Abstract
Recent advances allow aging-associated changes in B-cell function to be approached at a mechanistic level. Reduced expression of genes crucial to lineage commitment and differentiation yield diminished B-cell production. Moreover, intrinsic differences in the repertoire generated by B-cell precursors in aged individuals, coupled with falling B-cell generation rates and life-long homeostatic competition, result in narrowed clonotypic diversity. Similarly, reductions in gene products crucial for immunoglobulin class switch recombination and somatic hypermutation impact the efficacy of humoral immune responses. Together, these findings set the stage for integrated analyses of how age-related changes at the molecular, cellular and population levels interact to yield the overall aging phenotype.
Introduction
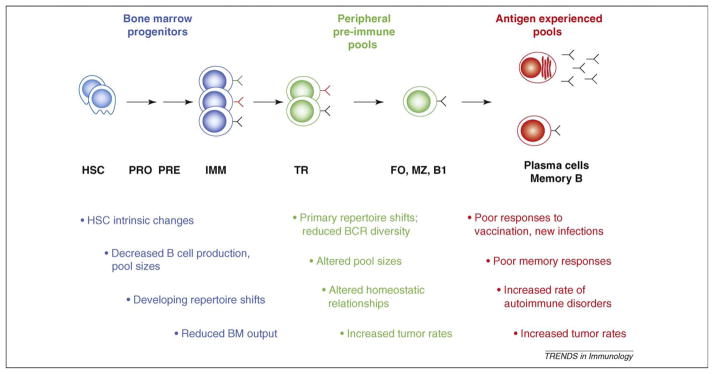
Advancing age yields numerous immune system changes, in aggregate termed immunosenescence [1–5]. These changes result in refractory responses to vaccination or infection, declines in previously established protective immunity and increased disease morbidity [5–8] (also see other articles in this special issue). B cells play central roles in the establishment and maintenance of protective immunity, including the generation of protective antibodies, antigen presentation, and more recently, appreciated regulatory functions [9]. Accordingly, assessments of how age impacts the production and behavior of B cells, as well as the accompanying effects on incipient and established humoral immunity, are fundamental to understanding immunosenescence. Early descriptive studies of age-associated changes in the B-cell lineage revealed reductions in the functional capacities of B cells and their progenitors, changes in the sizes of different subsets and shifts in the diversity and clonotypic composition of the antigen-responsive repertoire [10–14]. Recent advances in our understanding of the molecular and cellular mechanisms underlying B-cell differentiation, homeostasis and activation are now fostering analyses of the basis for the age-associated changes summarized in Figure 1.
Figure 1.
Aging-related changes in B-cell generation and function. The general timeline (left to right) of B-cell development and differentiation, from generation in the bone marrow (blue) to peripheral preimmune (green) and antigen-experienced (red) subsets. Colored Y-shaped molecules indicate B-cell receptors (BCRs) and corresponding antibodies of different antigenic specificities. Major aging-associated changes are listed below the corresponding subsets. FO, follicular B cell; HSC, hematopoietic stem cell; IMM, immature; MZ, marginal zone; PRE, pre-B cell stage; PRO, pro-B cell stage; TR, transitional B cell.
B-cell production wanes with age
In adults, B cells are generated continuously from bone marrow (BM) hematopoietic stem cells (HSCs) (Box 1). Descriptive studies have revealed substantial changes in the functional potential and sizes of developing B-cell subsets with age. For example, the frequency of precursors capable of generating B cells in vitro is reduced [13,15], and the pre-B and immature (IMM) BM pools are smaller [16]. These findings prompted the question of whether such changes reflect upstream shifts in B-lineage commitment; cell-intrinsic changes in mediators of key differentiation steps or deterioration of microenvironmental cues required for successful differentiation. Further, they suggested that B-cell production might wane with age, resulting in diminished BM output and altered turnover properties in mature B-cell subsets. Advances in the resolution of early B-lineage progenitors, insights into the genetic events required for B-lineage specification and the advent of tools to assess the dynamics of developing populations have allowed interrogation of these possibilities.
Box 1. Bone marrow B-cell development
Specification and commitment to the B-cell lineage involves key transcription factor networks [62], which in concert yield early B-cell progenitors. Lineage commitment is followed by recombination activating gene (RAG)-mediated IgH (heavy chain) gene rearrangement in the pro-B-cell stage. On successful IgH rearrangement, the Ig heavy chain is expressed on the cell surface associated with surrogate light chain (lambda-5/Vpre-B) and the Ig-α and Ig-β signaling complex. This initiates the pre-B-cell stage where, after brief proliferation, successful light chain rearrangement allows surface expression of a complete B-cell receptor, marking entrance to the immature marrow B-cell stage. At each stage, marrow stromal elements and products, such as interleukin 7, play key roles in sustaining differentiation.
There is increasing evidence that the differentiative potential of HSCs changes with age [17–20]. HSCs from aged mice show numerous changes in gene expression, resulting from an apparent breakdown of epigenetic regulation [21]. Other cell-intrinsic changes include increased HSC self-renewal and diminished lymphoid potential [18,20]. This is accompanied by downregulation of genes that mediate lymphoid specification and function – and enhanced expression of genes specifying myeloid development [18]. Together, these findings suggest epigenetic changes in HSCs that occur in aged individuals might impact all subsequent downstream subsets and differentiative events. Consistent with this, recent studies show that early B-cell progenitors (EBPs) are reduced with age [22]. Also in accord with this idea, the expression of transcriptional regulators essential to generating pro-B cells, including E2A gene products such as E47, are reduced [23–25]. Similarly, the expression of genes crucial to passage through the pro- and pre-B cell stages, including RAG (recombination activating gene) enzymes and lambda-5, is diminished in developing B cells from aged individuals [26–28]. Recent studies using a RAG reporter system coupled with flow cytometry demonstrated such reductions at the single cell level [29], strengthening the notion that intrinsic epigenetic changes in HSCs and developing B-cell subsets play a role in shifting the dynamics and quality of BM B-cell output. All of these findings suggest that both B-lineage commitment and transit through early developmental stages are compromised with advancing age, implying that BM B-cell output should fall. Indeed, in vivo labeling has confirmed that production rates in the pro-, pre- and immature BM B-cell pools are diminished with age [29–31], reflecting decreased ability to successfully complete each differentiation stage and transit to the next.
Determining the relative contributions of cell intrinsic versus microenvironmental changes has proven complex, because both mechanisms are involved. For example, BM stromal cells from aged mice provide less interleukin 7 (IL-7) in vitro, but B-cell progenitors from aged mice also respond less efficiently to IL-7 [32]. Similarly, reciprocal BM chimeras indicate that aged BM HSCs recapitulate young adult B-cell production kinetics when transferred in large numbers to young adults [29]; however, limiting the numbers of purified HSCs reveals cell-intrinsic reductions in B lineage–specified progenitors under the same conditions [22].
Together, these findings suggest interplay between intrinsic and microenvironmental factors, whereby the levels of gene products vital to lineage commitment and subsequent differentiation are altered. It is tempting to speculate that the overall B-cell aging phenotype derives from a ‘snowball’ effect, whereby failures in crucial upstream events – beginning with epigenetic changes in HSCs – yield consecutive, escalating downstream consequences. Accordingly, determining how levels of key gene products are controlled and how decreased BM output impacts the selection and homeostasis of pre-immune B-cell pools is a key next step.
An exciting possibility is that post-transcriptional mechanisms involved in mRNA and protein turnover regulate molecules involved in B-lineage development and function. In vitro studies indicate that the reduced E2A protein levels seen in B-cell precursors from aged hosts reflect increased protein turnover [33,34]. Under normal conditions, the turnover of E2A proteins is regulated by mitogen activated protein kinase (MAPK) activity and requires Notch [35]. B-cell precursors in aged hosts show increased extracellular signal-regulated kinase (ERK) MAPK activity, coinciding with heightened phosphorylation, ubiquitination and accelerated turnover of E2A (E47) proteins [34]. In contrast to E2A dysregulation in B-cell precursors from aged hosts, mature splenic B cells from aged mice and humans display reduced E2A due to increased mRNA degradation [9,36]. Therefore, expression of E2A, and possibly other key regulatory molecules, is compromised in aged B-lineage cells, but the mechanisms probably differ depending on their developmental or activation stage. Although decreased E2A levels will directly compromise lineage specification and developmental progression, the diminished expression of E2A regulated genes (e.g. RAGs or surrogate light chain) will further impair or alter B-cell repertoire establishment and selection, particularly at the pre-B-cell stage [37].
It is important to note that multiple pathways exist for B-cell generation in the adult BM, including the predominant B2 cell pathway, as well as a second pathway devoted to the B1 cell lineage [38]. B1 cells predominate in peritoneal and pleural cavities and represent a self-renewing pool that is established early in life. In addition, B1 cells express a specificity repertoire different from B2s that is generally characterized by polyspecificity and low-affinity self-reactivity [39]. B1 B cells are largely responsible for so-called natural antibodies and are skewed toward responses to parasite and bacterial antigens. The relative contributions of these two developmental pathways vary with age: in prenatal and neonatal life, the B1 pathway predominates, whereas in young adult life, the B2 pathway is dominant. However, as B2 production wanes with age, the proportional contribution of the B1 pathway again increases [40,41]. The exact consequences of this reversion in the proportional representation of B1 versus B2 output remain unclear but might contribute to some repertoire differences observed with advancing age.
Homeostatic relationships shift with age
Clearly, reduced BM output will impact the dynamics of mature, pre-immune B-cell pools. Moreover, studies in mice reveal that follicular (FO) B-cell numbers remain relatively constant with age, implying that they must turn over more slowly in the aged than in young mice [29,31]. In vivo labeling studies confirm this prediction, indicating two- to fivefold reductions in mature B-cell turnover in aged individuals [29–31]. Recent progress in our understanding of how primary B-cell selection and homeostasis are coupled (Box 2) provokes questions about how the interplay of decreased BM output, extended primary B-cell lifespan and life-long B-cell receptor (BCR)-driven selection yields the overall aging phenotype.
Box 2. Primary B-cell selection and homeostasis
After expression of a complete B-cell receptor (BCR), stringent specificity-based selection occurs at the immature (IMM) stage and again after exit from the bone marrow (BM) at the transitional (TR) stages. Cells that survive these events join the mature follicular (FO) and marginal zone (MZ) subsets, which constitute the major preimmune B-cell pools. Selective elimination at the IMM stage results from high avidity BCR engagement, whereas cells undergoing sustained, intermediate affinity BCR interactions persist to the TR stages but die before maturation. The notion of positive selection among B cells was more recently appreciated [63,64] and is characterized by a requisite for persistent low-level BCR signaling resulting in survival-promoting signals. Thus, primary B cells compete with one another in the periphery, yielding a fitness hierarchy based on BCR specificity. Freitas and colleagues [65–67] first proposed that interclonal competition underlies homeostatic control of primary B-cell pools, and this concept gained momentum from the discovery that B Lymphocyte Stimulator (BLyS, also termed BAFF) [68,69] is the limiting survival resource for which primary B cells compete [70]. Survival signals are delivered via BLyS receptor 3 (BR3, also termed BAFF-R) [71–73], which regulates crucial survival pathways. Moreover, cross-talk between BCR- and BR3-mediated signaling affords a ‘fulcrum’ mechanism for tipping the balance away from negative selection as B cells mature in the periphery. Finally, because these survival requisites and relationships are imposed at the TR stages, the stringency of BCR-mediated selection is plastic and can be adjusted based on marrow output and the size of the peripheral pool.
Decreased BM output might allow cells bearing auto-reactive specificities to enter the primary repertoire, given the propensity for relaxed selective stringency when interclonal competition is minimal. Indeed, in most inbred mouse strains, the spontaneous appearance of autoreactive serum antibodies and an increased incidence of humoral autoimmunity are associated with age. Alternatively, selection at the transitional (TR) differentiation stages might remain intact, or actually be more stringent, inasmuch as reduced BM output is superimposed on a mature pool of normal size that is maintained through extended lifespan. For example, the life-long selection of clonotypes with optimal tonic BCR signaling might underlie the longer average lifespan for primary B cells seen in aged individuals, thus imposing an increasingly competitive environment for newly formed cells. In addition to changes in the dynamics and composition of FO B cells, the relative representation of mature preimmune B-cell subsets can shift as well. Evidence from mouse models indicates that B1 cells might accumulate or expand with age as a result of chronic stimulation by environmental antigens [42]. Moreover, in some mouse strains (e.g. C57BL/6), the marginal zone (MZ) pool also enlarges with age [42], whereas in others (e.g. BALB/c), it decreases [43]. The basis for these fluctuations is unclear, but because MZ B cells display repertoire skewing similar to B1 cells, this might also contribute to the appearance of polyreactive and autoreactive antibodies in some cases.
BCR diversity truncates with age
Regardless of the exact manner in which homeostatic and selective parameters interact with advancing age, all potential outcomes predict that the clonotypic composition of primary B-cell pools will change as the aging phenotype emerges. Early findings in several mouse model systems suggested changes in the pre-immune B-cell repertoire with age [10,11,14,44]. The advent of technologies that allow more global repertoire assessments (e.g. spectratyping) have yielded additional insights, particularly in humans. Previous studies, concentrating on immunoglobulin heavy chain variable region (IGHV) gene use, have not always yielded significant age-related differences in the pre-immune B-cell repertoire [45–47]. Some studies have been limited in scope, either by sample size or breadth of the IGHV gene families analyzed. However, CDR3 spectratype analysis, which can interrogate large numbers of IGHV gene sequences over the entire repertoire, has demonstrated a significant loss of diversity in the peripheral blood of some older subjects [48]. Importantly, a loss of B-cell diversity revealed by this approach is correlated with poor health and survival [48]. It is unclear where repertoire skewing occurs. For example, age-related skewing of the naïve repertoire might result from intrinsic repertoire differences generated from ‘aged’ B-cell progenitors. Evidence for this possibility has recently come from HSC transfers in an Ig transgenic mouse model [20]. In addition, some repertoire skewing might reflect the combination of decreased marrow output and homeostatic alterations discussed above, which could in turn alter the ability to respond effectively to antigen challenge. Finally, some skewing might reflect expanded clones of memory cells that dominate the spectratype. Accordingly, similar analyses with specific B-cell subsets should reveal the relative contributions of each pool.
Repertoire skewing, regardless of source, would be expected to impact the nature and extent of humoral responses to pathogens. Indeed, many antibody responses can be characterized by patterns of IGHV gene use, and age-related changes have been described. For example, differences between IGHV gene use in young versus elderly patients have been observed in pneumococcal polysaccharide responses [49,50]. Further, experiments using young and old donors to reconstitute immune-deficient SCID mice suggest these differences arise from B-cell intrinsic factors, although the cytokine environment might also play a role [51]. Although causality remains difficult to establish, pneumococcal responses in the aged are severely compromised [51], and age brings a decrease in specific antibody production and a concomitant increase in cross-reactive and autoreactive antibodies after antigen challenge [52,53]. Accordingly, repertoire changes, in concert with other intrinsic and extrinsic alterations, might underlie functional failures in B-cell activation and the appropriate progression of humoral responses.
Key activation processes change with age
Age-related changes in the behavior of antigen-experienced B cells (Box 3) are generally evidenced by diminished responses to infection or vaccination, as well as declines in existing humoral immunity (also see the article by Chen et al in this special issue). The picture is complex, because it clearly involves changes intrinsic to B cells and the accessory cells that must act in concert to generate immune memory.
Box 3. Antigen-experienced B-cell subsets
Antigen-experienced B-cell subsets include recently activated B cells, as well as memory B cells and antibody-forming plasma cells. Humoral immune responses are either T independent (TI) or T dependent (TD), based on their requirement for cognate T-cell help. TI responses generate short-lived IgM-secreting plasma cells, lack substantial affinity maturation and yield little humoral memory. By contrast, TD responses produce germinal centers (GCs), where activated B cells undergo class switch (CSR) and somatic hypermutation (SHM), culminating in cells producing the high-affinity class-switched antibodies crucial to effective long-term humoral immunity. These processes require chromatin opening and cytokine-induced germline transcription at switch recombination (S) regions, as well as the activity of activation-induced cytidine deaminase (AID) [74]. This enzyme, whose expression is regulated by E47 [75], initiates CSR by converting S region cytidines to uracils via deamination, prompting the recognition and excision of base pair mismatches and yielding DNA double-strand breaks [74,76]. Mutations in AID yield hyper-IgM syndrome, which yields increased susceptibility to infections [77,78] and secondary antibody responses lacking characteristic class-switched, hypermutated antibodies.
Studies in humans have often focused on antigens that engender T-independent (TI) responses, reflecting interest in generating vaccines for bacterial infections in the elderly, whereas T-dependent (TD) responses have been studied more extensively in the mouse. It is well established that germinal center (GC) formation and kinetics are impaired in aged mice during both primary and secondary responses (reviewed in Ref [54]). Antibody affinity maturation, memory B-cell differentiation, and long-lived plasma cells in BM are correspondingly reduced [55,56]. Interestingly, the mucosal system of aged mice might be functionally intact, because GCs in Peyer’s patches of aged mice are similar in phenotype and occurrence to those of young mice [57], but detailed study of these pools in aged individuals is only now emerging (also see the article by Fujihashi and Kiyono in this issue).
This general breakdown in the ability to mount robust humoral responses probably reflects the combined results of myriad aging-related defects. For example, cell interactions required for effective antigen-driven B-cell activation are altered with age. Thus, the capacity for cognate T-cell help is decreased, possibly reflecting reduced CD154 (CD40 ligand) expression, and might contribute to the diminished efficacy of humoral responses [58]. In addition, follicular dendritic cells (FDCs) from aged mice are less effective at antigen trapping and dispersal, and this correlates with fewer GCs and smaller GC size. The underlying mechanism in part reflects downregulated Fc receptors, yielding reduced immune complex trapping and hence less BCR cross-linking [59]. As would be predicted by this mechanistic model, immunization with immune complexes significantly improved the GC response in aged mice [55].
The reduced efficacy of primary responses also reflects intrinsic class switch recombination (CSR) defects [9]. B cells from aged mice are deficient in CSR and secondary Ig production when stimulated in vitro [60], due to decreased induction of E47 and activation-induced cytidine deaminase (AID). In contrast to developing B cells, stability assays in activated B cells indicate E47 mRNA decay is accelerated in old B cells, whereas E47 protein degradation rates are comparable to those in young B cells. Thus, E47 expression in activated splenic B cells is primarily based on mRNA stability. This is mediated by TTP (tristetraprolin), which is more highly expressed in B cells from older mice [36]. A model for this is given in Figure 2.
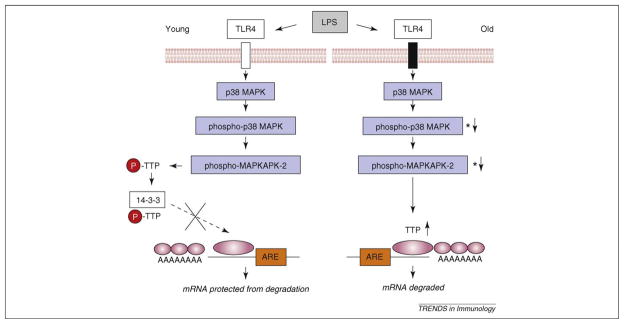
Figure 2.
Model for differential activation of E47 mRNA turnover in B cells in young versus old mice. In response to lipopolysaccharide and other stimuli, phospho-p38 mitogen activated protein kinase is lower in B cells from aged compared with those from young adults. This results in a decrease in phospho-tristetraprolin (TTP) that, together with increased TTP levels in old B cells, yields greater TTP binding to 3′ untranslated regions with AU-rich elements, as in E47 mRNA, resulting in increased degradation rates. See Frasca et al. [36,61]. LPS, lipopolysaccharide; MAPK, mitogen activated protein kinase; TTP, tristetraprolin. The large pink oval refers to the AU-rich sequences in the 3′UTR and the small pink ovals refer to the poly-A tail.
Overview and perspective
Conceptual and technical advances have permitted mechanistic studies aimed at understanding previously reported age-associated changes in B-cell populations and function. A common emerging feature is altered levels of crucial gene products involved in the genesis, survival and activation of B cells. These changes, coupled with the interplay of microenvironmental and homeostatic processes, generate the overall aging phenotype. Future studies will focus on the detailed interrogation of epigenetic and regulatory systems governing these gene products at the cellular level, as well as the downstream consequences and interactions in developing, mature and antigen-experienced populations.
References
- 1.Ben-Yehuda A, Weksler ME. Immune senescence: mechanisms and clinical implications. Cancer Invest. 1992;10:525–531. doi: 10.3109/07357909209024815. [DOI] [PubMed] [Google Scholar]
- 2.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi C, et al. Immunosenescence. Aging (Milano) 1998;10:153–154. [PubMed] [Google Scholar]
- 4.Malaguarnera L, et al. Immunosenescence: a review. Arch Gerontol Geriatr. 2001;32:1–14. doi: 10.1016/s0167-4943(00)00086-8. [DOI] [PubMed] [Google Scholar]
- 5.Gruver AL, et al. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kay MM, et al. Age-related changes in the immune system of mice of eight medium and long-lived strains and hybrids. II. Short- and long-term effects of natural infection with parainfluenza type 1 virus (Sendai) Mech Ageing Dev. 1979;11:347–362. doi: 10.1016/0047-6374(79)90010-1. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Yehuda A, Weksler ME. Host resistance and the immune system. Clin Geriatr Med. 1992;8:701–711. [PubMed] [Google Scholar]
- 8.Ginaldi L, et al. Immunosenescence and infectious diseases. Microbes Infect. 2001;3:851–857. doi: 10.1016/s1286-4579(01)01443-5. [DOI] [PubMed] [Google Scholar]
- 9.Frasca D, et al. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol. 2008;180:2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 10.Klinman NR, et al. The specificity repertoire of prereceptor and mature B cells. Ann N Y Acad Sci. 1983;418:130–139. doi: 10.1111/j.1749-6632.1983.tb18061.x. [DOI] [PubMed] [Google Scholar]
- 11.Riley SC, et al. Altered VH gene segment utilization in the response to phosphorylcholine by aged mice. J Immunol. 1989;143:3798–3805. [PubMed] [Google Scholar]
- 12.Nicoletti C, et al. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. [PubMed] [Google Scholar]
- 13.Riley RL, et al. B cell precursors are decreased in senescent BALB/c mice, but retain normal mitotic activity in vivo and in vitro. Clin Immunol Immunopathol. 1991;59:301–313. doi: 10.1016/0090-1229(91)90026-7. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti C, et al. Repertoire diversity of antibody response to bacterial antigens in aged mice. III. Phosphorylcholine antibody from young and aged mice differ in structure and protective activity against infection with Streptococcus pneumoniae. J Immunol. 1993;150:543–549. [PubMed] [Google Scholar]
- 15.Sherwood EM, et al. B cell precursors in senescent mice exhibit decreased recruitment into proliferative compartments and altered expression of Bcl-2 family members. Mech Ageing Dev. 2003;124:147–153. doi: 10.1016/s0047-6374(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 16.Stephan RP, et al. Stage-specific alterations in murine B lymphopoiesis with age. Int Immunol. 1996;8:509–518. doi: 10.1093/intimm/8.4.509. [DOI] [PubMed] [Google Scholar]
- 17.Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2009;130:46–53. doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi DJ, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A. 2005;102:9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Guerrettaz LM, et al. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci U S A. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers SM, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 23.Frasca D, et al. The age-related decrease in E47 DNA-binding does not depend on increased Id inhibitory proteins in bone marrow-derived B cell precursors. Front Biosci. 2003;8:a110–a116. doi: 10.2741/1059. [DOI] [PubMed] [Google Scholar]
- 24.Frasca D, et al. Age-related differences in the E2A-encoded transcription factor E47 in bone marrow-derived B cell precursors and in splenic B cells. Exp Gerontol. 2004;39:481–489. doi: 10.1016/j.exger.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Riley RL, et al. B cells, E2A, and aging. Immunol Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 26.Weksler ME. Immune senescence: deficiency or dysregulation. Nutr Rev. 1995;53:S3–S7. doi: 10.1111/j.1753-4887.1995.tb01513.x. [DOI] [PubMed] [Google Scholar]
- 27.Sherwood EM, et al. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. J Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- 28.Sherwood EM, et al. The reduced expression of surrogate light chains in B cell precursors from senescent BALB/c mice is associated with decreased E2A proteins. Mech Ageing Dev. 2000;118:45–59. doi: 10.1016/s0047-6374(00)00157-3. [DOI] [PubMed] [Google Scholar]
- 29.Labrie JE, 3rd, et al. Bone marrow microenvironmental changes underlie reduced RAG-mediated recombination and B cell generation in aged mice. J Exp Med. 2004;200:411–423. doi: 10.1084/jem.20040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kline GH, et al. B cell maintenance in aged mice reflects both increased B cell longevity and decreased B cell generation. J Immunol. 1999;162:3342–3349. [PubMed] [Google Scholar]
- 31.Johnson KM, et al. Aging and developmental transitions in the B cell lineage. Int Immunol. 2002;14:1313–1323. doi: 10.1093/intimm/dxf092. [DOI] [PubMed] [Google Scholar]
- 32.Stephan RP, et al. Development of B cells in aged mice: decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 33.Van der Put E, et al. Decreased E47 in senescent B cell precursors is stage specific and regulated posttranslationally by protein turnover. J Immunol. 2004;173:818–827. doi: 10.4049/jimmunol.173.2.818. [DOI] [PubMed] [Google Scholar]
- 34.King AM, et al. Accelerated Notch-dependent degradation of E47 proteins in aged B cell precursors is associated with increased ERK MAPK activation. J Immunol. 2007;178:3521–3529. doi: 10.4049/jimmunol.178.6.3521. [DOI] [PubMed] [Google Scholar]
- 35.Nie L, et al. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frasca D, et al. Tristetraprolin, a negative regulator of mRNA stability, is increased in old B cells and is involved in the degradation of E47 mRNA. J Immunol. 2007;179:918–927. doi: 10.4049/jimmunol.179.2.918. [DOI] [PubMed] [Google Scholar]
- 37.Alter-Wolf S, et al. Deviation of the B cell pathway in senescent mice is associated with reduced surrogate light chain expression and altered immature B cell generation, phenotype, and light chain expression. J Immunol. 2009;182:138–147. doi: 10.4049/jimmunol.182.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montecino-Rodriguez E, et al. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 39.Chumley MJ, et al. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol. 2002;169:1735–1743. doi: 10.4049/jimmunol.169.4.1735. [DOI] [PubMed] [Google Scholar]
- 40.Dorshkind K, et al. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9:57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 41.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 42.Johnson SA, et al. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002;168:5014–5023. doi: 10.4049/jimmunol.168.10.5014. [DOI] [PubMed] [Google Scholar]
- 43.Frasca D, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 44.Zharhary D, Klinman NR. The frequency and fine specificity of B cells responsive to (4-hydroxy-3-nitrophenyl)acetyl in aged mice. Cell Immunol. 1986;100:452–461. doi: 10.1016/0008-8749(86)90044-4. [DOI] [PubMed] [Google Scholar]
- 45.Kolar GR, et al. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand J Immunol. 2006;64:314–324. doi: 10.1111/j.1365-3083.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- 46.van Dijk-Hard I, et al. Age-related impaired affinity maturation and differential D-JH gene usage in human VH6-expressing B lymphocytes from healthy individuals. Eur J Immunol. 1997;27:1381–1386. doi: 10.1002/eji.1830270613. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Stollar BD. Immunoglobulin VH gene expression in human aging. Clin Immunol. 1999;93:132–142. doi: 10.1006/clim.1999.4781. [DOI] [PubMed] [Google Scholar]
- 48.Gibson KL, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8:18–25. doi: 10.1111/j.1474-9726.2008.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolibab K, et al. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun. 2005;73:7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smithson SL, et al. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable light chain repertoire. Infect Immun. 2005;73:7477–7484. doi: 10.1128/IAI.73.11.7477-7484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shriner AK, et al. Analysis of the young and elderly variable gene repertoire in response to pneumococcal polysaccharides using a reconstituted SCID mouse model. Vaccine. 2006;24:7159–7166. doi: 10.1016/j.vaccine.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 52.Huang YP, et al. The relationship between influenza vaccine-induced specific antibody responses and vaccine-induced nonspecific autoantibody responses in healthy older women. J Gerontol. 1992;47:M50–M55. doi: 10.1093/geronj/47.2.m50. [DOI] [PubMed] [Google Scholar]
- 53.Borghesi C, Nicoletti C. Increase of cross(auto)-reactive antibodies after immunization in aged mice: a cellular and molecular study. Int J Exp Pathol. 1994;75:123–130. [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng B, et al. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- 55.Zheng B, et al. Correction of age-associated deficiency in germinal center response by immunization with immune complexes. Clin Immunol. 2007;124:131–137. doi: 10.1016/j.clim.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 56.Han S, et al. Rectification of age-related impairment in Ig gene hypermutation during a memory response. Int Immunol. 2004;16:525–532. doi: 10.1093/intimm/dxh054. [DOI] [PubMed] [Google Scholar]
- 57.Rogerson BJ, et al. Germinal center B cells in Peyer’s patches of aged mice exhibit a normal activation phenotype and highly mutated IgM genes. Mech Ageing Dev. 2003;124:155–165. doi: 10.1016/s0047-6374(02)00115-x. [DOI] [PubMed] [Google Scholar]
- 58.Eaton SM, et al. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aydar Y, et al. Altered regulation of Fc gamma RII on aged follicular dendritic cells correlates with immunoreceptor tyrosine-based inhibition motif signaling in B cells and reduced germinal center formation. J Immunol. 2003;171:5975–5987. doi: 10.4049/jimmunol.171.11.5975. [DOI] [PubMed] [Google Scholar]
- 60.Frasca D, et al. Reduced Ig class switch in aged mice correlates with decreased E47 and activation-induced cytidine deaminase. J Immunol. 2004;172:2155–2162. doi: 10.4049/jimmunol.172.4.2155. [DOI] [PubMed] [Google Scholar]
- 61.Frasca D, et al. RNA stability of the E2A-encoded transcription factor E47 is lower in splenic activated B cells from aged mice. J Immunol. 2005;175:6633–6644. doi: 10.4049/jimmunol.175.10.6633. [DOI] [PubMed] [Google Scholar]
- 62.Singh H, Pongubala JM. Gene regulatory networks and the determination of lymphoid cell fates. Curr Opin Immunol. 2006;18:116–120. doi: 10.1016/j.coi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Gu H, et al. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lam KP, et al. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 65.Freitas AA, et al. The role of cellular competition in B cell survival and selection of B cell repertoires. Eur J Immunol. 1995;25:1729–1738. doi: 10.1002/eji.1830250636. [DOI] [PubMed] [Google Scholar]
- 66.Rosado MM, Freitas AA. The role of the B cell receptor V region in peripheral B cell survival. Eur J Immunol. 1998;28:2685–2693. doi: 10.1002/(SICI)1521-4141(199809)28:09<2685::AID-IMMU2685>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 67.De Boer RJ, et al. Resource competition determines selection of B cell repertoires. J Theor Biol. 2001;212:333–343. doi: 10.1006/jtbi.2001.2379. [DOI] [PubMed] [Google Scholar]
- 68.Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 69.Schneider P, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harless SM, et al. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 71.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 72.Schneider P, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–1697. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yan M, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 74.Nussenzweig MC, Alt FW. Antibody diversity: one enzyme to rule them all. Nat Med. 2004;10:1304–1305. doi: 10.1038/nm1204-1304. [DOI] [PubMed] [Google Scholar]
- 75.Sayegh CE, et al. E-proteins directly regulate expression of activation-induced deaminase in mature B cells. Nat Immunol. 2003;4:586–593. doi: 10.1038/ni923. [DOI] [PubMed] [Google Scholar]
- 76.Rada C, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol. 2002;12:1748–1755. doi: 10.1016/s0960-9822(02)01215-0. [DOI] [PubMed] [Google Scholar]
- 77.Durandy A. Hyper-IgM syndromes: a model for studying the regulation of class switch recombination and somatic hypermutation generation. Biochem Soc Trans. 2002;30:815–818. doi: 10.1042/bst0300815. [DOI] [PubMed] [Google Scholar]
- 78.Notarangelo LD, et al. Immunodeficiency with hyper-IgM (HIM) Immunodefic Rev. 1992;3:101–121. [PubMed] [Google Scholar]