10):1295–1305. doi: 10.1016/s0891-5849(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 95.Boado RJ, et al. Engineering and expression of a chimeric transferrin receptor monoclonal antibody for blood-brain barrier delivery in the mouse. Biotechnol Bioeng. 2009;102(4):1251–1258. doi: 10.1002/bit.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuo YM, et al. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Genet. 2005;14(1):49–57. doi: 10.1093/hmg/ddi005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17(9):1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 98.De S, et al. Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during Wallerian degeneration. Mol Cell Neurosci. 2003;24(3):753–765. doi: 10.1016/s1044-7431(03)00241-0. [DOI] [PubMed] [Google Scholar]