Abstract
Type I collagen is a major component of the hybrid layer, and improvement of its mechanical properties may be advantageous during bonding procedures.
Objective
To investigate the effect of three different cross-linking agents (Glutaraldehyde [GD], Grape seed extract [GSE], and Genipin [GE]) on the tensile bond strength (TBS) of resin-dentin bonds.
Materials and Methods
Sixty-four sound human molars were collected and their occlusal surfaces were ground flat to expose dentin. Dentin surfaces were etched using a phosphoric acid and then teeth were randomly divided according to the dentin treatment: Control group (no treatment), 5% GD, 6.5% GSE or 0.5% GE. Teeth were restored either with One Step Plus or Adper Single Bond Plus adhesive systems and resin composite. After 24 hours, teeth were sectioned to produce a cross-sectional surface area of 1.0 mm2 and tested for tensile bond strength. Data were statistically analyzed using ANOVA and Fisher's PLSD tests (p< 0.05). There was a statistically significant interaction between factors (treatment and adhesive p<0.001). Treatment affected TBS (p< 0.0001), while no differences were observed between the adhesive systems (p = 0.6961).
Conclusion
Chemical modification to the dentin matrix promoted by GD and GSE, but not GE, resulted in increased bond strength. The application of selective collagen cross-linkers during adhesive restorative procedures may be a new approach to improve dentin bond strength properties.
Keywords: Collagen, cross-linking agents, dentin, tensile bond strength, adhesive systems
Introduction
Current adhesive systems bond to dentin through a micromechanical mechanism based on the formation of a hybrid layer [1,2]. The hybrid layer, a collagen-resin interface, is the most vulnerable portion of the bonded interfaces where stress tends to concentrate and most failures take place [3,4]. While bonding to enamel substrate has been shown to be reliable over-time, bonding to dentin substrate is a great challenge [5,6]. Dentin represents the bulk of the tooth and a reliable long-term bond is essential for the success of adhesive restorations. In lieu of the challenges associated with degradation of the dentin-adhesive interface over time, continuous research has been done in order to improve the mechanical properties of the adhesive interface. Two main methods to increase the dentin/resin interface properties have to be considered: the continuing improvement/development of new adhesive systems and the establishment of tissue engineering/biomimetics approaches to improve the intrinsic properties of the substrate.
Intrinsic collagen cross-links provide the tensile properties of collagen molecules. The use of extrinsic collagen cross-linking agents can induce additional formation of inter and intra-molecular cross-links [7,8]. Selective cross-linking agents have been demonstrated to increase the ultimate tensile strength and elastic modulus of demineralized dentin [9,10]. Glutaraldehyde (GD), a synthetic cross-linking agent, is widely used as fixative agent [11] and has been reported to improve mechanical properties of various collagen-based tissues [9,10,12,13,14,15]. Genipin (GE), a natural occurring cross-linking agent, not only has shown to improve the mechanical properties of various protein-based biomaterials [10,16,17], but presents low toxicity when compared to GD [16,17]. Proanthocyanidin (PA), widely present in fruits, vegetables, nuts, seeds, flowers is a potent anti-oxidant cross-linking agent with vast biological activities. Recently, the use of a Grape seed extract, mainly composed of PA, has been shown to improve the mechanical properties of demineralized dentin. [9,10]
The aim of this study was to evaluate the effect of 3 collagen cross-link agents on the microtensile bond strength (μTBS) of two etch-and-rinse adhesive system to dentin. The null hypothesis tested was that the use of cross-linking agents in vitro would not increase the tensile bond strength when compared to a control (no treatment).
Materials and Methods
A. Teeth Preparation
The use of sixty-four sound extracted human molars in this investigation was approved by the Institutional Review Board Committee from the University of Illinois at Chicago (protocol #2006-0229). The teeth were collected, cleaned from debris and stored in distilled water with 0.5% thymol crystals solution. Teeth were ground flat and perpendicular to their long axis using 180, 320 and 600 grit Silicon Carbide (Buehler, Lake Bluff, IL, USA) paper respectively under running water to exposed dentin (Figure 1).
Figure 1.
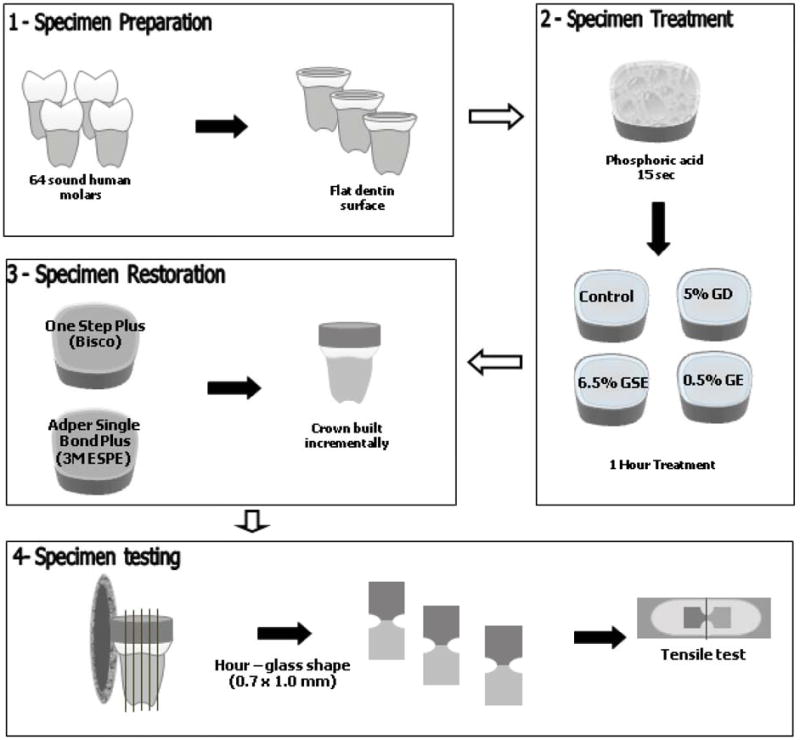
Illustration of specimen selection (1), treatment (2), restorative procedures (3) and testing (4). GD (Glutaraldehyde); GSE (Grape seed extract); GE (Genipin).
B. Dentin surface pre-treatment
Teeth were randomly divided according to the dentin treatment (n = 16): Group 1 - control group (Phosphate Buffer Solution, PBS); Group 2 - treated with 5% Glutaraldehyde in PBS (Fisher Scientific); Group 3 - treated with 6.5% Grape Seed Extract (MegaNatural- Polyphenolics Ind) in PBS, and group 4 treated with 0.5% Genipin (Wako Pure Chemical Ind) in PBS. All solutions had the pH adjusted to 7.4 using NaOH. Prior to treatment, the dentin surface was etched using a 37% and 35% phosphoric acid gel (3M ESPE, St Paul, USA or Bisco, Schaumburg, USA), respectively, for 15 seconds, then thoroughly rinsed with water for 15 seconds and kept moist. Teeth were immersed in their respective solutions for 1 hour. The concentrations of cross-linking agents were used based on previous studies [9,10].
C. Restorative procedures
After dentin treatment, the teeth were further divided into two subgroups (n = 8 teeth), according to the adhesive system used: ethanol-based Adper Single Bond Plus -SB (3M ESPE) or acetone-based One Step Plus - OS (Bisco). The adhesive systems were used following manufacturers instructions. A hybrid resin composite restorative material (Z250, 3M ESPE) was placed over the bonded surfaces incrementally (5 mm total thickness) to allow for gripping during the tensile testing. Increment thickness was limited to 2mm, and curing was accomplished for 40 seconds per increment (Demetron LC, SDS Kerr, Germany).
D. Microtensile bond strength testing (TBS)
For TBS evaluation, all restored teeth were stored in distilled water at 37° C for 24 hours. After that time, the restored teeth were sectioned perpendicular to the bonded interface into 0.7±0.2 mm2 thick slabs using a slow speed diamond saw (Buehler-Series 15LC Diamond, Lake Bluff, IL) under cooling water. The slabs were further trimmed at the interface using a fine diamond bur (no. 557D, Brasseler, Savannah, GA) in a high speed handpiece to produce a cross-sectional surface area of 1.0 mm2. The specimens were glued on a jig placed on a tensile tester machine (Bisco, Schaumburg, USA) and subjected to tensile force at a crosshead speed of 1 mm/min. Means and standard deviations were calculated and expressed in MPa. Data were statistically analyzed using a 2 way ANOVA and Fisher's PLSD with 95% confidence level.
E. Fracture pattern analysis
The debonded interfaces were visually classified as: adhesive failure at the interface, cohesive failure in composite, or in adhesive. After debonding, all fractured specimens were stored in 10% neutral buffered formalin solution (Fisher Scientific) and selected specimens were evaluated under scanning electron microscopy (SEM). Specimens were mounted on aluminum stubs, left to dry for 24 hours and gold sputter-coated (SEM Coating Unit E5150, Polaron Equipment Ltd., PA, USA). The micromorphology of the fractured interface was assessed using a scanning electron microscope (S-3000N Hitachi, Tokyo, Japan).
Results
The means and standard deviations (MPa, SD) were calculated and are shown in Table 1. There was a statistically significant interaction observed between factors (treatment and adhesive systems, p <0.0001). The different treatments resulted in statistically significant differences (p <0.0001), while the use of different adhesive systems had no effect on the bond strength (p =0.6961). The highest bond strength was observed for GSE treated groups (74.4 MPa, p <.0001), which was statistically higher than all the other experimental groups. GD treatment also resulted in a statistically increase in the TBS when compared to the control group (68.96 MPa, p <0.0001). There was no statistically significant difference between the TBS values of GE-treated samples and control groups (44.13 MPa and 43.70 MPa respectively, p =0 .7178).
Table 1.
Changes to the microtensile bond strength values [MPa, Mean (standard deviation)] for two adhesive systems following use of collagen cross-linkers.
| Adhesive systems | Dentin treatment | |||
|---|---|---|---|---|
| Control | GD | GSE | GE | |
| Adper Single Bond | 33.38 C (6.79) |
68.96 B (3.91) |
71.06 A (14.59) |
43.70 C (8.23) |
| One Step Plus | 44.13 C (8.54) |
65.46 B (8.06) |
74.40 A (10.08) |
34.80 C (4.09) |
Different letters indicate statistically significant (p<0.05) differences within each horizontal row.
GD (Glutaraldehyde); GSE (Grape seed extract); GE (Genipin)
The mode of fracture interface was visually assessed for every specimen. The majority of the fractured interfaces were at the interface. When analyzed under SEM, results showed distinct pattern for GD and GSE treated groups when compared to GE treated and control group. GD and GSE treated samples presented interface failures at the top of the hybrid layer (Figures 2 and 3). Hence, the morphology of the fractured interfaces is slighty atypical because the components of the bond have undergone excessive strain and plastic deformation. For instance, the fractured resin tags shown in Figure 2 have much larger diameters than normal due to elastic/plastic recoil. GE-treated and control samples showed similar pattern of interfacial fractures mostly present at the bottom of the hybrid layer (Figures 4 and 5).
Figure 2.

Representative SEM images of the debonded interfaces treated with Control group, One Step Plus adhesive system. The most common fractured surface was at the bottom of the hybrid layer. White arrows: Hybrid layer; T: Resin tags.
Figure 3.

Representative SEM images of the debonded interfaces treated with glutaraldehyde, One Step Plus system. Debonding pattern present at the adhesive layer/top hybrid layer. White arrows: Adhesive resin.
Figure 4.

Representative SEM images of the debonded interfaces treated with grape seed extract, One Step Plus adhesive system. Debonding pattern present at the adhesive layer/top hybrid layer. White arrows: hybrid layer; black arrows: Dentin tubules.
Figure 5.
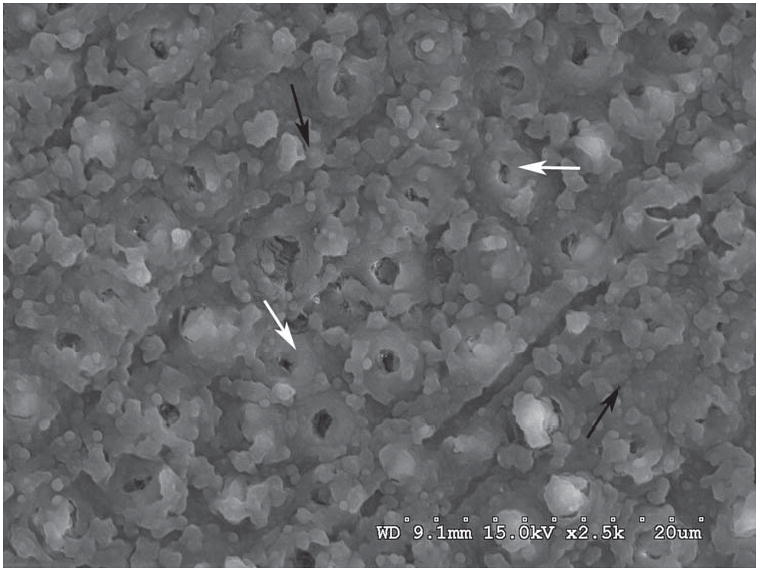
Representative SEM images of the debonded interfaces treated with Genipin, One Step Plus adhesive system. Samples fractured at the bottom of hybrid layer. White arrows: peritubular dentin; Black arrows: Hybrid layer.
Discussion
GD has been investigated in various studies as a potential collagen cross-linking agent [7,10,13]. Despite its ability to induce cross-links in collagen, it is known for its cytotoxicity [18,19,20]. In the present study, the use of GD increased the bond strength for both adhesives when compared to control (Table 1). Bedran-Russo et al., [9] used GD to evaluate the effect of cross-linking agents on undemineralized and demineralized dentin treated for 4 and 40 hours. Their results showed no difference in tensile strength of GD-treated group when compared to control. However, increased stiffness of demineralized dentin following use of 5% and 25% Glutaraldehyde was reported using a 3-point bending method [10]. The study reported that the modulus of elasticity of GD-treated samples increased as a function of exposure time and concentration [10]. The present study suggests that the presence of exogenous cross-links induced by GD were sufficient to positively affect the mechanical properties of the exposed dentin matrix and consequently increase the TBS. The increase of TBS was observed for both adhesive systems. GD reacts primarily with the ε-amino groups of lysyl (or hydrolysyl) residues by using its aldehyde functional groups [11,13,19]. An experiment proposed by Ritter et al. [13] on bovine teeth showed that the use of GD on demineralized dentin resulted in reduction of the free lysyl and hydrolysyl residues in collagen hydrolysates, which likely represent new formed cross-links.
On the other hand, GSE, a natural occurring compound, interacts with proteins to induce cross-links by four different mechanisms: covalent interaction, ionic interaction, hydrogen bonding interaction or hydrophobic interactions [7,21,22]. Therefore, GSE has far more interaction ability with collagen than GD and ability to affect the mechanical properties of dentin [10]. In the present study, treatment of the dentin surface using 6.5% GSE produced the highest TBS values that were statistically higher than the control group. It has been reported that the stiffness of demineralized dentin can be affected by the concentration and exposure time to GSE solution [9]. The results of the present study demonstrate that changes to the mechanical properties of dentin matrix promoted by GSE [9,10], resulted in increased dentin bond strength. In addition, the effect of GSE on TBS was statistically higher than all the other groups. A competitive binding assay studied the relative affinity of various proteins and PA showed that proline-rich proteins like collagen have an extremely high affinity for PA based components [7,23], forming a Proline-PA complex. The stabilization of collagen fibers through hydrogen bond formation resulted in an increase in the denaturation temperature of the fixed tissue [7,23]. Therefore, the high TBS of GSE treated groups are most likely due to changes to the dentin collagen which increased its mechanical properties and consequently the adhesive bonded interface components that incorporate collagen, i.e. hybrid layer.
In contrast, GE did not show a significant difference when compared to the control. The GE reaction mechanism with biological tissue is not clear yet. One proposition by Fujikawa et al., [17] states that GE reacts with amino acid to form a nitrogeniridoid spontaneously, which then undergoes dehydration to form an aromatic monomer. GE may react with free amino groups of lysine, hydroxylysine, or arginine to form intra or intermolecular cross-links within a collagen molecule or between adjacent collagen molecules [8,18]. The lack of increase in TBS in the GE-treated group could be explained by the fact that GE has a slower rate of cross-linking induction when compared to GD and GSE. GE-treated dentin matrix has been reported to increase only after 40 hours treatment [10]. Hence, the modulus of elasticity of demineralized dentin treated with GE increased only after 24 hours exposure (unpublished data). Therefore, one hour of GE treatment most likely did not affect the TBS of dentin due to limited treatment time for GE to induce exogenous cross-links and consequently affect the mechanical properties of the dentin collagen.
When the fractured interfaces were evaluated under SEM analysis, it was observed that the GD and GSE treated groups showed a more consistent failure mode. The failures were mainly in the adhesive layer and the top of the hybrid layer (Figures 2 and 3). On the control and GE treated groups, the fractured pattern was observed on the bottom of the hybrid layer (Figures 4 and 5). We suggest that changes to the fracture pattern of GD and GSE treated samples can be related to an increase in the properties of the hybrid layer, since the chemically modified collagen present improved properties. Control and GE presented similar patterns as GE did not affect the bond strength when compared to the control group. The findings support the ability of certain collagen cross-linkers to improve the dentin matrix properties, thus enhancing dentin bonded interfaces by increasing the hybrid layer properties. The adhesive bonding process to dentin takes place through a micromechanical mechanism with the formation of a hybrid layer [1,2]. The bond takes place by the impregnation of the dentin substrate through blends of resin monomers, where the formation of a compact and homogeneous hybrid layer is heavily dependent upon the stability of the bonded interface [24,25]. At the same time that the hybrid layer is essential for dentin bonding, it is also the weakest and most vulnerable component of the interface. Two dentin adhesive systems were employed in the study to assess possible effect of the bonding system components on the TBS after dentin treatment. One Step plus is an acetone-based system, while Adper Single Bond is a water/ethanol system. The present study observed that no statistically significant differences were observed between the bonding agents and dentin treatment. Thus changes to the dentin matrix promoted by GSE and GE, were not adhesive system dependent. Therefore, the null hypothesis must be rejected.
In conclusion, the use of GD and GSE, as collagen cross-linkers, increased the bond strength to dentin when compared to the control group. Treatment with GD and GSE more than doubled the TBS and SB and increased the TBS of OS by 47 and 69%, respectively. GE had no significant effect on TBS when compared to the control group. Modifications to the dentin matrix promoted by GD and GSE resulted in increased bond strength. The pretreatment time may be reduced by increasing the concentration of cross-linkers or by using several cross-linkers simultaneously to make it more clinical applicable. Thus, the application of collagen cross-linkers during adhesive restorative procedures may be a new approach to improve dentin bond strength properties.
Acknowledgments
This investigation was supported by research grant DE017740 from the National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD and UIC-COD Wach Research Fund Grant.
References
- 1.Nakabayashi N, Saimi Y. Bonding to intact dentin. J Dent Res. 1996;75:1706–1716. doi: 10.1177/00220345960750091401. [DOI] [PubMed] [Google Scholar]
- 2.Nakabayashi N, Pashley DH. Hybridization of dental hard tissues. Tokyo, Japan: Quintessence; 1998. pp. 64–68. [Google Scholar]
- 3.Van Noort R, Norooze S, Howard I, Cardew G. A critique of bond strength measurements. J Dent. 1989;17:61–67. doi: 10.1016/0300-5712(89)90131-0. [DOI] [PubMed] [Google Scholar]
- 4.Van Noort R, Cardew G, Howard I, Norooze S. The effect of local interfacial geometry on the measurements of the tensile bond strength to dentin. J Dent Res. 1991;70:889–893. doi: 10.1177/00220345910700050501. [DOI] [PubMed] [Google Scholar]
- 5.Van Meerbeek B, De Munck J, Yosida Y, Inoue S, Vargas M, Vijay P, et al. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003;28:215–235. [PubMed] [Google Scholar]
- 6.Toledano M, Osorio R, de Leonardi G, Rosales-Leal JI, Ceballos L, Cabrerizo Vilchez MA. Influence of self-etching primer on the resin adhesion to enamel and dentin. Am J Dent. 2001;14:205–210. [PubMed] [Google Scholar]
- 7.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res. 2003;65A:118–124. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 8.Sung HS, Chang WH, Ma CY, Lee MH. Cross-linking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res. 2003;64A:427–438. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 9.Bedran-Russo AK, Pashley DH, Agee K, Drummond JL, Miescke KJ. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J Biomed Mater Res B Appl Biomater. 2008;86B:330–4. doi: 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- 10.Bedran-Russo AK, Pereira PN, Duarte WR, Drummond JL, Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J Biomed Mater Res B: Appl Biomater. 2007;80:268–272. doi: 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- 11.Nimni ME, Cheung D, Startes B, Sheikh Kodama M. Bioprosthesis derived from cross-linked and chemically modified collagenous tissues. In: Nimni ME, editor. Collagen. III. Boca Raton, FL: CRC Press; 1988. pp. 1–38. [Google Scholar]
- 12.Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 2003;24:759–767. doi: 10.1016/s0142-9612(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 13.Ritter AV, Swift EJ, Jr, Yamauchi M. Effects of phosphoric acid and glutaraldehyde-HEMA on dentin collagen. Eur J Oral Sci. 2001;109:348–353. doi: 10.1034/j.1600-0722.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 14.Yannas IV. Tissue regeneration by use of collagen-glycosaminoglycan copolymers. Clin Mater. 1992;9:179–87. doi: 10.1016/0267-6605(92)90098-e. [DOI] [PubMed] [Google Scholar]
- 15.Silver FH. Wound dressings and skin replacement In: Silver FH, editor Biomaterials medical devices and tissue engineering an integral approach. London: Chapman & Hall; 1994. pp. 46–91. [Google Scholar]
- 16.Tsai TR, Tseng TY, Chen CF, Tsai TH. Identification and Determination of Geniposide Contained in Gardenia Jasminoides and in Two Preparations of Mixed Traditional Chinese Medicines. J Chromatogr A. 2002;961:83–88. doi: 10.1016/s0021-9673(02)00365-5. [DOI] [PubMed] [Google Scholar]
- 17.Frujikawa S, Yokota T, Konga K, Kumada J. The continuous hydrolysis of geniposide to genipin using immobilized beta-glucosidase on calcium alginate gel. J Biotechnol Lett. 1987;9:697–702. [Google Scholar]
- 18.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Crosslinking characteristics and mechnical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res. 1999;47:116–126. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Sung HW, Huang DM, Chang WH, Huang RN, Hsu JC. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhevises: In vitro study. J Biomed Mater Res. 1999;46:520–530. doi: 10.1002/(sici)1097-4636(19990915)46:4<520::aid-jbm10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Van Luyn MJA, van Wachem PB, Dijkstra PJ, olde Damink LHH, Feiijen J. Calcification of subcutaneously implanted collagen in relation to cytotoxicity, cellular interactions and crosslinking. J Mater Sci Mater Med. 1995;6:288–96. [Google Scholar]
- 21.Pierpoint WS. O-Quinones formed in plant extracts. Their reactions with amino acids and peptides. Biochem J. 1969;112:609–616. doi: 10.1042/bj1120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loomis WD. Overcoming problems of phenolics and quinines in the isolation of plant enzymes and organelles. Methods Enzymol. 1974;31:528–544. doi: 10.1016/0076-6879(74)31057-9. [DOI] [PubMed] [Google Scholar]
- 23.Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256:4494–4497. [PubMed] [Google Scholar]
- 24.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–273. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 25.Van Meerbeek B, Dhem A, Goret-Nicaise M, Braem M, Lambrechts P, Vanherle G. Comparative SEM and TEM examination of the ultrastructure of the resin–dentin interdiffusion zone. J Dent Res. 1993;72:495–501. doi: 10.1177/00220345930720020501. [DOI] [PubMed] [Google Scholar]


