Abstract
We recently demonstrated a novel effective therapeutic regimen for treating hamster heart failure based on injection of bone marrow mesenchymal stem cells (MSCs) or MSC-conditioned medium into the skeletal muscle. The work highlights an important cardiac repair mechanism mediated by the myriad of trophic factors derived from the injected MSCs and local musculature that can be explored for non-invasive stem cell therapy. While this therapeutic regimen provides the ultimate proof that MSC-based cardiac repair is mediated by the trophic actions independent of MSC differentiation or stemness, the trophic factors responsible for cardiac regeneration after MSC therapy remain largely undefined. Toward this aim, we took advantage of the finding that human and porcine MSCs exhibit species-related differences in expression of trophic factors. We demonstrate that human MSCs when compared to porcine MSCs express and secrete 5-fold less vascular endothelial growth factor (VEGF) in conditioned medium (40 ± 5 and 225 ± 17 pg/ml VEGF, respectively). This deficit in VEGF output was associated with compromised cardiac therapeutic efficacy of human MSC-conditioned medium. Overexpression of VEGF in human MSCs however completely restored the therapeutic potency of the conditioned medium. This finding indicates VEGF as a key therapeutic trophic factor in MSC-mediated myocardial regeneration, and demonstrates the feasibility of human MSC therapy using trophic factor-based cell-free strategies, which can eliminate the concern of potential stem cell transformation.
Keywords: VEGF, mesenchymal stem cell, heart failure, cell therapy
INTRODUCTION
Clinical trials of mesenchymal stem cell (MSC) therapies are currently being conducted for treating a broad spectrum of diseases [1]. Remarkably, although the multi-lineage differentiation potential (stemness) of MSCs was originally thought to mediate their therapeutic attributes, it has now become clear that the secretion of multiple growth factors and cytokines (trophic action) by MSCs is primarily responsible for many of the observed therapeutic benefits [2; 3]. Consistent with this notion, studies have attributed the cardiac repair function of MSCs to their trophic actions independent of their differentiation potentials [4; 5; 6]. We have in particular demonstrated that while MSCs injected into the hind limb muscle are trapped in the local musculature with no detectable migration to the heart, the administered MSCs exert a prominent cardiac repair function in a hamster heart failure model, and this therapeutic effect can be reproduced with a cell-free strategy based on intramuscular injections of MSC-conditioned medium [7]. These studies together illustrate that the trophic mediators secreted by MSCs improve cardiac function by a combination of multiple mechanisms such as attenuating tissue injury, inhibiting fibrotic remodeling, promoting angiogenesis, mobilizing host tissue stem cells, and reducing inflammation, although the bio-active factors remain largely undefined.
While clinical trials of MSCs have demonstrated no significant adverse side-effects even with multiple cell administrations [1; 8], long-term safety of MSC therapy remains to be evaluated. Notably, MSC transformation has been reported in prolonged ex vivo expansion [9; 10; 11], and over-expression of telomerase or inactivation of the Wnt pathway has been shown to provoke tumor formation from MSCs [12; 13]. MSC abnormalities have also been detected in patients with multiple myeloma [14; 15], suggesting that MSCs may be sensitive to precancerous or cancerous state of the host. Since MSC-derived trophic factors appear to underlie the cardiovascular therapeutic effects, they may be explored for development of trophic factor-based cell-free therapeutic strategies, thus eliminating the safety concern associated with cell transformation. Toward this end, we have begun to characterize the trophic actions of human and porcine bone marrow MSCs in relation to their therapeutic efficacy [7; 16; 17]. We report here that cardiac repair mediated by the trophic actions of MSCs can be critically influenced by the ability of MSCs to express and secrete adequate levels of vascular endothelial growth factor (VEGF). This finding bears significance to development of MSC-based therapeutic regimens for cardiac regeneration.
METHODS AND MATERIALS
Animals
Bio-F1B (normal) and Bio-TO2 (cardiomyopathic) male hamsters were obtained from Bio Breeders (Watertown, MA). All procedures and protocols conformed to institutional guidelines for the care and use of animals in research.
Echocardiography
Echocardiographic measurements were performed in a blind-folded fashion, and were described in our recent work [16; 18]. Cardiac function was assessed by left ventricular ejection fraction (LVEF).
MSC culture
Fresh human bone marrows were purchased from Lonza Walkersville (Gaithersburg, MD). Human MSCs (hMSCs) were prepared and expanded as described for porcine MSC (pMSCs) [16; 17; 19]. hMSCs and pMSCs were culture-expanded in MesenPro/RS medium (Invitrogen) and DMEM/F12 medium containing 10% fetal bovine serum (FBS), respectively. Both media were supplemented with 2 mM glutamine, 50 μg/ml gentamycin, and 0.125 μg/ml Fungizone. Only MSC cultures exhibiting robust growth capacity (typically less than 5 passages) were used for the study. To generate MSC-conditioned medium, MSCs were grown on fibronectin-coated dishes to ~90% confluency. Cells were then washed thoroughly with Hank’s balanced salt solution (HBSS), and maintained in serum- and phenol red-free Minimal Essential Medium (MEM) for 24 hours. The conditioned medium was harvested, and filtered prior to use. The protocol for viral infection of hMSCs using Ad-VEGF165 was described previously [17].
Intramuscular injections of MSCs and MSC-conditioned medium
MSCs (2 – 4 million cells per animal) were resuspended in 1 ml HBSS, and injected in equally divided doses into the left and right hamstrings of 4-month old TO2 cardiomyopathic hamsters as described [16]. For saline or medium injection, each hamstring was injected with 0.5 ml of saline or conditioned medium two to three times a week for four consecutive weeks as described [16]. Echocardiography was performed 1 month after the initiation of the therapeutic injections.
Real time qRT-PCR
RNA isolation and qRT-PCR protocols were as described [17]. β2-microglobulin (B2M) was used as the reference gene for calculations. Primer sequences are listed in Table 1.
Table 1.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Human B2M | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
| Human FGF2 | ACCCCGACGGCCGA | TCTTCTGCTTGAAGTTGTAGCTTGA |
| Human IGF1 | CAGCAGTCTTCCAACCCAAT | ACAGCGCCAGGTAGAAGAGA |
| Human IL6 | CGGGAACGAAAGAGAAGCTCTA | GGCGCTTGTGGAGAAGGAG |
| Human LIF | ACCAGATCAGGAGCCAACTG | GCCACATAGCTTGTCCAGGT |
| Human MCP1 | CATTGTGGCCAAGGAGATCTG | CTTCGGAGTTTGGGTTTGCTT |
| Human TGFb | CGAGAAGCGGTACCTGAAC | TGAGGTATCGCCAGGAATTGT |
| Human VEGF-A | GCACCCATGGCAGAAGG | CTCGATTGGATGGCAGTAGCT |
| Gene | Forward Primer | Reverse Primer |
| Porcine B2M | AAACGGAAAGCCAAATTACC | ATCCACAGCGTTAGGAGTGA |
| Porcine FGF2 | GGAGTGTGTGCAAACCGTTA | TCGTTTCAGTGCCACATACC |
| Porcine IGF1 | TGCTCTCCTTCACCAGCTCT | GCCTCCTCAGATCACAGCTC |
| Porcine IL6 | TTCACCTCTCCGGACAAAAC | TCTGCCAGTACCTCCTTGCT |
| Porcine LIF | GTCACCCATGTCACAGCAAC | CCCCTGGGCTGTGTAGTAGA |
| Porcine MCP1 | CACCAGCAGCAAGTGTCCTA | TCCAGGTGGCTTATGGAGTC |
| Porcine TGFb | AAGCGGCAACCAAATCTATG | CACGTGCTGCTCCACTTTTA |
| Porcine VEGF-A | CTACCTCCACCATGCCAAGT | ACACAGGACGGCTTGAAGAT |
ELISA Analysis
VEGF concentrations of MSC-conditioned medium were measured with a VEGF ELISA kit from R&D Systems (#DY293B DuoSet) following the manufacturer’s protocol.
Statistical Analysis
Data are expressed as means ± SEM. Comparisons between groups were based on Anova analysis. P<0.05 was considered statistically significant.
RESULTS
Preclinical and clinical studies of myocardial stem cell therapy often use invasive cell delivery approaches such as intramyocardial injection or intracoronary infusion, which can introduce harmful scar tissue, arrhythmia, calcification, or microinfarction in the heart [20; 21; 22; 23]. Aiming at developing a safer cell delivery regimen and taking advantage of the trophic actions of MSCs, we recently demonstrated a prominent cardiac repair mechanism achieved by injections of pMSCs or pMSC-conditioned medium into the hind limb muscle of TO2 cardiomyopathic hamsters [7; 18]. Intramuscularly injected pMSCs and pMSC-conditioned medium each significantly improved ventricular function one month post therapy. The functional improvement is associated with mobilization of bone marrow progenitor cells, increased myocardial capillary density, renewed cardiomyocyte populations, attenuated apoptosis, and decreased fibrosis [7].
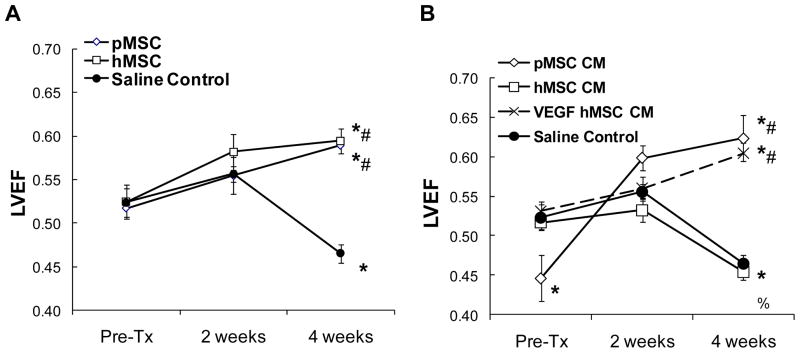
We were particularly encouraged by the finding that intramuscular injections of pMSC-conditioned medium could achieve significant cardiac repair as this strategy could be further optimized for a trophic factor-based cell-free therapeutic regimen, eliminating the concern of potential MSC transformation after cell administration [10; 11; 12; 13]. Since we noted species-related differences in the growth and trophic actions of MSCs during our characterizations of hMSCs and pMSCs [16], we first examined whether hMSCs delivered to the hind limb muscle of TO2 cardiomyopathic hamsters could also repair the failing hamster heart as demonstrated with pMSCs [7]. Figure 1A shows that hMSCs and pMSCs both significantly increased LVEF by ~30% four weeks after the cell therapy. We then compared the therapeutic potency of hMSC- and pMSC-conditioned medium (designated as hMSC CM and pMSC CM, respectively). Notably, although the injected hMSCs were therapeutically effective, hMSC CM failed to improve ventricular function of the failing hamster heart (Fig. 1B), suggesting that the hMSC-conditioned medium might not contain an adequate level of therapeutic trophic factors.
Figure 1.
Left ventricular functional improvement mediated by human and porcine MSCs and MSC-conditioned medium. Echocardiography was performed in a blind-folded manner. Left ventricular ejection fraction (LVEF) was measured pre-injection, 2 weeks, and 4 weeks after initiation of injections. As reference, normal age-matched F1B hamsters maintain a stable LVEF of ~70%. Panel A: TO2 hamsters were injected with hMSCs and pMSCs. A saline injection control group was also included. Panel B: TO2 hamsters were injected with MSC-conditioned medium. Conditioned medium was also prepared from hMSCs overexpressing VEGF (designated as VEGF hMSC CM). n=6 per animal group. Anova analysis was performed for group comparisons. *P<0.005 vs. pre-injection; %P<0.05 vs. pre-injection; #P<0.005 vs. saline control.
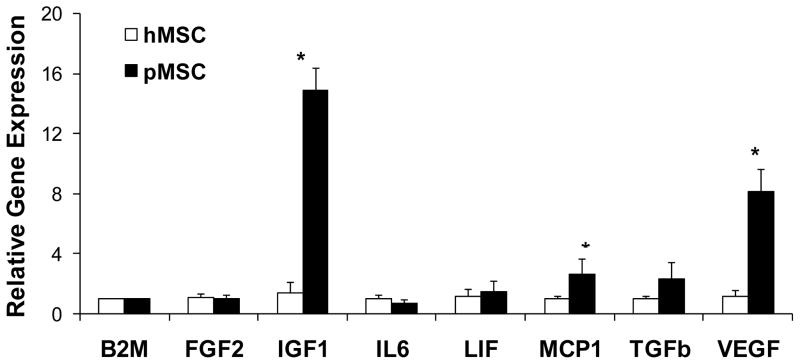
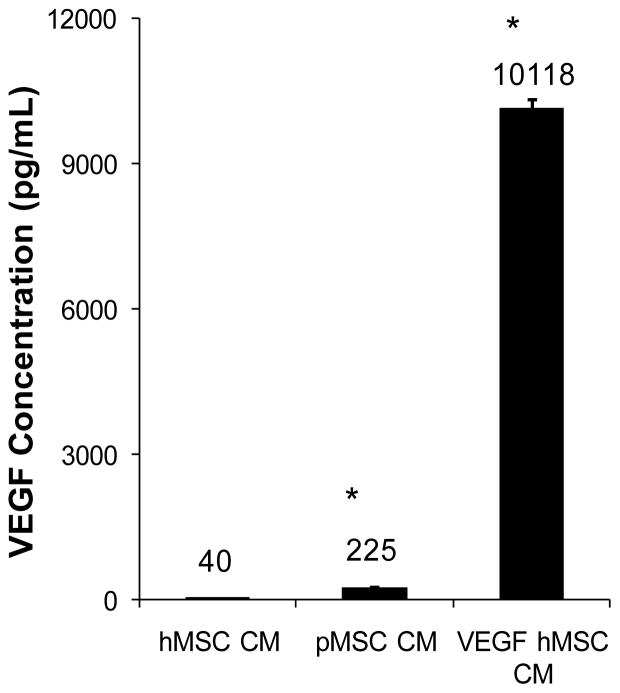
To further understand the difference in the trophic actions of hMSCs and pMSCs, we used qRT-PCR to quantify expression of growth factor and cytokine genes. Figure 2 shows that among the seven genes analyzed pMSCs significantly expressed higher levels of insulin-like growth factor 1 (IGF1), monocyte chemotactic protein 1 (MCP1), and vascular endothelial growth factor-A (VEGF) than hMSCs. Since VEGF has been used in clinical trials for heart disease [24; 25], the gene was singled out for further analysis. We further performed VEGF ELISA to validate the qRT-PCR data. Figure 3 shows that pMSC CM contained ~5 fold higher VEGF concentration than hMSC CM (225 ± 17 vs. 40 ± 5 pg/ml; P<0.001), which is in agreement with the qRT-PCR data presented in Figure 2.
Figure 2.
Expression profiles of hMSC and pMSC trophic factors analyzed by qRT-PCR. Primer sequences were listed in Table 1. Total RNA isolated from hMSCs and pMSCs was analyzed by qRT-PCR. Threshold cycles were normalized based on the reference gene β2-microglobulin (B2M). n=3. *P<0.05 between hMSCs and pMSCs.
Figure 3.
Measurement of VEGF concentrations by ELISA. Conditioned medium was prepared from hMSCs, pMSCs, and hMSCs overexpressing VEGF. The medium was assayed by a VEGF ELISA kit. VEGF concentrations were expressed as pg/ml shown above each bar. n=3. *P<0.001 compared to hMSC CM.
To determine whether the decreased VEGF output might be associated with the observed therapeutic deficit of hMSC CM, we overexpressed VEGF in hMSCs using a recombinant adenoviral vector as described previously [17; 26], and prepared conditioned medium (designated as VEGF hMSC CM) from the VEGF-expressing hMSCs. Figure 3 reveals that infection of hMSCs by the VEGF adenoviral vector caused a pronounced increase in VEGF concentration of the conditioned medium (10,118 ± 210 pg/ml). Injections of VEGF hMSC CM were subsequently found to completely restore the cardiac therapeutic potency of the hMSC-conditioned medium (Fig. 1B). Thus, although hMSCs exhibit a lower VEGF output compared to pMSCs, this deficit can be readily remedied by VEGF overexpression. These findings thus define VEGF as a key therapeutic trophic factor in MSC-mediated cardiac repair.
DISCUSSION
Despite recent recognition that the trophic actions of MSCs underlie their therapeutic effects in various preclinical and clinical studies [2; 3; 4], the trophic factors responsible for tissue functional improvement after MSC therapy remain largely undefined. While our study explored the feasibility of using a cell-free therapeutic strategy for cardiac repair based on MSC-conditioned medium, which can eliminate the concern of potential MSC transformation [9; 11; 12; 13], it further demonstrated VEGF as a key therapeutic trophic factor in MSC-mediated cardiac repair. This demonstration is consistent with a recent report showing that MSC-derived VEGF plays a critical role in attenuating acute kidney injury [27].
Development of trophic factor-based cell-free therapeutic regimens for tissue repair can be facilitated through analysis of the trophic actions of MSCs as demonstrated here. Using a cocktail of well-defined trophic factors for this purpose is not only pharmaceutically sound but also strategically advantageous since the trophic factor cocktail can be precisely modified to treat different degenerative conditions. Our comparative study of pMSC CM, hMSC CM, and VEGF hMSC CM suggests that a cocktail with a VEGF dosage greater than ~200 pg/ml is sufficient to elicit significant cardiac repair for the failing hamster heart in conjunction with other yet undefined MSC trophic factors. This VEGF dosage amounts to ~2 ng VEGF/kg body weight per intramuscular injection, which is three orders of magnitude lower than the typical VEGF dosages used in single growth factor therapeutic trials (>10 μg/kg body weight per injection) [28]. Interestingly, we have found that VEGF when administered alone (in the absence of MSC trophic factors) could also rescue the failing hamster heart at higher dosages (0.1–1 μg/kg body weight per intramuscular injection) [29]. Despite repeated intramuscular injections, side effects such as tissue edema, angioma formation, and inflammation, which have been associated with VEGF administration [30; 31], were not observed in our study. A prominent feature of the intramuscular VEGF or MSC delivery approach is that the local muscular bed produces additional beneficial soluble mediators in response to trophic factor signaling [7; 29]. Activation of the host muscle trophic factor network thus further amplifies the trophic actions of MSCs, culminating in an effective cardiac repair mechanism.
MSCs are thought to have a perivascular origin, and can be derived from multiple human organs including bone marrow, skeletal muscle, placenta, pancreas, and adipose tissue [32]. It is presently unknown whether the therapeutic potency of MSCs may be affected by the tissue source. In transitioning toward the clinical use of hMSCs, additional variables such as differences in donor gender, age, and life style can potentially complicate the logistic aspect of MSC therapy. For instance, limited information indicates that female stem cells may possess a more pronounced regenerative potential than male stem cells [33], which is in line with the finding that female patients typically exhibit certain cardioprotective phenomenon from acute myocardial infarction and better outcome after the incidence compared to male patients [34]. Although these gender influences are thought to be mediated through differential sex hormone receptor signaling, a recent study shows that female rodent MSCs produce a higher level of VEGF than male rodent MSCs in response to hypoxia [35]. Our demonstration here that VEGF hMSC CM, but not hMSC CM, improved cardiac function further highlights the critical role of VEGF in mediating the therapeutic function of MSCs. We suggest that the cardiac therapeutic potency of a given donor MSC may correlate with the cell’s ability to express and secrete an adequate level of VEGF, which can be readily assessed by qRT-PCR and ELISA as shown here.
The cardiovascular effects of VEGF appear to stem from its multiple biological attributes. VEGF is well known for its ability to mobilize bone marrow progenitor cells, and these recruited progenitor cells can participate in myogenesis and angiogenesis [36]. In addition to being an angiogenic growth factor, VEGF also possesses a nonangiogenic role important for the cardiovascular system [37]. VEGF can promote differentiation of stem cells into cardiomyocytes and endothelial cells [38; 39], and boost the function of MSCs and endothelial progenitor cells [40; 41]. We have demonstrated that MSCs engineered to overexpress VEGF exhibit more pronounced trophic factor outputs [17]. These trophic factors acting in concert can mediate cardiac repair through their progenitor cell-mobilizing, angiogenic, cytoprotective, myogenic, anti-fibrotic, and anti-inflammatory properties [7; 16]. Interestingly, an MSC-derived anti-inflammatory factor designated as TSG-6 has been found to mediate cardiac repair after myocardial infarction [6]. Given that tissue damage and degeneration is complex in nature, multiple MSC trophic factors acting in synergy are expected to be involved in tissue healing. Identification of these trophic factors can eventually lead to the development of cell-free trophic factor cocktails suitable for various clinical applications.
Acknowledgments
Contract grant sponsor: NIH HL84590; New York State Stem Cell Board
The work is supported by grants from NIH (HL84590) and New York State Stem Cell Board.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient’s bedside: an update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–24. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–3. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 4.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 6.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a non-invasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296:H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 9.Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, Westendorf JJ, McIvor RS, Hogendoorn PC, Szuhai K, Oseth L, Hirsch B, Yant SR, Kay MA, Peister A, Prockop DJ, Fibbe WE, Blazar BR. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–9. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 10.Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–9. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 11.Rubio D, Garcia S, Paz MF, De la Cueva T, Lopez-Fernandez LA, Lloyd AC, Garcia-Castro J, Bernad A. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One. 2008;3:e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns JS, Abdallah BM, Guldberg P, Rygaard J, Schroder HD, Kassem M. Tumorigenic heterogeneity in cancer stem cells evolved from long-term cultures of telomerase-immortalized human mesenchymal stem cells. Cancer Res. 2005;65:3126–35. doi: 10.1158/0008-5472.CAN-04-2218. [DOI] [PubMed] [Google Scholar]
- 13.Matushansky I, Hernando E, Socci ND, Mills JE, Matos TA, Edgar MA, Singer S, Maki RG, Cordon-Cardo C. Derivation of sarcomas from mesenchymal stem cells via inactivation of the Wnt pathway. J Clin Invest. 2007;117:3248–57. doi: 10.1172/JCI31377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garderet L, Mazurier C, Chapel A, Ernou I, Boutin L, Holy X, Gorin NC, Lopez M, Doucet C, Lataillade JJ. Mesenchymal stem cell abnormalities in patients with multiple myeloma. Leuk Lymphoma. 2007;48:2032–41. doi: 10.1080/10428190701593644. [DOI] [PubMed] [Google Scholar]
- 15.Wallace SR, Oken MM, Lunetta KL, Panoskaltsis-Mortari A, Masellis AM. Abnormalities of bone marrow mesenchymal cells in multiple myeloma patients. Cancer. 2001;91:1219–30. doi: 10.1002/1097-0142(20010401)91:7<1219::aid-cncr1122>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by non-autologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009;87:1275–1282. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin H, Shabbir A, Molnar M, Yang J, Marion S, Canty JMJ, Lee T. Adenoviral expression of vascular endothelial growth factor splice variants differentially regulate bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2008;216:458–468. doi: 10.1002/jcp.21414. [DOI] [PubMed] [Google Scholar]
- 18.Missihoun C, Zisa D, Shabbir A, Lin H, Lee T. Myocardial oxidative stress, osteogenic phenotype, and energy metabolism are differentially involved in the initiation and early progression of delta-sarcoglycan-null cardiomyopathy. Mol Cell Biochem. 2008;321:45–52. doi: 10.1007/s11010-008-9908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vacanti V, Kong E, Suzuki G, Sato K, Canty JM, Jr, Lee T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J Cell Physiol. 2005;205:194–201. doi: 10.1002/jcp.20376. [DOI] [PubMed] [Google Scholar]
- 20.Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–9. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 21.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–7. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 22.Widimsky P, Penicka M. Complications after intracoronary stem cell transplantation in idiopathic dilated cardiomyopathy. Int J Cardiol. 2006;111:178–9. doi: 10.1016/j.ijcard.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–4. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 24.Yla-Herttuala S, Rissanen TT, Vajanto I, Hartikainen J. Vascular endothelial growth factors: biology and current status of clinical applications in cardiovascular medicine. J Am Coll Cardiol. 2007;49:1015–26. doi: 10.1016/j.jacc.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 25.Testa U, Pannitteri G, Condorelli GL. Vascular endothelial growth factors in cardiovascular medicine. J Cardiovasc Med (Hagerstown) 2008;9:1190–221. doi: 10.2459/JCM.0b013e3283117d37. [DOI] [PubMed] [Google Scholar]
- 26.Lin H, Shabbir A, Molnar M, Lee T. Stem cell regulatory function mediated by expression of a novel mouse Oct4 pseudogene. Biochem Biophys Res Commun. 2007;355:111–6. doi: 10.1016/j.bbrc.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 27.Togel F, Zhang P, Hu Z, Westenfelder C. VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Post MJ, Laham R, Sellke FW, Simons M. Therapeutic angiogenesis in cardiology using protein formulations. Cardiovasc Res. 2001;49:522–31. doi: 10.1016/s0008-6363(00)00216-9. [DOI] [PubMed] [Google Scholar]
- 29.Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF Repairs the Failing Heart: Role of Host Derived Growth Factors and Mobilization of Progenitor Cells. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.00227.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz ER, Speakman MT, Patterson M, Hale SS, Isner JM, Kedes LH, Kloner RA. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat--angiogenesis and angioma formation. J Am Coll Cardiol. 2000;35:1323–30. doi: 10.1016/s0735-1097(00)00522-2. [DOI] [PubMed] [Google Scholar]
- 31.Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- 32.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Deasy BM, Lu A, Tebbets JC, Feduska JM, Schugar RC, Pollett JB, Sun B, Urish KL, Gharaibeh BM, Cao B, Rubin RT, Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray R, Novotny NM, Crisostomo PR, Lahm T, Abarbanell A, Meldrum DR. Sex steroids and stem cell function. Mol Med. 2008;14:493–501. doi: 10.2119/2008-00004.Ray. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crisostomo PR, Wang M, Herring CM, Markel TA, Meldrum KK, Lillemoe KD, Meldrum DR. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: role of the 55 kDa TNF receptor (TNFR1) J Mol Cell Cardiol. 2007;42:142–9. doi: 10.1016/j.yjmcc.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zachary I, Mathur A, Yla-Herttuala S, Martin J. Vascular protection: a novel nonangiogenic cardiovascular role for vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2000;20:1512–1520. doi: 10.1161/01.atv.20.6.1512. [DOI] [PubMed] [Google Scholar]
- 38.Song YH, Gehmert S, Sadat S, Pinkernell K, Bai X, Matthias N, Alt E. VEGF is critical for spontaneous differentiation of stem cells into cardiomyocytes. Biochem Biophys Res Commun. 2007;354:999–1003. doi: 10.1016/j.bbrc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 39.Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M, Werner C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–84. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 40.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, Silver M, Li T, Isner JM, Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–8. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Hu Q, Mansoor A, Lee J, Wang Z, Lee T, From AH, Zhang J. Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol. 2006;290:H1393–405. doi: 10.1152/ajpheart.00871.2005. [DOI] [PubMed] [Google Scholar]