Abstract
The appreciation that the inflammatory reaction does not ‘spontaneously’ finish, but rather that inflammatory resolution is an active phenomenon brought about by endogenous anti-inflammatory agonists opens multiple opportunities for a reassessment of the complexity of inflammation and its main mediators. This review dwells on one of these pathways, the one centred around the glucocorticoid-regulated protein Annexin A1 and its G protein-coupled receptor. In recent years, much of the knowledge detailing the processes by which Annexin A1 expresses its anti-inflammatory role on innate immunity has been produced. Moreover, the generation of the Annexin A1 null mouse colony has provided important proof-of-concept experiments demonstrating the inhibitory properties of this mediator in the context of inflammatory and/or tissue-injury models. Therefore, Annexin A1 acts as a pivotal homeostatic mediator, where if absent, inflammation would overshoot and be prolonged. This new understanding scientific information could guide us onto the exploitation of the biological properties of Annexin A1 and its receptor to instigate novel drug discovery programmes for anti-inflammatory therapeutics. This line of research relies on the assumption that anti-inflammatory drugs designed upon endogenous anti-inflammatory mediators would be burdened by a lower degree of secondary effects as these agonists would be mimicking specific pathways activated in our body for safe disposal of inflammation. We believe that the next few years will produce examples of such new drugs and the validity of this speculation could then be assessed.
This article is part of a themed issue on Mediators and Receptors in the Resolution of Inflammation. To view this issue visit http://www3.interscience.wiley.com/journal/121548564/issueyear?year=2009
Keywords: anti-inflammation, neutrophils, migration, trafficking, intravital microscopy, GPCR, induction, drug discovery, peptides
Introduction
The awareness of the complexity of the experimental inflammatory reaction has steadily increased in the past decade, in concomitance with the discovery of endogenous inflammatory and pro-resolving pathways (Nathan, 2002). Together with a few other laboratories worldwide, we have pioneered the concept that the inflammatory response follows – in an ideal fashion – a bell-shaped curve such that an acute strong inflammatory phase is followed by a pro-resolving phase with dampening of the response (Perretti, 1997; Serhan and Savill, 2005; Serhan et al., 2007). This sequence attained by an active involvement of inhibitory pathways and mediators (Gilroy et al., 2004; Serhan et al., 2007). Figure 1 illustrates the necessary balance between pro-inflammation and anti-inflammation in order to assure homeostasis is restored in the inflamed tissue after proper resolution. Furthermore, this balance is finely tuned so that alterations, either by an excess of pro-inflammatory mediator expression and/or function or by an augmentation of the endogenous anti-inflammatory arm, would lead to disease (Figure 1).
Figure 1.
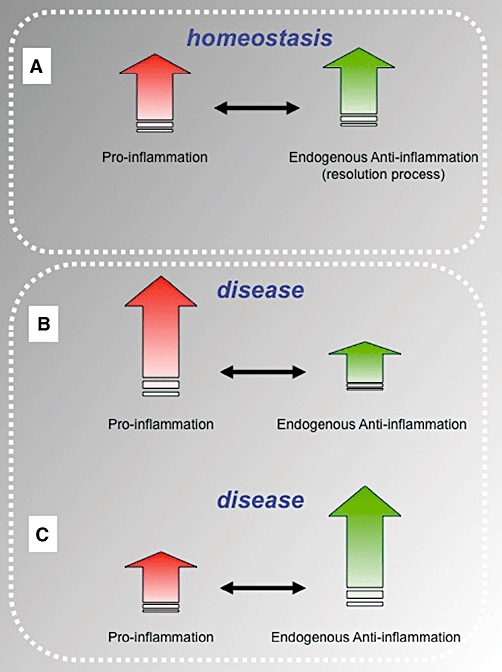
Schematic representation of the required balance between pro- and anti-inflammatory mediators and pathways. (A) An equilibrium between pro-inflammatory mediators and effectors of anti-inflammation is required to assure a prompt yet spatially and temporally restricted inflammatory reaction. This is required for a proper function of the inflammatory response the host mounts upon infection and encounter with xenobiotics, so that upon killing and/or disposal of the inflammogen, resolution of inflammation is completed with restoring of tissue physiology and homeostasis regain. (B and C) Pro-inflammation in association with a deficient endogenous anti-inflammatory response, hence with inadequate resolution phase, would lead to disease; the same holds true if an inappropriate pro-inflammatory is produced, so that resolution of inflammation would prevail (e.g. leukocyte adhesion deficiency, whereby lack of leukocyte trafficking is at the basis of patients' inability to fight infections, leading to a poor life expectancy). Acceptance of this important yin/yan in inflammation ensues that chronic inflammatory disease (various forms of arthritis, vasculitis, psoriasis but also perhaps atherosclerosis and reperfusion injury conditions) could be due, at least partly, to an insufficient activation of the endogenous anti-inflammatory response so that resolution pathways are not properly activated and/or operating. Along this line, potentiation of one or more endogenous pro-resolving pathways could be another approach to the therapeutic control of inflammatory diseases.
This review focuses on one of these mediators, the glucocorticoid-modulated protein Annexin A1. Recent reviews have covered multiple aspects of the biology of this protein spanning from the central nervous system (John et al., 2004; Solito et al., 2008) to action upon the adaptive immune system (Gerke and Moss, 2002;D'Acquisto et al., 2008b; Perretti and D'Acquisto, 2009). Here we summarize the actions of Annexin A1 in innate immunity, emphasizing the opportunity that a better appreciation of the mechanisms activated by this protein to inhibit the inflammatory reaction would represent for innovative drug discovery. In fact, as proposed before (Perretti, 1997), we hypothesize that targets activated by endogenous anti-inflammatory mediators could be exploited for novel drug discovery programmes; new molecules obtained and developed in this manner would likely be devoid of major side effects, as their application would be mimicking the way our body naturally disposes of inflammation. Development of adenosine or somatostatin analogues would fit with this approach (Perretti, 1997).
Annexin A1: generalities
Annexin A1 is a 37 kDa protein formed by 346 amino acids. It is the first member of 13 member of a protein family ‘The Annexins’, grouped together in view of their structural characteristics, including the presence of shared sequences for calcium binding (Gerke and Moss, 2002; Gerke et al., 2005). All annexins consist of a core, which is constituted by four repeats of 60–70 amino acids each, attached to a unique N-terminal region. The core represents the large majority (≥80%) of the protein, whereas the N-terminus likely confers specificity of action to each member of the annexin superfamily of proteins (Gerke et al., 2005).
An important feature of Annexin A1, also shared by other members of the family, is its ability to alter its conformation upon binding to calcium cations (Rosengarth et al., 2001a,b; Gerke et al., 2005). In the presence of a calcium concentration ≥1 mM (e.g. as in plasma or other biological fluids), Annexin A1 undergoes a conformational restructuring allowing phospholipid binding, in particular binding to acidic phospholipids (Rosengarth et al., 2001a,b;). Interaction with phospholipids via the core region, sustained by its calcium-binding motifs, is concomitant with a conformational rearrangement of the N-terminal region such that its amino acids are now exposed to the extracellular environment (Rosengarth et al., 2001a). These structural changes are likely to impact on the biology of the protein and, in particular on its ability to interact with potential receptors (discussed below).
Our major interest, in line with the functions of Annexin A1 in the context of inflammation, lies in the role that this protein plays once in the extracellular fluids, including plasma and inflammatory exudates. However we should not overlook the notion that Annexin A1 might be endowed with specific intracellular roles. These intracellular properties are also governed by its ability to interact with membranes and possibly again being regulated by the levels of cations in a specific subcellular compartment (Gerke et al., 2005).
An important aspect in the biology of Annexin A1, which will be only superficially covered in this review, is the modulation of protein expression as well as cellular localization by glucocorticoids (Mulla et al., 2005). Indeed, historically Annexin A1 was identified as a glucocorticoid-regulated protein, as earlier observations showed that this class of drugs would augment its levels both in macrophages, lung tissue and kidney mesangial cells (Flower and Blackwell, 1979; Blackwell et al., 1980; Flower, 1985; 1988;). It is now evident that the association between glucocorticoids and Annexin A1 is more complex than initially observed.
Dexamethasone and other steroids can rapidly increase cell surface localization of Annexin A1 (Croxtall et al., 1998; 2000;Solito et al., 2006); this process does not require de novo protein synthesis and is associated with rapid changes in the cellular localization of the protein. More delayed augmentation of cell surface expression of Annexin A1 is consequent to gene activation. Actinomycin D and cycloheximide could inhibit delayed Annexin A1 expression even though the Annexin A1 promotor region lacks a canonical glucocorticoid response element in the Annexin A1 promoter region (Solito et al., 1998a,b;). This observation does not exclude the possibility that such regions might be present upstream of the DNA sequences analysed so far, nonetheless it poses the problem of how glucocorticoids could increase Annexin A1 gene expression. In monocytic cells, involvement of specific transcription factors such as nuclear factor interleukin (IL)-6 has been invoked in regulating Annexin A1 gene expression (Solito et al., 1998b). This issue requires further investigation including the validation of such a mechanism in other cell types. Glucocorticouds may also down-regulate Annexin A1 gene expression under some conditions as recently demonstrated in T cells (D'Acquisto et al., 2008a). The role of Annexin A1 in the adaptive although immune response is not covered in the present review (see Perretti and D'Acquisto, 2009 for a recent review) and will not be described here.
Cell sources of Annexin A1
Annexin A1 is widely distributed in the body being found in white blood cells, stromal cells and biological fluids. In the circulation, granulocytes and monocytes are the largest cell source of Annexin A1, with the neutrophils being the predominant cell source in the granulocyte subgroup (Perretti et al., 1996b; Oliani et al., 2002). Lymphocytes have modest expression of Annexin A1, with T cells being positive for the protein (although with nearly a 1:100 the expression observed in neutrophils), whereas B cells (Morand et al., 1995; Mulla et al., 2005; D'Acquisto et al., 2007; Perretti et al., 2009) and platelets are negative for Annexin A1 expression.
In general terms, cell differentiation is associated with a higher expression of Annexin A1 (Kamal et al., 2005; Babbin et al., 2006) and this is evident when macrophages are compared with monocytes prepared from the same donor (Peers et al., 1993); mast cells also express Annexin A1 (Oliani et al., 2000; 2008; Sena et al., 2006; Silistino-Souza et al., 2007) and the same applies to stromal cells such as the fibroblast (Errasfa et al., 1985; Goulding et al., 1996a; Solito et al., 1998a; Dasuri et al., 2004; Tagoe et al., 2008). The latter cell type has been one of the first to be used for the identification of the biological function of the protein. Epithelial cells are also a major source of the protein in compartments such as the lung, the gut and the kidney (Vervoordeldonk et al., 1994; Solito et al., 1998a; Babbin et al., 2006). Kidney mesangial cells are also strongly positive for the protein. Therefore, in simple terms, fully differentiated cell types such as those found in tissues are strongly positive for Annexin A1. Furthermore, cell differentiation and in some cases also cell activation is a major stimulus for Annexin A1 synthesis and up-regulation, although the molecular processes behind this response have not been fully addressed. Examples of this important regulation of the protein expression are seen with tumour necrosis factor-stimulated fibroblasts (Tagoe et al., 2008) or responses that have been reported in epithelial cells (Croxtall and Flower, 1992).
Finally an unsolved mystery in the assessment of Annexin A1 biology is the mode of secretion of the protein. As stated above, we propose that in order to exert its actions and impact onto the inflammatory process, Annexin A1 must be externalized by its cellular sources. However the protein lacks a signal peptide and therefore cannot be secreted via a classical pathway (Muesch et al., 1990; Christmas et al., 1991). We have already mentioned that glucocorticoids can provoke rapid mobilization and externalization of the protein, possibly consequent to rapid phosphorylation by protein kinase C activation (Solito et al., 2006); however, other mechanisms might also exist. In the context of neutrophil biology it was observed that a large proportion of Annexin A1 was contained in subcellular granules and that this pool was externalized upon cell adhesion to endothelial monolayers: ∼60–70% of total Annexin A1 content in human neutrophils was lost in post-adherent cells (Perretti et al., 1996b). More recently, Annexin A1 externalization has been observed also in activated neutrophils, without the need of an endothelial monolayer (Vong et al., 2007). Moreover, a new mode of secretion of Annexin A1 from this cell type has been identified via microparticle release (Dalli et al., 2008). Microparticles (also called ectosomes) are organelles that spawn from activated cells by a mechanism involving flippase and scramblase activation, so that their lipid bilayer is inside-out, with phosphatidyl serine being exposed on the outside (Distler et al., 2005). We have found that neutrophil-derived microparticles (Gasser et al., 2003) can display Annexin A1 on their surface, and that this is instrumental to bring about their ability to inhibit neutrophil/endothelium interaction under flow in vitro (Dalli et al., 2008). Furthermore, Annexin A1-rich microparticles exerted anti-migratory effects, in line with what has been reported for the protein (see below); it is plausible that mobilization and externalization of Annexin A1 via microparticles or other forms of microvesicles could be a common feature for the release of this protein from different cell sources, similarly to the process of IL-1 secretion (MacKenzie et al., 2001).
Annexin A1 and inflammation
The generation of human recombinant (hr)-Annexin A1, at the time called lipocortin (Wallner et al., 1986), provided great input for the definition of its biological activities, allowing the availability of sufficient amounts for in vivo investigations. Annexin A1 was initially characterized for its ability to inhibit prostanoid release (Cirino et al., 1987), an effect that underlined its efficacy in the rat paw oedema model (Cirino et al., 1989). However, hr-Annexin A1 was unable to affect oedema responses elicited by stimuli provoking vasodilatation (e.g. histamine or serotonin), leading to the hypothesis that its mechanism required inhibition of phospholipase A2 and ensuing reduction in prostaglandin generation. Subsequently it became evident that the anti-inflammatory properties of hr-Annexin A1 were also relying on important inhibitory effects on the process of leucocyte migration. Movement of blood-borne cells to the site of inflammation, and therefore extravasation into the injured tissue, is a hallmark of the inflammatory response (Ley et al., 2007). Analysis of the effects of Annexin A1 in models of leukocyte migration indicated that this property was not reliant upon prostaglandin generation (Perretti and Flower, 1993), that is Annexin A1 inhibited neutrophil recruitment even when inhibitors of prostaglandin synthesis were without effect. These studies indicated that more than one mechanism of action could be advocated for the pharmacology of Annexin A1 in experimental inflammation.
It therefore soon appeared that the protein was able to produce macroscopic effects due to multiple molecular and cellular actions, in as much as hr-Annexin A1 was able to elicit an anti-pyretic response that was clearly associated with an inhibition of prostaglandin E2 production in the third ventricle (Carey et al., 1990). These initial observations were then followed up, over the years, by analysis of the tissue protective and anti-inflammatory actions of Annexin A1 using different models in rodents (see Table 1 for a non-exhaustive list of these studies). Efficacy in a given model of pathology could, clearly, guide the potential disease application(s) for Annexin A1 mimetics.
Table 1.
Non-exhaustive list of experimental systems where the anti-inflammatory actions of Annexin A1 and its fragments have been analysed
| Agent | Experimental model | Observed function | References |
|---|---|---|---|
| Annexin A1 | Poly I : C-induced pyrogenesis (rabbit) | ↓ Febrile response | Davidson et al. (1991) |
| Carrageenin paw oedema (rat) | Dose response inhibition | Cirino et al. (1989) | |
| Carrageenin oedema (adrenalectomized rat) | ↓ Oedema | Cirino et al. (1989) | |
| Bradykinin-, serotonin-, dextran- or PAF-induced oedema (rat) | Not effective at any dose tested | Cirino et al. (1989) | |
| Compound 48/80 oedema (rat) | ↓ Oedema | Cirino et al. (1989) | |
| PLA2 oedema (rat) | ↓ Oedema (∼85% at the top dose) | Cirino et al. (1989) | |
| Mesenteric microcirculation activated by zymosan (mouse) | ↓ Leukocyte adhesion and emigration (s.c.) | Lim et al. (1998) | |
| ↑ Detachment of adherent neutrophils (i.v.) | |||
| Heart ischaemia–reperfusion (rat) | ↓ Infarct size (≤50%) | D'Amico et al. (2000) | |
| Dorsal injection of polyacrylimide gel (mouse) | ↓ PMN migration | Errasfa and Russo-Marie (1989) | |
| ↓ PGE2 and LTB4 levels | |||
| ↓ PLA2 activity | |||
| Cerebral ischaemia–reperfusion (mouse) | ↓ Infarct volume, numbers of adherent and rolling leukocytes | Gavins et al. (2007) | |
| Improvement of neurological score | |||
| IL-1β inflamed air-pouch (mouse) | ↓ PMN migration | Perretti and Flower (1993) | |
| Neutrophil/endothelial interaction under flow (human cells) | ↓ PMN adhesion | Hayhoe et al. (2006) | |
| Neutrophil/endothelial interaction (human cells) | ↓ PMN transmigration | Walther et al. (2000); Zouki et al. (2000) | |
| Annexin A1 1-188 | Cerebral ischaemia (rat) | ↓ Infarct | Relton et al. (1991) |
| Pyrogenesis caused by central injection of interferon or IL-1β (rat) | ↓ Colonic temperature and oxygen consumption | Carey et al. (1990) | |
| Pyrogenesis caused by central injection of PGE2 (rat) | No change in oxygen consumption or colonic temperature. | Carey et al. (1990) | |
| Lung activation (guinea pig) | ↓ TXA2 induced by bolus injection of LTC4 or FMLP | Cirino et al. (1987) | |
| IL-1β inflamed air-pouch (mouse) | ↓ Leukocyte migration | Perretti et al. (1993) | |
| Peptide Ac2-26 | IL-1β inflamed air-pouch (mouse) | ↓ Leukocyte migration | Perretti et al. (1993) |
| IL-8 inflamed air-pouch (mouse) | ↓ Leukocyte migration | Perretti et al. (1993) | |
| FMLP-induced neutropenia (mouse) | ↓ Neutropenia | Perretti et al. (1993) | |
| Albumin extravasation in the skin (mouse) | ↓ Skin oedema | Perretti et al. (1993) | |
| Heart ischaemia–reperfusion (rat) | ↓ Infarct size by up to 50% | La et al. (2001a) | |
| ↓ IL-1β and MPO levels in infarcted hearts | |||
| Mesenteric microcirculation activated by ischaemia–reperfusion (mouse) | ↓ Leukocyte adhesion and emigration but not rolling | Gavins et al. (2003) | |
| ↓ Plasma protein extravasation | |||
| Carrageenan paw oedema (rat) | ↓ Oedema | Cirino et al. (1993) | |
| Carrageenan-induced arthritis (rat) | ↓ The disease severity (intra-articular injection) | Yang et al. (1997) | |
| Glacial acetic acid-induced gastric ulcers (mouse) | ↑ Ulcer healing upon a 4 day treatment | Martin et al. (2008) | |
| Contusive spinal cord injury (rat) | ↓ PLA2 and MPO activities | Liu et al. (2007) | |
| ↓ Glial fibrillary acidic protein (4 weeks post injury) | |||
| ↑ White matter sparing in vivo | |||
| Metabolic inhibition of cardiac myocytes (rat cells) | ↓ Cellular injury | Ritchie et al. (2005) | |
| Ovalbumin-induced pleurisy (rat) | ↓ Mast cell degranulation and plasma protein leakage | Bandeira-Melo et al. (2005) | |
| ↓ PMN and eosinophil accumulation | |||
| ↓ Eotaxin release in exudates | |||
| Splanchnic artery ischaemia–reperfusion (rat) | ↓ The progressive fall in blood pressure | Cuzzocrea et al. (1997) | |
| ↓ PMN accumulation | |||
| ↓ Bowel injury | |||
| Glycogen-induced peritonitis (mouse) | ↓ PMN accumulation | Teixeira et al. (1998) | |
| Ovalbumin-induced sensitization (mouse) | No effect on skin eosinophil recruitment | Teixeira et al. (1998) | |
| Zymosan-induced peritonitis (mouse) | ↓ PMN migration (4 h) | Getting et al. (1997) | |
| ↓ Monocyte migration (24 h) | |||
| In vitro model of septic shock (rat heart) | Abrogation of the fall in the inotropic response to isoprenaline | Ritchie et al. (2003) | |
| ↓ COX-2 mRNA | |||
| No effect NOS-2 mRNA | |||
| Mesenteric microcirculation activated by zymosan (mouse) | ↓ Leukocyte adhesion and emigration (s.c.) | Lim et al. (1998) | |
| ↑ Detachment of adherent leukocytes (i.v.) | |||
| Intestinal ischaemia–reperfusion (mouse) | ↓ Tissue injury | Souza et al. (2007) | |
| ↓ TNF-α levels | |||
| ↓ Lethality | |||
| Neutrophil/endothelial interaction under flow (human cells) | ↓ PMN adhesion | Hayhoe et al. (2006) | |
| Phagocytosis of apoptotic neutrophils (human cells) | ↑ Clearing by macrophages | Maderna et al. (2005) | |
| Peptide Ac2-12 | Zymosan inflamed air-pouch (mouse) | ↓ PMN recruitment | Perretti et al. (2002) |
| Heart ischaemia–reperfusion (rat) | ↓ Infarct size (≤35%) | La et al. (2001a) |
COX, cyclooxygenase; FMLP, formyl-Met-Leu-Phe; IL, interleukin; LPS, lipopolysaccharide; LT, leukotriene; MPO, myeloperoxydase; NOS, nitric oxide synthase; PAF, platelet-activating factor; PL, phospholipase; PMN, polymorphonuclear leukocyte; PG, prostaglandin; TNF, tumour necrosis factor; TX, thromboxane.
Parallel efforts were devoted to the characterization of the Annexin A1 pharmacophore, noting that the N-terminal region of the protein, which is unique to this protein among the Annexin super family, contains sequences that could reproduce most if not all of the effects of the full protein. Therefore, a peptide spanning the first part of the Annexin A1 N-terminus was synthesized, termed peptide acetyl-2-26 and tested for its ability to inhibit neutrophil recruitment into sites of inflammation (Perretti et al., 1993). This peptide was active in suppressing several aspects of the inflammatory response, including plasma protein extravasation (Perretti et al., 1993; Gavins et al., 2003), nociception (Ferreira et al., 1997) and eliciting, overall, inhibitory effects on neutrohil and monocyte trafficking (Miotla et al., 1995; Getting et al., 1997). It subsequently emerged, perhaps not surprisingly, that in affected tissues the overall tissue-protective effects of peptide acetyl-2-26 might derive from more than one single mechanism; for instance protective actions in myocardial infarct were likely due to a combination of local anti-inflammatory effect (La et al., 2001a,b;) as well as of a direct protective action on the cardiomyocyte (Ritchie et al., 2003).
Table 1 lists also the series of in vivo experimentations where the effects of peptide acetyl-2-26 have been studied.
Pharmacological properties aside, an important question is the relevance of the pathway centred around endogenous Annexin A1 on the outcome of the host response. Initial experiments addressing this aspect were conducted with neutralizing antisera raised in rabbits or sheep; these antibodies revealed a role for Annexin A1 on nociception (paw pressure test) and inflammatory (leukocyte trafficking, tumour necrosis factor and IL-1 secretion) responses. Moreover, these studies also determined the potential role that Annexin A1 might play in the anti-inflammatory, anti-nociceptive and anti-arthritic effects produced by animal treatment with glucocorticoids (Perretti et al., 1996a; Ferreira et al., 1997; Yang et al., 1999). However, it is evident that a major impetus to this complex aspect – the pathophysiology of Annexin A1 – could be obtained with the generation of Annexin A1 null mice.
Generated by a classical homologues recombination approach by Rod Flower, Bob Hannon et al., these animals bear a transgenic gene that disrupted the endogenous Annexin A1 gene, meanwhile having a LacZ gene under the control of the Annexin A1 promoter (Hannon et al., 2003). Therefore, this important tool could address on the one hand the function of Annexin A1 in a given biological process, and on the other hand determine the potential spatial and temporal regulation of the Annexin A1 gene promoter activity. Indeed, Annexin A1 null mice display an augmented inflammatory reaction and tissue damage when subjected to a given experimental protocol (see Table 2 for a list of experimental settings tested and their major outcome). Moreover, time-dependent induction of the Annexin A1 gene promoter could be monitored in the context of an ongoing inflammatory reaction (Damazo et al., 2005; 2006;). In particular, it was noted that extravasated neutrophils bear an activated Annexin A1 gene, possibly affecting the fate of the extravasated leukocytes by promoting apoptosis and phagocytosis of apoptotic cells (Maderna et al., 2005; Scannell et al., 2007). Time-dependent activation of the Annexin A1 gene promoters was observed in cell types other than the neutrophil, including the macrophage, the mast cell and the endothelial cell (Damazo et al., 2006), indicating that this mediator may indeed sustain multiple homeostatic functions. Moreover, systemic inflammation elicited by lipolysaccharide led to marked activation of this gene in lung epithelial cells too (Damazo et al., 2005). The flexibility of this system afforded the possibility to observe a remarkable phenotype in parallel to monitoring gene promoter activity. Absence of Annexin A1 led to animal mortality even when an otherwise non-lethal dose of lipolysaccharide was administered (Damazo et al., 2005), higher degree of cell migration and extravasation into the site of inflammation (Chatterjee et al., 2005; Damazo et al., 2006), higher levels of inflammatory markers in a model of localized joint inflammation (Yang et al., 2004), a faster degree of neurological damage in a model of stroke (Gavins et al., 2007) and a delayed repair in a model of colitis (Babbin et al., 2008) (Table 2).
Table 2.
Lessons from the Annexin A1 null mouse
| Experimental model | Outcome | References |
|---|---|---|
| Antigen-induced arthritis | ↑ Pannus formation (day 7) | Yang et al. (2004) |
| ↑ Synovial cytokine mRNAs | ||
| Stroke | ↑ Neurological score | Gavins et al. (2007) |
| ↑ Cell adhesion (pia mater vessels) | ||
| ↑ Brain cytokine mRNAs | ||
| Peritonitis | ↑ Neutrophil recruitment (4 and 24 h) | Damazo et al. (2006) |
| Lipolysaccharide Endotoxaemia | Mortality | Damazo et al., (2005) |
| ↑ TNF/IL-6 dysfunctional production from macrophages | ||
| ↑ Markers of organ injury | ||
| ↑ Organ infiltration with PMN | ||
| ↑ Peritoneal trafficking of leukocytes | ||
| Paw oedema | ↑ Paw volume (selected time points) | Hannon et al. (2003) |
| Cremaster microcirculation | ↑ Cell emigration | Chatterjee et al. (2005) |
| (PAF or zymosan-induced) | ||
| DSS Colitis | ↑ Susceptibility to DSS | Babbin et al. (2008) |
| Delayed resolution of the colitis | ||
| ↓ Fpr2 induction |
Reported are some of the most informative studies conducted with the Annexin A1 null mouse, showing the major outcome with respect to a selection of the markers under observation, and the respective bibliographic reference.
DSS, dodecylsulphate sodium; IL, interleukin; PAF, platelet-activating factor; PMN, polymorphonuclear leukocyte; TNF, tumour necrosis factor.
Annexin A1 target(s)
An important aspect in the biology of Annexin A1, at least in the context of inflammation, has been its mechanism of action, as its solution would have clear pharmacological benefit for drug discovery. Originally thought to act as an inhibitor of phospholipase A2 (Flower and Blackwell, 1979) with consequent inhibitory effects on the generation of prostaglandin and leukotrienes (Cirino et al., 1987; Flower, 1988; Goulding et al., 1990; 1992; 1996b;), the identification of binding sites for Annexin A1 on both peripheral blood neutrophils and monocytes indicated the possible existence of an Annexin A1 receptor(s) (Goulding et al., 1990; 1992; 1996b;). Binding sites for the protein have also been reported in both endothelial cells (Srikrishna et al., 2001) and U937 cell line (Solito et al., 2000).
The breakthrough came through the work of Volker Gerke and his lab, demonstrating that Annexin A1 and Annexin A1-derived peptides could produce responses in human neutrophils that were blocked by antagonists to the formyl peptide receptor (FPR) (Walther et al., 2000), the so-called Boc derivative (where Boc stands for buthyloxycarbonyl, a bulky group used to protect the N-terminal end of an amino acid sequence during the synthesis of peptides) (Dalpiaz et al., 1999; Paclet et al., 2004). FPR is the receptor for formylated peptides, a G protein-coupled receptor (GPCR) that was cloned in the mid 1980s (Boulay et al., 1990a,b; Becker et al., 1998). The study by Walther et al. (2000) demonstrated that addition of Annexin A1 or its peptides to neutrophils reduced the extent of cells transmigration across monolayers of endothelial cells. This effect was paired by a direct activation of the human neutrophils upon application of these peptides in single cell systems, characterized by a transient calcium flux and by shedding of L selectin (Walther et al., 2000).
This fundamental study therefore opened a new avenue of research in the field of annexin A1, allowing further investigations to characterize the functional and molecular links between Annexin A1 and this family of receptors. FPR is the prototype of a family of receptors of which three members have been described in the human system. FPR-like 1 (FPRL-1) and FPR-like 2 (FPRL-2) are structurally related to human FPR although display distinctions with respect to ligand binding: for instance, the original formylated peptide used to clone human FPR, which activates this receptor at low nanomolar concentrations, would activate FPRL-1 at concentrations that are 1000-fold higher, and does not activate FPRL-2 at all (Le et al., 2002; Fu et al., 2006).
Our own studies demonstrated, subsequently, that following adhesion of human neutrophils to endothelial monolayers, endogenous Annexin A1 could be immunoprecipitated together with FPRL-1 (Perretti et al., 2002). Moreover, this receptor was also activated by another endogenous anti-inflammatory mediator, the lipid termed lipoxin A4; Annexin A1 and lipoxin A4 compete for binding to this receptor. These data supported the interesting hypothesis that at least two endogenous effectors of anti-inflammation could share a specific GPCR. Because Annexin A1 and lipoxin A4 have historically been associated to, at least some of, the pharmacological effects produced by glucocorticoids and aspirin, the intriguing idea of this convergence between the most used classes of anti-inflammatory drugs on to this specific target was put forward (Perretti et al., 2002;Gilroy and Perretti, 2005).
The situation is likely more complex as subsequent studies have demonstrated that Annexin A1-derived peptides would activate all three members of the human FPR receptor family (Rescher et al., 2002; Ernst et al., 2004; Karlsson et al., 2005), with not much difference in terms of active concentrations, whereas the full-length protein was shown to bind to FPRL-1 but not to FPR (Hayhoe et al., 2006) (no data are currently available regarding the binding of Annexin A1 to FPRL-2). Altogether, we believe it is plausible that FPRL-1 would be the receptor responsible for transducing the inhibitory signals of Annexin A1 in the pathophysiology of inflammation; hence it could be targeted for novel anti-inflammatory drug discovery programmes (see below).
FPRL-1 is activated by Lipoxin A4, as stated above, and is characterized by a large degree of promiscuity as it binds to several other peptides, proteins and molecules that are apparently unrelated from a structural point of view (Su et al., 1999; Le et al., 2001; Le et al., 2002; Resnati et al., 2002). This observation that the active site accommodates a series of chemically unrelated agonists is difficult to explain in terms of receptor topology. It remains to be seen which of the agonist/receptor interactions reported in the literature is relevant in the context of an inflammatory reaction. In many studies, for instance, the indication that a given ligand would activate FPRL-1 emerged from individual experiments performed with cells overexpressing this GPCR, with little physiological support. In contrast, the interaction between endogenous Annexin A1 and this receptor, initially demonstrated with human neutrophils, has been confirmed also in vivo, at least in the mouse mesenteric tissue (Gastardelo et al., 2009).
This fact brings us to discuss the efforts made to identify the murine receptor(s) responsible for the anti-inflammatory effects of Annexin A1 and its peptides. The mouse FPR receptor family is more complex and difficult to describe, as several genes have been reported for this family at variance from three discovered in the human genome. According to the latest annotation of the mouse genome project data (see UCSC genome browser; mouse chromosome 17 section chr17:18,000,000-18,150,000), FPRL-1 corresponds to the mouse gene previously termed fpr-rs2 (and now referred to as fpr2); therefore the fpr1 and fpr2 genes are regarded as unequivocal. The terminology of gene fpr-rs1 (previously indicated as the orthologue of human FPRL-1) has been changed: it exists in two isoforms, one officially designated fpr3 (old fpr-rs3) and the other comprising an exon from fpr3 and one from the fpr-rs2. To add further confusion, genes spanning from fpr-rs3 to fpr-rs7 have been reported in the mouse receptor family. For the purposes of this review, we will restrict our analyses to mouse Fpr1 as the orthologue of the human FPR and to mouse Fpr2 as the orthologue of human FPRL-1.
Following the initial study of Volker Gerke proposing human FPR as the receptor activated by Annexin A1-derived peptides (Walther et al., 2000), a subsequent study was conducted in the mouse taking advantage of mouse Fpr1 knock out animals (Perretti et al., 2001). Absence of the murine orthologue of human FPR indicated that the large majority of the anti-migratory property of peptide acetyl-2-26 was lost in the absence of this receptor when tested using a mouse model of peritonitis. In contrast a good proportion of the inhibitory effect of hr-Annexin A1 was maintained. This indicates that the subsequent observations made for human FPR and human FPRL-1 in terms of their dichotomy in binding abilities to Annexin A1 and its peptides (Hayhoe et al., 2006) was somehow already hinted in the murine system.
The scenario that is currently emerging is that the large majority of properties displayed by hr-Annexin A1 and/or its bioactive peptides are somehow mediated by activation of members of the FPR family. This conclusion is mostly reached through the observation that Boc derivatives, known to act as non-selective antagonists to all members of this family when used at the appropriate concentrations and/or doses (Gavins et al., 2003; Paclet et al., 2004), prevent the expected biological outcomes. This is true for instance in models of myocardial infarct and/or stroke, where the protective effect of the Annexin A1 biologicals was abrogated by co-administration of a Boc derivative (10–50 µg per animal) whereas it was intact in mice nullified for Fpr1 (Gavins et al., 2005; 2007;); this would indicate the involvement of a receptor of this family, but not the prototype Fpr1, in bringing about the tissue protective actions of Annexin A1 and its peptides. The same holds true when the effects evoked by Annexin A1 and its peptides on the pituitary (adrenocorticotrophin release) are investigated: Fpr1 deletion did not modify the inhibitory effects produced by the protein (John et al., 2007).
In virtually all systems (with one exception; see below) antagonism of formyl peptide receptors was effective to inhibit a specific action of Annexin A1 and/or its peptides, in the whole animal as well as at the cellular level, in human and murine cells (Walther et al., 2000; Rescher et al., 2002; Ernst et al., 2004). We acknowledge that there is one exception, where the inhibitory signal produced by peptide acetyl-2-26 on human neutrophils was described to arise independently from an interaction with members of the FPR receptor family (only FPR and FPRL-1 are expressed on human granulocytes) (Karlsson et al., 2005). Collectively these studies indicate that receptors of the FPR family are responsible for the majority, if not all, of the effects produced by Annexin A1 in several human and murine biological systems related to inflammatory conditions. The next question would then be whether this information could be of use for the development of novel anti-inflammatory drug discovery programmes.
Annexin A1 receptor for novel drug discovery
Our current view, supported by a series of data produced by our as well as other laboratories, is that one specific receptor of the FPR family conveys the anti-inflammatory signals promoted by Annexin A1 to exert a tonic inhibitory function on the inflammatory reaction. The human system termed FPRL-1, is a GPCR that conveys anti-inflammatory signals promoted also by the Lipoxin A4 mediator (Chiang et al., 2006), as mentioned above. Nonetheless, other ligands for this receptor including serum amyloid protein A (Su et al., 1999) or fragments of the Beta-amyloid protein (Le et al., 2001), the deposition of which is the cause of Alzheimer's disease, appear to use the same receptor to initiate pro-inflammatory signal. Generations of mice nullified for the orthologue of human FPRL-1 would be one way to assess what function this receptor might have in a defined inflammatory condition. Another way to address the question of whether the real nature of FPRL-1, is it pro-inflammatory or anti-inflammatory in nature, is to determine the properties of ligands that are selective for this receptor, without the confounding effect of activating other receptors of this family or other receptors tout-court; such ligands have recently been generated.
Amgen have developed a programme to identify small chemical entities that bind and activate selectively FPRL-1 (Burli et al., 2006). At the cellular level, these ligands would promote ‘cell activation’, that is induce generation of IL-6 from human blood monocytes (Frohn et al., 2007). However, and of great importance, administration of some of these selective FPRL-1 agonists to the experimental animal produced inhibitory effects (ear swelling in response to prostaglandin E2 and leukotriene B4 application) (Burli et al., 2006), whereas related molecules able to bind to FPRL-1 without promoting a signalling response were inactive. This prompts us to propose that irrespective of the readouts monitored in ‘controlled’ cellular systems (often calcium fluxes have been used to monitor FPRL-1 activation upon agonist application), selective ligands to FPRL-1 would produce anti-inflammatory/inhibitory actions in in vivo integrated systems.
This conclusion is supported by an even more recent study whereby novel peptides were generated using computer modelling approaches (Shemesh et al., 2008); a peptide that displayed selective binding to FPRL-1, but did not bind to FPR or many other receptors investigated, was identified (Hecht et al., 2009). When given to animals, this peptide was able to inhibit neutrophil trafficking elicited in response to IL-1; of interest, no pro-inflammatory effect was produced by the FPRL-1 peptide ligand once injected into a 6-day-old mouse air-pouch, removing the possibility that – at least in these conditions – Fpr2 activation might convey pro-inflammatory signals and provoke leukocyte trafficking by itself. More importantly, and reminiscent of our own data generated with the Annexin A1 peptide acetyl-2-26 in models of myocardial infarct (La et al., 2001a), this new selective agonist for FPRL-1 was effective in inhibiting heart tissue injury when animals where subjected to an ischaemia–reperfusion procedure (Hecht et al., 2009). Such a protective effect was associated with reduced tissue infiltration by neutrophils, again in agreement with what was reported for hr-Annexin A1 (D'Amico et al., 2000). Therefore, this information is again in line with the notion that the Annexin A1 receptor, FPRL-1 (or ALX if the nomenclature used for lipid receptors – in this case the Lipoxin A4 receptor – is followed; Chiang et al., 2006), would be a target suitable for identifying selective agonists and develop novel anti-inflammatory therapeutics.
Conclusion
In summary these are exciting times as over 20 years of research on the physiology, pathology and pharmacology of Annexin A1 is now coming together in a coherent and integrated fashion, providing a platform for a promising future whereby this line of research could be effectively exploited for the development of novel anti-inflammatory drugs.
The philosophy that pervades the research approach in our laboratory as well as in many more worldwide suggests that the study of endogenous anti-inflammatory agonists could lead to the identification and development of better anti-inflammatory therapeutics. These drugs should be burdened to a lesser degree by side effects as they will be acting by mimicking the way our body disposes, safely, of the complex inflammatory process (Perretti, 1997; Gilroy et al., 2004; Serhan et al., 2007). A correlated concept we recently put forward in an announcement article (which stemmed from a 1 day workshop organized by the British Pharmacological Society in April 2006), and that should be reiterated here, is the need to determine, as part of drug discovery programmes, whether anti-inflammatory therapeutics under development are resolution-toxic or resolution-safe. Indeed, virtually 100% of new anti-inflammatory therapeutics are tested for their ability of inhibiting production and/or function of pivotal pro-inflammatory cytokines, whereas their potential modulation of endogenous anti-inflammatory mediators is scarcely, if at all, determined (Serhan et al., 2007). We therefore propose that besides attempting to develop novel therapeutics by mimicking specific effectors of endogenous anti-inflammation, we should also determine if old and new anti-inflammatory drugs are detrimental to the expression and/or function of one or more of these homeostatic effectors [e.g. Annexin A1, Lipoxin A4, prostaglandin D2, melanocortins and many more (Perretti, 1997; Gilroy et al., 2004)], hence display some resolution-toxic features that could potentially limit their overall therapeutic efficacy.
Acknowledgments
The work conducted in our laboratory and referred to in this review was funded by the Arthritis Research Campaign UK (senior fellowship 15755), the Wellcome Trust (programmes 069234 and 086867) and the British Heart Foundation (FS/2000076 and FS/03/100/16326). We apologize to the many colleagues whose work could not be quoted here for space limitations.
Glossary
Abbreviations:
- FPR
human formyl peptide receptor
- Fpr1
mouse formyl peptide receptor
- Fpr-rs
mouse formyl peptide receptor-related sequence
- FPRL-1
human FPR-like 1
- GPCR
G protein-coupled receptor
Conflict of interest
MP declares licensing of a patent on Annexin A1-derived anti-inflammatory peptides to Unigene Corp (Fairfield, NJ, USA). JD, no conflict to declare.
References
- Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem. 2006;281:19588–19599. doi: 10.1074/jbc.M513025200. [DOI] [PubMed] [Google Scholar]
- Babbin BA, Laukoetter MG, Nava P, Koch S, Lee WY, Capaldo CT, et al. Annexin A1 regulates intestinal mucosal injury, inflammation, and repair. J Immunol. 2008;181:5035–5044. doi: 10.4049/jimmunol.181.7.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira-Melo C, Bonavita AG, Diaz BL, e Silva PM, Carvalho VF, Jose PJ, et al. A novel effect for annexin 1-derived peptide ac2-26: reduction of allergic inflammation in the rat. J Pharmacol Exp Ther. 2005;313:1416–1422. doi: 10.1124/jpet.104.080473. [DOI] [PubMed] [Google Scholar]
- Becker EL, Forouhar FA, Grunnet ML, Boulay F, Tardif M, Bormann BJ, et al. Broad immunocytochemical localization of the formylpeptide receptor in human organs, tissues, and cells. Cell Tissue Res. 1998;292:129–135. doi: 10.1007/s004410051042. [DOI] [PubMed] [Google Scholar]
- Blackwell GJ, Carnuccio R, Di Rosa M, Flower RJ, Parente L, Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980;287:147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Boulay F, Tardif M, Brouchon L, Vignais P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochem Biophys Res Commun. 1990a;168:1103–1109. doi: 10.1016/0006-291x(90)91143-g. [DOI] [PubMed] [Google Scholar]
- Boulay F, Tardif M, Brouchon L, Vignais P. The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry. 1990b;29:11123–11133. doi: 10.1021/bi00502a016. [DOI] [PubMed] [Google Scholar]
- Burli RW, Xu H, Zou X, Muller K, Golden J, Frohn M, et al. Potent hFPRL1 (ALXR) agonists as potential anti-inflammatory agents. Bioorg Med Chem Lett. 2006;16:3713–3718. doi: 10.1016/j.bmcl.2006.04.068. [DOI] [PubMed] [Google Scholar]
- Carey F, Forder R, Edge MD, Greene AR, Horan MA, Strijbos PJLM, et al. Lipocortin 1 fragment modifies pyrogenic actions of cytokines in rats. Am J Physiol. 1990;259:R266–R269. doi: 10.1152/ajpregu.1990.259.2.R266. [DOI] [PubMed] [Google Scholar]
- Chatterjee BE, Yona S, Rosignoli G, Young RE, Nourshargh S, Flower RJ, et al. Annexin 1-deficient neutrophils exhibit enhanced transmigration in vivo and increased responsiveness in vitro. J Leukoc Biol. 2005;78:639–646. doi: 10.1189/jlb.0405206. [DOI] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Christmas P, Callaway J, Fallon J, Jonest J, Haigler HT. Selective secretion of annexin I, a protein without a signal sequence, by the human prostate gland. J Biol Chem. 1991;266:2499–2507. [PubMed] [Google Scholar]
- Cirino G, Flower RJ, Browning JL, Sinclair LK, Pepinsky RB. Recombinant human lipocortin 1 inhibits thromboxane release from guinea-pig isolated perfused lung. Nature. 1987;328:270–272. doi: 10.1038/328270a0. [DOI] [PubMed] [Google Scholar]
- Cirino G, Peers SH, Flower RJ, Browning JL, Pepinsky RB. Human recombinant lipocortin 1 has acute local anti-inflammatory properties in the rat paw edema test. Proc Natl Acad Sci USA. 1989;86:3428–3432. doi: 10.1073/pnas.86.9.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino G, Cicala C, Sorrentino L, Ciliberto G, Arpaia G, Perretti M, et al. Anti-inflammatory actions of an N-terminal peptide from human lipocortin 1. Br J Pharmacol. 1993;108:573–574. doi: 10.1111/j.1476-5381.1993.tb12843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Flower RJ. Lipocortin 1 mediates dexamethasone-induced growth arrest of the A549 lung adenocarcinoma cell line. Proc Natl Acad Sci USA. 1992;89:3571–3575. doi: 10.1073/pnas.89.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Flower RJ. Inhibitory effect of peptides derived from the N-terminus of lipocortin 1 on arachidonic acid release and proliferation in the A549 cell line: identification of EQEYV as a crucial component. Br J Pharmacol. 1998;123:975–983. doi: 10.1038/sj.bjp.0701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Tailor A, Zingarelli B, Salzman AL, Flower RJ, Szab¢ C, et al. Lipocortin 1 protects against splanchnic artery occlusion and reperfusion injury by affecting neutrophil migration. J Immunol. 1997;159:5089–5097. [PubMed] [Google Scholar]
- D'Acquisto F, Merghani A, Lecona E, Rosignoli G, Raza K, Buckley CD, et al. Annexin-1 modulates T-cell activation and differentiation. Blood. 2007;109:1095–1102. doi: 10.1182/blood-2006-05-022798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Acquisto F, Paschalidis N, Raza K, Buckley CD, Flower RJ, Perretti M. Glucocorticoid treatment inhibits annexin-1 expression in rheumatoid arthritis CD4+ T cells. Rheumatology (Oxford) 2008a;47:636–639. doi: 10.1093/rheumatology/ken062. [DOI] [PubMed] [Google Scholar]
- D'Acquisto F, Perretti M, Flower RJ. Annexin-A1: a pivotal regulator of the innate and adaptive immune systems. Br J Pharmacol. 2008b;155:152–169. doi: 10.1038/bjp.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amico M, Di Filippo C, La M, Solito E, McLean PG, Flower RJ, et al. Lipocortin 1 reduces myocardial ischaemia-reperfusion injury by affecting local leukocyte recruitment. FASEB J. 2000;14:1867–1869. doi: 10.1096/fj.99-0602fje. [DOI] [PubMed] [Google Scholar]
- Dalli J, Norling LV, Renshaw D, Cooper D, Leung KY, Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- Dalpiaz A, Ferretti ME, Pecoraro R, Fabbri E, Traniello S, Scatturin A, et al. Phe-D-Leu-Phe-D-Leu-Phe derivatives as formylpeptide receptor antagonists in human neutrophils: cellular and conformational aspects. Biochim Biophys Acta. 1999;1432:27–39. doi: 10.1016/s0167-4838(99)00081-3. [DOI] [PubMed] [Google Scholar]
- Damazo AS, Yona S, D'Acquisto F, Flower RJ, Oliani SM, Perretti M. Critical protective role for annexin 1 gene expression in the endotoxemic murine microcirculation. Am J Pathol. 2005;166:1607–1617. doi: 10.1016/S0002-9440(10)62471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damazo AS, Yona S, Flower RJ, Perretti M, Oliani SM. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasuri K, Antonovici M, Chen K, Wong K, Standing K, Ens W, et al. The synovial proteome: analysis of fibroblast-like synoviocytes. Arthritis Res Ther. 2004;6:R161–R168. doi: 10.1186/ar1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J, Flower RJ, Milton AS, Peers SH, Rotondo D. Antipyretic actions of human recombinant lipocortin-1. Br J Pharmacol. 1991;102:7–9. doi: 10.1111/j.1476-5381.1991.tb12122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler JH, Pisetsky DS, Huber LC, Kalden JR, Gay S, Distler O. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 2005;52:3337–3348. doi: 10.1002/art.21350. [DOI] [PubMed] [Google Scholar]
- Ernst S, Lange C, Wilbers A, Goebeler V, Gerke V, Rescher U. An annexin 1 N-terminal Peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J Immunol. 2004;172:7669–7676. doi: 10.4049/jimmunol.172.12.7669. [DOI] [PubMed] [Google Scholar]
- Errasfa M, Russo-Marie F. A purified lipocortin shares the anti-inflammatory effect of glucocorticosteroids in vivo in mice. Br J Pharmacol. 1989;97:1051–1058. doi: 10.1111/j.1476-5381.1989.tb12561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errasfa M, Rothhut B, Fradin A, Billardon C, Junien JL, Bure J, et al. The presence of lipocortin in human embryonic skin fibroblasts and its regulation by anti-inflammatory steroids. Biochim Biophys Acta. 1985;847:247–254. doi: 10.1016/0167-4889(85)90027-8. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Cunha FQ, Lorenzetti BB, Michelin MA, Perretti M, Flower RJ, et al. Role of lipocortin-1 in the analgesic actions of glucocorticoids. Br J Pharmacol. 1997;121:883–888. doi: 10.1038/sj.bjp.0701211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RJ. Background and discovery of lipocortins. Agents Actions. 1985;17:255–262. doi: 10.1007/BF01982616. [DOI] [PubMed] [Google Scholar]
- Flower RJ. Lipocortin and the mechanism of action of the glucocorticoids. Br J Pharmacol. 1988;94:987–1015. doi: 10.1111/j.1476-5381.1988.tb11614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RJ, Blackwell GJ. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278:456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- Frohn M, Xu H, Zou X, Chang C, McElvaine M, Plant MH, et al. New ‘chemical probes’ to examine the role of the hFPRL1 (or ALXR) receptor in inflammation. Bioorg Med Chem Lett. 2007;17:6633–6637. doi: 10.1016/j.bmcl.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, Dahlgren C. Ligand recognition and activation of formyl peptide receptors in neutrophils. J Leukoc Biol. 2006;79(2):247–256. doi: 10.1189/jlb.0905498. [DOI] [PubMed] [Google Scholar]
- Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243–257. doi: 10.1016/s0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Gastardelo TS, Damazo AS, Dalli J, Flower RJ, Perretti M, Oliani SM. Functional and ultrastructural analysis of annexin A1 and its receptor in extravasating neutrophils during acute inflammation. Am J Pathol. 2009;174:177–183. doi: 10.2353/ajpath.2009.080342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavins FN, Yona S, Kamal AM, Flower RJ, Perretti M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- Gavins FN, Kamal AM, D'Amico M, Oliani SM, Perretti M. Formyl-peptide receptor is not involved in the protection afforded by annexin 1 in murine acute myocardial infarct. FASEB J. 2005;19:100–102. doi: 10.1096/fj.04-2178fje. [DOI] [PubMed] [Google Scholar]
- Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21:1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Flower RJ, Perretti M. Inhibition of neutrophil and monocyte recruitment by endogenous and exogenous lipocortin 1. Br J Pharmacol. 1997;120:1075–1082. doi: 10.1038/sj.bjp.0701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW, Perretti M. Aspirin and steroids: new mechanistic findings and avenues for drug discovery. Curr Opin Pharmacol. 2005;5:405–411. doi: 10.1016/j.coph.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Goulding NJ, Luying P, Guyre PM. Characteristics of lipocortin 1 binding to the surface of human peripheral blood leucocytes. Biochem Soc Trans. 1990;18:1237–1238. doi: 10.1042/bst0181237. [DOI] [PubMed] [Google Scholar]
- Goulding NJ, Jefferiss CM, Pan L, Rigby WF, Guyre PM. Specific binding of lipocortin-1 (annexin I) to monocytes and neutrophils is decreased in rheumatoid arthritis. Arth Rheum. 1992;35:1395–1397. doi: 10.1002/art.1780351126. [DOI] [PubMed] [Google Scholar]
- Goulding NJ, Dixey J, Morand EF, Dodds RA, Pitsillides AA, Edwards JCW. Differential distribution of annexins-I, -II, -IV, and -VI in synovium. Ann Rheumatic Dis. 1996a;54:841–845. doi: 10.1136/ard.54.10.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding NJ, Pan L, Wardwell K, Guyre VC, Guyre PM. Evidence for specific annexin I-binding proteins on human monocytes. Biochem J. 1996b;316:593–597. doi: 10.1042/bj3160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon R, Croxtall JD, Getting SJ, Roviezzo F, Yona S, Paul-Clark MJ, et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1-/- mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- Hayhoe RP, Kamal AM, Solito E, Flower RJ, Cooper D, Perretti M. Annexin 1 and its bioactive peptide inhibit neutrophil-endothelium interactions under flow: indication of distinct receptor involvement. Blood. 2006;107:2123–2130. doi: 10.1182/blood-2005-08-3099. [DOI] [PubMed] [Google Scholar]
- Hecht I, Jiang R, Sampaio AL, Hermesh C, Rutledge C, Shemesh R, et al. A novel peptide agonist of FPRL1 (ALX) displays anti-inflammatory and cardioprotective effects. J Pharmacol Exp Ther. 2009;328:426–434. doi: 10.1124/jpet.108.145821. [DOI] [PubMed] [Google Scholar]
- John CD, Christian HC, Morris JF, Flower RJ, Solito E, Buckingham JC. Annexin 1 and the regulation of endocrine function. Trends Endocrinol Metab. 2004;15:103–109. doi: 10.1016/j.tem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- John CD, Sahni V, Mehet D, Morris JF, Christian HC, Perretti M, et al. Formyl peptide receptors and the regulation of ACTH secretion: targets for annexin A1, lipoxins, and bacterial peptides. FASEB J. 2007;21:1037–1046. doi: 10.1096/fj.06-7299com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal AM, Flower RJ, Perretti M. An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem Inst Oswaldo Cruz. 2005;100(Suppl. 1):39–47. doi: 10.1590/s0074-02762005000900008. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Fu H, Boulay F, Dahlgren C, Hellstrand K, Movitz C. Neutrophil NADPH-oxidase activation by an annexin AI peptide is transduced by the formyl peptide receptor (FPR), whereas an inhibitory signal is generated independently of the FPR family receptors. J Leukoc Biol. 2005;78:762–771. doi: 10.1189/jlb.0305153. [DOI] [PubMed] [Google Scholar]
- La M, D'Amico M, Bandiera S, Di Filippo C, Oliani SM, Gavins FN, et al. Annexin 1 peptides protect against experimental myocardial ischemia-reperfusion: analysis of their mechanism of action. FASEB J. 2001a;15:2247–2256. doi: 10.1096/fj.01-0196com. [DOI] [PubMed] [Google Scholar]
- La M, Tailor A, D'Amico M, Flower RJ, Perretti M. Analysis of the protection afforded by annexin 1 in ischaemia-reperfusion injury: focus on neutrophil recruitment. Eur J Pharmacol. 2001b;429:263–278. doi: 10.1016/s0014-2999(01)01325-5. [DOI] [PubMed] [Google Scholar]
- Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, et al. Amyloid {beta}42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J Neurosci. 2001;21:RC123, 121–125. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Lim LH, Solito E, Russo-Marie F, Flower RJ, Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci USA. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Zhang YP, Han S, Pei J, Xu LY, Lu PH, et al. Annexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injury. J Neuropathol Exp Neurol. 2007;66:932–943. doi: 10.1097/nen.0b013e3181567d59. [DOI] [PubMed] [Google Scholar]
- MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac2-26. J Immunol. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- Martin GR, Perretti M, Flower RJ, Wallace JL. Annexin-1 modulates repair of gastric mucosal injury. Am J Physiol Gastrointest Liver Physiol. 2008;294:G764–G769. doi: 10.1152/ajpgi.00531.2007. [DOI] [PubMed] [Google Scholar]
- Miotla JM, Perretti M, Flower RJ, Jeffery PK, Hellewell PG. Suppression of experimental acute lung injury in the mouse by dexamethasone and the role of lipocortin-1. Br J Pharmacol. 1995;114:62P. [Google Scholar]
- Morand EF, Hutchinson P, Hargreaves A, Goulding NJ, Boyce NW, Holdsworth S. Detection of intracellular lipocortin 1 in human leukocyte subsets. Clin Immunol Immunopathol. 1995;76:195–202. doi: 10.1006/clin.1995.1115. [DOI] [PubMed] [Google Scholar]
- Muesch A, Hartmann E, Rohde K, Rubartelli A, Sitia R, Rapoport TA. A novel pathway for secretory proteins? Trends Biochem Sci. 1990;15:86–88. doi: 10.1016/0968-0004(90)90186-f. [DOI] [PubMed] [Google Scholar]
- Mulla A, Leroux C, Solito E, Buckingham JC. Correlation between the antiinflammatory protein annexin 1 (lipocortin 1) and serum cortisol in subjects with normal and dysregulated adrenal function. J Clin Endocrinol Metab. 2005;90:557–562. doi: 10.1210/jc.2004-1230. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Christian HC, Manston J, Flower RJ, Perretti M. An immunocytochemical and in situ hybridization analysis of annexin 1 expression in rat mast cells: modulation by inflammation and dexamethasone. Lab Invest. 2000;80:1429–1438. doi: 10.1038/labinvest.3780150. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Damazo AS, Perretti M. Annexin 1 localisation in tissue eosinophils as detected by electron microscopy. Med Inflamm. 2002;11:287–292. doi: 10.1080/09629350210000015683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliani SM, Ciocca GA, Pimentel TA, Damazo AS, Gibbs L, Perretti M. Fluctuation of annexin-A1 positive mast cells in chronic granulomatous inflammation. Inflamm Res. 2008;57:450–456. doi: 10.1007/s00011-008-7222-7. [DOI] [PubMed] [Google Scholar]
- Paclet MH, Davis C, Kotsonis P, Godovac-Zimmermann J, Segal AW, Dekker LV. N-Formyl peptide receptor subtypes in human neutrophils activate L-plastin phosphorylation through different signal transduction intermediates. Biochem J. 2004;377:469–477. doi: 10.1042/BJ20031114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers SH, Smillie F, Elderfield AJ, Flower RJ. Glucocorticoid- and non-glucocorticoid induction of lipocortins (annexins) 1 and 2 in rat peritoneal leucocytes in vivo. Br J Pharmacol. 1993;108:66–72. doi: 10.1111/j.1476-5381.1993.tb13441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M. Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol Sci. 1997;18:418–425. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol. 1993;150:992–999. [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A, Harris JG, Goulding NJ, Flower RJ. Lipocortin-1 fragments inhibit neutrophil accumulation and neutrophil-dependent edema in the mouse: a qualitative comparison with an anti-CD11b monoclonal antibody. J Immunol. 1993;151:4306–4314. [PubMed] [Google Scholar]
- Perretti M, Ahluwalia A, Harris JG, Harris HJ, Wheller SK, Flower RJ. Acute inflammatory response in the mouse: exacerbation by immunoneutralization of lipocortin 1. Br J Pharmacol. 1996a;117:1145–1154. doi: 10.1111/j.1476-5381.1996.tb16709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Croxtall JD, Wheller SK, Goulding NJ, Hannon R, Flower RJ. Mobilizing lipocortin 1 in adherent human leukocytes downregulates their transmigration. Nat Med. 1996b;22:1259–1262. doi: 10.1038/nm1196-1259. [DOI] [PubMed] [Google Scholar]
- Perretti M, Getting SJ, Solito E, Murphy PM, Gao JL. Involvement of the receptor for formylated peptides in the in vivo anti- migratory actions of annexin 1 and its mimetics. Am J Pathol. 2001;158:1969–1973. doi: 10.1016/S0002-9440(10)64667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A(4) receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton JK, Strijbos PJLM, O'Shaughnessy CT, Carey F, Forder RA, Tilders FJ, et al. Lipocortin-1 is an endogenous inhibitor of ischemic damage in the rat brain. J Exp Med. 1991;174:305–310. doi: 10.1084/jem.174.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescher U, Danielczyk A, Markoff A, Gerke V. Functional activation of the formyl Peptide receptor by a new endogenous ligand in human lung a549 cells. J Immunol. 2002;169:1500–1504. doi: 10.4049/jimmunol.169.3.1500. [DOI] [PubMed] [Google Scholar]
- Resnati M, Pallavicini I, Wang JM, Oppenheim J, Serhan CN, Romano M, et al. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 2002;99:1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RH, Sun X, Bilszta JL, Gulluyan LM, Dusting GJ. Cardioprotective actions of an N-terminal fragment of annexin-1 in rat myocardium in vitro. Eur J Pharmacol. 2003;461:171–179. doi: 10.1016/s0014-2999(03)01314-1. [DOI] [PubMed] [Google Scholar]
- Ritchie RH, Gordon JM, Woodman OL, Cao AH, Dusting GJ. Annexin-1 peptide Anx-1(2-26) protects adult rat cardiac myocytes from cellular injury induced by simulated ischaemia. Br J Pharmacol. 2005;145:495–502. doi: 10.1038/sj.bjp.0706211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengarth A, Gerke V, Luecke H. X-ray structure of full-length Annexin 1 and implications for membrane aggregation. J Mol Biol. 2001a;306:489–498. doi: 10.1006/jmbi.2000.4423. [DOI] [PubMed] [Google Scholar]
- Rosengarth A, Rosgen J, Hinz HJ, Gerke V. Folding energetics of ligand binding proteins II. Cooperative binding of Ca2+ to annexin I. J Mol Biol. 2001b;306:825–835. doi: 10.1006/jmbi.2000.4358. [DOI] [PubMed] [Google Scholar]
- Scannell M, Flanagan MB, deStefani A, Wynne KJ, Cagney G, Godson C, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. J Immunol. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- Sena AA, Provazzi PJ, Fernandes AM, Cury PM, Rahal P, Oliani SM. Spatial expression of two anti-inflammatory mediators, annexin 1 and galectin-1, in nasal polyposis. Clin Exp Allergy. 2006;36:1260–1267. doi: 10.1111/j.1365-2222.2006.02570.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh R, Toporik A, Levine Z, Hecht I, Rotman G, Wool A, et al. Discovery and validation of novel peptide agonists for G-protein-coupled receptors. J Biol Chem. 2008;283:34643–34649. doi: 10.1074/jbc.M805181200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silistino-Souza R, Rodrigues-Lisoni FC, Cury PM, Maniglia JV, Raposo LS, Tajara EH, et al. Annexin 1: differential expression in tumor and mast cells in human larynx cancer. Int J Cancer. 2007;120:2582–2589. doi: 10.1002/ijc.22639. [DOI] [PubMed] [Google Scholar]
- Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. Human annexin 1 is highly expressed during differentiation of the epithelial cell line A549: involvement of nuclear factor interleukin 6 in phorbol ester induction of annexin 1. Cell Growth Differ. 1998a;9:327–336. [PubMed] [Google Scholar]
- Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. IL-6 stimulates annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998b;10:514–521. doi: 10.1006/cyto.1997.0325. [DOI] [PubMed] [Google Scholar]
- Solito E, Romero IA, Marullo S, Russo-Marie F, Weksler BB. Annexin 1 binds to U937 monocytic cells and inhibits their adhesion to microvascular endothelium: involvement of the α4β1 integrin. J Immunol. 2000;165:1573–1581. doi: 10.4049/jimmunol.165.3.1573. [DOI] [PubMed] [Google Scholar]
- Solito E, Christian HC, Festa M, Mulla A, Tierney T, Flower RJ, et al. Post-translational modification plays an essential role in the translocation of annexin A1 from the cytoplasm to the cell surface. FASEB J. 2006;20:1498–1500. doi: 10.1096/fj.05-5319fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solito E, McArthur S, Christian H, Gavins F, Buckingham JC, Gillies GE. Annexin A1 in the brain – undiscovered roles? Trends Pharmacol Sci. 2008;29:135–142. doi: 10.1016/j.tips.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, et al. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179:8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Panneerselvam K, Westphal V, Abraham V, Varki A, Freeze HH. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J Immunol. 2001;166:4678–4688. doi: 10.4049/jimmunol.166.7.4678. [DOI] [PubMed] [Google Scholar]
- Su SB, Gong W, Gao JL, Shen W, Murphy PM, Oppenheim JJ, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagoe CE, Marjanovic N, Park JY, Chan ES, Abeles AM, Attur M, et al. Annexin-1 mediates TNF-alpha-stimulated matrix metalloproteinase secretion from rheumatoid arthritis synovial fibroblasts. J Immunol. 2008;181:2813–2820. doi: 10.4049/jimmunol.181.4.2813. [DOI] [PubMed] [Google Scholar]
- Teixeira MM, Das AM, Miotla JM, Perretti M, Hellewell PG. The role of lipocortin 1 in the inhibitory action of dexamethasone on eosinophil trafficking in cutaneous inflammatory reactions in the mouse. Br J Pharmacol. 1998;123:538–544. doi: 10.1038/sj.bjp.0701625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoordeldonk MJBM, Schalkwijk CG, Vishwanath BS, Aarsman AJ, van den Bosch H. Levels and localization of group II phospholipase A2 and annexin I in interleukin- and dexamethasone-treated rat mesangial cells: evidence against annexin mediation of the dexamethasone-induced inhibition of group II phospholipase A2. Biochim Biophys Acta. 1994;1224:541–550. doi: 10.1016/0167-4889(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Vong L, D'Acquisto F, Pederzoli-Ribeil M, Lavagno L, Flower RJ, Witko-Sarsat V, et al. Annexin 1 cleavage in activated neutrophils: a pivotal role for proteinase 3. J Biol Chem. 2007;282:29998–30004. doi: 10.1074/jbc.M702876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner BP, Mattaliano RJ, Hession C, Cate RL, Tizard R, Sinclair LK, et al. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986;320:77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- Walther A, Riehemann K, Gerke V. A novel ligand of the formyl peptide receptor: annexin I regulates neutrophil extravasation by interacting with the FPR. Mol Cell. 2000;5:831–840. doi: 10.1016/s1097-2765(00)80323-8. [DOI] [PubMed] [Google Scholar]
- Yang Y, Leech M, Hutchinson P, Holdsworth SR, Morand EF. Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation. 1997;21:583–596. doi: 10.1023/a:1027330021479. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hutchinson P, Morand EF. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 1999;42:1538–1544. doi: 10.1002/1529-0131(199907)42:7<1538::AID-ANR29>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Yang YH, Morand EF, Getting SJ, Paul-Clark MJ, Liu DL, Yona S, et al. Modulation of inflammation and response to dexamethasone by annexin-1 in antigen-induced arthritis. Arthritis Rheum. 2004;50:976–984. doi: 10.1002/art.20201. [DOI] [PubMed] [Google Scholar]
- Zouki C, Ouellet S, Filep JG. The anti-inflammatory peptides, antiflammins, regulate the expression of adhesion molecules on human leukocytes and prevent neutrophil adhesion to endothelial cells. FASEB J. 2000;14:572–580. doi: 10.1096/fasebj.14.3.572. [DOI] [PubMed] [Google Scholar]


