Abstract
Palmitoylation is a key post-translational modification mediated by a family of DHHC-containing palmitoyl acyl-transferases (PATs). Unlike other lipid modifications, palmitoylation is reversible and thus often regulates dynamic protein interactions. We find that the mouse hair loss mutant, depilated, (dep) is due to a single amino acid deletion in the PAT, Zdhhc21, resulting in protein mislocalization and loss of palmitoylation activity. We examined expression of Zdhhc21 protein in skin and find it restricted to specific hair lineages. Loss of Zdhhc21 function results in delayed hair shaft differentiation, at the site of expression of the gene, but also leads to hyperplasia of the interfollicular epidermis (IFE) and sebaceous glands, distant from the expression site. The specific delay in follicle differentiation is associated with attenuated anagen propagation and is reflected by decreased levels of Lef1, nuclear β-catenin, and Foxn1 in hair shaft progenitors. In the thickened basal compartment of mutant IFE, phospho-ERK and cell proliferation are increased, suggesting increased signaling through EGFR or integrin-related receptors, with a parallel reduction in expression of the key differentiation factor Gata3. We show that the Src-family kinase, Fyn, involved in keratinocyte differentiation, is a direct palmitoylation target of Zdhhc21 and is mislocalized in mutant follicles. This study is the first to demonstrate a key role for palmitoylation in regulating developmental signals in mammalian tissue homeostasis.
Author Summary
During embryonic development, growth and patterning are regulated at many levels. Signals that mediate transcriptional activity, where and when genes are expressed, are a primary level of regulation. However, developmental signals can be further fine-tuned by modulating protein stability, localization, and activity via post-translational modifications. One such modification is the reversible addition of the fatty acid palmitate to proteins. This modification mediates dynamic trafficking of target proteins to specific subdomains of the cell. A large family of enzymes carries out this palmitoylation process, where each family member has specificity towards particular targets. However, the functional significance of palmitoylation during mammalian development is unclear. We present evidence of a critical role for palmitoylation during mouse development using a mutation of a specific palmitoylating enzyme, whose loss of function leads to hair loss and skin defects in depilated (dep) mice. Despite its restricted expression in hair follicles, loss of function of this enzyme results in developmental defects in nearby structures. We show that palmitoylation plays an important regulatory role in hair growth and epidermal homeostasis.
Introduction
Palmitoylation (or protein S-acylation) is a reversible post-translational lipid modification which involves addition of the fatty acid palmitate onto specific cysteine residues [1]. Some post-translational lipid modifications such as myristoylation and prenylation serve to localize otherwise soluble proteins to the cytoplasmic surfaces of cellular membranes. In contrast, palmitoylation substrates are proteins that are already membrane associated, and the modification acts to increase or stabilise membrane affinity or to traffic the protein to specific membrane domains. In particular, palmitoylation results in localization of the protein to lipid rafts; domains of the plasma membrane rich in cholesterol and sphingolipids. Furthermore, as palmitoylation is reversible, it allows for membrane localization or trafficking to be dynamically regulated. This has best been demonstrated in synapses, where palmitoylation regulates membrane localization and activity of the AMPA receptor [2] and GABAA receptor [3]. Palmitoylation of the post-synaptic density protein PSD95 permits clustering of the protein at synapses and regulates synaptic strength [4]. A recent global study of the neural palmitoyl-proteome highlights the breadth of targets that are rapidly modulated by palmitoylation [5], further emphasizing the importance of this modification in dynamic biological processes.
Members of the zinc finger, DHHC containing (ZDHHC) protein family have recently been shown to promote palmitoylation of intracellular proteins in yeast and in mammalian cells [6]–[8]. These palmitoyl-acyl transferases (PATs) are predicted membrane proteins possessing a cysteine-rich domain and a putative zinc finger with a characteristic Asp-His-His-Cys (DHHC) motif, required for activity. This family is encoded by 24 genes in both mouse and humans, of which 23 are orthologous pairs. Assaying individual target proteins against the entire repertoire of PATs indicates that there is substrate specificity; each substrate is primarily modified by a subgroup of structurally similar ZDHHC proteins [9].
Although some human ZDHHC genes have been implicated in cancer [10],[11], genetic evidence for function of these genes is limited to neurological disorders. ZDHHC8 shows association with schizophrenia in humans and neurophysiological deficits in mice [12]–[14]. X-linked mental retardation is associated in a few patients with loss of expression of ZDHHC15 [15] and in others with frameshifts, splice or missense mutations of ZDHHC9 [16]. Recently, the Drosophila ortholog of Zdhhc8 (App) was shown to play a key role in patterning and growth control of imaginal discs [17]. However, very little is known about specific palmitoylation functions during normal mammalian development.
Several lineage-restricted stem cell populations exist in the adult skin and contribute to renewal of only their own specific niche under normal steady-state conditions [18]. Their progeny proliferate, migrate and terminally differentiate along the lineages of the interfollicular epidermis (IFE), hair follicle and sebaceous gland [reviewed in 19]. The cornified layer of postnatal skin is constantly shed and replenished by progeny of the epidermal stem cells in the basal IFE, which proliferate, differentiate and migrate suprabasally. Similarly, hair shafts are shed and replaced in a cycle of regression (catagen), rest (telogen) and regeneration (anagen). During each anagen, stem cells, residing in the permanent bulge region, are mobilized to provide hair follicle progenitors, which differentiate into eight different lineages that make up the hair shaft (consisting of the medulla, cortex and hair shaft cuticle), the inner root sheath (IRS) (consisting of the inner root sheath cuticle, Huxley's and Henle's layers), the companion layer cells and the outer root sheath (ORS). Also within the permanent portion of the follicle is the sebaceous gland which produces lipid-rich sebum to lubricate the skin and hair, in addition to providing antibacterial activity. Sebum is released by disintegrating sebocytes that are continuously replaced from progenitors in the periphery of the gland. These three stem cell lineages require a precise balance of self-renewal and differentiation of their committed progeny. However under certain experimental conditions or genetic manipulations, stem cells from one niche can contribute to hair, IFE and sebaceous gland lineages [20],[21], highlighting the interdependence of these epidermal compartments in maintaining homeostasis.
The depilated mutation (dep, MGI:94884) results in a recessive phenotype characterized by variable hair loss, with thinner and shorter hairs remaining in a greasy coat. Recombination experiments show that the phenotype is due to a defect in the epidermis, rather then the dermis [22]. Here, we genetically map and further characterize the dep mutant and show that it carries a single amino acid deletion in Zdhhc21, resulting in loss of PAT activity. A detailed study of the phenotype demonstrates that lack of palmitoylation by Zdhhc21 results in hyperplasia of the IFE and sebaceous glands and delayed differentiation of the hair shaft. Furthermore, we identify Fyn, a member of the Src family of tyrosine protein kinases required for keratinocyte differentiation, as a direct palmitoylation target of Zdhhc21 and demonstrate its mislocalization within dep mutant follicles.
Results/Discussion
Mutation in Zdhhc21 causes the dep phenotype
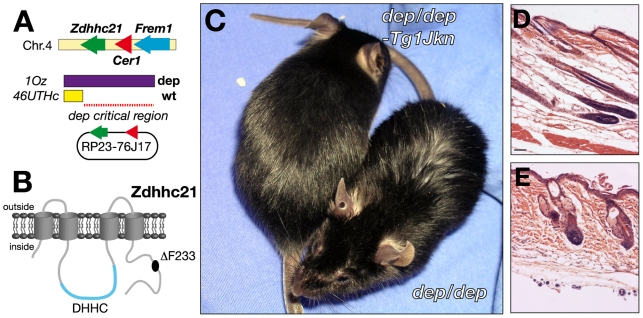
The location of the dep mutation has previously been defined by complementation against a set of chromosomes bearing deletions centred on the Tyrp1 gene [23]. The endpoints of those deletions defining the proximal and distal boundaries of the candidate interval were further refined using polymorphic markers on mice carrying the deletion chromosome opposite a Mus spretus chromosome [24],[25, data not shown]. The candidate location of dep, defined by the deletions 46UThc proximally and 1OZ distally, is only 160kb in length and contains all or part of just 3 genes: Zdhhc21, Cer1 and Frem1 (Figure 1A). Two of these have existing established mutations.
Figure 1. Identification and transgenic rescue of the dep mutation.
(A) Mapping the dep interval against the b-del complex. When dep is crossed with the b-IOZ deletion mutant (purple), offspring exhibit the hairloss phenotype. When crossed with the b-46UTHc deletion mutant (yellow), the hairloss phenotype disappears, indicating that the dep mutation lies within the genomic interval between the distal breakpoints of the 2 deletions. (B) Schematic of Zdhhc21 protein with dep C-terminal 3bp deletion resulting in the loss of a single highly conserved residue, phenylalanine (F) at position 233. The cysteine-rich domain containing a conserved DHHC motif is shown in blue on the cytoplasmic side. (C) The BAC clone RP23-76J17, which harbors the intact genomic sequences of Zdhhc21 and Cer1 successfully rescues the dep phenotype, shown at 6.5 weeks. (D) Transgenic dep mutant skin appears histologically normal and correct timing hair follicle differentiation is also restored. (E) Non-transgenic mutant littermate control.
Frem1 is associated with 2 ENU-induced alleles and the classical mutation head blebs (heb) [26] which result in an embryonic blebbing phenotype, and is a mouse model for Frasers Syndrome. Furthermore, a genetic complementation analysis between a Frem1 mutant (bfd) and dep produces normal mice (personal communication, Monica Justice), indicating Frem1 is not allelic to dep. There are several knockout mutant alleles of Cer1 but none of these exhibit the dep phenotype [27]–[29]. We have sequenced all known exons of both Frem1 and Cer1 in dep DNA have found no mutations. Additionally no non-coding RNAs are annotated or predicted within this interval (miRBase: microrna.sanger.ac.uk, Ensembl: www.ensembl.org, VEGA: vega.sanger.ac.uk).
However, sequencing of the 7 exons of Zdhhc21 (MGI:1915518) in dep mutants revealed a 3-bp deletion which results in the deletion of a single, highly conserved, phenylalanine residue (del-233F) close to the C terminus of the protein (Figure 1B).
Although this deletion was the only coding alteration found in the candidate interval, it remained possible that an undetected non-coding mutation could affect expression of genes outside the interval. To establish the causative link between Zdhhc21 and the dep phenotype, we generated transgenic mice containing the bacterial artificial chromosome, RP23-76J17, containing only Zdhhc21 and Cer1 (Figure 1A). When crossed onto a dep background, this transgene rescues the hair phenotype to a smooth and shiny dorsal coat, indistinguishable from wild-type, whilst the hair of nontransgenic littermates retains the greasy and disorderly dep phenotype (Figure 1C). Later in life, non-transgenic mutant littermates lose their hair, whilst the transgenic mice do not. Skin sections of transgenic rescued mice show a normal histological appearance, confirming that the dep phenotype is fully rescued (Figure 1D and 1E).
Zdhhc21-del233F is mislocalised and lacks PAT function
Zdhhc21 has previously been demonstrated to have palmitoyl transferase (PAT) activity. Among 23 Zdhhc members tested, endothelial nitric oxide synthase (eNOS, Nos3) [30] and lymphocyte-specific protein tyrosine kinase (Lck) [31] were found to be robustly palmitoylated by Zdhhc21. Using these substrates, we examined whether the dep mutant Zdhhc21 protein retains PAT function.
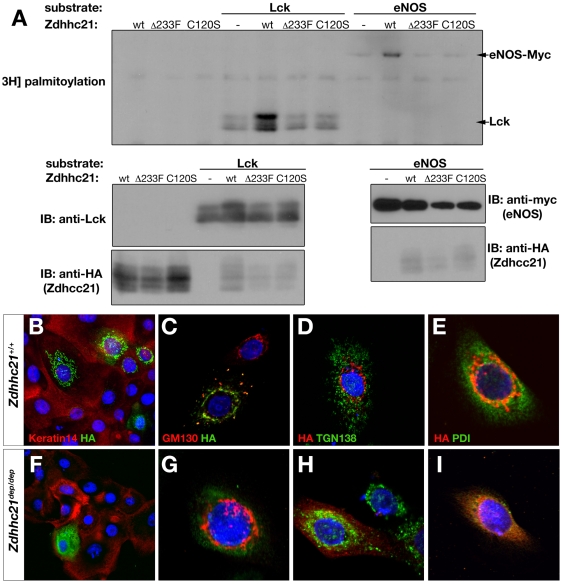
To test PAT activity, plasmids encoding tagged wild-type and mutant Zdhhc21 proteins were cotransfected with plasmids expressing Lck or eNOS (Nos3). Palmitoylation of substrates was assessed by metabolic labeling with [3H]palmitate followed by SDS-PAGE and fluorography [8],[9]. Wild-type Zdhhc21 protein enhanced both eNOS and Lck palmitoylation, whilst the del233F protein showed no enhancement over background palmitoylation. A second mutant protein, C120S, in which the cysteine residue in the conserved DHHC motif was mutated, was also inactive in this assay (Figure 2A).
Figure 2. dep mutation disrupts PAT activity and localization of Zdhhc21.
(A) [3H]Palmitate fluorography of individual Zdhhc21 (wild type, dep and C120S) HA-tagged constructs co-transfected with eNOS or Lck into HEK293 cells. Increased incorporation of [3H]palmitate into targets is observed with the wild type construct. Neither mutant shows palmitoylation activity above background. Immunoblots using anti-HA (Zdhhc21 constructs), anti-myc (eNOS) and anti-Lck control for loading. (B–I) Immunofluorescence of primary keratinocytes transfected with wild type (B–E) and dep (F–I) HA-tagged Zdhhc21 cDNAs. (B,F) Epidermal marker Keratin 14 (red) and anti-HA (green) antibody staining. While wild type protein shows discrete and compartmentalized localization, the mutant protein is diffuse. (C,G) cis-Golgi network marker GM130 (red) and anti-HA (green) antibody staining. (D,H) trans-Golgi marker Tgn138 (green) and anti-HA (red). (E,I) ER marker PDI (green) and anti-HA (red).
As mislocalisation of the mutant protein could affect its function in vivo, we examined the cellular localization of tagged variants of Zdhhc21 proteins in cell culture. In primary keratinocytes, HA-tagged wild type Zdhhc21 localizes to highly specific cytoplasmic structures, which co-localise with the cis-Golgi marker GM130, consistent with previous studies showing localization of other Zdhhc proteins to the Golgi (Figure 2C) [8],[30]. In contrast, Zdhhc21-del233F colocalizes with the endoplasmic reticulum (ER) marker, protein disulfide isomerase (PDI), demonstrating that dep mutant protein is unable to target specifically to the Golgi and appears to be trapped in the ER. (Figure 2I). We further verified these observations by transfection in NIH-3T3 cells, and demonstrated the mislocalisation and lack of PAT activity of additional mutant forms of the protein (Figure S1)
Zdhhc21 is a PAT expressed in epithelial tissues
To define the target tissue in which PAT function is required for normal hair development, Zdhhc21 mRNA and protein expression were analyzed at embryonic and postnatal time-points related to hair follicle morphogenesis and cycling.
In the developing skin, Zdhhc21 expression could not be detected prior to hair follicle induction (E13.5) or early morphogenesis (E14.5) (data not shown). Expression of Zdhhc21 is initially detected in the inner root sheath (IRS) of developing vibrissae follicles at E16.5 (Figure S2) and later in the developing IRS of E18.5 pelage follicles (data not shown).
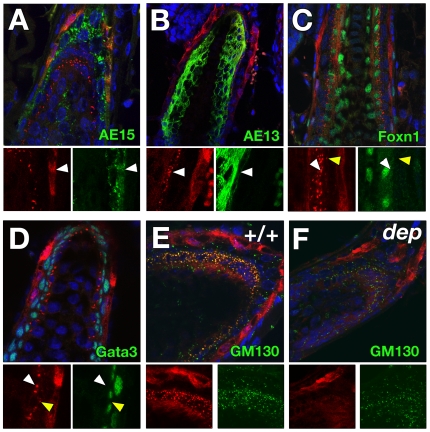
Postnatally, Zdhhc21 exhibits two patterns of expression in distinct layers of more distal post-mitotic lineages in the hair bulb. Strong ubiquitous cellular expression of Zdhhc21 is detected in a single layer of the IRS (Figure 3A). Double immunofluorescence with antibodies against trichohyalin (AE15) (Figure 3A) or Gata3, which is expressed only in Huxley's layer and the IRS cuticle (Figure 3D), demonstrated partial co-localization with trichohyalin but not Gata3, indicating Zdhhc21 is expressed in Henle's layer, the outermost IRS layer. A second Zdhhc21 expression domain, marked by punctate staining, is found predominantly in the outermost layer of cells expressing hair cortex keratins (AE13-positive) (Figure 3B, white arrowhead) and Foxn1-positive cells (Figure 3C, white arrowhead), indicative of the hair shaft cuticle. A less prominent but similarly punctate pattern is found in the adjacent Gata3-positive IRS cuticle cells (Figure 3C and 3D, yellow arrowhead). As in cell culture, these Zdhhc21-positive punctae colocalize with cis-Golgi marker GM130 in vivo suggesting that the protein in these cells is active in palmitoylation (Figure 3E). Importantly, while Zdhhc21 transcript expression is not altered in dep follicles (Figure S2D and S2E), mutant Zdhhc21 protein is mislocalized in both cuticle lineages where it shows diffuse staining (Figure 3F, Figure S3). Together, the loss of in vivo Golgi localization of Zdhhc21 in dep mutants and the resulting mutant hair shaft phenotype suggest that Zdhhc21 function is primarily required in the cuticle layer. Both patterns of hair follicle expression are hair cycle dependent; expression of Zdhhc21 cannot be detected in telogen (Figure S2J) or very early anagen follicles, but it is first expressed in nested layers of the IRS and cuticle of anagen and catagen follicles (Figure S2). Comparable cyclic expression of Zdhhc21 during this postnatal hair cycle is also observed in dep mutant skin. Notably, the onset of expression in differentiating lineages in the anagen follicles correlates with the first sign of abnormal morphology (Figure S3).
Figure 3. Zdhhc21 expression is restricted to differentiated post-mitotic lineages of hair follicles.
Confocal slices (1µm) of Zdhhc21 protein expression in wild type (A–E) and dep (F) P28 anagen follicles. (A) Ubiquitous Zdhhc21 (red) expression colocalizes with the outermost layer of AE15 (green) expression. (B) Punctate Zdhhc21 staining (red) colocalizes with the outermost layer of AE13 expression (green: weaker than inner cortex expression) and (C) Foxn1 expression (white arrowhead). (D) Single foci of Zdhhc21 staining (red: yellow arrowhead) per cell of the IRS cuticle is seen in the innermost Gata3-positive layer (green). (E) In wild type skin, punctae of Zdhhc21 (red) colocalize with GM130 (green), whilst diffuse staining is observed in dep mutants (F). Arrowheads mark same region in single channels.
Outside the cycling portion of the hair follicle, we find specific cellular Zdhhc21 protein strongly present in the degenerated remains of the IRS surrounding the isthmus, in the permanent portion of the follicle (Figure S2I). Importantly, expression of Zdhhc21 mRNA or protein cannot be detected in the bulge, IFE or in the sebaceous gland at any stage of the hair cycle.
Zdhhc21 is required for epithelial homeostasis
The dep phenotype can be identified macroscopically within the first postnatal week as a greasy and disorderly hair distribution, as previously reported [22]. To determine the cellular basis of the observed abnormalities, we conducted histological and molecular analyses of skin samples at a range of developmental stages.
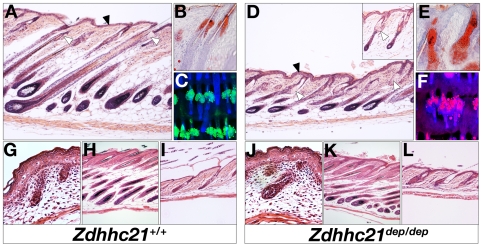
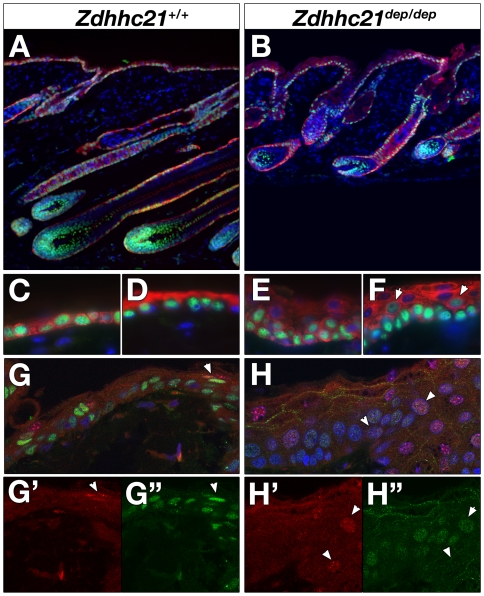
Dorsal skin from dep embryos at E14.5 and E18.5 have follicle morphology and numbers comparable to wild type (Figure 4G and 4J and data not shown), indicating that Zdhhc21 function is dispensable for hair follicle patterning and morphogenesis. The first abnormalities in dep mice are observed shortly after birth where mild sebaceous gland hyperplasia and slight thickening of the IFE develop at P5. While dep mutants appear to progress through the first hair cycle normally (Figure 4H and 4K), by telogen, defects in the permanent portions of dep skin are apparent and include thickening of the IFE and a dilated infundibulum (Figure 4I and 4L). By the onset of the second hair cycle around P28, the dep follicles are growth retarded and immature compared to littermates (Figure 4A and 4D) coincident with the onset of Zdhhc21 expression (Figure S3). In addition, the thickening of IFE and sebaceous gland hyperplasia appear more prominent (Figure 4D, arrowed). Staining for lipids reveals enlarged sebaceous glands with an excess of sebum (Figure 4E and 4F), underlying the greasy appearance of the coat at this stage. In some dep animals, from P28 onwards, small epidermal cysts containing keratinized material can be observed in the upper portion of the dermis (not shown). Given the hyperplastic changes observed in the upper portions of dep follicles, we asked whether the closely associate bulge stem cell niche was also perturbed. Keratin 15 (K15) is a marker for these cells, and indeed, the K15-positive population is expanded in dep mutants, although its expression remains restricted to the bulge niche, suggesting that changes in the size and shape of the dep bulge during the hair cycle could impact progenitor allocation to various epidermal compartments (Figure S5F and S5L, data not shown).
Figure 4. Zdhhc21 is required for epidermal differentiation and patterning.
dep mutant skin displays pronounced defects postnatally associated with anagen stages of hair cycle including abnormal hair follicle differentiation, and interfollicular and sebaceous hyperplasia. Stages shown: anagen P28 (A–F), late embryonic morphogenesis E18.5 (G,J), early catagen P14 (H,K) and telogen P21 (I,L). Hematoxylin and eosin staining (A,D,G–L). Oil-Red-O staining of cryosections (B,E). Nile red wholemount staining of P28 tail skin(C,F). Insert in (A) shows histology of a “hairless” dorsal region at P28: note the more severe follicular phenotype. Filled arrowheads mark the interfollicular epidermis and open arrowheads point to examples of sebaceous glands.
The hyperplastic phenotype of dep IFE and sebaceous glands is most prominent during anagen in younger skin, when growth stages of the hair cycle are highly synchronized. To determine whether this hyperplastic phenotype was due to increased proliferation of these non-follicular compartments, we carried out BrdU pulse labeling cohorts of P32 gender-matched animals. These studies revealed a small but significant increase in the fraction of BrdU positive dep IFE cells (8.314±1.493, n = 2, p<0.005) compared with heterozygous (6.790±1.8223, n = 2) or wild type controls (6.686±1.711, n = 2) (Figure S4L). A greater increase in percentage BrdU positive cells was observed in dep sebaceous glands (11.46±2.784, n = 2, p<0.05) compared to controls (heterozygous: 6.881±2.499; wild type: 7.882±2.868). A concomitant decrease of BrdU labeling is observed in dep mutant follicles during anagen (Figure S4K). Additional proliferation markers, including the M-phase marker phospho-histone H3 and a general marker Ki67 identifying all phases of the cell cycle, confirm this change in proliferation is relatively small and is restricted to the basal compartment (Figure S4A, S4B, S4C, S4D, S4E, S4F, S4G, S4H, S4I, S4J).
We asked whether aberrant terminal differentiation of keratinocytes in the IFE also contributes to the dep phenotype, such that an expanded progenitor pool contributing to the IFE could result in an increase in cell number in the stratified layers. Immunolabelling of basal cell markers p63 and keratin 5 (K5) showed an expansion of this progenitor compartment (Figure 5A, 5B, 5C, and 5E). Furthermore, p63-positive cells were found in thickened K10-positive spinous layer in dep skin (Figure 5D and 5F, arrowed). The terminal differentiation markers loricrin and filaggrin were only slightly expanded in dep mutants (Figure S5A, S5B, S5G, S5H) indicating that differentiation in dep mutants occured but was significantly delayed. Interestingly, the transcription factor Gata3, which is normally expressed in the basal and suprabasal layers of the IFE where it directs keratinocyte and lipid based barrier differentiation programs [32],[33], is strongly reduced in dep IFE (Figure 5G and 5H, arrowed) consistent with the observed delay in differentiation. Reduced levels of Gata3 in the dep IFE during anagen may contribute to defects in lipid biosynthesis required for barrier function, which may give rise indirectly to the hyperproliferative phenotype observed [34]. However, unlike Gata3 knock-out skin [32], delays in the establishment of embryonic barrier function by dye penetration assays were not seen and keratinocyte terminal differentiation program in embryonic skin occurred normally (Figure S6). Furthermore, phenotypes associated with impaired barrier function, including failure to thrive or red shiny skin, were not observed in dep neonates. These observations suggest that any barrier defects present in dep mutants are likely quite subtle and limited to a postnatal window. In contrast to the decrease in Gata3 and altered terminal differentiation, an increase in phospho-ERK staining, indicative of growth factor and integrin signaling linked to increased proliferation in the basal layer of the IFE [35], is observed in dep mutants (Figure 5G and 5H, Figure S5D, S5D′, S5D″, S5E, S5E′, S5J, S5J′, S5J″, S5K, S5K′, arrowed). These observations together suggest that the thickening of the IFE observed during anagen in dep mutants is due to continued division and delayed differentiation of the expanded basal progenitor compartment after leaving the basement membrane.
Figure 5. Loss of Zdhhc21 disrupts balance between proliferation and differentiation in anagen interfollicular epidermis.
P28 wild type (A,A′,C,D,G,H,K,L) and dep (B,B′,E,F,I,J,M,N) skin. dep mutants show expansion of K5+ (red), p63+ (green) progenitor compartment (A–D). p63+ progenitors (green) are expanded into terminally differentiating K10+ (red) layers of dep IFE (D,F). Expression of transcriptional regulator of differentiation Gata3 in the basal and suprabasal keratinocytes is greatly reduced or absent in dep IFE with a parallel increase in proliferative basal phospho-ERK signals (green; Gata3, red; phospho-p42/44, G,H). (G–H″) single channels of region of interest in merge shown in (G,H).
The restricted expression of Zdhhc21 in the IRS and cuticle of the hair follicle is hard to reconcile with a direct effect on proliferation and differentiation in the IFE and sebaceous gland. One possibility is that the physiologically relevant palmitoylation targets are highly diffusible signals, or are regulators of such signals. Alternatively, Zdhhc21 may act locally in the follicle to indirectly impact non-follicular lineages as a consequence of hair abnormalities in dep mice. Such a phenomenon is seen in K14-Cre-induced knockout of the hair-follicle specific,transcription factor Dlx3, where the resultant abnormal and undifferentiated hair shafts are accompanied by hyperplastic sebaceous glands [36].
Hair cycle signal transduction defects in dep mutants
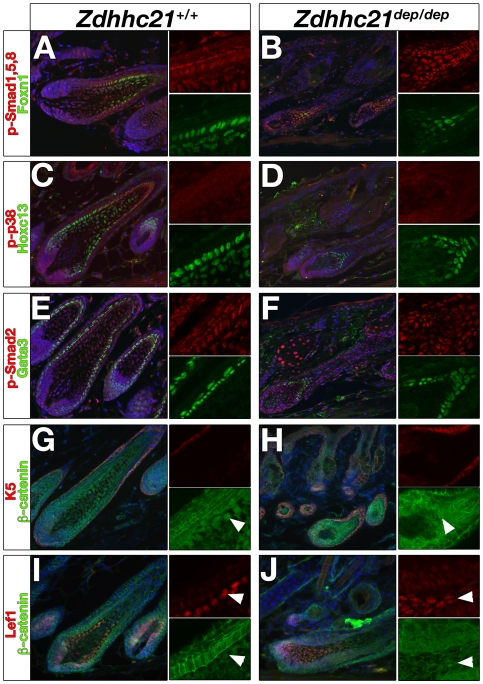
As palmitoylation is usually involved in the regulation of dynamic processes, we investigated whether key signalling events throughout the postnatal hair cycle were affected in dep mutant skin. Bone morphogenetic protein (BMP) signalling is required for embryonic hair follicle development and postnatal hair cycling [37]. Furthermore, conditional epidermal ablation of receptor BMPR1a [38]–[40] result in a hair loss phenotype associated with poorly differentiated hair follicles, and thickened IFE. However, no difference in expression of activated phospho-Smad1/5/8, mediators of canonical BMP signalling, was detected in mutant skin at various stages of the hair cycle (Figure 6A and 6B, data not shown). Transforming growth factor beta (TGF-β) signalling also plays a key role in hair follicle development and cycling, as well as keratinocyte differentiation [41],[42]. No difference in expression of activated canonical intracellular mediator phospho-Smad2 was observed in dep mutant follicles or IFE (Figure 6E and 6F, data not shown). Recent studies have suggested a key role for palmitoylation in BMP- [43] or TGF- mediated signalling events [44] via the non-canonical p38 MAPK arm; however, no alterations in phospho-p38 staining could be detected in dep mutants (Figure 6C and 6D). These results suggest that despite the profound follicular phenotype of dep mutant mice, these key developmental signals required for adult hair cycle are not broadly affected.
Figure 6. Anagen signalling is delayed in dep follicles.
Wild type (A,C,E,G,I) and dep (B,D,F,H,J) P28 dorsal follicles. Several key signalling pathways required for postnatal hair development are not affected in dep mutants. Canonical BMP signalling (red; phospho-Smad-1/-5/-8, A,B), non-canonical BMP/TGF-β signalling (red; phospho-p38, C,D) and canonical TGF-β signalling (red: phospho-Smad-2/-3 E,F) are expressed in delayed dep follicles. Expression and nuclear localization of downstream canonical Wnt effectors β-catenin (green; β-catenin, G–J) and Lef-1 (red; Lef-1, I,J) are reduced in dep hair matrix and shaft (arrowed). Of the transcription factors regulating differentiation of anagen hair follicle lineages, expression of Wnt transcriptional target Foxn1 is reduced or absent in dep follicles (green; Foxn1 A,B), while Hoxc13 is expressed in all dep follicles (green; Hoxc13 C, D). Gata3 is expressed in all dep follicles although levels of expression may be slightly reduced. (green; Gata3 E,F). Along side merge panel are shown single channel of regions of interest to highlight staining.
The range of phenotypes seen in dep animals is reminiscent of a reduction of Wnt signalling, which plays many important roles during hair development. Precise levels of β-catenin activation are required for differentiation into specific epidermal lineages. High levels of β-catenin signaling promote hair follicle formation [45],[46] and normal differentiation of the hair shaft [47]. Low levels of Wnt/β-catenin signaling promote terminal differentiation of the IFE and sebaceous glands [48],[49]. To determine whether a reduction in Wnt signalling is seen in dep mutant skin, we analyzed Wnt responses in embryonic and adult skin by immunohistochemistry. Wnt responses during embryonic hair follicle morphogenesis appear normal in dep embryos (data not shown). At the initiation of the first, synchronized, anagen phase (P24), prior to expression of Zdhhc21, both control and dep littermates show nuclear Lef1 in the dermal papilla and surrounding secondary hair germ (Figure S7A, S7B, S7C, S7D). However, in dep mice, propagation of this anagen response appears defective and differentiation of the hair shaft and cortex is significantly delayed. By P28, at the onset of Zdhhc21 expression when wild type hair is well established in anagen, the delayed dep hair follicles fail to expand strong Lef1 and nuclear β-catenin expression in the matrix and precortex (Figure 6H and 6J). Accordingly, the Lef1 transcriptional target, Foxn1, which regulates expression of hair specific keratins, is strongly reduced or absent from mutant follicles (Figure 6A and 6B, Figure S7I and S7K) as are acidic hair shaft keratins (AE13), (Figure S7J and S7L) consistent with the delayed state of development. By contrast expression of homeodomain transcription factor Hoxc13, which also regulates expression of several hair shaft keratins, is still detected in all dep mutant follicles at this stage of anagen (Figure 6C and 6D). Surprisingly, unlike the profound reduction in Gata3 expression observed in the dep IFE and similarities between follicular phenotype observed in conditional Gata3 mutant mice [50], Gata3 is still detected in all dep follicles although at slightly reduced levels throughout anagen (Figure 6E and 6F, Figure S3). By P35, many dep follicles express levels of Lef1 and hair-shaft keratins comparable to controls, although the morphology of dep follicles remain misshapen and misoriented (Figure S7M, S7N, S7O, S7P). Interestingly, some regions in dep mice continue to remain visibly “hairless”, although histological analysis reveals normal numbers of retarded follicles which fail to proceed through anagen and form functional hairs (Figure 4D, insert).
Our data suggest that a number of signalling pathways required for epidermal homeostasis are disrupted in the absence of Zdhhc21 PAT activity. During the anagen phase of the hair cycle there is a reduction of Wnt responses in the hypoproliferative dep follicles and an increase in phospho-ERK signalling in the hyperplastic mutant IFE. Which of these phenotypes are direct or indirect consequences of the loss of Zdhhc21 palmitoylation remains to be addressed. Given we cannot detect Zdhhc21 expression outside the follicle, we suggest the follicular phenotype observed in dep mutants is the primary cause where defects in hair shaft differentiation during anagen perturb processes at a distance in the IFE and sebaceous glands. Importantly, palmitoylation may influence the quality and the quantity of a signalling event rather than acting as an absolute ON/OFF switch. This is in keeping with the observation that the amplification of Wnt responses during early anagen is very delayed, and not completely blocked, suggesting some threshold could be operating and is eventually met in mutant follicles.
In vivo palmitoylation targets for Zdhhc21
At the synapse, palmitoylation mediates dynamic changes in membrane associations of pools of target proteins involved in signaling, cell adhesion and trafficking [51]. Given the rapid remodeling observed in the hair cycle, it is tempting to speculate that similar processes are involved in the skin and to ask what are the biologically relevant targets of palmitoylation. It should be noted, that while several targets have been identified for each of the 23 Zdhhc PATs, each of these target so far is palmitoylated by multiple PATs, at least in vitro. This suggests a level of functional redundancy in the palmitoylation machinery exists. It also suggests that the dep phenotype could result from the loss of palmitoylation of one or more targets.
We reasoned that any direct target of PAT activity must be expressed in the same cells in which we detect Zdhhc21 expression. One possibility is the known Zdhhc21 target, eNOS [30], which is expressed in the skin [52]. However, observation of eNOS mutant mice indicates that this is not required for normal skin and hair development [53, data not shown], suggesting it is unlikely to be the key palmitoylation target of Zdhhc21 in skin.
Given that Zdhhc21 expression is restricted to hair follicles but multiple epidermal lineages are affected in dep mutants, we asked whether diffusible Wnt proteins could be functional palmitoylation targets for Zdhhc21. Wnt proteins are known to be palmitoylated, and this modification is essential for their function [54]. However, this is believed to be mediated by the ER protein porcupine (PORC), a PAT unrelated to the Zdhhc family [55]. Nevertheless, we tested three candidate Wnts (Wnt3a, 5a and 10a), which are expressed in domains that overlap with Zdhhc21 expression [56]. Although these Wnts are predicted to have multiple palmitoylation sites (CSS-Palm, data not shown) [57], none are directly palmitoylated by Zdhhc21 (data not shown). Trafficking of Wnt ligands from the Golgi to endosomes requires the cargo receptor Wntless/Evi (Wls), a seven-transmembrane protein expressed in the Golgi [58]. Sustained Wnt signaling also requires that this cargo receptor be recycled via the retromer complex. Similar cargo proteins have been shown to require DHHC-dependent palmitoylation for retrograde sorting [59]. However, no palmitoylation of Wls by Zdhhc21 was detected in our co-transfection assay (data not shown). While it remains possible that Zdhhc21 acts locally in a subset of Wnt-responding cells in the hair follicle required for proper hair shaft differentiation (i.e. through modulation of receptor complexes or intracellular signal transduction), we have been unable to establish a direct link between palmitoylation and Wnt responses in this present study.
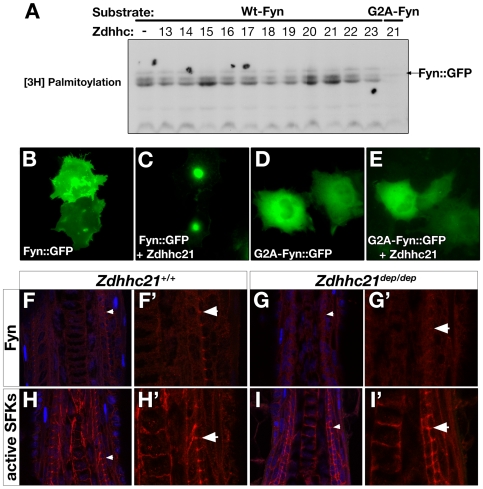
While the Src family kinase, Lck, is a known target of Zdhhc21, it is not required for keratinocyte differentiation nor do Lck mutants have any gross skin phenotype [60]. We therefore considered whether other related kinases that are epidermally expressed could be potential palmitoylation targets. Fyn is indeed expressed in the skin where it plays a role in keratinocyte differentiation in vitro and in vivo [60], in part through down-regulating EGFR signaling [61]. The role for Fyn in hair follicle development and cycling remains unclear. Aged Fyn −/− Fak+/− mice develop progressive hair loss with IFE and sebaceous gland hyperplasia, but this is not observed in Fyn −/− mutants [62]. We therefore tested GFP-tagged Fyn with a panel of Zdhhc PATs by co-transfection and metabolic labelling. Fyn is palmitoylated in our in vitro assay by Zdhhc2, 3, 7, 10, 15, 20 and 21 (Figure 7A, data not shown). Palmitoylation of Fyn by Zdhhc21 results in efficient targeting of Fyn to the perinuclear region in HEK cells (Figure 7B and 7C). Fyn is also subject to myristoylation, an irreversible covalent lipid modification involved in membrane targeting and signaling. Interestingly, we show a mutant Fyn construct lacking the myristoylation site (Fyn-G2A) cannot be palmitoylated by Zdhhc21 or correctly targeted (Figure 7D and 7E), suggesting palmitoylation of Fyn is downstream of the myristoylation event.
Figure 7. Zdhhc21 palmitoylation of Fyn is required for localization in vitro and in vivo.
(A) [3H]Palmitate fluorography of subset of panel of all mammalian Zdhhc HA-tagged constructs co-transfected with Fyn::GFP into HEK293 cells. Increased incorporation of [3H]palmitate into targets is observed with Zdhhc15, −20 and −21. A myristoylation-deficient Fyn mutant is not palmitoylated following cotransfection with Zdhhc21 (G2A, final lane). (B–E) Immunofluorescence of HEK293 cotransfected with Fyn::GFP alone (B), Fyn::GFP and wild type HA-tagged Zdhhc21 cDNA (C), G2A mutant Fyn::GFP alone (D) and G2A mutant Fyn::GFP and HA-tagged Zdhhc21 (E). Zdhhc21 causes increased perinuclear accumulation of Fyn::GFP, a domain associated with localization of other palmitoylated targets including PSD-95 and GAP-43. This localization is not observed with G2A mutant Fyn::GFP (D). (F–I) Confocal images (0.5µm) of immunostaining of Fyn (red: F,G) and active SFKs (red: H–I) in P32 anagen dorsal hair follicles in wild type (F,H) and dep mutants (G,I). Arrowheads mark region of interest in IRS cuticle that are enlarged in single channel images (F′–I′) where Fyn localization to membranes between IRS cuticle cells is lost in dep mutants.
To test whether Fyn was a viable in vivo target of Zdhhc21, we examined localization Fyn in wild type and dep follicles. In wild type anagen (P32) follicles, Fyn is initially expressed diffusely in the hair bulb becoming very discretely localized to membranes at junctions between cells of the IRS cuticle with differentiation (Figure 7F and 7F′, arrowed). This localization is weaker and more diffuse in dep follicles (Figure 7G and 7G′, Figure S8A and S8B). These results were confirmed using an antibody which recognizes the active, phosphorylated forms of all Src-family kinases (SFKs) in addition to Fyn. The active SFKs show a broader expression pattern with striking membrane localization, including the junctions between cells of the IRS cuticle (Figure 7H, Figure S8C). In contrast, while general expression of active SFKs is not altered in dep mutant follicles, uniform active SFK is seen around cells of the IRS cuticle (Figure 7I, Figure S8D). Given that the Zdhhc21 Golgi-localization observed in the IRS cuticle of wild type follicles is lost in dep mutants and that Golgi-localization is dependent on auto-palmitoylation via the PAT activity of wild type Zdhhc21, our results suggest that Zdhhc21-mediated palmitoylation of Fyn is required in vivo for Fyn's discrete localization in the differentiating IRS cuticle. It is interesting to note that in the despite the proliferation and differentiation defects observed in the dep mutant IFE, and Fyn's established role in keratinocyte differentiation, the localization of Fyn in the dep IFE is normal, although it is delayed as expected given the expanded basal compartment in dep mutants (Figure S8E and S8F).
Our data demonstrates that Fyn is a direct palmitoyaltion target for Zdhhc21 in vitro and dysregulation of Fyn occurs in vivo in dep mutant follicles. In assessing the dep phenotype, it is worth noting that the consequences of dysregulated palmitoylation may not mirror those of gene ablation studies, as palmitoylation has the potential to modulate cell signaling in a complex manner. Furthermore, the phenotypic features of dep mice extend beyond those likely caused by the loss of Fyn activity, correlating with the broad substrate specificity of different Zdhhc proteins. To comprehensively tackle the functional requirement of Zdhhc21, the use of global approaches, recently applied in yeast, will be necessary to compare the palmitoylated proteome of dep mutant and wild type cells [63].
This study is the first to highlight a role for palmitoylation in mammalian development and homeostasis. We have demonstrated that loss of Zdhhc21 function in dep mutants results in defects in all three epidermal lineages, including hyperplasia of the IFE and sebaceous glands with a delay in hair follicle differentiation. Given the highly restricted pattern of Zdhhc21 expression to the differentiating hair follicle, our results demonstrate that defective palmitoylation can have far-reaching effects disrupting epidermal homeostasis by altering the balance between proliferation and differentiation. Although the full identity of direct and biologically relevant palmitoylation targets in the skin remains unknown, we show Zdhhc21 can directly palmitoylate Fyn in vitro and this modification affects Fyn localization both in vitro and in vivo. Future studies into the distinct and overlapping roles of additional Zdhhc members will help to fully understand the role of palmitoylation in modulating key signals during development.
Materials and Methods
Ethics statement
Mice were maintained in accordance with MRC Guidelines “Responsibility in the Use of Animals for Medical Research” (July 1993) and research licenced by the UK Home Office under the Animals (Scientific Procedures) Act 1986.
Mouse husbandry and BAC transgenics
Animals were maintained in SPF environment and on a C57BL/6J background. Genomic DNA extracted from ear clips or tail biopsies was used for PCR genotyping. For dep, exon 7 of Zdhhc21 was amplified by standard PCR to yield a 249bp fragment that was run on the ABI310 genetic analyzer to detect the deletion.
The dep genotyping primer sequences were: 5′-FAM-AGCTGACTGAAGGGCACC-3′ (Exon 7F) and 5′-AAAACCTGTAACGCATTTCCA-3′ (Exon 7R).
For transgenic rescue, purified RP23-76J17 BAC DNA (BAC PAC Resource Center (BPRC), the Children's Hospital Oakland Research Center Institute, CA) was injected into homozygous dep embryos. The presence of the BAC was genotyped using three markers, including CmR specific to the plasmid, as well as two markers at both ends of the BACs, amplifying the border between the BAC carrier plasmid and BAC genomic region. For timed matings for embryonic samples, the morning of vaginal plug was counted as 0.5 (E0.5). For postnatal timepoints, a set of gender-matched wild type, heterozygous and mutant littermates were aged accordingly from the day of birth 0 (P0).
Deletion mapping
Mapping of the deletion endpoints defining dep was described by Smyth et al [24].
Palmitoylation assay
cDNA encoding wild type and mutant Zdhhc21 were transfected into HEK293 cells, along with candidate palmitoylation substrates. Cells were labelled with [3H]palmitate for 4 hours as previously described [8],[9]. After metabolic labeling, palmitoylated proteins were analysed by SDS-PAGE. Transfection efficiency and translation of substrates was assessed by Western blotting.
Preparation of mammalian expression plasmids and cell culture
The mammalian expression vector of wild-type Zdhhc21, pEFBos-HA-Zdhhc-21 (with EF1-alpha promoter), was provided by Masaki Fukata. It was then modified by using the QuikChange® Site-Directed Mutagenesis Kit (Stratagene), to introduce single nucleotide changes for the following Zdhhc21 alleles: L91F, C95S and C106S. For the dep mutation, del-233F, was modified from pEFBos-HA-Zdhhc-21 by subcloning dep cDNA (Access RT-PCR System, Promega) into the BamHI sites flanking the insert.
Full-length mouse Wnt cDNAs (kindly provided by Jeremy Nathans (Wnt3a), Wnt5a (Yingzi Yang) and Wnt10a (Takano Yamamoto)) were introduced into a pCMX2GFPFLAGSTOP vector (kindly provided by Nick Gilbert) to express double FLAG-tagged full-length proteins. Constructs were verified by direct DNA sequencing, using primers:
5′-CAAATGGGCGGTAGGCGTGT-3′ (Pcmxgfp2fg-seqF)
5′-TTGTCCAATTATGTCACACCA-3′ (Pcmxgfp2fg-seqR)
The Myc-tagged full length Wls cDNA used in these studies has accension number BC018381 (Catalog No: MR207034: Origene, MD).
Human foreskin keratinocytes [64] and NIH 3T3 cells (ATCC) were maintained as described. DNA plasmids were transfected into cells using Lipofectamine 2000 (Invitrogen) as per manufacturer's specifications.
Zdhhc21 RNA in situ probe and antibody generation
Zdhhc21 cDNA product was generated using Access RT-PCR kit (Promega) and cloned into p-GEM-T. The RT-PCR primers used were: 5′-CATGGGCTTGATTGTCTTTGT-3′ and 5′-ACGTGATTGGCAAAGTGGTAG-3′. DIG-labeled RNA section in situ protocol was performed, details available on request. Custom rabbit polyclonal antibodies to Zdhhc21 were generated using a peptide comprising residues 73–87 (GRLPENPKIPHAERE+C)(Eurogentec). Pre-incubation of the Zdhhc21 antibody with its immunizing peptide blocked all signal in immunohistochemistry (Figure S2L).
Histological and marker gene analysis
Tail epidermis wholemount preparations
Whole tail skin was peeled off connective tissue and bone, then incubated in 5mM EDTA/PBS for 4 hours at 37°C. Forceps were used to separate epidermis from dermis which was then fixed in 4% paraformaldehyde/PBS.
Alkaline phosphatase staining of dermal papilla and staging hair cycle
Paraffin embedded sections of 6µm thickness were dewaxed and rehydrated, and rinsed in PBS. Sections were incubated 15 minutes in APB (100mM NaCl, 100mM Tris pH9.5, 50mM MgCl2, 0.3% Tween-20), then either BM Purple (Roche) or Vector Red Alkaline Phosphatase kit (Vector Labs) color substrate was added. Slides were incubated at room temperature in the dark until staining developed. Sections were rinsed in water and counterstained with eosin (in the case of BM Purple), then dehydrated and mounted in DePex mounting media (BDH). Staging of hair follicles in hair cycle was done according to characteristics described in [65].
Oil-Red-O staining for lipids
Cryosections of P24 skin of 10µm thickness were rinsed in water, followed by 50% EtOH and stained with Oil-Red-O (60% stock in water). Slides were rinsed in 50% EtOH, followed by water and counterstained with haematoxylin. Samples were blued and washed in water and mounted in Aquamount (BDH). Oil-Red-O stock solution was prepared by dissolving 0.5g Oil-Red-O in 100ml 99% Isopropyl Alcohol.
Barrier function assay
Dye penetration assays were performed on late E16.5 litters as previously described [66] with the following modifications. Embryos were dissected out in cold PBS and permeabilized by taking them up and down a methanol/PBS series (25%, 50%, 75%, 100%) with 5 minute washes. Embryos were stained in 0.1% toludine blue solution for 3 minutes, then rinsed through 4 rapid PBS washes. Embryos were immediately photographed on a Nikon AZ100 macroscope using a Nikon APO 0.5× lens in PBS on 2% agarose plates.
Immunohistochemistry and immunocytochemistry
Paraffin sections were dewaxed and rehydrated, followed by washes in TBST +0.5% Tx-100). Microwave antigen retrieval was carried out using 1mM EDTA (pH8) or citrate buffer (1.8mM citric acid, 8.2mM sodium citrate, pH6) for 20–30 minutes depending on the antigen at 900W. Cryosections samples were allowed to come to room temperature and post-fixed in acetone (−20’C for 10 minutes) followed by rinsing in water. No antigen retrieval step was required for cryosections. Slides were cooled to room temperature and washed in TBST. Slides were blocked in 10% donkey serum/TBST, followed by TBST washes. Primary antibodies were diluted in 1% donkey serum/TBST incubated on slides overnight at 4°C (Table 1). After TBST washes, secondary antibodies were diluted in 1% donkey serum/TBST and added to the slides for 60 minute incubation (Table 2). Following stringent TBST washes, nuclei were stained with DAPI (Sigma) or TOTO-3 (Molecular Probes). In the case of TOTO-3, slides were pre-incubated with RNAse A during primary antibody incubation. Slides were mounted with Vectashield (Vector) or Prolong Gold (Molecular Probes) antifade media and coverslips. Brightfield and fluorescent images were acquired using a Coolsnap HQ CCD camera (Photometrics Ltd, Tucson, AZ) Zeiss Axioplan II fluorescence microscope with Plan-neofluar objectives. Image capture and analysis were performed using in-house scripts written for IPLab Spectrum (Scanalytics Corp, Fairfax, VA). For colocalization studies, 0.5–1 µm optical slice images in Z-stacks were acquired with a Zeiss LSM510 confocal microscope and Zeiss Plan Apochromat lenses (Carl Zeiss, Welwyn Garden City, UK). LSM software was used for analysis (Carl Zeiss, Welwyn Garden City, UK).
Table 1. Details of primary antibodies used in this study.
| Antigen | Clone Name | Host Species | Dilution | Source | Notes |
| AE13 | N/A | Mouse | 1∶10 | T.T. Sun | P-IHC |
| AE15 | N/A | Mouse | 1∶2 | T.T. Sun | P-IHC |
| β-catenin | 15B8 | Mouse IgG1 | 1∶500 | Sigma | P-IHC |
| β-catenin | C2206 | Rabbit | 1∶2000 | Sigma | P-IHC |
| β-galactosidase | CR7001RP2 | Mouse IgG1 | 1∶1000 | Cortex | P-IHC |
| BrDU | B44 | Mouse IgG1 | 1∶50 | BD Biosciences | P-IHC |
| Cleaved Caspase3 (Asp175) | 9661 | Rabbit | 1∶400 | Cell Signaling | P-IHC |
| Filaggrin | PRB-417P | Rabbit | 1∶1000 | Covance | P-IHC |
| Foxn1 | G-20 | Goat | 1∶100 | Santa Cruz | P-IHC |
| Fyn | Y303 | Rabbit IgG | 1∶50 | Abcam | P-IHC |
| Gata3 | HG3-31 | Mouse | 1∶75 | Santa Cruz | P-IHC |
| GM130 | 35 | Mouse IgG1 | 1∶200 | BD Biosciences | IF, P-IHC |
| HA | 05-904 | Mouse IgG3 | 1∶200–400 | Upstate | IF, WB |
| HA | HA-7 | Mouse IgG1 | 1∶25 | Sigma | IF |
| Hoxc13 | 10D4 | Mouse IgG1 | 1∶50 | abnova | P-IHC |
| Keratin 5 | PRB-160 | Rabbit | 1∶1000 | Covance | P-IHC |
| Keratin 6 | PRB-169 | Rabbit | 1∶500 | Covance | P-IHC |
| Keratin 10 | PRB-159 | Rabbit | 1∶500 | Covance | P-IHC |
| Keratin 14 | AF64 | Rabbit | 1∶500 | Covance | IF |
| Keratin 15 | sc-56520 | Mouse IgG2a | 01∶50 | Santa Cruz | P-IHC |
| Ki67 | TEC-3 | Rat | 1∶50 | DakoCytomation | P-IHC |
| Lck | 3A5 | Mouse IgG2bk | 1∶600 | Chemicon | WB |
| Lef1 | N/A | Rabbit | 1∶500 | R. Grosschedl | P-IHC |
| Lef1 | C12A5 | Rabbit | 1∶1000 | Cell Signaling | P-IHC |
| Loricrin | PRB-145 | Rabbit | 1∶500 | Covance | P-IHC |
| p63 | BC4A4 | Mouse IgG2a | 1∶100 | Abcam | P-IHC |
| PDI | RL90 | Mouse IgG2a | 1∶100 | Abcam | IF |
| phospho-p38 (Thr180/Tyr182) | 12F8 (4631) | Rabbit IgG | 1∶50 | Cell Signaling | P-IHC |
| phospho-p42/44 (Thr202/Tyr204) | E10 (9106) | Mouse IgG1 | 1∶400 | Cell Signaling | P-IHC |
| phospho-p42/44 (Thr202/Tyr204) | 20G11 (9106) | Rabbit IgG | 1∶100 | Cell Signaling | P-IHC |
| phospho-histone H3 (Ser10) | 9701 | Rabbit | 1∶100 | Cell Signaling | P-IHC |
| phospho-Smad1/5/8 | 9511 | Rabbit | 1∶50 | Cell Signaling | P-IHC |
| phospho-Smad2 | 3101 | Rabbit | 1∶50 | Cell Signaling | P-IHC |
| phospho-Src Family (Tyr416) | 2101 | Rabbit | 1∶25 | Cell Signaling | P-IHC |
| Tgn138 | 2F7.1 | Mouse IgG1 | 1∶50 | Affinity Bioreagents | IF |
| Zdhhc21 | 299 | Rabbit | 1∶100–500 | Custom Eurogentec | P-IHC, IF, WB |
Table 2. Details of secondary antibodies used in this study.
| Antigen | Host Species | Dilution | Source | Notes |
| ECL α-Mouse IgG, HRP-conjugated | Sheep | 1∶10000 | GE Healthcare UK Ltd | WB |
| ECL α-Rabbit IgG, HRP-conjugated | Sheep | 1∶10000 | GE Healthcare UK Ltd | WB |
| TRITC-conjugated α-Rabbit IgG | Donkey | 1∶250 | Jackson Immunoresearch | P-IHC, IF |
| FITC-conjugated α-Rabbit IgG | Donkey | 1∶250 | Jackson Immunoresearch | P-IHC, IF |
| Alexa 488-conjugated-α-Mouse | Donkey | 1∶500 | Molecular Probes | P-IHC, IF |
| Alexa 594-conjugated-α-Mouse | Donkey | 1∶500 | Molecular Probes | P-IHC, IF |
| Alexa 594-conjugated-α-Rabbit | Donkey | 1∶500 | Molecular Probes | P-IHC, IF |
| Alexa 488-conjugated-α-Rat | Donkey | 1∶500 | Molecular Probes | P-IHC, IF |
| Biotin-conjugated-α-Rabbit | Donkey | 1∶400 | Jackson Immunoresearch | P-IHC, IF |
BrdU labeling
For BrdU labeling experiments, 2 age- and gender-matched mice of each genotype were injected with 50 µg BrdU/g body weight and sacrificed after 2 hrs. Skin sections were dewaxed, subjected to proteinase K antigen retrieval, followed by HCl denaturation and neutralization, before incubation with anti-BrdU antibody (BD). For indirect colorimetric visualization, a biotinylated donkey anti-mouse secondary antibody (Jackson Labs) and Vectastain Universal Elite ABC Kit (Vector Laboratories) were used, followed by NovaRed substrate (Vector Laboratories) according to manufacturer's protocol. A proliferative index was calculated by counting the number of positive cells divided by the total number of nuclei within the epidermal compartment, in each of ten fields at 10× magnification, and the average index per field was calculated. Statistical significance was calculated using a two-tailed Student's t-test.
Supporting Information
Localization and function of Zdhhc21 is altered by mutations of cysteines within DHHC consensus core. (A) Schematic of mutations in Zdhhc21. In addition to the dep deletion in C-terminal intracellular tail, several point mutations were generated by disrupting key cysteine residues within the DHHC domain. Another mutation, L91F, close to the DHHC domain was identified from an archive of ENU-mutagenised sperm from Harwell. However unlike mutations in the critical cysteines, this mutant protein was correctly localized and exhibited normal PAT activity. Mice homozygous for this mutation had normal hair. (B–G) Localization of HA-tagged Zdhhc21 cDNAs transfected into NIH-3T3 cells (anti-HA red) compared to cis-Golgi marker GM130 (green). Wild type and L91F strongly co-localize with GM130, whereas mutations within DHHC domain disrupt localization similar to dep. (H) Zdhhc21 protein variants which disrupt localization abrogate autopalmitoylation responses using ABE chemistry and pulled down by streptavidin agarose beads and resolved by SDS-PAGE [47]. Portions not pulled down were also resolved by SDS-PAGE as loading control (I).
(1.66 MB TIF)
Characterization of Zdhhc21 expression in skin. Expression of Zdhhc21 mRNA (B,D,E,G,J) and protein (A,C,F,H,I,K,). (A) E16.5 vibrissae follicle (Zdhhc21: green, p63: red). (B,C) P24 dorsal control skin. (D–F) P35 dorsal follicles of dep (D) and wild type (E), show similar levels and patterns of transcript, as observed with Zdhhc21 antibody (F). (G–I) While Zdhhc21 mRNA and protein expression is similar in the lower portions of P63 dorsal follicles (G,H), only protein can be detected in the upper (I) portions of the isthmus (I) but not in the bulge, sebaceous glands or IFE. (J–L) In telogen, (P21) wild-type dorsal skin shows no expression of Zdhhc21 mRNA (J) while some antibody staining is detected in the isthmus (K), which is specifically blocked by pre-incubating the antibody with the blocking peptide (L).
(4.99 MB TIF)
Cyclic expression of Zdhhc21 during postnatal hair cycle in wild-type and dep mutant follicles. Expression of Zdhhc21 (red) and Gata3 (green) during catagen (P14 A,B), telogen (P21 C,D), initiation of anagen (P24 E,F), early anagen (P28 G,H) and late anagen (P35 I,J) in wild-type (A,C,E,G,I) and dep follicles (B,D,F,H,J). Expression of Zdhhc21 is limited to the post-mitotic lineages of IRS and cuticle of both control and dep anagen and catagen follicles.
(6.63 MB TIF)
Aberrant epidermal proliferation during anagen contributes to dep hyperplastic interfollicular epidermis and sebaceous glands. Hematoxylin and eosin (A–D). Phosphohistone H3 (red, E–J) with Ki67 (green; I,J,). Significant differences in proliferation were not readily detectable at telogen (P21; A,B,E,F), or early (P28; C,D,G–J) anagen. However, quantitative BrDU labelling studies during anagen (P32) revealed a small but significant increase in proliferation in dep sebaceous glands and IFE (L), with a parallel decrease in proliferation in dep hair follicles (K). (**p<0.005, *p<0.05)
(4.18 MB TIF)
Aberrant epidermal differentiation in dep mutant skin. Wild-type (A–F) and dep (G–,L) P28 dorsal follicles. Expression of terminal differentiation markers (loricrin (red), p63 (green) (A,G); filaggrin (red) (B,H) is delayed in dep mutant skin. Ectopic Keratin 6 expression (K6 (red), Ki67 (green) (C,I) is not observed in dep interfollicular epidermis, but expression remains restricted to the infundibulum and inner root sheath of the hair follicle. Imbalance of proliferative and differentiation signals in dep basal IFE where increased nuclear phospho-ERK (phospho-P42/44 (red), Gata3 (green), (D,–D′,J–J′) is observed with reduced expression of Gata3, in contrast to wild type skin where high suprabasal phospho-ERK is associated with strong Gata3 expressing cells (D–D′, arrowheads). Aberrant elevated basal p42/44 signalling was confirmed with a second antibody (I–I′,K–K′). Despite expanded bulge region below the dilated infundibulum and overgrown sebaceous glands, the expression of K15 (green) remains restricted to the bulge (F,L). Nuclei were labelled with DAPI (blue:C,I) or TOTO-3 (blue:D–F,J–L).
(4.65 MB TIF)
Loss of Zdhhc21 function does not result in delays in selective barrier acquisition or keratinocyte terminal differentiation defects in embryonic dep epidermis. Wild-type (A–E) and dep mutant (F–J) late E16.5 embryos and E18.5 embryonic skins (C–E, H–J). (A,B,F,G) Dye exclusion assay showing similar range of barrier acquisition in a litter with wild-type and dep littermates from less advanced (A,F) to more established stages of barrier development (B,G). No difference in expression of terminal differentiation markers loricrin (C,H) and filaggrin (D,I) is detected between wild type and dep neonatal skin. Comparable Gata3 expression is observed in developing hair follicles and IFE of wild-type and dep neonatal skin (E–J).
(2.42 MB TIF)
Initiation of Wnt-dependent anagen responses is normal in dep mice but subsequent propagation is affected. Alkaline phosphatase staining (A,C,E,G) marks dermal papillae. Induction of first anagen at P24 (A–D) with strong dermal papilla Lef1 staining (red) (B,D) and few adjacent positive cells in epidermal hair germ is observed in both wild-type (A,B) and mutant (C,D) skin. Subsequent propagation of anagen responses is defective at P28 (E–L) where retarded dep follicles show little Lef1 signal in matrix (F,H) as well as reduced or absent Foxn1 (green,Zdhhc21 red; J,K) and AE13 (green, Zdhhc21 red; J,L) in hair shaft precursors. By P35 (M–P), although misshapen and misoriented, many dep follicles (O,P) are similar to control littermates (M,N) as shown by AE13 (green, Zdhhc21 red; M,O) and beta-catenin (green, K5 red; N,P).
(5.73 MB TIF)
Effective membrane targeting of Fyn during keratinocyte differentiation is compromised in dep mutant skin. Fyn localization in P32 anagen follicles (A–B′) and IFE (E–F′) with localization of active Src family kinases (including Src, Fyn, Yes and Lck) (C–D′,G–H′). Fyn expression is detected diffusely in the wild type bulb and becomes restricted to the membrane of differentiating IRS cuticle and some Henle's layers at the junction of the hair bulb and shaft. High levels of membrane associated active SFKs are seen throughout anagen hair follicle including dermal papilla, proliferative matrix, ORS and IRS lineages. In dep mutants, this membrane association of Fyn is greatly reduced/absent whilst active Src family kinase expression is largely unchanged. Fyn expression in the control IFE and IF becomes membrane restricted in suprabasal, differentiating keratinocytes, whilst membrane associated active Src family kinases can be seen throughout the basal and suprabasal IFE. Membrane associated Fyn and active SFKs is delayed in dep mutants. Arrowheads indicated areas of interest in merge and single channels.
(4.72 MB TIF)
Acknowledgments
We thank A. Weiss (Lck cDNA), W. Sessa (eNOS cDNA), Jeremy Nathans (Wnt3a cDNA), Yingzi Yang (Wnt5a cDNA), Takano Yamamoto (Wnt10a cDNA), Nick Gilbert (pCMX2GFPFLAGSTOP vector), T.T.Sun (AE13 and AE15 antibodies), and R. Grosschedl (Lef1 antibody) for sharing reagents. We are grateful to Paul Perry and Brendan Doe for technical assistance and to Tilo Kunath and Richard Mort for critical comments and discussions.
Footnotes
The authors have declared that no competing interests exist.
This work was funded by the Medical Research Council (UK), a National Sciences and Engineering Research Council of Canada Fellowship to PM, a Caledonian Research Foundation Fellowship to PM, a Wellcome Trust Travelling Fellowship to IS, an R. Douglas Wright Fellowship to IS, an Australian Research Council grant to IS, and a Human Frontiers Science Program grant to YF and MF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Smotrys JE, Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. 2004;73:559–587. doi: 10.1146/annurev.biochem.73.011303.073954. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 3.Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, et al. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Husseini A, Schnell E, Dakoji S, Sweeney N, Zhou Q, et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 5.Kang R, Wan J, Arstikaitis P, Takahashi H, Huang K, et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lobo S, Greentree WK, Linder ME, Deschenes RJ. Identification of a Ras palmitoyltransferase in Saccharomyces cerevisiae. J Biol Chem. 2002;277:41268–41273. doi: 10.1074/jbc.M206573200. [DOI] [PubMed] [Google Scholar]
- 7.Roth AF, Feng Y, Chen L, Davis NG. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J Cell Biol. 2002;159:23–28. doi: 10.1083/jcb.200206120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Fukata Y, Iwanaga T, Fukata M. Systematic screening for palmitoyl transferase activity of the DHHC protein family in mammalian cells. Methods. 2006;40:177–182. doi: 10.1016/j.ymeth.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Oyama T, Miyoshi Y, Koyama K, Nakagawa H, Yamori T, et al. Isolation of a novel gene on 8p21.3–22 whose expression is reduced significantly in human colorectal cancers with liver metastasis. Genes Chromosomes Cancer. 2000;29:9–15. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1001>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Ducker CE, Stettler EM, French KJ, Upson JJ, Smith CD. Huntingtin interacting protein 14 is an oncogenic human protein: palmitoyl acyltransferase. Oncogene. 2004;23:9230–9237. doi: 10.1038/sj.onc.1208171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukai J, Liu H, Burt RA, Swor DE, Lai WS, et al. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat Genet. 2004;36:725–731. doi: 10.1038/ng1375. [DOI] [PubMed] [Google Scholar]
- 13.Faul T, Gawlik M, Bauer M, Jung S, Pfuhlmann B, et al. ZDHHC8 as a candidate gene for schizophrenia: analysis of a putative functional intronic marker in case-control and family-based association studies. BMC Psychiatry. 2005;5:35. doi: 10.1186/1471-244X-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansouri MR, Marklund L, Gustavsson P, Davey E, Carlsson B, et al. Loss of ZDHHC15 expression in a woman with a balanced translocation t(X;15)(q13.3;cen) and severe mental retardation. Eur J Hum Genet. 2005;13:970–977. doi: 10.1038/sj.ejhg.5201445. [DOI] [PubMed] [Google Scholar]
- 16.Raymond FL, Tarpey PS, Edkins S, Tofts C, O'Meara S, et al. Mutations in ZDHHC9, which encodes a palmitoyltransferase of NRAS and HRAS, cause X-linked mental retardation associated with a Marfanoid habitus. Am J Hum Genet. 2007;80:982–987. doi: 10.1086/513609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, et al. A single type of progenitor cell maintains normal epidermis. Nature. 2007;446:185–189. doi: 10.1038/nature05574. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci U S A. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer TC, Kleiman NJ, Green MC. Depilated (dep), a mutant gene that affects the coat of the mouse and acts in the epidermis. Genetics. 1976;84:59–65. doi: 10.1093/genetics/84.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinchik EM. Molecular genetics of the brown (b)-locus region of mouse chromosome 4. II. Complementation analyses of lethal brown deletions. Genetics. 1994;137:855–865. doi: 10.1093/genetics/137.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth IM, Wilming L, Lee AW, Taylor MS, Gautier P, et al. Genomic anatomy of the Tyrp1 (brown) deletion complex. Proc Natl Acad Sci U S A. 2006;103:3704–3709. doi: 10.1073/pnas.0600199103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson EH, Suffolk R, Bell JA, Jordan SA, Johnson DK, et al. A comparative transcript map and candidates for mutant phenotypes in the Tyrp1 (brown) deletion complex homologous to human 9p21–23. Mamm Genome. 2000;11:58–63. doi: 10.1007/s003350010011. [DOI] [PubMed] [Google Scholar]
- 26.Smyth I, Du X, Taylor MS, Justice MJ, Beutler B, et al. The extracellular matrix gene Frem1 is essential for the normal adhesion of the embryonic epidermis. Proc Natl Acad Sci U S A. 2004;101:13560–13565. doi: 10.1073/pnas.0402760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shawlot W, Min DJ, Wakamiya M, Behringer RR. The cerberus-related gene, Cerr1, is not essential for mouse head formation. Genesis. 2000;26:253–258. [PubMed] [Google Scholar]
- 28.Stanley EG, Biben C, Allison J, Hartley L, Wicks IP, et al. Targeted insertion of a lacZ reporter gene into the mouse Cer1 locus reveals complex and dynamic expression during embryogenesis. Genesis. 2000;26:259–264. doi: 10.1002/(sici)1526-968x(200004)26:4<259::aid-gene70>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Belo JA, Bachiller D, Agius E, Kemp C, Borges AC, et al. Cerberus-like is a secreted BMP and nodal antagonist not essential for mouse development. Genesis. 2000;26:265–270. [PubMed] [Google Scholar]
- 30.Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, et al. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:369–377. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsutsumi R, Fukata Y, Noritake J, Iwanaga T, Perez F, et al. Identification of G-protein alpha subunit palmitoylating enzyme. Mol Cell Biol. 2009;29:435–447. doi: 10.1128/MCB.01144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Guzman Strong C, Wertz PW, Wang C, Yang F, Meltzer PS, et al. Lipid defect underlies selective skin barrier impairment of an epidermal-specific deletion of Gata-3. J Cell Biol. 2006;175:661–70. doi: 10.1083/jcb.200605057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Candi E, Terrinoni A, Rufini A, Chikh A, Lena AM, et al. p63 is upstream of IKK alpha in epidermal development. J Cell Sci. 2006;119:4617–22. doi: 10.1242/jcs.03265. [DOI] [PubMed] [Google Scholar]
- 34.Proksch E, Feingold KR, Man MQ, Elias PM. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991;87:1668–1673. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haase I, Hobbs RM, Romero MR, Broad S, Watt FM. A role for mitogen-activated protein kinase activation by integrins in the pathogenesis of psoriasis. J Clin Invest. 2001;108:527–36. doi: 10.1172/JCI12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang J, Mehrani T, Millar SE, Morasso MI. Dlx3 is a crucial regulator of hair follicle differentiation and cycling. Development. 2008;135:3149–59. doi: 10.1242/dev.022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol. 2003;120:36–47. doi: 10.1046/j.1523-1747.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- 38.Kobielak K, Pasolli HA, Alonso L, Polak L, Fuchs E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J Cell Biol. 2003;163:609–23. doi: 10.1083/jcb.200309042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–68. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 40.Yuhki M, Yamada M, Kawano M, Iwasato T, Itohara S, et al. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development. 2004;131:1825–33. doi: 10.1242/dev.01079. [DOI] [PubMed] [Google Scholar]
- 41.Foitzik K, Lindner G, Mueller-Roever S, Maurer M, Botchkareva N, et al. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2001;14:752–60. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- 42.Descargues P, Sil AK, Sano Y, Korchynskyi O, Han G, et al. IKKalpha is a critical coregulator of a Smad4-independent TGFbeta-Smad2/3 signaling pathway that controls keratinocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:2487–92. doi: 10.1073/pnas.0712044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leong WF, Zhou T, Lim GL, Li B. Protein palmitoylation regulates osteoblast differentiation through BMP-induced osterix expression. PLoS One. 2009;4:e4135. doi: 10.1371/journal.pone.0004135. doi: 10.1371/journal.pone.0004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo W, Chen YG. Specific activation of mitogen-activated protein kinase by transforming growth factor-beta receptors in lipid rafts is required for epithelial cell plasticity. Mol Biol Cell. 2009;20:1020–9. doi: 10.1091/mbc.E08-09-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gat U, DasGupta R, Degenstein L, Fuchs E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 46.Silva-Vargas V, Lo CC, Giangreco A, Ofstad T, Prowse DM, et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DasGupta R, Rhee H, Fuchs E. A developmental conundrum: a stabilized form of beta-catenin lacking the transcriptional activation domain triggers features of hair cell fate in epidermal cells and epidermal cell fate in hair follicle cells. J Cell Biol. 2002;158:331–344. doi: 10.1083/jcb.200204134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 50.Kurek D, Garinis GA, van Doorninck JH, van der Wees J, Grosveld FG. Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development. 2007;134:261–72. doi: 10.1242/dev.02721. [DOI] [PubMed] [Google Scholar]
- 51.Kang R, Wan J, Artikaitis P, Takahashi H, Huang K, et al. Neural palmitoyl proteomics reveals dynamic synaptic palmitoylation. Nature. 2008;456:904–909. doi: 10.1038/nature07605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sowden HM, Naseem KM, Tobin DJ. Differential expression of nitric oxide synthases in human scalp epidermal and hair follicle pigmentary units: implications for regulation of melanogenesis. Br J Dermatol. 2005;153:301–309. doi: 10.1111/j.1365-2133.2005.06718.x. [DOI] [PubMed] [Google Scholar]
- 53.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 55.Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- 56.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 57.Ren J, Wen L, Gao X, Jin C, Xue Y, et al. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Engineering, Design and Selection. 2008;21:639–644. doi: 10.1093/protein/gzn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 59.McCormick PJ, Dumaresq-Doiron K, Pluviose AS, Pichette V, Tosato G, et al. Palmitoylation controls recycling in lysosomal sorting and trafficking. Traffic. 2008;9:1984–1997. doi: 10.1111/j.1600-0854.2008.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calautti E, Missero C, Stein PL, Ezzell RM, Dotto GP. fyn tyrosine kinase is involved in keratinocyte differentiation control. Genes Dev. 1995;9:2279–91. doi: 10.1101/gad.9.18.2279. [DOI] [PubMed] [Google Scholar]
- 61.Cabodi S, Calautti E, Talora C, Kuroki T, Stein PL, et al. A PKC-eta/Fyn-dependent pathway leading to keratinocyte growth arrest and differentiation. Mol Cell. 2000;6:1121–9. doi: 10.1016/s1097-2765(00)00110-6. [DOI] [PubMed] [Google Scholar]
- 62.Iliæ D, Kanazawa S, Nishizumi H, Aizawa S, Kuroki T, et al. Skin abnormality in aged fyn−/− fak+/− mice. Carcinogenesis. 1997;18:1473–6. doi: 10.1093/carcin/18.8.1473. [DOI] [PubMed] [Google Scholar]
- 63.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, et al. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DiColandrea T, Karashima T, Maatta A, Watt FM. Subcellular distribution of envoplakin and periplakin: insights into their role as precursors of the epidermal cornified envelope. J Cell Biol. 2000;151:573–586. doi: 10.1083/jcb.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, et al. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 66.Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Localization and function of Zdhhc21 is altered by mutations of cysteines within DHHC consensus core. (A) Schematic of mutations in Zdhhc21. In addition to the dep deletion in C-terminal intracellular tail, several point mutations were generated by disrupting key cysteine residues within the DHHC domain. Another mutation, L91F, close to the DHHC domain was identified from an archive of ENU-mutagenised sperm from Harwell. However unlike mutations in the critical cysteines, this mutant protein was correctly localized and exhibited normal PAT activity. Mice homozygous for this mutation had normal hair. (B–G) Localization of HA-tagged Zdhhc21 cDNAs transfected into NIH-3T3 cells (anti-HA red) compared to cis-Golgi marker GM130 (green). Wild type and L91F strongly co-localize with GM130, whereas mutations within DHHC domain disrupt localization similar to dep. (H) Zdhhc21 protein variants which disrupt localization abrogate autopalmitoylation responses using ABE chemistry and pulled down by streptavidin agarose beads and resolved by SDS-PAGE [47]. Portions not pulled down were also resolved by SDS-PAGE as loading control (I).
(1.66 MB TIF)
Characterization of Zdhhc21 expression in skin. Expression of Zdhhc21 mRNA (B,D,E,G,J) and protein (A,C,F,H,I,K,). (A) E16.5 vibrissae follicle (Zdhhc21: green, p63: red). (B,C) P24 dorsal control skin. (D–F) P35 dorsal follicles of dep (D) and wild type (E), show similar levels and patterns of transcript, as observed with Zdhhc21 antibody (F). (G–I) While Zdhhc21 mRNA and protein expression is similar in the lower portions of P63 dorsal follicles (G,H), only protein can be detected in the upper (I) portions of the isthmus (I) but not in the bulge, sebaceous glands or IFE. (J–L) In telogen, (P21) wild-type dorsal skin shows no expression of Zdhhc21 mRNA (J) while some antibody staining is detected in the isthmus (K), which is specifically blocked by pre-incubating the antibody with the blocking peptide (L).
(4.99 MB TIF)
Cyclic expression of Zdhhc21 during postnatal hair cycle in wild-type and dep mutant follicles. Expression of Zdhhc21 (red) and Gata3 (green) during catagen (P14 A,B), telogen (P21 C,D), initiation of anagen (P24 E,F), early anagen (P28 G,H) and late anagen (P35 I,J) in wild-type (A,C,E,G,I) and dep follicles (B,D,F,H,J). Expression of Zdhhc21 is limited to the post-mitotic lineages of IRS and cuticle of both control and dep anagen and catagen follicles.
(6.63 MB TIF)
Aberrant epidermal proliferation during anagen contributes to dep hyperplastic interfollicular epidermis and sebaceous glands. Hematoxylin and eosin (A–D). Phosphohistone H3 (red, E–J) with Ki67 (green; I,J,). Significant differences in proliferation were not readily detectable at telogen (P21; A,B,E,F), or early (P28; C,D,G–J) anagen. However, quantitative BrDU labelling studies during anagen (P32) revealed a small but significant increase in proliferation in dep sebaceous glands and IFE (L), with a parallel decrease in proliferation in dep hair follicles (K). (**p<0.005, *p<0.05)
(4.18 MB TIF)
Aberrant epidermal differentiation in dep mutant skin. Wild-type (A–F) and dep (G–,L) P28 dorsal follicles. Expression of terminal differentiation markers (loricrin (red), p63 (green) (A,G); filaggrin (red) (B,H) is delayed in dep mutant skin. Ectopic Keratin 6 expression (K6 (red), Ki67 (green) (C,I) is not observed in dep interfollicular epidermis, but expression remains restricted to the infundibulum and inner root sheath of the hair follicle. Imbalance of proliferative and differentiation signals in dep basal IFE where increased nuclear phospho-ERK (phospho-P42/44 (red), Gata3 (green), (D,–D′,J–J′) is observed with reduced expression of Gata3, in contrast to wild type skin where high suprabasal phospho-ERK is associated with strong Gata3 expressing cells (D–D′, arrowheads). Aberrant elevated basal p42/44 signalling was confirmed with a second antibody (I–I′,K–K′). Despite expanded bulge region below the dilated infundibulum and overgrown sebaceous glands, the expression of K15 (green) remains restricted to the bulge (F,L). Nuclei were labelled with DAPI (blue:C,I) or TOTO-3 (blue:D–F,J–L).
(4.65 MB TIF)
Loss of Zdhhc21 function does not result in delays in selective barrier acquisition or keratinocyte terminal differentiation defects in embryonic dep epidermis. Wild-type (A–E) and dep mutant (F–J) late E16.5 embryos and E18.5 embryonic skins (C–E, H–J). (A,B,F,G) Dye exclusion assay showing similar range of barrier acquisition in a litter with wild-type and dep littermates from less advanced (A,F) to more established stages of barrier development (B,G). No difference in expression of terminal differentiation markers loricrin (C,H) and filaggrin (D,I) is detected between wild type and dep neonatal skin. Comparable Gata3 expression is observed in developing hair follicles and IFE of wild-type and dep neonatal skin (E–J).
(2.42 MB TIF)
Initiation of Wnt-dependent anagen responses is normal in dep mice but subsequent propagation is affected. Alkaline phosphatase staining (A,C,E,G) marks dermal papillae. Induction of first anagen at P24 (A–D) with strong dermal papilla Lef1 staining (red) (B,D) and few adjacent positive cells in epidermal hair germ is observed in both wild-type (A,B) and mutant (C,D) skin. Subsequent propagation of anagen responses is defective at P28 (E–L) where retarded dep follicles show little Lef1 signal in matrix (F,H) as well as reduced or absent Foxn1 (green,Zdhhc21 red; J,K) and AE13 (green, Zdhhc21 red; J,L) in hair shaft precursors. By P35 (M–P), although misshapen and misoriented, many dep follicles (O,P) are similar to control littermates (M,N) as shown by AE13 (green, Zdhhc21 red; M,O) and beta-catenin (green, K5 red; N,P).
(5.73 MB TIF)
Effective membrane targeting of Fyn during keratinocyte differentiation is compromised in dep mutant skin. Fyn localization in P32 anagen follicles (A–B′) and IFE (E–F′) with localization of active Src family kinases (including Src, Fyn, Yes and Lck) (C–D′,G–H′). Fyn expression is detected diffusely in the wild type bulb and becomes restricted to the membrane of differentiating IRS cuticle and some Henle's layers at the junction of the hair bulb and shaft. High levels of membrane associated active SFKs are seen throughout anagen hair follicle including dermal papilla, proliferative matrix, ORS and IRS lineages. In dep mutants, this membrane association of Fyn is greatly reduced/absent whilst active Src family kinase expression is largely unchanged. Fyn expression in the control IFE and IF becomes membrane restricted in suprabasal, differentiating keratinocytes, whilst membrane associated active Src family kinases can be seen throughout the basal and suprabasal IFE. Membrane associated Fyn and active SFKs is delayed in dep mutants. Arrowheads indicated areas of interest in merge and single channels.
(4.72 MB TIF)