Abstract
Objective
To investigate whether elevated C-reactive protein (CRP) levels and HIV infection are independently associated with acute myocardial infarction (AMI) among patients receiving care in a large US health care system.
Methods
Analyses included patients receiving care in the Partners HealthCare System between January 1997 and December 2006, with a most recent CRP less than 3 years and more than 1 week before AMI. Over this period, 70,357 (487 HIV and 69,870 non-HIV) patients met these criteria, from the background population of 1,648,687 patients followed in the system. We included both CRP and high-sensitivity CRP and defined elevated CRP based on the normal range of the assay used. We used multivariate logistic regression analysis to test the association of elevated CRP and HIV with AMI after adjustment for demographic and other cardiovascular covariates, including hypertension, diabetes, and dyslipidemia.
Results
In univariate analyses, elevated CRP and HIV were each significantly associated with AMI [odds ratio (OR) 2.51; 95% confidence interval (CI) 2.27 to 2.78; P < 0.0001 for elevated CRP and OR 2.07; 95% CI 1.31 to 3.10; P = 0.001 for HIV]. In a combined model including CRP category and HIV status, elevated CRP (OR 2.50; 95% CI 2.26 to 2.77; P < 0.0001) and HIV (OR 1.74; 95% CI 1.10 to 2.61; P = 0.01) were both independently associated with AMI. In a fully adjusted model controlling for age, sex, race, hypertension, diabetes, and dyslipidemia, both elevated CRP (OR 2.13; 95% CI 1.92 to 2.37; P < 0.0001) and HIV (OR 1.93; 95% CI 1.21 to 2.93; P = 0.004) remained independently associated with AMI. Compared with patients with normal CRP and without HIV, the OR for AMI was increased more than 4-fold among patients with HIV and elevated CRP.
Conclusions
Elevated CRP and HIV are independently associated with increased AMI risk, and patients with HIV with increased CRP have a markedly increased relative risk of AMI. Measurement of CRP may be useful in the cardiovascular risk assessment of patients with HIV.
Keywords: C-reactive protein, coronary disease, HIV, myocardial infarction, risk factors
Introduction
Patients with HIV demonstrate increased rates of coronary heart disease compared with patients without HIV.1–4 Although traditional cardiovascular risk factors have been shown to be common among patients with HIV,4–9 the role of inflammation and the utility of related biomarkers have not been studied. In the general population, there is strong evidence that inflammation plays a role in the development of acute myocardial infarction (AMI),10,11 and elevated levels of C-reactive protein (CRP) have been significantly associated with coronary events in a number of studies.12–14 CRP levels have been shown to be elevated in patients with HIV compared with general populations.6,15,16 However, it remains unknown whether CRP adds prognostic information with respect to cardiovascular events in the HIV population. In this preliminary study, we used a clinical care data registry to investigate whether elevated CRP and HIV are independently associated with AMI among patients followed in a large US health care system.
Methods
Data Source and Study Sample
Patient groups were identified by query of the Research Patient Data Registry, a clinical data warehouse for more than 2 million patients from Brigham and Women's Hospital and Massachusetts General Hospital, comprising Partners Health-Care System. Patients who were between the ages of 18 and 84 years at the time of database query and who had at least 1 encounter at either Brigham and Women's Hospital or Massachusetts General Hospital over a 10-year period from January 1, 1997 to December 31, 2006 were included in the initial sample of 1,648,687 patients (Fig. 1). Of these, there were 77,835 patients with CRP test data available. Patients were further censored if their most recent CRP value was after the AMI event, less than 1 week before the AMI event, or more than 3 years before the AMI event. The final analysis included 70,357 patients, of which 487 were HIV infected and 69,870 were not HIV infected. Patients with multiple encounters were counted only once, and for those with an AMI event, only the first event was considered. The study was approved by the Partners Human Research Committee.
FIGURE 1.
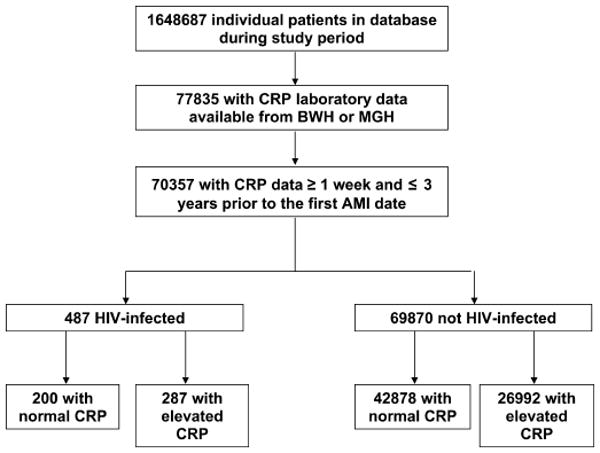
Study sample. The initial query generated 1,648,687 patients. Of these, 77,835 had CRP test data available during the study period from Brigham and Women's Hospital or Massachusetts General Hospital. After excluding patients whose most recent CRP value was after the first AMI, less than 1 week before the first AMI, or more than 3 years before the first AMI, there were 70,357 patients. Of these patients, 487 were HIV infected and 69,870 were not HIV infected. The distribution of normal vs. elevated CRP is shown in the Figure.
AMI Event Ascertainment
Patients were classified as having the primary outcome of AMI if they had at least 1 documented code of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 410.xx (AMI). ICD-based AMI diagnoses were validated with formal medical record review on a subset of patients by an experienced clinical nurse researcher. A single ICD code of 410.xx was found to have 98% sensitivity and 85% specificity for clinically defined AMI.
CRP Test Classification
The most recent CRP value for each patient before AMI or before the end of the study period was used in all analyses. All CRP assays were included. Elevated CRP was defined as a value exceeding the upper limit of the normal reference range for standard CRP assays, or the highest quintile for high-sensitivity CRP assays. A specific numerical value could not be used to define elevated CRP because reference ranges varied depending on the specific assay. Although patients in the study were included if they had a CRP test result available between 1997 and 2006, more than 90% of both patients with HIV and patients without HIV had their CRP drawn in 2002 or later.
Clinical Exposure Definitions
Patients with at least 1 recorded ICD-9-CM code of either 042 (HIV disease) or V08 (asymptomatic HIV infection status) before first AMI or end date of the study period were classified as HIV infected. ICD-based HIV disease ascertainment was found to have 100% sensitivity and 100% specificity for clinical HIV definition based on trained research nurse review of a randomly selected subset of medical records.
Standard risk factors for AMI were identified using ICD-9-CM codes 401 (essential hypertension), 250 (diabetes mellitus), and 272 (disorders of lipoid metabolism) recorded before the first AMI or before the end of the study period. The sensitivity of ICD-based diagnoses was 85% for hypertension, 96% for diabetes, and 88% for dyslipidemia based on trained research nurse medical record review. The specificity was 87% for hypertension, 93% for diabetes, and 85% for dyslipidemia.
Medical Record Review
Detailed medical record review of 200 patients with HIV and 200 patients without HIV (100 with elevated CRP and 100 with normal CRP in each group) was performed to assess variables not uniformly available by electronic data capture. Patients were classified as smokers if there was documentation of current or past smoking at any time in the medical record. The most recent HIV viral load and CD4 count within 6 months before the index CRP date (most recent CRP test) were used. Antiretroviral (ARV) medication and 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitor (statin) use were characterized by prescription of medication within a year before the index CRP date.
Statistical Analysis
χ2 P values were used to compare the prevalence of cardiovascular risk factors in patients with CRP available compared with the background population, within HIV and non-HIV groups. We used logistic regression to estimate the odds ratio (OR) and 95% confidence intervals (CIs) for the outcome of AMI in models containing CRP category (high vs. not high) and HIV status (HIV vs. non-HIV), adjusting for demographic factors and other cardiovascular risk factors, to test the hypothesis that CRP and HIV are significant independent risk factors for AMI. In secondary sensitivity analyses, regression models were repeated varying the maximum allowed length of time between most recent CRP test date and first AMI date. Statistical analysis was conducted using JMP statistical software version 5.0.1 (1989–2003; SAS Institute, Cary, NC) and a P value <0.05 was considered to indicate statistical significance.
Results
Patient Characteristics According to CRP Testing Status
Table 1 shows demographic and clinical characteristics for patients with HIV and patients without HIV comparing those with CRP data available to the background population during the study period. Among patients with HIV, sex, age, and race patterns were similar for patients with CRP available compared with the background population. Demographic patterns were also comparable among the non-HIV group comparing patients with CRP available to the background population. Rates of cardiovascular risk factors were significantly higher in patients with CRP available compared with background patients for both patients with HIV and patients without HIV (P < 0.01 for all comparisons).
TABLE 1.
Characteristics of Patients by CRP Test Availability*
| HIV | Non-HIV | |||
|---|---|---|---|---|
| Characteristic | Background (n = 7099) | CRP Available (n = 487) | Background (n = 1,641,588) | CRP Available (n = 69,870) |
| Sex | ||||
| Female | 2351 (33.1) | 181 (37.2) | 937,748 (57.1) | 38,019 (54.4) |
| Age (yrs) | ||||
| 18–34 | 978 (13.8) | 42 (8.6) | 409,637 (25.0) | 6773 (9.7) |
| 35–44 | 2303 (32.4) | 145 (29.8) | 347,291 (21.2) | 10,230 (14.6) |
| 45–54 | 2472 (34.8) | 180 (37.0) | 305,919 (18.6) | 15,399 (22.0) |
| 55–64 | 997 (14.0) | 74 (15.2) | 260,344 (15.9) | 17,688 (25.3) |
| 65–74 | 252 (3.5) | 32 (6.6) | 175,498 (10.7) | 12,153 (17.4) |
| 75–84 | 99 (1.4) | 14 (2.9) | 142,896 (8.7) | 7627 (10.9) |
| Race | ||||
| White | 3522 (49.6) | 283 (58.1) | 1,039,761 (63.3) | 53,919 (77.2) |
| Nonwhite† | 3020 (42.5) | 184 (37.8) | 306,951 (18.7) | 9249 (13.2) |
| Not recorded | 558 (7.9) | 20 (4.1) | 294,667 (18.0) | 6702 (9.6) |
| Cardiac risk factor | ||||
| Hypertension | 1689 (23.8) | 183 (37.6) | 257,257 (15.7) | 32,848 (47.0) |
| Diabetes | 1140 (16.1) | 105 (21.6) | 108,765 (6.6) | 11,662 (16.7) |
| Dyslipidemia | 1893 (26.7) | 184 (37.8) | 244,175 (14.9) | 36,701 (52.5) |
Data are n (%) of patients, unless otherwise indicated.
Nonwhite includes all races other than white for whom race data were recorded.
The median time from most recent CRP to first AMI was not significantly different for patients with HIV vs. patients without HIV (199 vs. 176 days; P = 0.5). The mean duration of observation from first to last encounter was 6.0 years for patients with HIV and 5.8 years for patients without HIV (P = 0.25).
Rates of Elevated CRP
Among patients with CRP data available, the most recent CRP was classified as high in 287 patients with HIV (59%) and 26,992 patients without HIV (39%; P < 0.0001).
Association of Elevated CRP and HIV With AMI
We evaluated the association between elevated CRP, HIV, and AMI in sequential regression models to examine the effect of each risk factor on AMI individually and in combination (Table 2). Elevated CRP and HIV were individually associated with AMI in univariate analyses (OR 2.51; 95% CI 2.27 to 2.78; P < 0.0001 for elevated CRP and OR 2.07; 95% CI 1.31 to 3.10; P = 0.001 for HIV). When CRP category (high vs. not high) and HIV status (HIV vs. non-HIV) were included in the same model, both remained significantly associated with AMI (OR 2.50; 95% CI 2.26 to 2.77; P < 0.0001 for elevated CRP and OR 1.74; 95% CI 1.10 to 2.61; P = 0.01 for HIV).
TABLE 2.
Adjusted ORs for AMI in Multivariate Regression Modeling*
| Recent CRP High vs. Not | HIV vs. Non-HIV | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Model 1 (CRP) | 2.51 (2.27 to 2.78) | <0.0001 | ||
| Model 2 (HIV) | 2.07 (1.31 to 3.10) | 0.0009 | ||
| Model 3 (CRP, HIV) | 2.50 (2.26 to 2.77) | <0.0001 | 1.74 (1.10 to 2.61) | 0.0122 |
| Model 4 (CRP, HIV, age) | 2.31 (2.08 to 2.56) | <0.0001 | 2.29 (1.44 to 3.45) | 0.0002 |
| Model 5 (CRP, HIV, age, sex) | 2.34 (2.11 to 2.59) | <0.0001 | 2.15 (1.35 to 3.25) | 0.0006 |
| Model 6 (CRP, HIV, age, sex, race) | 2.27 (2.05 to 2.52) | <0.0001 | 1.86 (1.16 to 2.81) | 0.0056 |
| Model 7 (CRP, HIV, age, sex, race, hypertension) | 2.23 (2.01 to 2.47) | <0.0001 | 1.97 (1.23 to 2.99) | 0.0025 |
| Model 8 (CRP, HIV, age, sex, race, diabetes) | 2.14 (1.93 to 2.38) | <0.0001 | 1.83 (1.15 to 2.77) | 0.0068 |
| Model 9 (CRP, HIV, age, sex, race, dyslipidemia) | 2.43 (2.19 to 2.70) | <0.0001 | 1.91 (1.20 to 2.89) | 0.0037 |
| Model 10 (CRP, HIV, age, sex, race, hypertension, diabetes, dyslipidemia) | 2.13 (1.92 to 2.37) | <0.0001 | 1.93 (1.21 to 2.93) | 0.0035 |
Adjusted ORs and associated CIs and P-values are from logistic regression analysis; P value <0.05 was considered significant. Covariates included in the model are listed after the model number in parentheses.
In a model controlling for age, sex, race, hypertension, diabetes, dyslipidemia, CRP category, and HIV status as covariates, both elevated CRP (OR 2.13; 95% CI 1.92 to 2.37; P < 0.0001) and HIV (OR 1.93; 95% CI 1.21 to 2.93; P = 0.004) remained independently associated with AMI. Having both elevated CRP and HIV increased the odds of an AMI by 4.11 compared with having neither risk factor present in the fully adjusted model. We tested for an interaction and found no evidence that there was a statistically significant interaction between CRP and HIV (P = 0.12).
Analysis of Additional Cardiovascular Risk Factors and HIV Disease Markers by Medical Record Review
We conducted a detailed medical record review of 400 patients to assess variables not uniformly available by electronic data capture, including smoking status, family history of cardiovascular disease, HIV viral load, absolute CD4 count, ARV medication class, statin use, and reason for CRP testing. Smoking rates were higher in patients with HIV compared with patients without HIV (57% of patients with HIV vs. 35% of patients without HIV; P < 0.0001). However, there was no significant difference in smoking rates in patients with elevated CRP compared with normal CRP, within either patients with HIV (smoking rate 57% in patients with elevated CRP vs. 57% in patients with normal CRP; P = 0.92) or patients without HIV (smoking rate 38% in patients with elevated CRP vs. 32% in patients with normal CRP; P = 0.44). Rates of family history of cardiovascular disease were similar for the 200 patients with HIV compared with the 200 patients without HIV assessed. In patients with HIV compared with patients without HIV, 34% compared with 38% had a family history of cardiovascular disease (P = 0.55). There was no significant association between statin use and elevated CRP, among either the HIV or the non-HIV groups (8% on statins for elevated CRP vs. 11% for normal CRP; P = 0.54 among HIV; 19% on statins for elevated CRP vs. 14% for normal CRP; P = 0.29 among non-HIV).
There was no significant association between CRP test status (elevated vs. normal) and HIV viral load (34% with undetectable viral load in patients with elevated CRP vs. 38% in patients with normal CRP; P = 0.67), CD4 count (43% with absolute CD4 count less than 200 in patients with elevated CRP vs. 28% in patients with normal CRP; P = 0.14), nucleoside reverse transcriptase inhibitor use (94% in patients with elevated CRP vs. 87% in patients with normal CRP; P = 0.32), or nonnucleoside reverse transcriptase inhibitor use (42% in patients with elevated CRP vs. 43% in patients with normal CRP; P = 0.89). There was an association between CRP test status and protease inhibitor use (67% in patients with elevated CRP vs. 39% in patients with normal CRP; P = 0.04). The lack of association between CRP and CD4 count persisted after stratification for undetectable (P = 0.11) and detectable (P = 0.21) HIV viral load.
Among the HIV records reviewed in detail, 76% were drawn during hospital admission or outpatient work-up of active problem (35% during an inpatient noncardiovascular admission, 2% during an inpatient cardiovascular admission, and 38% during an outpatient evaluation of a clinical noncardiovascular problem, primarily work-up of infectious or autoimmune problems). In addition, 8% of CRP tests were obtained coincident with outpatient visits for which there was no apparent clinical problem prompting the test. Elevated CRP was seen in 58% of patients with CRP drawn during a hospitalization or in follow-up of an active problem vs. 32% in whom it was not (P = 0.002). When excluding patients whose CRP was drawn during a cardiovascular hospital admission, these proportions were essentially unchanged (58% and 33%, respectively, P = 0.003). Time from CRP to AMI was within 2 years regardless of whether CRP was drawn during work-up for an active problem.
Secondary Sensitivity Analyses
We performed subsidiary regression analyses that varied with respect to the maximum time allowed between the most recent CRP test and the first AMI. In all analyses, patients were censored if the most recent CRP test occurred less than a week before the first AMI. In secondary analyses, patients were also censored if their most recent CRP test occurred more than 1 or 2 years before the first AMI (vs. 3 years in the primary analysis). The results of the sensitivity analyses were similar, with elevated CRP and HIV each remaining significantly associated with AMI in fully adjusted models, for both the 1- and 2-year periods (data not shown).
Discussion
In this study, we demonstrate that elevated CRP and HIV infection are independently associated with AMI among patients followed in a large US health care system. Increased CRP and HIV infection were each associated with an approximately 2-fold increased risk for AMI. The presence of both elevated CRP and HIV infection increased the odds of AMI to more than 4-fold compared with patients with neither elevated CRP nor HIV infection. These independent effects remained significant in an adjusted model accounting for demographics, hypertension, diabetes, and dyslipidemia.
This study is consistent with data from other studies demonstrating high prevalence rates of elevated CRP in patients with HIV compared with control groups.6,15,16 However, the role of CRP testing with respect to cardiovascular events has not been explored in relation to HIV infection. Patients with HIV merit aggressive cardiovascular risk assessment and modification given their increased risk for cardiovascular disease,17–19 yet it is not clear whether CRP, a biomarker useful for cardiovascular prediction in non-HIV populations,12,13 is useful in the setting of HIV infection. Specifically, it is unknown whether CRP would have independent prognostic power for the prediction of AMI beyond that of HIV status itself, which has been shown to be associated with AMI4 and might serve as a general index to identify increased inflammatory risk. Moreover, it is unknown whether CRP would remain associated with AMI after accounting for increased rates of traditional risk factors that are seen in the HIV population. We employed data from patients with HIV and patients without HIV followed in a large US health care system to show that elevated CRP is in fact associated with AMI, independent of HIV itself, and remains associated with AMI after accounting for the increased rates of traditional cardiovascular risk factors seen in the HIV population.
We used several strategies to verify data accuracy and standardize the timing of CRP test data. The outcome event and all exposures were validated to verify the accuracy of ICD-based diagnoses. Patients were excluded from the analysis if the most recent CRP test data occurred after the first AMI, within a week before the first AMI, or more than 3 years before the first AMI. Censoring patients with CRP tests after or immediately preceding the AMI event decreased the likelihood that the elevated CRP was a consequence of the event. The timing of 3 years between CRP test and AMI was selected based on previous literature that showed a mean of 3 years' follow-up in assessing the prognostic value of increased CRP in relation to AMI among patients without HIV.13 Moreover, the timing from most recent CRP test to first AMI was similar for patients with HIV and patients without HIV.
Through detailed medical record review, we were able to investigate the relationship of CRP to several important variables not uniformly available by electronic data capture. The equal distribution of smoking as stratified across CRP test status suggests that smoking is not likely to be a confounding variable in this analysis. Similarly, the lack of association between elevated CRP with HIV viral load, absolute CD4 count, or nucleoside reverse transcriptase inhibitor and nonnucleoside reverse transcriptase inhibitor medication classes suggests that these HIV-specific variables—reflective of HIV disease status and treatment category—were not likely to have contributed to the association between elevated CRP and AMI. The association observed between CRP test status and protease inhibitor use suggests that the use of this ARV medication class could contribute to the association between increased CRP and AMI within the HIV group, and further studies should address this question.
CRP testing may have occurred for the purposes of cardiovascular risk prediction or to evaluate an inflammatory or infectious process. Indeed, our detailed review of a subset of clinical records suggests that CRP was most often not drawn for cardiovascular risk stratification among the HIV group. Regardless of the reason for testing, the association between elevated CRP and AMI remained significant even after accounting for HIV infection, suggesting that elevated CRP and HIV are each independently associated with cardiovascular risk. The results of the Strategies for Management of Antiretroviral Therapy (SMART) study, which demonstrated higher cardiovascular event rates in patients whose ARV treatment was interrupted20 and increased levels of several inflammatory markers after treatment interruption,21 suggest a possible association between acute episodic inflammation and cardiovascular events. Further investigation of the association of elevated CRP and cardiovascular events in patients with HIV will help to elucidate this mechanism, but our data suggest that elevated CRP values drawn during work-up of acute noncardiovascular medical problems still lend prognostic significance for cardiovascular risk prediction.
The study has some limitations. First, we did not have an adequate number of patients to study the effects of individual drugs nor the effects of discontinuation of ARV drugs in the data set of patients with CRP available. Second, it is possible that events occurred outside the Partners HealthCare System were not captured by the database. However, the mean duration from first to last encounter of approximately 6 years for both patients with HIV and patients without HIV suggests both a long period of follow-up and similar continuity for patients with HIV and patients without HIV within the system. Thus, any loss-to-follow-up was likely similar in each study group.
Our results suggest that elevated CRP and HIV are independently associated with AMI and that having both an elevated CRP and HIV infection further increases the risk for AMI. Our data suggest that patients with HIV with increased CRP are at 4-fold increased risk for AMI compared with patients with neither risk factor. Although these data cannot establish causality, they highlight the need for further in-depth evaluation of the prognostic value of CRP for AMI in the HIV population. Developing HIV-specific cardiovascular risk assessment strategies is important for the long-term health of the HIV population.
Acknowledgments
The authors are grateful to Hang Lee for assistance with statistical analysis, Nancy Wong for assistance with data management and programming, and Jo Ann David-Kasdan for assistance with validation substudies. The authors also wish to thank Shawn Murphy and the Partners HealthCare Research Patient Data Registry group for facilitating use of their database.
Funded in part by the MGH Clinical Research Program, T32 AI07387 (V.A.T.); K01 AI073109 (V.A.T.); K24 DK080140 (J.B.M.); and K24 DK064545 (S.K.G.).
References
- 1.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Klein D, Hurley LB, Quesenberry CP, Jr, et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Klein D, Hurley L, Silverberg M, et al. Surveillance data for myocardial infarction hospitalizations among HIV+ and HIV- Northern Californians: 1994–2006. Paper presented at: 14th Conference on Retroviruses and Opportunistic Infections; February 27, 2007; Los Angeles, CA. [Google Scholar]
- 4.Triant VA, Lee H, Hadigan C, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 6.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 7.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. see comment. [DOI] [PubMed] [Google Scholar]
- 8.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 9.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–1193. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 10.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. see comment. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148(Suppl 1):S19–S26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Arinola OG, Adedapo KS, Kehinde AO, et al. Acute phase proteins, trace elements in asymptomatic human immunodeficiency virus infection in Nigerians. Afr J Med Med Sci. 2004;33:317–322. [PubMed] [Google Scholar]
- 16.Noursadeghi M, Miller RF. Clinical value of C-reactive protein measurements in HIV-positive patients. Int J STD AIDS. 2005;16:438–441. doi: 10.1258/0956462054094006. [DOI] [PubMed] [Google Scholar]
- 17.Grinspoon SK, Grunfeld C, Kotler DP, et al. State of the science conference: initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS executive summary. Circulation. 2008;118:198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsue PY, Squires K, Bolger AF, et al. Screening and assessment of coronary heart disease in HIV-infected patients. Circulation. 2008;118:e41–e47. doi: 10.1161/CIRCULATIONAHA.107.189626. [DOI] [PubMed] [Google Scholar]
- 19.Schambelan M, Wilson PW, Yarasheski KE, et al. Development of appropriate coronary heart disease risk prediction models in HIV-infected patients. Circulation. 2008;118:e48–e53. doi: 10.1161/CIRCULATIONAHA.107.189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 21.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]


