Abstract
Transient receptor potential vanilloid (TRPV) channels, which include the thermosensitive TRPV1–V4, have large cytoplasmic regions flanking the transmembrane domain, including an N-terminal ankyrin repeat domain. We show that a multiligand binding site for ATP and calmodulin previously identified in the TRPV1 ankyrin repeat domain is conserved in TRPV3 and TRPV4, but not TRPV2. Accordingly, TRPV2 is insensitive to intracellular ATP, while, as previously observed with TRPV1, a sensitizing effect of ATP on TRPV4 required an intact binding site. In contrast, ATP reduced TRPV3 sensitivity and potentiation by repeated agonist stimulations. Thus, ATP and calmodulin, acting through this conserved binding site, are key players in generating the different sensitivity and adaptation profiles of TRPV1, TRPV3, and TRPV4. Our results suggest that competing interactions of ATP and calmodulin influence channel sensitivity to fluctuations in calcium concentration and perhaps even metabolic state. Different feedback mechanisms likely arose because of the different physiological stimuli or temperature thresholds of these channels.
Introduction
Transient receptor potential channels, including the six vanilloid (TRPV)3 channels in warm-blooded vertebrates, have many physiological functions in neuronal and non-neuronal cells (1). TRPV5 and TRPV6 are calcium channels in the gut and kidney important for Ca2+ homeostasis (2), whereas TRPV1–V4 are nonselective cation channels that contribute to temperature sensation (3). TRPV1 and TRPV2 activate at noxious temperatures above 42 and 52 °C, respectively, whereas TRPV3 and TRPV4 activate at warm temperatures ∼33–39 and 25–34 °C, respectively.
Thermosensitive TRPVs are polymodal channels activated by physical stimuli (e.g. temperature) and chemical agonists. For instance, capsaicin and low extracellular pH activate TRPV1 (4); thymol, carvacrol and eugenol activate TRPV3 (5); and extracellular hypotonicity, phorbol esters, and arachidonic acid metabolites activate TRPV4 (6–9). 2-Aminoethyl diphenylborinate (2-APB) is promiscuous and activates TRPV1, TRPV2, and TRPV3 (10).
Remaining questions include whether TRPV channels have maintained common regulatory mechanisms. Thermosensitive TRPV channels are modulated intracellularly by Ca2+, calmodulin (CaM), and phosphoinositides (11–13). TRPV1 desensitization depends on intracellular Ca2+ and CaM (14, 15). Similarly, TRPV4 is first potentiated and then inactivated by intracellular Ca2+, again likely through CaM (16). Like TRPV1, TRPV4 desensitizes after repeated or prolonged stimulations (17). In contrast, TRPV3 currents increase with repeated stimulation (18–20), and while TRPV3 sensitivity also depends on Ca2+ and CaM, the effects differ from TRPV1 and TRPV4 (21). The nature of these differences in homologous temperature-sensitive TRPVs has yet to be determined.
TRPVs have a channel domain homologous to Shaker K+ channels and cytosolic N- and C-terminal domains, including a conserved N-terminal ankyrin repeat domain (ARD) (22). TRPV1-, TRPV2-, and TRPV6-ARD structures have been reported (15, 23–25). The crystal structure of TRPV1-ARD revealed a bound ATP molecule, and it was shown that ATP and Ca2+-CaM compete for a common binding site on TRPV1-ARD (15). Intracellular ATP sensitizes TRPV1, while both Ca2+-CaM and its binding site on the ARD are necessary to inactivate TRPV1 (15).
We investigated whether the modulatory binding site found on TRPV1-ARD exists in other TRPV channels. We demonstrate that TRPV3- and TRPV4-ARD also bind ATP and Ca2+-CaM. Similar to TRPV1, TRPV4 is sensitized by intracellular ATP and a binding site mutation eliminates this sensitization. In contrast, intracellular ATP prevents TRPV3 sensitization to 2-APB, and binding site mutations confirm a role for the ARD in regulating TRPV3 sensitivity. Moreover, the ARD is key to the previously reported sensitivity of TRPV3 to intracellular Ca2+ and CaM (21). Potential physiological roles of this multiligand binding site conserved on several thermosensitive TRPV channels include setting channel responsiveness to stimuli and adaptation to the metabolic state.
MATERIALS AND METHODS
Cloning of Expression Vectors
cDNA fragments encoding ARDs (human TRPV3-ARD residues 115–367 and chicken TRPV4-ARD residues 132–383) and full-length protein (human TRPV3 and chicken TRPV4) were cloned into the NdeI and NotI sites of pET21-C6H (23) and pFastBac-CFLAG (15) vectors, respectively. Baculovirus stocks were generated and used to infect Sf21 cells as described in the Bac-to-Bac manual (Invitrogen). Full-length TRPVs in pcDNA3 were provided by Michael Caterina (Johns Hopkins School of Medicine; rat TRPV2), David Clapham (Harvard Medical School; human TRPV3) and Stefan Heller (Stanford University; chicken TRPV4). All mutants were generated by mutagenesis, and all clones were verified by DNA sequencing.
Expression and Purification of TRPV ARDs
The ARDs were expressed in Escherichia coli BL21(DE3) by induction with 0.4 mm isopropyl-β-d-thiogalactopyranoside overnight at room temperature after the cells reached A600 = 0.6. Cells were resuspended in lysis buffer (20 mm Tris-HCl (pH 8.0), 300 mm NaCl, 20 mm imidazole (pH 8.0), and 1 mm phenylmethylsulfonyl fluoride) with 0.1% Triton X-100, 0.2 mg/ml lysozyme, 50 μg/ml RNase A, and 25 μg/ml DNase I and lysed by sonication. The cleared lysate was loaded onto nickel-nitrilotriacetic acid (Qiagen) and eluted by a step gradient containing 50, 100, 150, and 200 mm imidazole (pH 8) in lysis buffer. Ten mm EDTA (pH 8.0) and 1 mm dithiothreitol (DTT) were added after elution. The fractions containing TRPV3-ARD or TRPV4-ARD were pooled and further purified on Q or SP Sepharose FF (GE Healthcare), respectively, in 20 mm Tris (pH 8.0), 5 mm DTT using a linear gradient of 0–0.4 M NaCl. Size exclusion chromatography on a Superdex 75 column (GE Healthcare) in 10 mm Tris-HCl (pH 8.0), 200 mm NaCl, and 1 mm DTT was used for further purification of TRPV3-ARDs, whereas the TRPV4-ARDs were dialyzed in 20 mm Tris-HCl (pH 8.0), 300 mm NaCl, 10% glycerol, and 1 mm DTT. All proteins were concentrated to >7 mg/ml in a Vivaspin centrifugal filter (10,000 molecular weight cut off; Sartorius AG, Goettingen, Germany), flash frozen, and stored at −80 °C. TRPV1-ARD, TRPV2-ARD, TRPV5-ARD, and TRPV6-ARD were purified as described previously (15, 23, 25).
ATP- and CaM-Agarose Pulldown Assays
All assays were carried out at 4 °C as described previously (25). The ATP-agarose assays were performed in the absence of divalent ions except otherwise noted, in binding buffer (10 mm Tris-HCl (pH 7.5), 50 mm NaCl, 1 mm DTT, and 0.15% n-decyl-β-d-maltopyranoside; except 150 mm NaCl was used for TRPV4-ARD mutant analyses to preserve protein solubility). For ATP competition assays, competing compounds were added to reaction mixtures prior to the agarose slurry. All nucleotides used where sodium salts diluted from 0.5 m stocks adjusted to pH 7 with NaOH. The CaM-agarose assays were performed in binding buffer supplemented with 2 mm CaCl2 or 5 mm EGTA (pH 7.5). In each load lane, the volumes loaded corresponded to 2 μg of protein. Gels were quantified using ImageJ (26), and shown are the average ± S.D. for at least three independent experiments.
Insect and Mammalian Cell Culture and Full-length TRPV Protein Expression
Sf21 insect cells were maintained in Hink's TNM-FH (Mediatech, Manassas, VA), supplemented with 10% fetal bovine serum, 0.1% pluronic F-68, and 10 μg/ml gentamycin. Cells at 5 × 105 cells/ml were adhered to glass coverslips in medium without pluronic F-68 and infected with baculovirus. HEK293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, GlutaMAX (Invitrogen), 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were co-transfected with pNEGFP and pcDNA3 containing the appropriate full-length TRPV using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer directions.
Electrophysiology
Insect cells were tested 44–48 h postinfection and HEK293 cells were tested 20–25 h post-transfection under continuous perfusion using a multichamber perfusion apparatus for agonist application. 2-APB and thymol were dissolved in dimethyl sulfoxide and 4α-phorbol 12,13-didecanoate (4αPDD) in ethanol prior to dilution in bath solution. Currents were recorded and analyzed as described (15). Data are presented as mean ± S.E. The intracellular/pipette solution contained 140 mm NaMethanesulfonate, 10 mm HEPES, and either (4 mm NaCl and 10 mm EGTA) for EGTA conditions or (0.6 mm MgCl2 and 10 mm BAPTA, resulting in 0.4 mm free Mg2+ according to MaxChelator (27)) for BAPTA conditions. The BAPTA conditions were very similar to those used in Ref. 21. The pH was adjusted to 7.2 with NaOH, and the final osmolarity was ∼315 mOsm. As indicated, the intracellular solution was supplemented with 4 mm ATP (sodium salt) or ATPγS (lithium salt) from 0.5 m stocks (pH adjusted to ∼7 with NaOH). In EGTA conditions, all ATP should be free ATP, whereas in BAPTA conditions, the presence of 0.6 mm MgCl2 results in 0.001 mm free Mg2+, 0.58 mm Mg-ATP, and 3.42 mm free ATP (27). For CaM depletion experiments, the intracellular solution was supplemented with 2 μg/ml CaM85 or an isotype-matched control antibody (Invitrogen). The extracellular/perfusion solution was 150 mm NaCl, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 10 mm HEPES, and 10 mm d-glucose (pH adjusted to 7.4 with NaOH; ∼315 mOsm), except for TRPV3 dose-response experiments and TRPV4 voltage step experiments in insect cells, where the extracellular solution was 150 mm NaGluconate, 10 mm NaCl, 2 mm CaCl2, 10 mm HEPES, and 10 mm d-glucose (pH adjusted to 7.2 with NaOH; ∼ 315 mOsm), which produced more stable seals with less leak current at high agonist concentrations.
Data Analysis
EC50 values were calculated by fitting the average normalized current at −100 mV for a range of agonist concentrations to the Hill Equation, I(S) = 1 − (Kn/(Kn + Sn)), where I is the current, K is the EC50, S is the agonist concentration, and n is the Hill coefficient. Tail currents from voltage step experiments in HEK293 cells used for the determination of TRPV4 V½ were measured during the first millisecond of a step to a voltage of −160 mV and normalized to the maximum current. Average tail currents were fit to a modified Boltzmann function: G(V) = Gmax − (Gmax − Gmin)/(1 + exp(zF/RT*(V − V½))), where z is the valence of the gating charge and F/RT is 25 mV−1. Statistical analyses were performed using a two-tailed t test, with p < 0.05 being considered statistically significant. Data are presented as mean ± S.E.
RESULTS
TRPV3-ARD and TRPV4-ARD Bind ATP and Ca2+-CaM
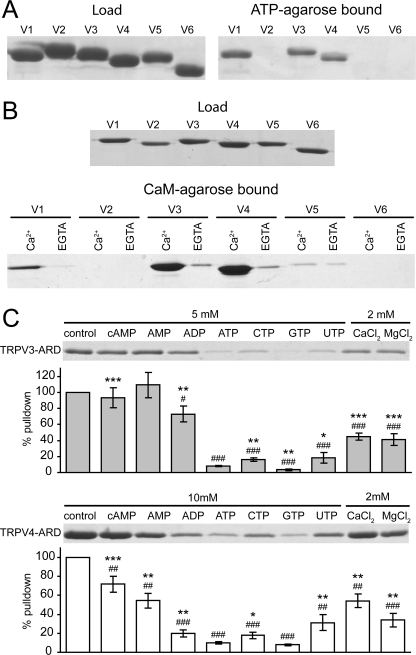
To determine whether the ATP/CaM-binding site on the TRPV1-ARD is conserved in other TRPV channels, the ARDs from all six TRPV channels common to warm-blooded vertebrates were tested for ATP binding in pulldown assays (Fig. 1). As previously observed (15, 25), the TRPV1-ARD bound ATP-agarose, while the TRPV2-, TRPV5- and TRPV6-ARDs did not. Both TRPV3-ARD and TRPV4-ARD were precipitated by ATP-agarose (Fig. 1A), suggesting that the TRPV1-ARD ATP-binding site is conserved in TRPV3 and TRPV4. Furthermore, the three ARDs that interact with ATP, the TRPV1-, TRPV3- and TRPV4-ARDs, were also precipitated with CaM-agarose in the presence of Ca2+, and this interaction was eliminated in the presence of EGTA, a Ca2+-chelator (Fig. 1A). As previously determined, the TRPV2-, TRPV5-, and TRPV6-ARDs interacted either very weakly or not at all with CaM-agarose (Fig. 1B) (15, 25).
FIGURE 1.
Interactions of TRPV ARDs with ATP and CaM. A, Coomassie-stained gel of an ATP-agarose pulldown assay with the six TRPV ARDs, showing loaded (left) and ATP-agarose-bound (right) proteins. B, Coomassie-stained gels of a CaM-agarose pulldown assay of the six TRPV ARDs showing loaded protein (top) and protein bound in the presence of Ca2+ or EGTA (bottom). C, nucleotide specificity of the TRPV3- and TRPV4-ARD. Coomassie-stained gels of wild type TRPV3-ARD (top) or TRPV4-ARD (bottom) bound to ATP-agarose in the presence of the indicated concentration of competing compounds. The histogram below each representative gel shows the average amount of protein recovered (±S.D.) in the absence or presence of nucleotide and divalent cations over four experiments. The statistical significance with respect to control (#, p < 0.05; ##, p < 0.01; ###, p < 0.001) and ATP (*, p < 0.05; **, p < 0.01; ***, p < 0.001) was determined using two-tailed t tests.
The ATP and Ca2+-CaM Binding Site Is Conserved in TRPV3-ARD and TRPV4-ARD
To further characterize the properties of the ATP-binding site on TRPV3 and TRPV4, we tested its specificity in competition assays with other nucleotides. As previously reported with TRPV1 (15), free GTP and ATP most efficiently competed for binding to ATP-agarose for both TRPV3-ARD and TRPV4-ARD (Fig. 1C). Furthermore, Ca2+ and Mg2+ also reduced binding to ATP-agarose (Fig. 1C). Therefore, the ATP-binding sites on the ankyrin repeats of TRPV3 and TRPV4 have the highest affinity for divalent-free triphosphate nucleotides, with a small preference for purines over pyrimidines, a specificity profile comparable to TRPV1-ARD (15).
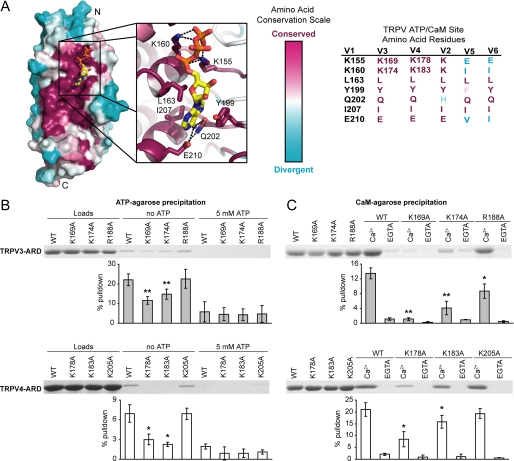
The similar nucleotide specificities of TRPV3-ARD, TRPV4-ARD, and TRPV1-ARD strongly suggest that ATP interacts with these domains at a conserved site. The overall sequence conservation of TRPV1, TRPV3, and TRPV4 (supplemental Fig. 1) was mapped onto the structure of TRPV1-ARD bound to ATP (Fig. 2A). The most conserved surface encompasses the ATP-binding site. We used mutagenesis to confirm that the conserved phosphate-binding residues are important for the interaction of TRPV3-ARD and TRPV4-ARD with ATP. Lys155 and Lys160 interact with the triphosphate moiety of ATP in the TRPV1-ARD structure and are important for ATP and CaM binding (15). The corresponding lysines, Lys169 and Lys174 in TRPV3-ARD and Lys178 and Lys183 in TRPV4-ARD, were mutated to alanine. Arg188 in TRPV3 and Lys205 in TRPV4, predicted to lie on the opposite face of the ARDs, were also mutated to alanine and used as negative controls. In pulldown assays, the TRPV3-ARD and TRPV4-ARD lysine mutants showed reduced binding to both ATP and CaM compared with the wild type proteins and negative control mutants (Fig. 2B and 2C). In summary, the lysines homologous to Lys155 and Lys160 in TRPV1 are also necessary for TRPV3 and TRPV4 interactions with ATP and CaM, indicating that the multiligand binding site previously identified in TRPV1 (15) is conserved in TRPV3 and TRPV4.
FIGURE 2.
A conserved ATP/CaM binding site in the ARDs of TRPV1, TRPV3, and TRPV4. A, The amino acid conservation between these three ARDs was calculated and mapped onto the surface of the TRPV1-ARD structure (Protein Data Bank code 2PNN) using Consurf (44) based on the alignment in supplemental Fig. 1. The most conserved and divergent residues are purple and cyan, respectively. The ATP binding site is magnified to show the amino acid side chains that contact ATP. The identity of the TRPV1 site and corresponding residues in the other five TRPVs is shown on the right. B, Coomassie-stained gels of wild type and mutant TRPV3-ARD (top) or TRPV4-ARD (bottom) loaded (left) and bound to ATP-agarose in the absence (middle) or presence (right) of competing free ATP. C, Coomassie-stained gels show wild type and mutant TRPV3-ARD (top) or TRPV4-ARD (bottom) loaded (left) and bound to CaM-agarose in the presence of Ca2+ or EGTA. In B and C, the average percentage of protein recovered (±S.D.) is plotted below. The statistical significance of the reduction in binding to ATP-agarose or Ca2+-CaM-agarose with respect to wild type (WT) was determined by one-tailed t tests, with p < 0.05 and p < 0.01 indicated by * and **, respectively.
TRPV2 Is Insensitive to Intracellular ATP
Electrophysiology experiments demonstrated that intracellular ATP can sensitize TRPV1 and prevent its desensitization to repeated applications of capsaicin (15). Experiments with the K155A and K160A mutants of TRPV1 also indicated that these effects of ATP were through its direct interaction with the TRPV1-ARD. Rat TRPV2 was hypothesized to be a natural negative control; ATP and Ca2+-CaM were not expected to affect its sensitivity because its ARD did not bind either. Rat TRPV2 expressed in HEK293 cells responded to 2-APB in whole cell patch clamp recordings as reported previously (10, 28). TRPV2 exhibited similar currents when stimulated with 2-APB in the absence or presence of intracellular ATP (Fig. 3). Furthermore, no significant desensitization or tachyphylaxis was observed in response to repeated 2-APB applications. Therefore, TRPV2 activity was not affected by the presence of intracellular ATP, correlating with the lack of interaction between ATP and the TRPV2-ARD.
FIGURE 3.
TRPV2 is insensitive to intracellular ATP. A, sample whole cell patch clamp recordings from TRPV2 expressing HEK293 cells with (right) and without (left) 4 mm ATP in the intracellular solution. Currents at +80 mV (gray) and −80 mV (black) were extracted from linear voltage ramps. Gray bars indicate perfusion with 0.4 mm 2-APB, and black lines indicate zero current. B, average maximum current density evoked during the first 2-APB application. B, TRPV2 does not undergo tachyphylaxis. Currents evoked by multiple 2-APB applications at ±80 mV were normalized to the maximum current from the first 2-APB application. For both B and C, control cells (n = 6) are colored gray and cells with intracellular solution supplemented with 4 mm ATP are colored white (n = 6).
We attempted to generate a TRPV2-ARD mutant that could bind ATP and/or CaM. We looked at two mutations: D78N, which neutralizes a negatively charged side chain which maps in close proximity of the phosphate-interaction site, and H165Q, to attempt to restore the adenine-binding pocket (supplemental Fig. 2). Neither of the single mutants bound to ATP- or CaM-agarose in our assays. The D78N/H165Q mutant bound weakly but significantly to ATP, but not CaM. Because the TRPV2-ARD is only 50% identical to the TRPV1-ARD, it is difficult to determine which other sequence differences may be responsible for the differences in biochemical properties.
TRPV4 Is Sensitized by Intracellular ATP
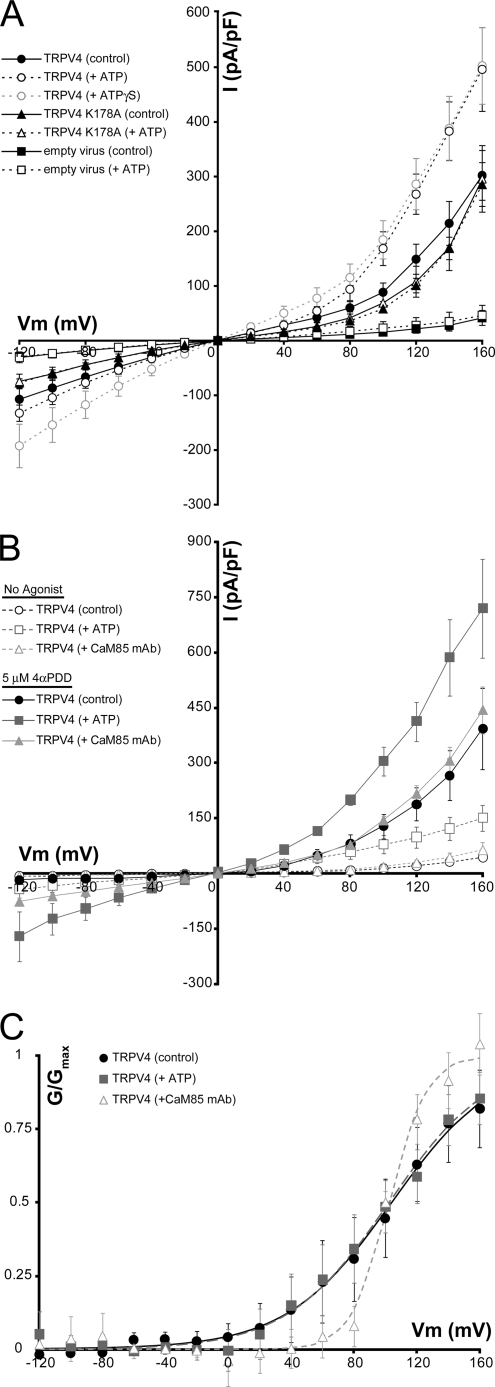
We used whole cell patch clamp electrophysiology to determine the effect of intracellular ATP on the sensitivity of TRPV4 expressed in insect and HEK293 cells. TRPV4 showed constitutive basal activity in both cell types (Fig. 4 and supplemental Fig. 3), similar to previous observations (e.g. Refs. 6, 7). In voltage step experiments in insect cells, TRPV4 currents were significantly increased in the presence of intracellular ATP or the nonhydrolyzable ATP analog ATPγS (Fig. 4A). Furthermore, the K178A mutation, which reduces ATP binding, abolished sensitization by ATP (Fig. 4A).
FIGURE 4.
TRPV4 is sensitized by intracellular ATP. A, average current density from voltage step experiments in insect cells plotted against holding potential for cells recorded in the absence (solid symbols) or presence of intracellular ATP (black, open symbols) or ATPγS (gray, open symbols). Data from control cells infected with empty virus (n = 4 each; squares), wild type (WT) TRPV4 data (n = 7 each; circles), and TRPV4 K178A data (n = 7 each; triangles) are shown. ATP and ATPγS cause a significant current increase (p < 0.05 at Vm > 100 mV). B, average current density plotted against holding potential from voltage step experiments in TRPV4-expressing HEK293 cells. Data were collected on unstimulated (open symbols) and 4αPDD-perfused cells (5 μm; filled symbols) with control intracellular solution (black circles, n = 6), 4 mm ATP (dark gray squares, n = 7) or an anti-CaM monoclonal antibody (CaM85, light gray triangles, n = 6). C, activation curves from TRPV4-expressing HEK293 cells calculated from the average, normalized tail currents measured in the first milliseconds after a step to −160 mV from the cells in B (control, black circles; ATP, dark gray squares; CaM85 mAb, light gray triangles). Lines represent the fit of a modified Boltzmann function to the data.
Similar results were obtained from basal TRPV4 currents in HEK293 cells (Fig. 4B), although the lower constitutive activity in HEK293 cells enabled us to also look at 4αPDD-stimulated activity. Currents observed after perfusion with 4αPDD were also significantly increased by the addition of ATP to the recording solution (Fig. 4B). The effect of ATP was similar in both 4αPDD-stimulated and constitutive conditions (at +100 mV, constitutive currents increased 1.9-fold and 3.0-fold in insect and HEK293 cells, respectively, while 4αPDD-stimulated currents increased 2.4-fold). Furthermore, depleting HEK293 cells of CaM by including a monoclonal anti-CaM antibody in the intracellular solution, as was previously done in TRPV1-expressing cells (15), did not affect the voltage response of unstimulated TRPV4, but did significantly increase inward currents in TRPV4 expressing HEK293 cells treated with 4αPDD (p < 0.05 at Vm ≤ −20 mV).
Similar to TRPV1 (15), this increased current density for TRPV4 in the presence of intracellular ATP appears to be a result of increased whole cell conductance, rather than a shift in the current-voltage relationship. Tail-current analyses from voltage step experiments in TRPV4-expressing HEK293 cells with control, 4 mm ATP, or anti-CaM antibody intracellular solutions (Fig. 4C and supplemental Fig. 3) show that the V½ of TRPV4 is not altered by ATP or CaM with V½ values of 104 ± 36.4, 102 ± 8.3, and 102 ± 13.6 mV for the control, ATP and anti-CaM experiments, respectively. Of note, the anti-CaM antibody increases the steepness of the G/V curve, suggesting that CaM may affect the intersubunit cooperativity of TRPV4. Overall, these results strongly suggest that the previously observed potentiation or desensitization by binding of intracellular ATP or CaM, respectively, to the N-terminal ankyrin repeats of TRPV1 is conserved in TRPV4.
ATP Lowers the Agonist Sensitivity of TRPV3
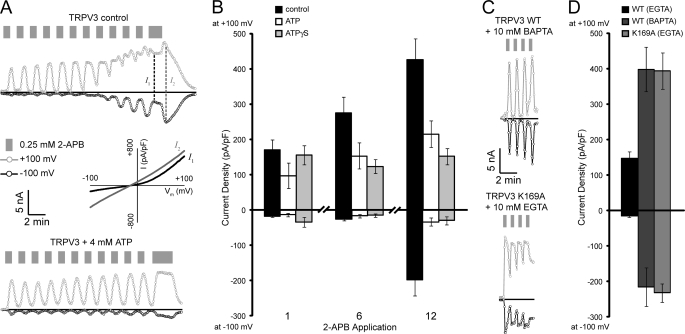
Similar to previously published reports using mammalian cells (21, 29), TRPV3 expressed in insect cells is sensitized by repeated applications of 2-APB (Fig. 5A). Once sensitized, TRPV3 also showed biphasic currents (Fig. 5A) where the initial outward rectified current (I1) is followed by an off-response with the appearance of a less rectified, higher amplitude current that is slower to inactivate (I2), similar to the currents reported in HEK293 cells and primary keratinocytes overexpressing TRPV3 (30). The sensitization of TRPV3 to repeated agonist applications is in contrast to what is observed with TRPV1, which is desensitized by repeated agonist applications (14, 15). Also unlike TRPV1 and TRPV4, intracellular ATP blocked the sensitization of TRPV3 to repeated 2-APB applications (Fig. 5B). The same effect was observed when ATPγS was used, supporting the idea that it is ATP binding, not an ATP hydrolysis-dependent process, that prevents TRPV3 sensitization. There is no significant difference between the currents observed during the first and twelfth 2-APB applications in presence of intracellular ATP or ATPγS. Furthermore, the currents observed on the twelfth 2-APB application with the control cells are significantly larger than in cells with intracellular ATP or ATPγS (Fig. 5B). Additionally, while biphasic currents and off-responses were observed for seven of the nine control cells tested, none of the ATP (0/6) or ATPγS (0/7) cells showed biphasic currents or off-responses.
FIGURE 5.
Sensitization of TRPV3 in insect cells. A, sample whole cell patch clamp recordings from baculovirus-infected insect cells expressing wild type TRPV3. Shown are currents at +100 (gray circles) or −100 mV (black circles) extracted from linear voltage ramps from a control cell (top) and cells with intracellular ATP (bottom). Applications of 0.25 mm 2-APB are indicated by gray bars and zero current by black lines. For the control cell the dashed lines indicate the time points for the I–V traces (plotted as current density versus membrane voltage) from type 1 (I1) and off-response type 2 (I2) currents, which are shown below the control. B, control cells are sensitized by repeat applications of 2-APB, and this is blocked by ATP and ATPγS. Average current densities (pA/pF) at +100 and −100 mV are shown for the first, sixth, and twelfth applications of 2-APB to TRPV3-expressing cells with intracellular solutions containing no nucleotide (control; black bars, n = 9), ATP (white, n = 6) or ATPγS (gray, n = 7). C, sample whole cell recordings from insect cells expressing wild type TRPV3 with BAPTA as the intracellular calcium buffer (top) and TRPV3 K169A with EGTA as the intracellular calcium buffer (bottom) collected and displayed as in A. D, average maximum current density at +100 and −100 mV from a 30 s application of 0.25 mm 2-APB for wild type (WT) TRPV3 with EGTA (black bars) or BAPTA (dark gray bars), and K169A TRPV3 with EGTA (gray bars). Note that C and D are on the same scales as A and B, respectively.
The sensitization of TRPV3 is dependent on the strength of the intracellular Ca2+ buffer. When BAPTA, a more rapid and specific Ca2+ buffer, was used in place of EGTA, TRPV3 was pre-sensitized, showing large responses to the first application of 2-APB and little increased sensitivity to subsequent 2-APB applications (21). This behavior could also be reproduced in our insect cell system (Fig. 5, C and D). Also, TRPV3 K169A (one of the ATP/CaM site mutants that no longer bound ATP or CaM) (Fig. 2) showed initial current densities similar to those of wild type TRPV3 in the presence of BAPTA, even when EGTA was used as the Ca2+ buffer (Fig. 5). The TRPV3 K169A currents were similar to the I2 currents observed with sensitized wild type TRPV3, with large amplitudes, little rectification, and slower deactivation after removal of 2-APB. Consistent with a sensitized state, the average current density from the first 2-APB application for TRPV3 K169A was as large as that for wild type TRPV3 either from the twelfth 2-APB application in experiments with EGTA as the Ca2+ buffer, or the first 2-APB application when presensitized with BAPTA as the Ca2+ buffer (Fig. 5). These results show that disruption of ATP/CaM binding eliminates the sensitivity of TRPV3 to intracellular Ca2+ levels and indicate that interaction of ATP and/or Ca2+-CaM on the N-terminal ankyrin repeats regulates TRPV3 sensitization.
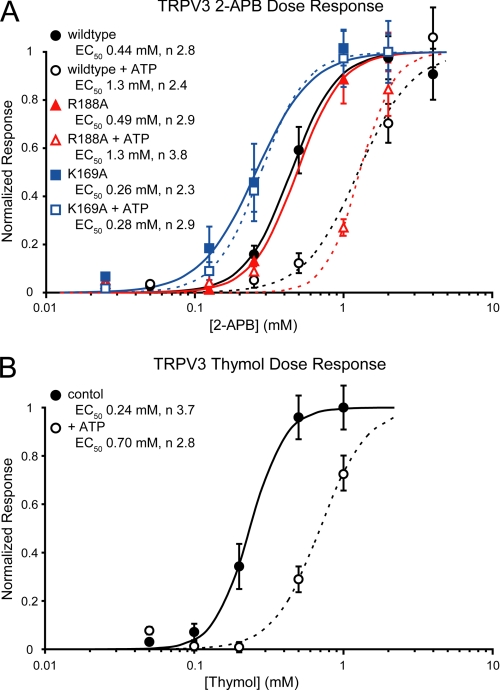
To further characterize the mechanism by which intracellular ATP regulates TRPV3 sensitivity, dose-response relationships were measured for two different TRPV3 agonists, 2-APB and thymol, in the absence or presence of intracellular ATP (Fig. 6). Dose-response experiments were carried out with BAPTA to presensitize TRPV3 and remove any confounding effects on the dose response from repeated agonist applications. Additionally, the chloride ions in the extracellular solution were replaced with gluconate. Replacing chloride with gluconate lowers the agonist-induced TRPV3 currents, allowing us to also determine the dose response of TRPV3 K169A, which was otherwise difficult to inactivate after the first agonist application (supplemental Fig. 4). Thymol concentrations above 1 mm were toxic and as a result saturated currents could not be recorded in the presence of ATP. The responses of TRPV3 to thymol in the absence or presence of ATP were both normalized to the maximum current density from the experiments without ATP. ATP increases the EC50 of both agonists by ∼3-fold (Fig. 6), indicating that intracellular ATP reduces the sensitivity of TRPV3 to its agonists.
FIGURE 6.
ATP lowers the sensitivity of TRPV3 to chemical agonists. A, dose response of TRPV3 to 2-APB. The dose response of wild type (black circles), R188A (red triangles), and K169A (blue squares) TRPV3 to 2-APB were determined from control cells (filled symbols) and cells with intracellular ATP (open symbols). Normalized responses (based on the average maximum current density at −100mV) are plotted against the concentration of 2-APB. Fits of the data to the Hill equation are shown as solid (control cells) or dashed lines (+ ATP), and the resulting EC50 and Hill coefficients (n) values are listed for each sample. B, dose response of wild type TRPV3 currents to thymol, measured as in A, showing control cells (filled circles; solid line) and cells with intracellular ATP (open circles; dashed line).
TRPV3 K169A showed an increased sensitivity to 2-APB with an EC50 ∼2-fold lower than wild type TRPV3, and it is unchanged in the presence of ATP (Fig. 6). The changes in sensitivity between K169A and wild type TRPV3 are not due to differences in expression (supplemental Fig. 5) and instead could result from the loss of ATP and CaM binding in K169A. In contrast, the behavior of the TRPV3 R188A control mutant is indistinguishable from wild type either in the presence or absence of intracellular ATP (Fig. 6). These results strongly support the role of ATP binding to the TRPV3-ARD in altering agonist sensitivity.
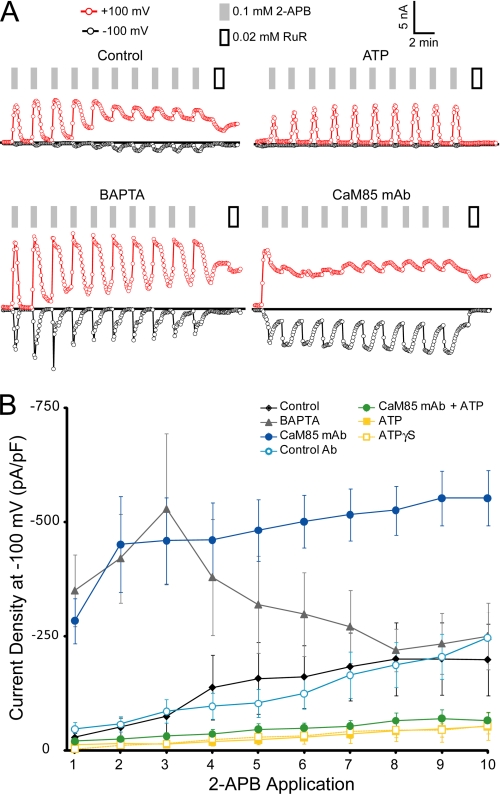
TRPV3 behaved similarly in HEK293 cells. Intracellular ATP or ATPγS reduced sensitization compared with control (EGTA), whereas intracellular BAPTA caused significantly increased current densities for the first three 2-APB applications (Fig. 7). In HEK293 cells, we could also test the effects of CaM depletion using an anti-CaM monoclonal antibody. Addition of anti-CaM antibody into the intracellular solution led to immediate sensitization and significantly larger current densities for all 2-APB applications, while currents in the presence of an isotype-matched control antibody were indistinguishable from control (Fig. 7B). Furthermore, the 2-APB-induced currents from CaM-depleted cells were similar to those observed with the K169A mutant in insect cells in that they did not inactivate upon agonist removal and showed little rectification (compare Fig. 5C with Fig. 7A). Taken together, the results from HEK293 and insect cells indicate that there is direct role for CaM binding to the conserved ARD site in TRPV3 inactivation and that ATP binding to the same site can maintain TRPV3 in a low sensitivity state. This is in agreement with a previous report that sensitization of TRPV3 results from a loss of CaM binding (21) and further demonstrates a role for the ARD in this CaM-mediated regulatory mechanism.
FIGURE 7.
Ca2+-CaM and ATP decrease the sensitivity of TRPV3 in HEK293 cells. A, sample whole cell patch clamp recordings from transiently transfected HEK293 cells expressing wild type TRPV3. Shown are currents at +100 (red circles) or −100 mV (black circles) extracted from linear voltage ramps from cells with different intracellular solutions; control (top left), 4 mm ATP (top right), 10 mm BAPTA (lower left), and 2 μg/ml anti-CaM antibody (Ab) (CaM85, lower right). Application of 0.1 mm 2-APB to the cells is shown by gray bars. White bars indicate application of 20 μm ruthenium red (RuR), a channel blocker. B, average current density at −100 mV (in pA/pF or picoampere per picofarad) from 10 consecutive applications of 2-APB for cells with control intracellular solution (black diamonds), 10 mm BAPTA (gray triangles), CaM85 monoclonal antibody (mAb; dark blue circles), isotype matched control antibody (light blue circles), ATP (yellow squares), ATPγS (open yellow squares), and both ATP and CaM85 monoclonal antibody (green circles).
DISCUSSION
We find that the binding of ATP and Ca2+-CaM to the N-terminal ARD observed in TRPV1 (15) is conserved in two of the three other thermo TRPVs, TRPV3, and TRPV4. Intracellular ATP increased TRPV4 currents in response to voltage steps, indicating a sensitizing effect, similar to the effect of intracellular ATP on TRPV1, increasing currents in response to capsaicin. In contrast, the response of TRPV3 to agonists is reduced in the presence of intracellular ATP. More precisely, intracellular ATP prevented the sensitization of TRPV3 to repeated applications of 2-APB and increased the EC50 for agonists. Furthermore, mutagenesis and electrophysiology data support a role for the ATP/CaM binding site on the ARD in regulating both TRPV3 and TRPV4 sensitivity. Therefore, although the biochemical properties of the TRPV1-, TRPV3-, and TRPV4-ARDs are similar, there are marked differences in functional consequences of modulatory interactions with the ARD.
Of the four thermosensitive TRPVs, only TRPV2 did not bind to ATP or Ca2+-CaM through its ARD. This is likely a result of several amino acid substitutions within and around the conserved ATP/CaM binding site (Fig. 2 and supplemental Fig. 2) (15, 23, 24). Accordingly, we saw no significant effects of intracellular ATP on TRPV2 currents (Fig. 3). Although TRPV2-ARD does not interact with ATP or CaM, it is still important for TRPV2 function, since deletions of parts of the ARD impair activation by 2-APB or heat and surface localization (28).
Our data do not directly demonstrate a physical interaction between ATP and the TRPV ARDs under the patch clamp conditions where the effects of intracellular ATP on channel sensitivity were observed. It is therefore difficult to rule out an indirect effect of intracellular ATP. However, several observations support a direct binding of ATP to the ARDs. First, similar results are obtained in two different cell types, HEK293 and insect cells, ruling out factors that are not conserved in both cell types. Second, the effects of ATP can be observed in the absence of divalent cations and/or presence of chelator in the intracellular solution and are reproduced by ATPγS, a poorly hydrolyzable ATP analog. This argues against an ATP-hydrolysis-dependent process (e.g. phosphoinositide synthesis). Third, the disruption of the ligand-binding site on the ARD by mutagenesis, confirmed biochemically, eliminated the effect of ATP on channel function in TRPV1 (15), TRPV3, and TRPV4. This supports a direct role for ATP binding to the ARD in regulating TRPV channel sensitivity.
What might be the physiological purpose of intracellular ATP-meditated regulation of TRPV ion channels? As suggested above, the overall role of the ATP/CaM binding site on the ARD may be to tune the sensitivity of TRPV channels. Regulation by intracellular ATP has also been observed in other ion channels, including TRP channels TRPC5 (31), TRPM4 (32), and TRPM6 (33). KATP channels use several nucleotide-binding sites to sense nucleotide levels and have been implicated in sensing metabolic levels in tissues ranging from muscles to the pancreas to neurons, tying membrane potential to the metabolic level of the cell (34). Furthermore, the C-terminal domain of ClC-type chloride channels binds adenine nucleotides (35), and, at least under some circumstances, intracellular adenine nucleotides inhibit ClC channels, although the ATP-mediated regulation of ClCs remains controversial (36). Hence, intracellular ATP may play an important role in modulating physiological functions of multiple channel families including TRPV channels. The data on fluctuations of nucleotide concentration in cellular physiology are still sparse, but some studies suggest that such variations may be important (37). Thus, changes in cellular nucleotide concentrations reflecting the metabolic state, either local or global, could directly affect TRPV channel sensitivity.
Alternatively, ATP may act as a cofactor in sensing Ca2+ levels. ATP binding to TRPV ARDs is sensitive to the divalent cation concentration: only free ATP has high affinity for the binding site. High concentrations of Ca2+ disrupt the interaction with ATP, presumably through Ca2+ chelation by the triphosphate moiety and favor the interaction with Ca2+-CaM. Of note, although most ATP is chelated by Mg2+ in vivo, the cellular concentration of free ATP is still significant, ranging from 0.3–0.7 mm (Ref. 38 and references therein). It was suggested that the competition of ATP with CaM for the same binding site on the ARD could provide sensitivity to global Ca2+ levels while making the channel less sensitive to transient local Ca2+ concentration changes that rapidly dissipate (39). That is, the competition between ATP and CaM affects the kinetic and thermodynamic parameters of the channel modulation by Ca2+. In such a scenario, ATP could be considered a cofactor tuning the sensitivity of TRPV channels to intracellular Ca2+.
The different modulatory effects of the ATP/CaM binding site on TRPV3 versus TRPV1 and TRPV4 may have arisen to provide different basal sensitivity and/or feedback mechanisms. That is, the physiological roles of these channels, which are still being uncovered (see Ref. 40 for a recent review), likely require different adaptation and potentiation mechanisms. TRPV3, unlike TRPV1 and TRPV4, is sensitized by repeated agonist applications. The data presented here (Fig. 7) and by others (21) clearly show that TRPV3 is sensitized by the removal of CaM. Here we further show that these effects are mediated through the conserved ATP/CaM site in the TRPV3-ARD (Fig. 5). Moreover, ATP binding maintains the TRPV3 channel in a low sensitivity state, even though it also prevents CaM binding. We hypothesize that TRPV3 undergoes a conformational change in the open state that decreases the ability of TRPV3 to bind CaM, making TRPV3 easier to open and slower to close. The channel is slow to revert back to the CaM-binding state, and therefore further stimulations result in an increased population of the sensitized TRPV3 state. On the other hand, according to our model, ATP binding to the ARD holds TRPV3 in a lower sensitivity state, requiring higher agonist concentrations to activate the channel (Fig. 6) and preventing the transition to the sensitized state.
The structural similarity of the ligand-free TRPV2-ARD (23, 24) and ATP-bound TRPV1-ARD (15) suggests that ligand binding causes little conformational change in the ankyrin repeats. This is supported by a recent survey of ankyrin repeat structures; ligand binding typically imposes little conformational change on ankyrin repeats (22). The molecular basis for the differences between TRPV3 and its close homologs, TRPV1 and TRPV4, may instead originate from distinct pathways within the protein to decode the bound regulatory ligand on the ARD and communicate to the channel gate. Notably, the TRPV3-ARD sequence diverges from both the TRPV1-ARD and TRPV4-ARD sequences, with several deviations on the side opposite the conserved binding site (supplemental Fig. 1). Hence, these sequence variations may contribute to the different responses to intracellular ATP in TRPV1 and TRPV3 by engaging distinct interactions with other regions of the channel. Differences outside of the ARD likely contribute as well. In fact, mutagenesis screens on TRPV1 (41) and TRPV3 (42) identified other regions involved in channel sensitivity and activation, which might act in concert with the ARDs to regulate channel activity (Fig. 8). In TRPV3, the pore region has been implicated in both heat activation (42) and regulation by extracellular calcium (21). The intracellular membrane proximal regions at both the N and C termini were implicated in 2-APB activation (43). Further experiments will be required to determine how all these different local interactions converge to effect channel gating. The conserved biochemical properties but distinct modulatory outputs of ATP binding to the ARD of TRPV1 and TRPV4 versus TRPV3 provide a starting point to design such experiments.
FIGURE 8.
Topology of TRPV3 and location of functionally important sites. The ARD is important in the tuning of TRPV3 sensitivity and interacts with ATP (square) and Ca2+-CaM (starred triangle; see also Ref. 21). The previously identified sites of heat activation (Ile644, Asn647, and Tyr661 marked as white stars; Ref. 42), activation by 2-APB (cytoplasmic residues His426 and Arg696, circles; Ref. 43), and calcium sensitivity (intracellular site Arg696; Ref. 42) and extracellular site Asp641; Ref. 21) are also indicated.
Supplementary Material
Acknowledgments
We thank current and former lab members for technical help and discussions.
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM081340. This work was also supported by American Heart Association Grant (Scientist Development Grant 0335134N) and a Klingenstein Award and a McKnight Scholar Award (to R. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- TRPV
- transient receptor potential vanilloid
- ARD
- ankyrin repeat domain
- 2-APB
- 2-aminoethyl diphenylborinate
- CaM
- calmodulin
- DTT
- dithiothreitol
- 4αPDD
- 4α-phorbol 12,13-didecanoate
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
REFERENCES
- 1.Venkatachalam K., Montell C. (2007) Annu. Rev. Biochem. 76, 387–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nijenhuis T., Hoenderop J. G., Bindels R. J. (2005) Pflugers Arch. 451, 181–192 [DOI] [PubMed] [Google Scholar]
- 3.Caterina M. J. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R64–R76 [DOI] [PubMed] [Google Scholar]
- 4.Tominaga M., Caterina M. J. (2004) J. Neurobiol. 61, 3–12 [DOI] [PubMed] [Google Scholar]
- 5.Xu H., Delling M., Jun J. C., Clapham D. E. (2006) Nat. Neurosci. 9, 628–635 [DOI] [PubMed] [Google Scholar]
- 6.Liedtke W., Choe Y., Martí-Renom M. A., Bell A. M., Denis C. S., Sali A., Hudspeth A. J., Friedman J. M., Heller S. (2000) Cell 103, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strotmann R., Harteneck C., Nunnenmacher K., Schultz G., Plant T. D. (2000) Nat. Cell Biol. 2, 695–702 [DOI] [PubMed] [Google Scholar]
- 8.Watanabe H., Davis J. B., Smart D., Jerman J. C., Smith G. D., Hayes P., Vriens J., Cairns W., Wissenbach U., Prenen J., Flockerzi V., Droogmans G., Benham C. D., Nilius B. (2002) J. Biol. Chem. 277, 13569–13577 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. (2003) Nature 424, 434–438 [DOI] [PubMed] [Google Scholar]
- 10.Hu H. Z., Gu Q., Wang C., Colton C. K., Tang J., Kinoshita-Kawada M., Lee L. Y., Wood J. D., Zhu M. X. (2004) J. Biol. Chem. 279, 35741–35748 [DOI] [PubMed] [Google Scholar]
- 11.Lee H., Caterina M. J. (2005) Pflugers Arch. 451, 160–167 [DOI] [PubMed] [Google Scholar]
- 12.Nilius B., Owsianik G., Voets T. (2008) EMBO J. 27, 2809–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M. X. (2005) Pflugers Arch. 451, 105–115 [DOI] [PubMed] [Google Scholar]
- 14.Koplas P. A., Rosenberg R. L., Oxford G. S. (1997) J. Neurosci. 17, 3525–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lishko P. V., Procko E., Jin X., Phelps C. B., Gaudet R. (2007) Neuron 54, 905–918 [DOI] [PubMed] [Google Scholar]
- 16.Strotmann R., Schultz G., Plant T. D. (2003) J. Biol. Chem. 278, 26541–26549 [DOI] [PubMed] [Google Scholar]
- 17.Güler A. D., Lee H., Iida T., Shimizu I., Tominaga M., Caterina M. (2002) J. Neurosci. 22, 6408–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peier A. M., Reeve A. J., Andersson D. A., Moqrich A., Earley T. J., Hergarden A. C., Story G. M., Colley S., Hogenesch J. B., McIntyre P., Bevan S., Patapoutian A. (2002) Science 296, 2046–2049 [DOI] [PubMed] [Google Scholar]
- 19.Xu H., Ramsey I. S., Kotecha S. A., Moran M. M., Chong J. A., Lawson D., Ge P., Lilly J., Silos-Santiago I., Xie Y., DiStefano P. S., Curtis R., Clapham D. E. (2002) Nature 418, 181–186 [DOI] [PubMed] [Google Scholar]
- 20.Smith G. D., Gunthorpe M. J., Kelsell R. E., Hayes P. D., Reilly P., Facer P., Wright J. E., Jerman J. C., Walhin J. P., Ooi L., Egerton J., Charles K. J., Smart D., Randall A. D., Anand P., Davis J. B. (2002) Nature 418, 186–190 [DOI] [PubMed] [Google Scholar]
- 21.Xiao R., Tang J., Wang C., Colton C. K., Tian J., Zhu M. X. (2008) J. Biol. Chem. 283, 6162–6174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudet R. (2008) Mol. Biosyst. 4, 372–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin X., Touhey J., Gaudet R. (2006) J. Biol. Chem. 281, 25006–25010 [DOI] [PubMed] [Google Scholar]
- 24.McCleverty C. J., Koesema E., Patapoutian A., Lesley S. A., Kreusch A. (2006) Protein Sci. 15, 2201–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phelps C. B., Huang R. J., Lishko P. V., Wang R. R., Gaudet R. (2008) Biochemistry 47, 2476–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramoff M. D., Magelhaes P. J., Ram S. J. (2004) Biophotonics International 11, 36–42 [Google Scholar]
- 27.Patton C., Thompson S., Epel D. (2004) Cell Calcium 35, 427–431 [DOI] [PubMed] [Google Scholar]
- 28.Neeper M. P., Liu Y., Hutchinson T. L., Wang Y., Flores C. M., Qin N. (2007) J. Biol. Chem. 282, 15894–15902 [DOI] [PubMed] [Google Scholar]
- 29.Chung M. K., Lee H., Mizuno A., Suzuki M., Caterina M. J. (2004) J. Neurosci. 24, 5177–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung M. K., Güler A. D., Caterina M. J. (2005) J. Biol. Chem. 280, 15928–15941 [DOI] [PubMed] [Google Scholar]
- 31.Dattilo M., Penington N. J., Williams K. (2008) Mol. Pharmacol. 73, 42–49 [DOI] [PubMed] [Google Scholar]
- 32.Nilius B., Prenen J., Tang J., Wang C., Owsianik G., Janssens A., Voets T., Zhu M. X. (2005) J. Biol. Chem. 280, 6423–6433 [DOI] [PubMed] [Google Scholar]
- 33.Thébault S., Cao G., Venselaar H., Xi Q., Bindels R. J., Hoenderop J. G. (2008) J. Biol. Chem. 283, 19999–20007 [DOI] [PubMed] [Google Scholar]
- 34.Bryan J., Muñoz A., Zhang X., Düfer M., Drews G., Krippeit-Drews P., Aguilar-Bryan L. (2007) Pflugers Arch. 453, 703–718 [DOI] [PubMed] [Google Scholar]
- 35.Meyer S., Savaresi S., Forster I. C., Dutzler R. (2007) Nat. Struct. Mol. Biol. 14, 60–67 [DOI] [PubMed] [Google Scholar]
- 36.Accardi A. (2008) J. Gen. Physiol. 131, 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ataullakhanov F., Vitvitsky V. (2002) Bioscience Reports 22, 501–511 [DOI] [PubMed] [Google Scholar]
- 38.Taylor J. S., Vigneron D. B., Murphy-Boesch J., Nelson S. J., Kessler H. B., Coia L., Curran W., Brown T. R. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 6810–6814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tadross M. R., Dick I. E., Yue D. T. (2008) Cell 133, 1228–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vennekens R., Owsianik G., Nilius B. (2008) Curr. Pharm. Des. 14, 18–31 [DOI] [PubMed] [Google Scholar]
- 41.Myers B. R., Bohlen C. J., Julius D. (2008) Neuron 58, 362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grandl J., Hu H., Bandell M., Bursulaya B., Schmidt M., Petrus M., Patapoutian A. (2008) Nat. Neurosci. 11, 1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu H., Grandl J., Bandell M., Petrus M., Patapoutian A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 1626–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landau M., Mayrose I., Rosenberg Y., Glaser F., Martz E., Pupko T., Ben-Tal N. (2005) Nucleic Acids Res. 33, W299–W302 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.