Abstract
Recently, our understanding of hematopoiesis and the development of the immune system has fundamentally changed, leading to significant discoveries with important clinical relevance. Hematopoiesis, once described in terms of irreversible and discrete developmental branch points, is now understood to exist as a collection of alternative developmental pathways capable of generating functionally identical progeny. Developmental commitment to a particular blood-cell lineage is gradually acquired and reflects both cell intrinsic and extrinsic signals. Chief among the extrinsic factors are the environmental cues of hematopoietic microenvironments that comprise specific “developmental niches” that support hematopoietic stem and progenitor cells. Most of this new understanding comes from the study of normal, steady-state hematopoiesis, but there is ample reason to expect that special developmental and/or differentiative mechanisms operate in response to inflammation. For example, both stem and progenitor cells are now known to express Toll-like receptors that can influence hematopoietic cell fates in response to microbial products. Likewise, pro-inflammatory cytokines mobilize hematopoietic stem cells to peripheral tissues. In this Perspective, we review inflammation’s effects on central and extramedullary B lymphopoiesis and discuss the potential consequences of peripheral B-cell development in the context of systemic autoimmune diseases.
Introduction
B lymphocytes and antibodies are crucial components of both innate and adaptive immunity and provide for the specific removal of pathogens and/or their toxins. Indeed, the generation of the memory B-cell compartments and long-lived serum antibody provide the basis for protective immunity elicited by the great majority of contemporary vaccines. B cells and their development in the primary lymphoid tissues have been the focus of intense study at the molecular and cellular level over the past several decades [for reviews, see (Defrance et al., 2002; Hardy and Hayakawa, 2001)]. These investigations have sharpened our understanding of how B-cell antigen receptors are generated and function and the role of B lymphocytes in the organization and distribution of secondary (Gonzalez et al., 1998) and tertiary (Lorenz et al., 2003) lymphoid tissues. Similarly, over the past 15 years our knowledge of antigen-driven B-cell differentiation and especially the germinal center reaction has grown exponentially leading to a basic understanding of just how the humoral immune response achieves its specificity and affinity (Bachmann, 1998; Berek, 1993; Kelsoe, 1996).
Despite this hard-won, new knowledge, immunologists have held tightly to a classic notion that divides B-cell development and differentiation into distinct phases that are antigen-independent or -dependent. Simply put, antigens are generally not thought to affect in any significant way the early phases of B-cell development and maturation in the bone marrow (Defrance et al., 2002). This classic view places the earliest interaction between B cells and exogenous antigen in the spleen or other peripheral sites where expansions or contractions of B-cell populations are induced. This view also implies that the tempo of B-lymphopoiesis does not respond to external cues as does erythropoiesis (Mide et al., 2001) and granulopoiesis (Basu et al., 2000; Hirai et al., 2006). However, recent studies indicate that this view may no longer be tenable and that infection, and even sterile inflammation, control the site and rate of B lymphopoiesis (Ueda et al., 2005; Ueda et al., 2004). In this review, we shall provide a concise overview of B-cell development and differentiation in the bone marrow and periphery with a focus on the impact that acute and chronic inflammation has on these processes.
The process of B lymphopoiesis can be divided roughly into four temporal and spatial phases: early development in the bone marrow; maturation of immature/transitional B cells during their transit to the periphery; entry into the mature B-cell compartments; and antigen-dependent differentiation into antibody-secreting cells and/or memory B cells (Carsetti et al., 2004; Hardy and Hayakawa, 2001).
During their early development in the bone marrow, distinct stages of B-cell development can be characterized by the rearrangement status of immunoglobulin genes (Hardy and Hayakawa, 2001; Hartley et al., 1991; Meffre et al., 2000). Commitment to the B-lineage occurs prior to the initial rearrangements of immunoglobulin gene segments that are necessary to construct a functional B-cell antigen receptor (BCR) (Allman et al., 1999; Hardy et al., 1991) and is identified as the pre-pro-B cell compartment (Hardy et al., 1991). These earliest committed progenitors express low levels of the RAG1/2 recombinase (Oettinger et al., 1990; Schatz et al., 1989), but have immunoglobulin gene loci in an unrearranged, germline configuration (Hardy et al., 1991). Subsequently, pro-B cells highly express RAG1/2 (Hardy et al., 1991; Li et al., 1993) and initiate DH-to-JH rearrangements on both Igh alleles (Alt et al., 1984; Ehlich et al., 1994). After DHJH rearrangement, VH→DHJH recombination follows, but this event does not occur simultaneously at both alleles (Alt et al., 1984). Initial VHDHJH rearrangements that are out-of-frame and therefore, non-functional, can be compensated by second VH→DHJH rearrangement attempts on the alternative allele (Sonoda et al., 1997; ten Boekel et al., 1998). In-frame VHDHJH rearrangements lead to the expression of functional μH polypeptides and their association with the surrogate light chain (SLC), a light-chain-like chaperone complex comprising the VpreB and λ5 polypeptides (Karasuyama et al., 1990; Karasuyama et al., 1993). This point in B-lineage development defines the pre-B cell stage (Goldsby et al., 2003) and the μH and SLC polypeptides associate with the Igα/Igβ heterodimer to form a pre-B cell receptor (pre-BCR) (Karasuyama et al., 1996) that provides constitutive survival and proliferation signals. Following the assembly of pre-BCR, pre-B cells undergo several rounds of cell division and a coincident reduction in RAG1/2 expression (Hardy et al., 1991; Li et al., 1996; Lin and Desiderio, 1993) as large pre-B cells; after their arrest in the G1 phase of the cell cycle (Li et al., 1993) the cells are known as small pre-B cells. RAG1/2 expression becomes elevated in small pre-B cells to drive Vκ-to-Jκ light (L)-chain rearrangements (Reth et al., 1987; Schlissel and Baltimore, 1989). L-chain expression leads to the assembly of mature BCR by the replacement of SLC with Igκ or Igλ polypeptide (Alt et al., 1987; Rajewsky, 1996). These immature B cells are the first in the B-lineage to express the BCR on their surfaces and to become capable of recognizing exogenous antigens (Hardy et al., 1991; Hardy and Hayakawa, 2001).
The generation of functional antigen-receptors - the BCR - by genetic rearrangement and the combinatorial association of gene segments ensures a diverse repertoire of antibody and B-cell specificities but also the generation of self-reactive clones (Nemazee and Weigert, 2000). Thus, the newly generated pool of immature B cells is purged of self-reactivity by at least three mechanisms of immunological tolerance: apoptotic deletion, inactivation by anergy, and receptor editing, the replacement of autoreactive BCR by secondary V(D)J rearrangement (Nemazee and Weigert, 2000). B cells that survive this negative selection may enter one of the mature B-cell compartments, including the mature follicular pool that recirculates through peripheral tissues (Gray et al., 1982) and the fixed splenic marginal zone compartment (Berland et al., 2006; Lopes-Carvalho and Kearney, 2004; Martin and Kearney, 2002). These mature compartments respond to antigens by distinct pathways of differentiation to produce specific antibody and/or establish pools of high affinity memory B cells that persist for long periods of time. The memory pathway appears to be largely reserved for the mature follicular compartment whereas marginal zone B cells provide rapid but transient antibody responses (Oliver et al., 1999).
Early B-cell Development
In adult mice and humans, the bone marrow (BM) is the primary site of B lymphopoiesis and the screening of newly formed B lymphocytes for self-reactivity. Many of the characteristic events of B-cell development, including the rearrangement of the Igh and Igκ/λ gene loci, correlate with expression of surface markers, allowing the definition of specific stages in B-cell development by multiparameter flow cytometry (Osmond et al., 1998). That several phenotypic definitions have been used by different laboratories to describe B-cell development has led to slightly different models/nomenclature for B lymphopoiesis (Osmond et al., 1998). To avoid confusion, and for the sake of simplicity, in this review we shall focus on the molecular events that define B-cell development.
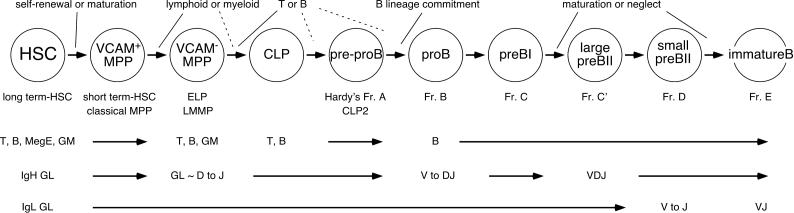
B cells derive from multipotent, self-renewing hematopoietic stem cells (HSC) and transit through a series of maturational steps and developmental checkpoints in the bone marrow (Hardy et al., 2007; Kondo et al., 2003). The initial developmental checkpoint that defines the B lineage is the lymphoid/myeloid branch point which is thought to occur at or after HSC differentiate into non-renewing multipotent progenitors (MPP) that express the adhesion molecule VCAM-1 (Fig. 1) (Kondo et al., 2003). Functional analyses suggest that MPP first lose the potential for megakaryocyte/erythroid (MegE) differentiation, followed by a diminished capacity to generate granulocytes and macrophages (GM), and the eventual commitment to the lymphoid lineages (Adolfsson et al., 2005; Lai and Kondo, 2006). B lineage specification begins as early as the VCAM-1− MPP stage of development, also known as the early lymphoid progenitor (ELP), where cells express B-cell specific transcription factors together with other lineage specific molecules (Igarashi et al., 2002; Lai et al., 2005). The more differentiated, common lymphoid progenitors (CLP) give rise to pre-pro-B cells (also known as CLP-2) and completely lose T-and NK cell potential upon reaching the pro-B stage (Fig. 1) (Tudor et al., 2000).
Figure 1. B cell development in bone marrow.
The step-wise differentiation of hematopoietic stem cells (HSC) into immature B cells in the bone marrow is depicted. Hematopoietic lineage potential gradually decreases as HSC mature through multipotent progenitors (MPP) to common lymphoid progenitors (CLP). The differentiation potential of each progenitor type to give rise to T cells (T), B cells (B), megakaryocyte/erythroid cells (MegE), and granulocytes/macrophages (GM) is indicated. Cells that commit to the B-lineage progress through a series of additional developmental stages defined by the rearrangement of immunoglobulin genes. The stages at which the immunoglobulin heavy chain genes (IgH) and light chain genes (IgL) rearrange are shown (GL, germline configuration). For reference, Hardy’s nomenclature for murine B-cell development is shown [Ref (Hardy, 1991 #7958)].
Extracellular factors are necessary for the expression of B-cell specific transcription factors, including EBF (early B-cell factor), LRF (liver regeneration factor), and Pax5 (paired box). In mice, IL-7 receptor (IL-7Rα) signaling is necessary for the expression of EBF, at least at the transition of CLP to pre-pro-B cells/CLP-2, as B-cell development is blocked at the pre-pro-B stage in IL-7 and IL-7Rα deficient mice as a consequence of low EBF expression (Dias et al., 2005; Kikuchi et al., 2005). Similarly, lack of the transcriptional repressor LRF (also known as Pokemon) arrests B-cell development at the pre-pro-B/CLP-2 stage and results in extrathymic T-cell development in bone marrow (Maeda et al., 2007), presumably as the result of altered Notch signaling (Pui et al., 1999; Radtke et al., 1999). Another B-lineage transcription factor, Pax5, is also expressed in CLP, even though one of its targets, the B-lineage signal transducer, CD19, is not (Hsu et al., 2006). Presumably, Pax5 levels are insufficient in CLP to induce this critical target and establish B-cell commitment (Hsu et al., 2006). B-cell specific transcription factors form transcriptional networks along with more generally expressed transcription factors including PU.1 and E2A (Nutt and Kee, 2007). In pro-B cells, Pax5 and Stat5 interact and critically regulate the accessibility of the Igh gene loci to the RAG1/2 recombinase and thereby control the VH-to-DHJH rearrangements necessary for the production of μH polypeptides (Bertolino et al., 2005; Fuxa et al., 2004). Pax5 and E2A are also important in regulating the expression of the SLC polypeptides, Vpre-B and λ5 (Melchers, 2005); in addition, E2A plays a crucial role in the regulation of Igk rearrangement (Lazorchak et al., 2006) and consequently, the generation of immature B cells that express mature BCR capable of interacting with exogenous and self-antigens.
Roughly 90% of the cells that commit to the B lineage do not reach the immature B cell stage (Rolink et al., 1998). Many, perhaps as many as 75% of developing B cells express BCR that recognize self-antigens (Wardemann et al., 2003). Immature B cells that encounter their antigen ligands in the bone marrow undergo apoptosis (Nemazee and Buerki, 1989), or are rendered non-functional by the process known as anergy (Goodnow et al., 1988), or reactivate Ig gene rearrangement to replace the self-reactive BCR (Gay et al., 1993; Radic et al., 1993; Tiegs et al., 1993). This last tolerance mechanism, receptor editing, is unusual in that it operates on the autoreactive receptor rather than the autoreactive cell (Nemazee and Weigert, 2000). Once past this initial tolerance checkpoint, the residual immature B cell compartment begins a series of maturation steps as they emigrate from the bone marrow to the spleen or other peripheral lymphoid tissues where they complete their development.
Peripheral B-cell development
The final stages of B-cell development take place in the spleen where transitional B cells (Carsetti et al., 1995), essentially intermediate forms between the immature and mature phenotypes, undergo selection into the peripheral B-cell compartments (Loder et al., 1999). Type 1 transitional (T1) B cells continue to mature through the transitional 2 (T2) B cell stage, which in turn completes B-cell development/maturation as mature follicular or marginal zone B cells. Tolerance continues to operate during the T1 and T2 stages of B-cell development, as the frequency of self-reactive clones continues to decrease through the immature, T1, and T2 stages of B-cell development (Wardemann et al., 2003). However, unlike immature B cells, T1 B cells do not appear able to undergo receptor editing on exposure to self-antigen ligands (Wang et al., 2007). Instead, T1 cells undergo apoptosis following BCR ligation whereas BCR ligation on T2 B cells induces proliferation and differentiation to antibody secretion similar to mature B cells (Petro et al., 2002). Though the mechanism is still unclear, studies of the frequencies of VH genes in immature and mature B cell populations have uncovered biases in VH gene usage, suggesting that positive selection also occurs in the transitional stages of B-cell development (Gu et al., 1991; Levine et al., 2000).
In addition to tonic BCR signaling, extracellular factors are required for transitional B cells to complete their development. B-cell activating factor of the TNF family (BAFF) is an important regulator of transitional B-cell maturation, and controls the T1→T2 transition. Mice deficient for BAFF have severely reduced numbers of B cells beyond the T1 stage of development but normal numbers of developing B cells in the BM (Gorelik et al., 2003; Schiemann et al., 2001; Shulga-Morskaya et al., 2004). Three receptors for BAFF have been identified, transmembrane activator and calcium-modulator and cyclophilin-ligand interactor (TACI), B-cell maturation antigen (BCMA), and BAFF receptor (BAFF-R) (Mackay and Browning, 2002). However, BAFF-R is the principal receptor for controlling peripheral B-cell development, as mice deficient in this receptor exhibit a phenotype comparable to BAFF deficient mice (Sasaki et al., 2004). BAFF-R signaling activates several transcription factors, including NF-κB (nuclear factor of κ B cells) (Kanakaraj et al., 2001), as well as expression of anti-apopototic genes, including Bcl-2, Bcl-xL, and Mcl-1 (Do et al., 2000; Hatada et al., 2003; Hsu et al., 2002; Woodland et al., 2008). Signaling through BAFF-R also upregulates the expression of CD21 and CD23, B-cell associated receptors (for complement and IgE, respectively) that distinguish T1 B cells from more mature T2 and mature B cells (Gorelik et al., 2004).
A recent study identified a subset of T2 B cells that are highly sensitive to proliferative signals through BAFF-R, suggesting that BAFF targets this population as part of the homeostatic regulation of peripheral B-cell development (Meyer-Bahlburg et al., 2008). Further evidence for BAFF as a homeostatic regulator of peripheral B lymphopoiesis is based on observations that patients exhibit significant increases in serum BAFF concentrations during B cell depletion therapy (Cambridge et al., 2006; Pers et al., 2007), suggesting that BAFF concentrations mediate the size of the peripheral B-cell pool. Interestingly, transgenic mice that express high levels of BAFF exhibit large increases in the numbers of T2 and mature B cells, and typically show evidence of systemic autoimmune disease (Batten et al., 2000; Khare et al., 2000; Mackay et al., 1999). Presumably, BAFF overexpression allows for the survival of T1 B cells that would normally be purged by apoptosis (Thien et al., 2004). Consistent with this hypothesis, transgenic B cells bearing self-reactive receptors are more dependent on BAFF for survival than are alloreactive B cells (Lesley et al., 2004).
Elevated serum BAFF has also been associated with several autoimmune diseases, including lupus, rheumatoid arthritis, and Sjogren’s syndrome (Becker-Merok et al., 2006; Cheema et al., 2001; Groom et al., 2002; Jonsson et al., 2005; Zhang et al., 2001), garnering much attention for BAFF as a potential therapeutic target.
Though BAFF production is typically attributed to myeloid cells, experiments with reciprocal BM chimeras demonstrated that BAFF production by radiation-resistant (presumably non-hematopoietic) cells is necessary for peripheral B-cell development and sufficient for humoral immune responses (Gorelik et al., 2003). However, BAFF-deficient mice reconstituted with BAFF-sufficient bone marrow mount antibody responses in response to immunization with T-cell dependent antigens, indicating that hematopoietic cell-derived BAFF contributes significantly to B-cell maintenance and their differentiation to antibody-secreting cells. Hematopoietic sources of BAFF include dendritic cells (Schneider et al., 1999), macrophages (Craxton et al., 2003), neutrophils (Scapini et al., 2003), T cells (Yoshimoto et al., 2006), and even B cells themselves (Chu et al., 2007; Daridon et al., 2007).
BAFF is required for normal peripheral B-cell development, but is it necessary for mature B cells during an immune response? A role for BAFF in the maintenance of germinal centers has been proposed based on the observation that BAFF-deficient and BAFF-R-deficient mice have attenuated germinal center responses (Rahman et al., 2003; Vora et al., 2003). However, a recent study found that freshly isolated germinal center B cells have no detectable BAFF bound to their surface receptors, though they express receptors capable of binding exogenous BAFF (Darce et al., 2007). Follicular B cells, on the other hand, have BAFF bound to their surface receptors. Together these findings suggest that the germinal center is a BAFF-poor environment, consistent with the concept that the survival of germinal center B cells is determined by competition for antigen (Kelsoe, 1996). It seems possible, therefore, that attenuated germinal center responses in BAFF-deficient mice may be explained better by the indirect effects of BAFF on B-cell development. We note that the few B cells that reach maturity in BAFF-deficient mice do not express CD21, a receptor for the complement activation products C3d and C3b (Carroll, 2004). The binding of CD21 to C3-decorated antigen strongly amplifies BCR signaling (Fearon and Carroll, 2000) and mice deficient in CD21 exhibit decreased antibody responses to T-dependent antigens (Ahearn et al., 1996; Molina et al., 1996), at least in part due to the inability of CD21 deficient B cells to persist in germinal centers (Fischer et al., 1998).
Although a direct role for BAFF in germinal centers can be debated, other B-cell compartments may rely on BAFF to generate effective humoral responses. BAFF has been implicated in T-independent antibody responses to infection and has been shown to promote class-switching independently of T-cell derived CD40 ligand(Castigli et al., 2005; Litinskiy et al., 2002), observations that suggest a direct link between B cells and innate immune cells in T-independent humoral responses. Finally, BAFF production by blood dendritic cells also appears to be required for the differentiation of marginal zone B cells into plasmacytes secreting low-affinity antibody during the early humoral response to bacterial infections (Balazs et al., 2002).
Inflammation and B lymphopoiesis
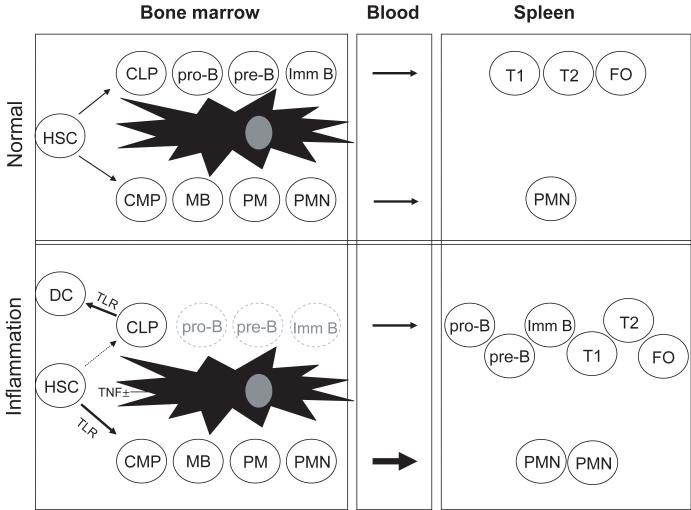
While infection and inflammation have long been known to affect leukocyte production (Apperley et al., 1989; Nagaoka et al., 2000; Young et al., 1989), it has only been in recent years that the molecular and cellular details of inflammatory hematopoiesis have been explored (Fig. 2). Several recent studies demonstrated that inflammation alters hematopoietic output of bone marrow by favoring myelopoiesis, especially granulopoiesis, over lymphopoiesis (Nagai et al., 2006; Nagaoka et al., 2000; Ueda et al., 2005; Ueda et al., 2004). Our current understanding of how inflammation affects B lymphopoiesis is based on three mechanisms: i) diversion of lymphoid progenitors from the B lineage; ii) induction of apoptosis in developing B cells; and iii) mobilization of developing B cells to the periphery.
Figure 2. Inflammation induces extramedullary B lymphopoiesis.
A simplified schematic of hematopoiesis during normal and inflamed conditions is depicted, with B-cell development progressing from hematopoietic stem cells (HSC) through common lymphoid progenitors (CLP), pro-B cells (pro-B), pre-B cells (pre-B), immature B cells (imm B), transitional 1 B cells (T1), transitional 2 B cells (T2), and mature B cells (Mat B). From HSC, granulocyte differentiation occurs through common myeloid progenitors (CMP), myeloblasts (MB), promyelocytes (PM), and polymorphonuclear neutrophils (PMN). Toll-like receptor (TLR) signals induce HSC to develop into myeloid cells and CLP to develop into dendritic cells (DC). TNFα reduces CXCL12 and stem cell factor (SCF) expression by bone marrow stromal cells, resulting in the mobilization of developing B-lineage cells to peripheral lymphoid tissues such as the spleen, where they continue to mature.
Remarkably, microbial products can have direct effects on the differentiation of hematopoietic progenitors. Nagai and his colleagues (Nagai et al., 2006) have demonstrated that hematopoietic progenitors express at least some Toll-like receptors (TLR) and that TLR ligation induces their differentiation into innate immune cells. CLP incubated with TLR ligands differentiate into dendritic cells in vitro. Redirected differentiation of CLP into dendritic cells as a result of TLR ligation could reduce the B-cell progenitor pool, limiting B lymphopoiesis during infection; whether or not this potential reduction is manifest during infections and/or chronic inflammation is unknown but may represent a significant aspect of clinical management. Hopefully, we will soon understand how signaling through TLR overrides the fate decisions that are already established in CLP, such as the expression of Pax5 and the RAG1/2 recombinase.
Hematopoiesis is also affected by inflammatory cytokines. TNFα, perhaps the premiere pro-inflammatory cytokine (Beutler and Cerami, 1989), is known to play a major role in modulating B lymphopoiesis. For example, the bone marrow of mice infected with influenza have significantly lower numbers of developing B cells due to the anti-proliferative effects of TNFα and lymphotoxin (LTα) (Sedger et al., 2002). However, this study did not demonstrate whether the proliferative suppression by TNFα and LTα was direct or acted indirectly by altering the bone marrow environment.
More recently, TNFα has been shown to affect B lymphopoiesis by regulating the expression of chemokines and growth factors in the bone marrow. The CXCL12/CXCR4 axis is crucial to hematopoiesis (Nagasawa, 2006; Nagasawa et al., 1996) and is a target of inflammatory signals (Fedyk et al., 2001). The importance of this chemokine in B lymphopoiesis is evident in CXCL12-deficient mice, which have severely reduced B-cell numbers (Nagasawa et al., 1996). Similarly, blocking CXCR4 with antagonists in vivo mobilizes B cells from the bone marrow into the circulation (Martin et al., 2003). On a finer scale, CXCL12 gradients in the bone marrow may guide developing B cells to different microenvironments; Martin and colleagues proposed that CXCL12 is a necessary component of the stepwise progression of developing B cells through specialized bone marrow “niches” (Tokoyoda et al., 2004). They observed that early B lineage cells (pre-pro-B/CLP-2 cells) associate with CXCL12-expressing reticular cells whereas B-lineage cells in the subsequent, more mature developmental stage (i.e., pro-B cells) associate with reticular cells that express IL-7 but not CXCL12.
Mice immunized with sterile adjuvants such as alum also exhibit significant decreases in the numbers of developing B cells in the bone marrow. This loss of developing B cells correlates with a reduction in bone marrow CXCL12 (Ueda et al., 2004); indeed adjuvant-induced reductions in CXCL12 expression and the concomitant mobilization of B cells from the bone marrow to the blood circulation are attenuated in TNFα deficient mice, demonstrating that TNFα can modulate B lymphoiesis by reducing the ability of the BM to retain developing B cells. This premature emigration may reflect the physiologic process for removing transitional B cells into the periphery (Fedyk et al., 1999) but results in a transient loss of central B lymphopoiesis (Ueda et al., 2005; Ueda et al., 2004).
Inflammation also affects the expression of stem cell factor (SCF) in the bone marrow. The receptor for SCF, c-Kit, is expressed by hematopoietic stem cells (Ikuta et al., 1991; Ogawa et al., 1991), myeloid progenitors (Akashi et al., 2000), common lymphoid progenitors (Kondo et al., 1997), and early B-cell progenitors (Hunte et al., 1998). Mice with inactive c-Kit exhibit age-dependent blockades in B-cell development (Waskow et al., 2002), indicating that B lymphopoiesis in mice more than 10 days old requires SCF. Adjuvant immunization decreases SCF expression in BM, which, like CXCL12 expression, coincides with the mobilization of developing B cells to the circulation (Ueda et al., 2005). However, the inflammatory mediator(s) that alters SCF production in BM is not known.
The reduction in B-cell production during an inflammatory response is accompanied by increased production of neutrophils, a process referred to as “emergency granulopoiesis” (Basu et al., 2000; Hirai et al., 2006). The reciprocal production of B cells and granulocytes suggests that the progenitors of each compartment utilize a common developmental niche (Fig. 2). This hypothesis is supported by the observation that developing B cells and granulocytes co-localize beneath BM stromal cells in vitro to form mixed “cobblestone clusters” (Ueda et al., 2005). Interestingly, the intravenous administration of recombinant TNFα reduces CXCL12 in the bone marrow and mobilizes developing B cells to the periphery, but has only a modest effect on granulopoiesis. On the other hand, administration of IL-1 to mice, a proinflammatory cytokine that has been linked to granulopoiesis (Hestdal et al., 1992; Moore and Warren, 1987; Stork et al., 1988; Ueda et al., 2005), modestly increases BM neutrophil numbers but does not affect B cells. However, co-administration of TNFα and IL-1β recapitulates all of the effects of adjuvant-induced inflammation, mobilizing BM B cells to the periphery and significantly enhancing granulopoiesis, suggesting that these two proinflammatory cytokines synergize to redirect hematopoiesis in favor of granulopoiesis (Ueda et al., 2005).
Inflammation-induced reductions in BM B cells are accompanied by increases in the number of developing B cells in the spleen, indicating that inflammation mobilizes developing B cells from the BM to the periphery (Nagaoka et al., 2000; Ueda et al., 2005; Ueda et al., 2004). The fact that inflammation mobilizes developing B cells instead of inducing apoptosis leads to an important question: do developing B cells that have mobilized to the periphery continue their development outside of the bone marrow? The primary implication of extramedullary lymphopoiesis is that B cells could develop in a “selection-light” environment (Sandel et al., 2001). Development in such an environment might allow for the survival of self-reactive B cells that would normally be deleted in the bone marrow, thereby raising the potential for autoimmune pathology. This hypothesis provides a potential mechanism to explain the strong linkage between inflammation and the induction of systemic autoimmune disease.
On the other hand, the possibility of immature B cells playing a role in immune responses to pathogens cannot be excluded. Immature B cells are prominent in the spleens of adjuvant-immunized mice, placing them in an ideal position to interact with blood-borne antigens. Support for this model was provided in a recent report that focused on antibody production by immature/T1 B cells (Ueda et al., 2007). In this study, the authors observed that the number of splenic T1 B cells significantly increased during inflammation as a result of extramedullary lymphopoiesis. Though signaling through the BCR alone induces apoptosis in T1 B cells (Petro et al., 2002), LPS stimulation in vitro induces proliferation and secretion of class-switched antibody, an effect significantly augmented by the inclusion of BAFF. This observation indicates that T1 B cells are competent to respond to positive BCR signals and participate in T-independent humoral responses. The production of class-switched antibody requires the activity of activation-induced cytosine deaminase (AID). Surprisingly enough, T1 B cells, but not T2 B cells, have been found to express functional levels of AID as part of their developmental program, rather than in response to TLR ligation or T-cell help (Ueda et al., 2007). In addition, T1 B cells express low but significant amounts of Blimp-1 (B-lymphocyte maturation promoting), a transcription factor required for differentiation into ASC (Angelin-Duclos et al., 2000; Turner et al., 1994). Together, these observations suggest that T1 B cells, like marginal zone B cells, may be poised to respond rapidly to antigens as part of the “innate” humoral response. This notion is supported by the recent observation that human transitional B cells differentiate into plasmacytes upon encounter with TLR ligands (Capolunghi et al., 2008).
What is the physiologic role of antigen-responsive T1 B cells? Since T1 B cells have not completed developmental selection, they are enriched for self-reactive clones compared to mature follicular B cells (Wardemann et al., 2003). Normally, the production of low-affinity, polyreactive antibody during the early immune responses is attributed to B-1 and marginal zone B cells (Martin et al., 2001) but the ability of T1 B cells to respond quickly to antigen (Ueda et al., 2007) suggests that these developmentally immature cells may also contribute to early humoral defense.
The observation that LPS-stimulated T1 B cells are dependent on BAFF for survival (Ueda et al., 2007) raises the possibility that BAFF serves as the limiting factor in the recruitment of T1 B cells into immune responses. In this model, the amount of BAFF available in normal, uninfected individuals would be sufficient for B-cell development, maturation, and homeostasis, but not high enough to allow the survival of T1 B cells that encounter self- or exogenous antigens. Conversely, increased BAFF production during infection would promote the survival of T1 cells that encounter antigen, allowing their participation in immune responses. Indeed, the observation that dendritic cells upregulate BAFF production in response to inflammatory cytokines suggests that modulated BAFF production during infection may represent a physiological and protective response to abet humoral immunity (Litinskiy et al., 2002).
Chronic inflammation and B lymphopoiesis
If acute inflammation, even the modest inflammation that results from immunization with adjuvant, affects central hematopoiesis, what are the effects of persistent inflammation? Generally, inflammation is regarded as a beneficial, self-limiting, healing process, allowing for the clearance of the initiating stimuli via localized recruitment of immune effector cells. However, when the inflammatory stimulus persists, the resulting chronic inflammation can be protective or pathogenic depending on the circumstances. For example, granulomas can wall off persistent inflammatory agents when the normal mechanisms of antigen clearance fail or are otherwise insufficient (Chattopadhyay, 1994).
We and many others have used pristane (2,6,10,14-tetramethylpentadecane), a natural, saturated 19-C alkane, to induce chronic peritoneal inflammation (Potter, 2003; Richards et al., 1999). When injected into the peritoneal cavity, pristane is either phagocytosed by macrophages or surrounded by infiltrating leukocytes to form oil-cell complexes. These complexes adhere to peritoneal surfaces and preferentially to the mesenteric membranes (Potter and Maccardle, 1964). With time, the infiltrating leukocyte complexes expand to form structures known as oil granulomas.
Chronic inflammation induces tertiary lymphoid tissues via the same cellular and molecular signals that direct the organization and formation of secondary lymphoid tissues (Drayton et al., 2006). Mice deficient for TNFα (TNFα−/−), LTα (LTα−/−), or mature B cells (μMT) exhibit impaired abilities to form normal secondary lymphoid tissues including lymph nodes and Peyer’s patches (De Togni et al., 1994; Fu et al., 1998; Gonzalez et al., 1998; Pasparakis et al., 1996). The generation of organized oil granulomas under conditions of chronic inflammation led us to question whether these granulomas might be considered a sort of tertiary lymphoid tissue (Nacionales et al., 2006; Potter and Maccardle, 1964). If so, mutations that affect the development of secondary lymphoid tissues might also be expected to interfere with oil granuloma development and/or organization. To investigate this potential relationship, we injected C57BL/6 mice that were genetically deficient for genes [TNFα (Pasparakis et al., 1996), LTα (De Togni et al., 1994), μMT (Kitamura et al., 1991), and LAT (Zhang et al., 1999)] known to be important in the development and organization of secondary lymphoid tissues. Remarkably, the mutation with the strongest effect on oil granuloma formation was μMT, an IgCμ mutation that prevents complete B cell development (Kitamura et al., 1991). The μMT mutation is effective in interfering with the development of secondary lymphoid tissues - but these tissues contain large numbers of B cells (Ngo et al., 2001; Tumanov et al., 2004). We note that the μMT mutation also blocks the formation of oil granulomas even though very few B cells are present in the granuloma structure (manuscript in preparation). TNFα−/− mice exhibit modestly impaired granuloma formation after pristane whereas oil granuloma development in LTα deficient mice is comparable to normal controls (manuscript in preparation). From these observations, we conclude that pristane induced granulomas likely represent an inflammatory tissue that is organized, at least in part, by the genetic pathways that also organize secondary and tertiary lymphoid tissues. B cells, though rare in the oil granulomas of C57BL/6 mice, appear to exert a powerful effect on the development of these tissues.
Chronic inflammation may also contribute to the development of B-cell tumors. Pristane-induced oil granulomas contain macrophages and fibroblasts that secrete IL-6 (Fagarasan et al., 2000; Potter, 2003), an inflammatory cytokine that promotes the differentiation of B cells into plasmacytes (Roldan and Brieva, 1991). Mice that overexpress IL-6 develop spontaneous myelomas and lymphomas at much higher rates than control mice (Kovalchuk et al., 2002). Plasmacytes bearing the chromosomal translocations t(12;15) or t(6;15) proliferate and survive within the IL-6-rich environment of pristane-induced oil granulomas, resulting in the development of plasmacyte tumors called plasmacytomas or myelomas (Fagarasan et al., 2000; Potter, 2003). Thus, granulomatous tissues associated with chronic inflammation promote specific B-cell tumors by providing an environment where neoplastic B cells or plasmacytes escape normal regulatory cues.
Interestingly, the development of pristane-induced plasmacytomas is strain dependent; BALB/c mice are susceptible whereas C57BL/6 mice are resistant, indicating that the genetic differences between the strains affect both granuloma development and predisposition for plasmacytomas. Indeed, oil granulomas in C57BL/6 and BALB/c mice differ in their anatomical location on the mesentery; in C57BL/6 mice, pristane elicits oil granulomas at the gut/mesentery boundary whereas in BALB/c mice granulomas are centripetally distributed on the mesentery [(Potter and Maccardle, 1964) and manuscript in preparation]. The identification of genes that regulate oil granuloma formation is required to understand better the differential susceptibilities to pristane-induced plasmacytomas. At least two genes that confer resistance to plasmacytomas elicited by pristane have been identified (Potter et al., 1994), but their roles in oil granuloma development are unknown.
Chronic inflammation is also associated with the induction or exacerbation of autoimmunity. Pristane-induced autoimmunity is observed in many strains of mice (Mizutani et al., 2005; Satoh et al., 2003; Satoh et al., 2000) and has been linked to the induction of apoptosis by peritoneal cells, which provides the autoantigen substrate necessary to break tolerance (Calvani et al., 2005; Kirou et al., 2005; Potter and Maccardle, 1964; Zhuang et al., 2005). Sequestration of pristane by granuloma formation may act to inhibit autoimmunity; C57BL/6 mice wall off injected pristane much more efficiently than do BALB/c mice and C57BL/6 mice are less prone to develop severe autoimmune disease in response to pristane than are BALB/c mice (Nacionales et al., 2006; Potter and Maccardle, 1964; Wooley et al., 1989).
Conclusions
There is much more to hematopoiesis and B-cell development than the normal, steady-state hematopoietic pathways that have been described in naïve mice and humans. Inflammation redirects central hematopoiesis by altering the bone marrow environment to favor myelopoiesis and by mobilizing developing B cells to the blood and peripheral lymphoid sites where exposure to antigens and inflammatory agents are common. The utility, consequences, and potential dangers of extramedullary B lymphopoiesis are not yet known but deserve additional study and consideration, especially in the light of the persistent inflammation associated with chronic infection and autoimmunity. In the periphery, B-cell tolerance may be less efficient and the capacity of AID+ T1 B cells to respond to antigenic stimuli suggests a pathway for generating and expanding clones of B cells reactive with self-antigens. A particularly attractive test of this hypothesis may be found in the epitope spreading associated with the desmoglein antibodies responsible for pemphigoid disease (Amagai et al., 1999; Futei et al., 2000; Ishii et al., 1997; Miyagawa et al., 1999; Tsunoda et al., 2002).
Abbreviations
- ASC
Antibody Secreting Cell
- BM
Bone Marrow
- CLP
Common Lymphoid Progenitor
- HSC
Hematopoietic Stem Cell
- MPP
Multipotent Progenitor
- TLR
Toll-like Receptors
References
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Allman D, Li J, Hardy RR. Commitment to the B lymphoid lineage occurs before DH-JH recombination. J Exp Med. 1999;189:735–740. doi: 10.1084/jem.189.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt FW, Blackwell TK, Yancopoulos GD. Development of the primary antibody repertoire. Science. 1987;238:1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- Alt FW, Yancopoulos GD, Blackwell TK, Wood C, Thomas E, Boss M, Coffman R, Rosenberg N, Tonegawa S, Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. Embo J. 1984;3:1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40:167–170. doi: 10.1016/s0190-9622(99)70183-0. [DOI] [PubMed] [Google Scholar]
- Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–5471. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons PJ, Gordon M, Goldman JM. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol. 1989;17:38–45. [PubMed] [Google Scholar]
- Bachmann MF. The role of germinal centers for antiviral B cell responses. Immunol Res. 1998;17:329–344. doi: 10.1007/BF02786455. [DOI] [PubMed] [Google Scholar]
- Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–3733. [PubMed] [Google Scholar]
- Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15:570–576. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- Berek C. Somatic mutation and memory. Curr Opin Immunol. 1993;5:218–222. doi: 10.1016/0952-7915(93)90007-f. [DOI] [PubMed] [Google Scholar]
- Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Bertolino E, Reddy K, Medina KL, Parganas E, Ihle J, Singh H. Regulation of interleukin 7-dependent immunoglobulin heavy-chain variable gene rearrangements by transcription factor STAT5. Nat Immunol. 2005;6:836–843. doi: 10.1038/ni1226. [DOI] [PubMed] [Google Scholar]
- Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Calvani N, Caricchio R, Tucci M, Sobel ES, Silvestris F, Tartaglia P, Richards HB. Induction of apoptosis by the hydrocarbon oil pristane: implications for pristane-induced lupus. J Immunol. 2005;175:4777–4782. doi: 10.4049/jimmunol.175.7.4777. [DOI] [PubMed] [Google Scholar]
- Cambridge G, Stohl W, Leandro MJ, Migone TS, Hilbert DM, Edwards JC. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, Cagliuso M, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- Carroll MC. The complement system in B cell regulation. Mol Immunol. 2004;41:141–146. doi: 10.1016/j.molimm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. 1995;181:2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A. The granulomatous response and oral cavity. Indian J Dent Res. 1994;5:15–18. [PubMed] [Google Scholar]
- Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179:5947–5957. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003;101:4464–4471. doi: 10.1182/blood-2002-10-3123. [DOI] [PubMed] [Google Scholar]
- Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J Immunol. 2007;178:5612–5622. doi: 10.4049/jimmunol.178.9.5612. [DOI] [PubMed] [Google Scholar]
- Daridon C, Devauchelle V, Hutin P, Le Berre R, Martins-Carvalho C, Bendaoud B, Dueymes M, Saraux A, Youinou P, Pers JO. Aberrant expression of BAFF by B lymphocytes infiltrating the salivary glands of patients with primary Sjogren’s syndrome. Arthritis Rheum. 2007;56:1134–1144. doi: 10.1002/art.22458. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Defrance T, Casamayor-Palleja M, Krammer PH. The life and death of a B cell. Adv Cancer Res. 2002;86:195–225. doi: 10.1016/s0065-230x(02)86006-7. [DOI] [PubMed] [Google Scholar]
- Dias S, Silva H, Jr., Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Ehlich A, Martin V, Muller W, Rajewsky K. Analysis of the B-cell progenitor compartment at the level of single cells. Curr Biol. 1994;4:573–583. doi: 10.1016/s0960-9822(00)00129-9. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Watanabe N, Honjo T. Generation, expansion, migration and activation of mouse B1 cells. Immunol Rev. 2000;176:205–215. doi: 10.1034/j.1600-065x.2000.00604.x. [DOI] [PubMed] [Google Scholar]
- Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol. 2001;166:5749–5754. doi: 10.4049/jimmunol.166.9.5749. [DOI] [PubMed] [Google Scholar]
- Fedyk ER, Ryyan DH, Ritterman I, Springer TA. Maturation decreases responsiveness of human bone marrow B lineage cells to stromal-derived factor 1 (SDF-1) J Leukoc Biol. 1999;66:667–673. doi: 10.1002/jlb.66.4.667. [DOI] [PubMed] [Google Scholar]
- Fischer MB, Goerg S, Shen L, Prodeus AP, Goodnow CC, Kelsoe G, Carroll MC. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–585. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futei Y, Amagai M, Sekiguchi M, Nishifuji K, Fujii Y, Nishikawa T. Use of domain-swapped molecules for conformational epitope mapping of desmoglein 3 in pemphigus vulgaris. J Invest Dermatol. 2000;115:829–834. doi: 10.1046/j.1523-1747.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E, Busslinger M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsby RA, Kindt TJ, Osborne BA, Kuby J. Immunology. Fifth edn W. H. Freeman and Company; New York: 2003. [Google Scholar]
- Gonzalez M, Mackay F, Browning JL, Kosco-Vilbois MH, Noelle RJ. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J Exp Med. 1998;187:997–1007. doi: 10.1084/jem.187.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Cutler AH, Thill G, Miklasz SD, Shea DE, Ambrose C, Bixler SA, Su L, Scott ML, Kalled SL. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function. J Immunol. 2004;172:762–766. doi: 10.4049/jimmunol.172.2.762. [DOI] [PubMed] [Google Scholar]
- Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D, MacLennan IC, Bazin H, Khan M. Migrant mu+ delta+ and static mu+ delta- B lymphocyte subsets. Eur J Immunol. 1982;12:564–569. doi: 10.1002/eji.1830120707. [DOI] [PubMed] [Google Scholar]
- Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Tarlinton D, Muller W, Rajewsky K, Forster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- Hatada EN, Do RK, Orlofsky A, Liou HC, Prystowsky M, MacLennan IC, Caamano J, Chen-Kiang S. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- Hestdal K, Jacobsen SE, Ruscetti FW, Dubois CM, Longo DL, Chizzonite R, Oppenheim JJ, Keller JR. In vivo effect of interleukin-1 alpha on hematopoiesis: role of colony-stimulating factor receptor modulation. Blood. 1992;80:2486–2494. [PubMed] [Google Scholar]
- Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- Hsu CL, King-Fleischman AG, Lai AY, Matsumoto Y, Weissman IL, Kondo M. Antagonistic effect of CCAAT enhancer-binding protein-alpha and Pax5 in myeloid or lymphoid lineage choice in common lymphoid progenitors. Proc Natl Acad Sci U S A. 2006;103:672–677. doi: 10.1073/pnas.0510304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunte BE, Capone M, Zlotnik A, Rennick D, Moore TA. Acquisition of CD24 expression by Lin-CD43+B220(low)ckit(hi) cells coincides with commitment to the B cell lineage. Eur J Immunol. 1998;28:3850–3856. doi: 10.1002/(SICI)1521-4141(199811)28:11<3850::AID-IMMU3850>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Gregory S, Yokota T, Sakaguchi N, Kincade P. Transcription from the RAG1 Locus Marks the Earliest Lymphocyte Progenitors in Bone Marrow. Immunity. 2002;17:117. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Ingolia DE, Friedman J, Heimfeld S, Weissman IL. Mouse hematopoietic stem cells and the interaction of c-kit receptor and steel factor. Int J Cell Cloning. 1991;9:451–460. doi: 10.1002/stem.1991.5530090503. [DOI] [PubMed] [Google Scholar]
- Ishii K, Amagai M, Hall RP, Hashimoto T, Takayanagi A, Gamou S, Shimizu N, Nishikawa T. Characterization of autoantibodies in pemphigus using antigen-specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010–2017. [PubMed] [Google Scholar]
- Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren’s syndrome. J Clin Immunol. 2005;25:189–201. doi: 10.1007/s10875-005-4091-5. [DOI] [PubMed] [Google Scholar]
- Kanakaraj P, Migone TS, Nardelli B, Ullrich S, Li Y, Olsen HS, Salcedo TW, Kaufman T, Cochrane E, Gan Y, et al. BLyS BINDS TO B CELLS WITH HIGH AFFINITY AND INDUCES ACTIVATION OF THE TRANSCRIPTION FACTORS NF-kappaB AND ELF-1. Cytokine. 2001;13:25–31. doi: 10.1006/cyto.2000.0793. [DOI] [PubMed] [Google Scholar]
- Karasuyama H, Kudo A, Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990;172:969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H, Rolink A, Melchers F. A complex of glycoproteins is associated with VpreB/lambda 5 surrogate light chain on the surface of mu heavy chain-negative early precursor B cell lines. J Exp Med. 1993;178:469–478. doi: 10.1084/jem.178.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H, Rolink A, Melchers F. Surrogate light chain in B cell development. Adv Immunol. 1996;63:1–41. doi: 10.1016/s0065-2776(08)60853-6. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. The germinal center: a crucible for lymphocyte selection. Semin Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Kovalchuk AL, Kim JS, Park SS, Coleman AE, Ward JM, Morse HC, 3rd, Kishimoto T, Potter M, Janz S. IL-6 transgenic mouse model for extraosseous plasmacytoma. Proc Natl Acad Sci U S A. 2002;99:1509–1514. doi: 10.1073/pnas.022643999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Lin SM, Kondo M. Heterogeneity of Flt3-expressing multipotent progenitors in mouse bone marrow. J Immunol. 2005;175:5016–5023. doi: 10.4049/jimmunol.175.8.5016. [DOI] [PubMed] [Google Scholar]
- Lazorchak AS, Schlissel MS, Zhuang Y. E2A and IRF-4/Pip promote chromatin modification and transcription of the immunoglobulin kappa locus in pre-B cells. Mol Cell Biol. 2006;26:810–821. doi: 10.1128/MCB.26.3.810-821.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- Levine MH, Haberman AM, Sant’Angelo DB, Hannum LG, Cancro MP, Janeway CA, Jr., Shlomchik MJ. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci U S A. 2000;97:2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Dordai DI, Lee J, Desiderio S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity. 1996;5:575–589. doi: 10.1016/s1074-7613(00)80272-1. [DOI] [PubMed] [Google Scholar]
- Lin WC, Desiderio S. Regulation of V(D)J recombination activator protein RAG-2 by phosphorylation. Science. 1993;260:953–959. doi: 10.1126/science.8493533. [DOI] [PubMed] [Google Scholar]
- Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loder F, Mutschler B, Ray R, Paige C, Sideras P, Torres R, Lamers M, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- Lorenz RG, Chaplin DD, McDonald KG, McDonough JS, Newberry RD. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin beta receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Merghoub T, Hobbs RM, Dong L, Maeda M, Zakrzewski J, van den Brink MR, Zelent A, Shigematsu H, Akashi K, et al. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Meffre E, Casellas R, Nussenzweig MC. Antibody regulation of B cell development. Nat Immunol. 2000;1:379–385. doi: 10.1038/80816. [DOI] [PubMed] [Google Scholar]
- Melchers F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat Rev Immunol. 2005;5:578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg A, Andrews SF, Yu KO, Porcelli SA, Rawlings DJ. Characterization of a late transitional B cell population highly sensitive to BAFF-mediated homeostatic proliferation. J Exp Med. 2008;205:155–168. doi: 10.1084/jem.20071088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mide SM, Huygens P, Bozzini CE, Fernandez Pol JA. Effects of human recombinant erythropoietin on differentiation and distribution of erythroid progenitor cells on murine medullary and splenic erythropoiesis during hypoxia and post-hypoxia. In Vivo. 2001;15:125–132. [PubMed] [Google Scholar]
- Miyagawa S, Amagai M, Iida T, Yamamoto Y, Nishikawa T, Shirai T. Late development of antidesmoglein 1 antibodies in pemphigus vulgaris: correlation with disease progression. Br J Dermatol. 1999;141:1084–1087. doi: 10.1046/j.1365-2133.1999.03209.x. [DOI] [PubMed] [Google Scholar]
- Mizutani A, Shaheen VM, Yoshida H, Akaogi J, Kuroda Y, Nacionales DC, Yamasaki Y, Hirakata M, Ono N, Reeves WH, Satoh M. Pristane-induced autoimmunity in germ-free mice. Clin Immunol. 2005;114:110–118. doi: 10.1016/j.clim.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MA, Warren DJ. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987;84:7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacionales DC, Kelly KM, Lee PY, Zhuang H, Li Y, Weinstein JS, Sobel E, Kuroda Y, Akaogi J, Satoh M, Reeves WH. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane) Am J Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Garrett KP, Ohta S, Bahrun U, Kouro T, Akira S, Takatsu K, Kincade PW. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka H, Gonzalez-Aseguinolaza G, Tsuji M, Nussenzweig MC. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T. Microenvironmental niches in the bone marrow required for B-cell development. Nat Rev Immunol. 2006;6:107–116. doi: 10.1038/nri1780. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nemazee D, Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci U S A. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo VN, Cornall RJ, Cyster JG. Splenic T Zone Development Is B Cell Dependent. J Exp Med. 2001;194:1649–1660. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Matsuzaki Y, Nishikawa S, Hayashi S, Kunisada T, Sudo T, Kina T, Nakauchi H. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–7207. [PubMed] [Google Scholar]
- Osmond DG, Rolink A, Melchers F. Murine B lymphopoiesis: towards a unified model. Immunol Today. 1998;19:65–68. doi: 10.1016/s0167-5699(97)01203-6. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pers JO, Devauchelle V, Daridon C, Bendaoud B, Le Berre R, Bordron A, Hutin P, Renaudineau Y, Dueymes M, Loisel S, et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjogren’s syndrome. Arthritis Rheum. 2007;56:1464–1477. doi: 10.1002/art.22603. [DOI] [PubMed] [Google Scholar]
- Petro JB, Gerstein RM, Lowe J, Carter RS, Shinners N, Khan WN. Transitional type 1 and 2 B lymphocyte subsets are differentially responsive to antigen receptor signaling. J Biol Chem. 2002;277:48009–48019. doi: 10.1074/jbc.M200305200. [DOI] [PubMed] [Google Scholar]
- Potter M. Neoplastic development in plasma cells. Immunol Rev. 2003;194:177–195. doi: 10.1034/j.1600-065x.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- Potter M, Maccardle RC. Histology Of Developing Plasma Cell Neoplasia Induced By Mineral Oil In Balb/C Mice. J Natl Cancer Inst. 1964;33:497–515. [PubMed] [Google Scholar]
- Potter M, Mushinski EB, Wax JS, Hartley J, Mock BA. Identification of two genes on chromosome 4 that determine resistance to plasmacytoma induction in mice. Cancer Res. 1994;54:969–975. [PubMed] [Google Scholar]
- Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- Radic MZ, Erikson J, Litwin S, Weigert M. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–1173. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- Rahman ZS, Rao SP, Kalled SL, Manser T. Normal Induction but Attenuated Progression of Germinal Center Responses in BAFF and BAFF-R Signaling-Deficient Mice. J Exp Med. 2003 doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Reth M, Petrac E, Wiese P, Lobel L, Alt FW. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. Embo J. 1987;6:3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards HB, Satoh M, Shaheen VM, Yoshida H, Reeves WH. Induction of B cell autoimmunity by pristane. Curr Top Microbiol Immunol. 1999;246:387–392. doi: 10.1007/978-3-642-60162-0_47. discussion 393. [DOI] [PubMed] [Google Scholar]
- Roldan E, Brieva JA. Terminal differentiation of human bone marrow cells capable of spontaneous and high-rate immunoglobulin secretion: role of bone marrow stromal cells and interleukin 6. Eur J Immunol. 1991;21:2671–2677. doi: 10.1002/eji.1830211105. [DOI] [PubMed] [Google Scholar]
- Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28:3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Sandel PC, Gendelman M, Kelsoe G, Monroe JG. Definition of a novel cellular constituent of the bone marrow that regulates the response of immature B cells to B cell antigen receptor engagement. J Immunol. 2001;166:5935–5944. doi: 10.4049/jimmunol.166.10.5935. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- Satoh M, Kuroda Y, Yoshida H, Behney KM, Mizutani A, Akaogi J, Nacionales DC, Lorenson TD, Rosenbauer RJ, Reeves WH. Induction of lupus autoantibodies by adjuvants. J Autoimmun. 2003;21:1–9. doi: 10.1016/s0896-8411(03)00083-0. [DOI] [PubMed] [Google Scholar]
- Satoh M, Richards HB, Shaheen VM, Yoshida H, Shaw M, Naim JO, Wooley PH, Reeves WH. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin Exp Immunol. 2000;121:399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, Cassatella MA. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedger LM, Hou S, Osvath SR, Glaccum MB, Peschon JJ, van Rooijen N, Hyland L. Bone marrow B cell apoptosis during in vivo influenza virus infection requires TNF-alpha and lymphotoxin-alpha. J Immunol. 2002;169:6193–6201. doi: 10.4049/jimmunol.169.11.6193. [DOI] [PubMed] [Google Scholar]
- Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP, Kalled SL, Scott ML. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol. 2004;173:2331–2341. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- Sonoda E, Pewzner-Jung Y, Schwers S, Taki S, Jung S, Eilat D, Rajewsky K. B cell development under the condition of allelic inclusion. Immunity. 1997;6:225–233. doi: 10.1016/s1074-7613(00)80325-8. [DOI] [PubMed] [Google Scholar]
- Stork LC, Peterson VM, Rundus CH, Robinson WA. Interleukin-1 enhances murine granulopoiesis in vivo. Exp Hematol. 1988;16:163–167. [PubMed] [Google Scholar]
- ten Boekel E, Melchers F, Rolink AG. Precursor B cells showing H chain allelic inclusion display allelic exclusion at the level of pre-B cell receptor surface expression. Immunity. 1998;8:199–207. doi: 10.1016/s1074-7613(00)80472-0. [DOI] [PubMed] [Google Scholar]
- Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Tsunoda K, Ota T, Suzuki H, Ohyama M, Nagai T, Nishikawa T, Amagai M, Koyasu S. Pathogenic autoantibody production requires loss of tolerance against desmoglein 3 in both T and B cells in experimental pemphigus vulgaris. Eur J Immunol. 2002;32:627–633. doi: 10.1002/1521-4141(200203)32:3<627::AID-IMMU627>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Tudor KS, Payne KJ, Yamashita Y, Kincade PW. Functional assessment of precursors from murine bone marrow suggests a sequence of early B lineage differentiation events. Immunity. 2000;12:335–345. doi: 10.1016/s1074-7613(00)80186-7. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Kuprash DV, Mach JA, Nedospasov SA, Chervonsky AV. Lymphotoxin and TNF produced by B cells are dispensable for maintenance of the follicle-associated epithelium but are required for development of lymphoid follicles in the Peyer’s patches. J Immunol. 2004;173:86–91. doi: 10.4049/jimmunol.173.1.86. [DOI] [PubMed] [Google Scholar]
- Turner CA, Jr., Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation Controls B Lymphopoiesis by Regulating Chemokine CXCL12 Expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, Kalled SL. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–551. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- Wang H, Feng J, Qi CF, Li Z, Morse HC, 3rd, Clarke SH. Transitional B cells lose their ability to receptor edit but retain their potential for positive and negative selection. J Immunol. 2007;179:7544–7552. doi: 10.4049/jimmunol.179.11.7544. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- Waskow C, Paul S, Haller C, Gassmann M, Rodewald H. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, Hilbert DM, Thompson CB. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Takahashi Y, Ogasawara M, Setoyama Y, Suzuki K, Tsuzaka K, Abe T, Takeuchi T. Aberrant expression of BAFF in T cells of systemic lupus erythematosus, which is recapitulated by a human T cell line, Loucy. Int Immunol. 2006;18:1189–1196. doi: 10.1093/intimm/dxl053. [DOI] [PubMed] [Google Scholar]
- Young NS, Baranski B, Kurtzman G. The immune system as mediator of virus-associated bone marrow failure: B19 parvovirus and Epstein-Barr virus. Ann N Y Acad Sci. 1989;554:75–80. doi: 10.1111/j.1749-6632.1989.tb22411.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Roschke V, Baker KP, Wang Z, Alarcon GS, Fessler BJ, Bastian H, Kimberly RP, Zhou T. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- Zhuang H, Narain S, Sobel E, Lee PY, Nacionales DC, Kelly KM, Richards HB, Segal M, Stewart C, Satoh M, Reeves WH. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005;117:238–250. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]