Abstract
Directional migration is an important component of cell motility. Although the basic mechanisms of random cell movement are well characterized, no single model explains the complex regulation of directional migration. Multiple factors operate at each step of cell migration to stabilize lamellipodia and maintain directional migration. Factors such as topography of the extracellular matrix, the cellular polarity machinery, receptor signalling, integrin trafficking and co-receptors, and actin–myosin contraction converge on regulation of the Rho family of GTPases and control of lamellipodial protrusions to promote directional migration.
Introduction
Cell migration is important for embryogenesis, immune surveillance and wound healing. The basic mechanisms of cell motility are relatively well understood. To migrate efficiently, cells must possess an asymmetric morphology with defined leading and trailing edges. Polarized intracellular signalling orients protrusion of the leading edge, integrin-mediated adhesion to the underlying substrate, contraction and detachment at distinct regions of the cell to orchestrate cell motility 1, 2. This sequence of steps –– known as the cell motility cycle –– occurs in a wide range of epithelial and mesenchymal cells that migrate in different environments in response to a variety of factors. It is less clear how this basic motility machinery is coupled to a steering mechanism that integrates environmental cues with polarized signalling, adhesion and cytoskeleton remodelling to promote directionally persistent migration.
Conceptually, directional cell migration has two sources: intrinsic cell directionality of migration and external regulation. Intrinsic directionality is observed when cells respond to a non-directional motogenic signal 3, such as the uniform application of platelet-derived growth factor (PDGF) 4, that triggers the basic motility machinery in the absence of any external guiding factor (Box 1). Random migration occurs when a cell possesses relatively low intrinsic directionality. If the motogenic stimulus is presented as an external gradient or with another external guidance cue, a steering or compass mechanism coupled to the basic motility machinery responds to the asymmetric environmental factor. The cell then undergoes directed migration 5, 6. The nature of the asymmetric cue will often define the type of directed migration. Cells undergo chemotaxis in response to soluble cues, haptotaxis in response to graded adhesion in the underlying substrate or other guidance cues anchored within the extracellular matrix (ECM) 7, electrotaxis in response to electric fields 8, and durotaxis in response to mechanical signals in the environment 9.
Box 1. Chemokinesis, chemotaxis and directional migration.
Chemokinesis occurs when a factor, applied to the cell either symmetrically or asymmetrically, stimulates cell migration without determining the direction of migration. Chemotaxis occurs when a soluble factor is applied asymmetrically and dictates the direction of cell migration. The behaviour of a motile cell exposed to these different treatments can be quantified. The example depicts two cells at three time points as they migrate in a uniform concentration (see figure part a) or a gradient (see figure part b) of motogen. At each time point, the migration can be defined by the centre of the cell mass, the distance travelled between positions (path length), the turning angle (θ) and the net displacement. This information can be used to describe the rate and directionality of migration. Directionality is defined as the displacement divided by the total path length of the cell. If a cell is migrating more randomly, directionality decreases and vice versa. It can also be quantified by calculating the mean square displacement 134. Prior to stimulation of migration or during chemokinesis, these parameters describe intrinsic cell directionality. During chemotaxis, they characterize directed migration. Factors that increase directionality during chemokinesis can promote chemotaxis 13, 14, whereas other factors that decrease directionality can inhibit chemotaxis 14, 64, 68. More studies will be required to determine whether this relationship is universal.
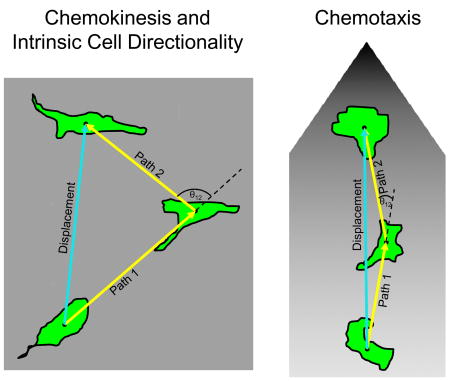
Both intrinsic and externally directed migration can be characterized quantitatively by the velocity and directional persistence of migration 10. Factors can change the velocity of migration by perturbing the basic mechanism of cell motility. For example, inhibiting the Ena/VASP family of actin-binding proteins slows lamellipodial dynamics and increases the rate of migration 11. Factors that affect the steering mechanism can alter the degree of directional persistence. For example, when phospholipase A2 (PLA2) and phosphatidylinositol 3-kinase (PI3K) isoforms 1 and 2 are deleted from Dictyostelium discoideum, the cells migrate at near normal rates but they no longer chemotax effectively 12. As will be highlighted throughout this Review, agents that increase random intrinsic migration will often diminish directed migration. Conversely, factors that increase directional persistence during intrinsic motility can sometimes promote directed migration 13, 14.
Recent studies on random versus directionally persistent migration during intrinsic motility appear to converge on a fundamental mechanism underlying directional migration. Cells achieve directionally persistent migration by forming and stabilizing actin-rich protrusions or lamellipodia that maintain the orientation of the leading edge 5, 15. As we will review, multiple factors can influence this process, including the topography of the ECM, cell polarity and cell adhesion. Understanding how these factors are integrated to regulate directional migration remains challenging. It is clear, however, that intracellular signalling, often mediated at the leading edge by the Rho family of small GTPases (Box 2), operates at each step of the cell motility cycle to promote directional migration by regulating leading edge formation.
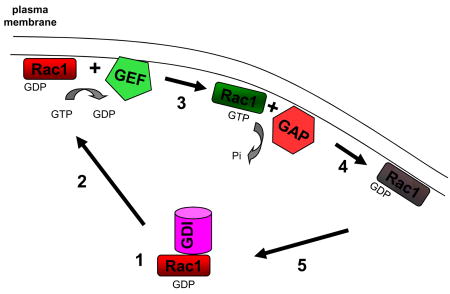
Box 2. Regulatory proteins and the Rho GTPase cycle of activation.
Small GTPases function as molecular switches in which the exchange of GDP for GTP triggers a conformational change that allows binding and activation of downstream effectors to direct cytoskeleton remodelling and adhesion formation 135. The Rho family of GTPases cycle between GTP-bound (active) and GDP-bound (inactive) states (see the figure). The activity of Rho-family GTPases is regulated by three classes of proteins, guanine nucleotide dissociation inhibitors (GDIs), guanine nucleotide-exchange factors (GEFs) and GTPase-activating proteins (GAPs) 136. The subcellular localization and protein binding partners of a particular GEF or GAP can specify where Rho GTPase regulation occurs and link their regulation to particular signalling pathways 137. By utilizing unique combinations of GEFs and GAPs, specific plasma membrane receptors can generate unique activation profiles of the Rho GTPase family with different functional outputs. The inactive GDP-bound GTPase, such as Rac1 (see figure), forms a complex with a GDI in the cytosol (1). The GDI regulates the interaction of the GTPase with intracellular membranes and blocks its binding to downstream effectors. Dissociation of the GDI and delivery to the appropriate intracellular membrane permits binding by a GEF (2). The GEF catalyses the release of the GDP and its replacement by GTP because of the higher concentration of GTP in the cytosol (3). Once active, the GTPase binds and activates downstream effectors by virtue of the conformational shift induced by GTP binding. GAPs bind the active GTPase and accelerate their intrinsic activity to convert GTP to GDP and inactivate the protein (4). The inactive GTPase is bound by the GDI, removed from the membrane, and sequestered in the cytoplasm (5). Members of the Rho family that play a prominent role regulating directed cell migration include Rac1, Cdc42 and RhoA.
This Review will first discuss the link between the stability of protrusions at the leading edge and directional cell migration. We then address how the topography of the ECM contributes to polarization and directional migration. Finally, we examine how the molecular mechanisms that drive each step of the basic motility cycle –– polarization, protrusion, integrin dynamics, or contraction and detachment can regulate directionally persistent migration. A recurring theme is that these processes regulate the number and orientation of lamellipodia to regulate directional migration (FIG. 1).
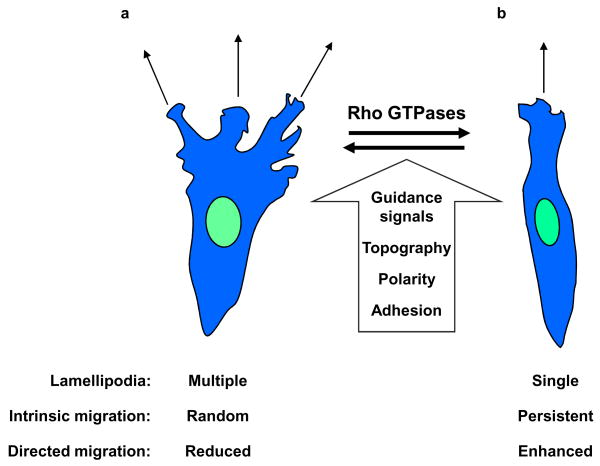
Figure 1. Control of lamellipodial protrusions promotes directional migration.
Directional migration is a result of regulated formation of lamellipodia during both intrinsic and directed cell motility. A variety of signals including external guidance cues, topography of the extracellular matrix, the intracellular polarity machinery and adhesion receptors can converge on the Rho GTPases to direct the adhesion and cytoskeleton remodelling necessary for lamellipodium formation. Increased lateral lamellipodia can result in random intrinsic migration and a reduced capacity to respond to external cues during directed cell migration (see the figure part a). Restricting lateral lamellipodia formation results in a single dominant leading edge, directionally persistent intrinsic cell migration, and enhanced directed migration during chemotaxis (see figure part b).
Stable protrusions guide migration
Cells differ in their intrinsic levels of directionally persistent cell migration, a property that can be quantified during chemokinesis 10. New protrusions are characteristically generated from the pre-existing leading edge, rather than in different directions around the cell 15. This process restricting lateral protrusions underlies directional migration in fibroblasts, leukocytes and D. discoideum 5, 15. Some cells can migrate without lamellipodia using bleb-based motility 16 but its role in random versus directional motility is not yet clear. Due to space restrictions, this Review will focus primarily on motility studies of mesenchymal and epithelial cells. For recent reviews of neutrophil and D. discoideum directional migration, see Refs 17–19.
Local signalling within a protrusion in response to an external guidance cue can direct the formation of a new protrusion 5 in vitro and in vivo. For example, the leading edge of neurons migrating within the central nervous system (CNS) consists of multiple extending and retracting branches 20. Similarly, endothelial tip cells at the growing ends of new blood vessels have several protrusions at their leading edge that direct cell trajectory 21. In both cases, the direction of migration is determined by the orientation of the most stable branch, which is regulated by external guidance cues and internal signalling 20, 21.
ECM topography guides migration
Cell adhesion can guide the directionality of migration; for example, adhesion to the underlying substratum stabilizes lamellipodial protrusions during chemotaxis and chemokinesis 22, 23. The topography of the ECM can also provide important regulation of cell motility through physical cues that geometrically constrain adhesion sites to guide directional migration (FIG. 2). During durotaxis, where the pliability of the underlying ECM affects rates of migration, fibroblasts migrate towards a rigid surface or a local region of higher local tension within an elastic polyacrylamide gel 9. Consequently, when cells probe their physical surroundings, they acquire mechanical information or signals that help determine the direction of migration –– e.g., in cell migration toward an increased ECM adhesive gradient during haptotaxis 7.
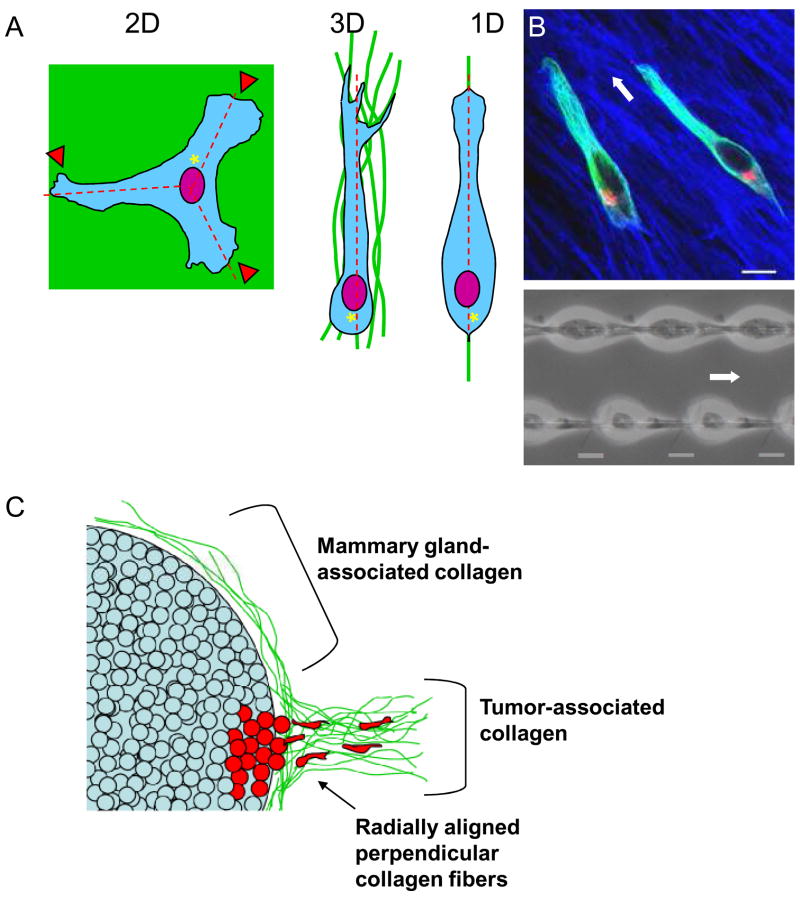
Figure 2. Topographical control of directional migration.
A. Representation of how different topographical cues (2D, 3D and 1D) result in different cell morphologies and migration. When plated on 2D surfaces, cells demonstrate multiple lamellae (arrowheads) compared to the single lamella and uni-axial or spindle morphology associated with cells in an oriented 3D matrix or on 1D lines. Centrosome and Golgi (asterisks) are oriented towards the posterior of the cell in 3D and 1D but towards the anterior of the cell in 2D. Cells in both 3D and 1D demonstrate a single directional axis of travel (dashed lines), whereas the 2D surface promotes multiple axes and reduced directional migration. B. 1D mimics 3D. The upper panel shows a confocal image of NIH/3T3 fibroblasts migrating through 3D cell-derived matrix (fibronectin is shown in blue) demonstrating a uni-axial phenotype and a posterior-oriented Golgi complex (red, with microtubules in green). Fibroblasts migrating on 1D lines have similar morphology (lower panel). White arrows indicate the direction of migration. Scale bar, 10 μm. C. Schematic of differences in stroma associated with normal mammary gland (top) and malignant mammary tumours (bottom). Collagen (pink) associated with mammary tissue often tightly surrounds the epithelial cells and is oriented along the axis of the gland. By contrast, invasive tumour cells (blue) reorient the collagen fibres perpendicular to the gland and may use these structures as highways for migration to initiate metastasis.
Classical studies of cells interacting with the fibrillar protein network of fibrin clots established that cells can re-orient the ECM, which in turn can alter mesenchymal cell morphology and migration 24, 25. In this process termed contact guidance, the physical structure of the surrounding ECM helps control cell shape and migration. Similar effects of ECM topography are found during single-cell mesenchymal migration 26 and embryogenesis 27–29. In amphibian gastrulation, aligned ECM fibrils facilitate mesodermal cell migration towards the animal pole. 27 Alignment of the fibrillar matrix in vitro can control migration, consistent with a role for ECM orientation in promoting the directional migration of these cells in vivo 28, 29.
Surface topography influences polarity and migration
‘Natural’ cell-derived environments contain multiple components, including other molecules besides the oriented ECM fibrils that might affect directionality (e.g., growth factors bound to the ECM). Consequently, a number of bioengineering studies have tested the effects of grooved or etched physical patterns in inorganic substrata. Mesenchymal cells from fish explanted onto parallel grooves in quartz coated solely with denatured type I collagen show cell elongation, polarization and migration along the grooved longitudinal axis 30. This polarizing effect of topographic patterns has been observed for a wide variety of cell types including oligodendrocytes 31, hippocampal neurons 32 and epithelial cells 33. Tests of nanotopographic patterns reveal that fibroblasts can respond to a grooved pattern with a depth and width of 35 nm and 100 nm, respectively 34, which is similar to the width of a single collagen fibril (~30–100 nm in width). These studies suggest that cell interactions with physical structures can induce cell responses and signalling independent of chemical factors to promote directional migration.
3D ECM structures promote directional migration
Cell migration and the regulation of directionally persistent migration have been studied primarily in vitro on two-dimensional (2D) surfaces. However, three-dimensionality can substantially affect fibroblast cell morphology, signalling and migration 35. Single fibroblasts migrating in 3D cell-derived matrix often display a spindle-shaped or uni-axial morphology (FIG. 2B) 24, 25. Mechanical flattening of 3D cell-derived matrices or coating 2D surfaces with solubilized 3D matrix molecules mimic simple 2D substrata with respect to cell morphology, adhesion and random migration. Interestingly, the spindle-shaped uni-axial morphology of cells in 3D can be induced by sandwiching fibroblasts between two 2D elastic polyacrylamide gels coated with collagen 36. These results indicate that dimensionality, or at least both dorsal and ventral matrix contact, can help regulate the shape and mode of migration of fibroblasts. It should be noted that the cellular response to fibrillar 3D structures may be cell-type specific and dependent on the mode of cell migration (i.e., single cells versus sheets). Further, amoeboid cells undergoing integrin-independent migration may respond only to physical constraints of the ECM rather than to fibrillar ECM structures 37.
1D topography underlies migration on 3D fibrils
The tissue and ECM environments of cells can differ with respect to orientation of the ECM; for example, certain human fibroblasts can produce highly oriented 3D matrices 38 while other matrices show little orientation. Cell migration along highly oriented matrix is highly directional and rapid 39 with many cells ‘streaming’ one after the other along fibronectin fibrils (A.D.D., unpublished observations). These oriented matrix fibrils can be mimicked by single 1.5 micron lines generated by micro photoablation and coated by matrix. These essentially one-dimensional (1D) fibrils also force cells into a uni-axial morphology with a single lamella 39 (FIG. 2B). Migration along 1D patterns is rapid (>1.5-fold higher than 2D), unidirectional, highly ordered as shown by coordinated protrusion-retraction cycles, and independent of ligand density; these properties match those of cells migrating through oriented 3D cell-derived matrices.
Other similarities between 1D and oriented 3D models not shared by 2D models include distinctive localization of key adhesion components (α5 and activated β1 integrin), presence of stabilized Glu-tubulin in an axon-like pattern, rearward-oriented Golgi apparatus and centrosome –– both of which point toward the leading edge in 2D wound-healing models (see below) — and sensitivity of cell migration to inhibition of cellular contractility and disruption of microtubules. Consequently, cell association with fibrillar structures appears to provide important physical cues to initiate cell polarization by regulating cell shape and orientation of cellular organelles, resulting in unidirectional cell migration (FIG. 2A).
Nanofibre topography can guide cell migration in vivo
Fibrillar topographical cues in the form of 1D nanofibres can guide axonal growth and glial cell migration in vivo 40, 41. After spinal cord injury, failure of axons to regenerate results in paralysis. This clinical problem is due partially to the inability of axons to traverse scar tissue generated locally by glial cell infiltration into the wound that physically blocks axon regeneration 41. Immediately after a spinal cord injury in an animal model, introduction of peptide amphiphile molecules that self-assemble into nanofibres reduces glial scarring and promotes motor and sensory neuron outgrowth through the wounded region. While more investigation is required to understand how topographic physical cues are involved in directional migration in vivo, it is clear that association of cells with ECM with a defined structure, whether a 2D surface, a 3D matrix, or a 1D line/nanofibre, can strongly affect cell polarity, cell morphology and cell migration.
Topography of ECM fibrils and cancer invasion
Cell migration can now be studied in native in vivo environments using new imaging approaches. For example, studies of in vivo explants to analyse breast cancer metastasis reveal metastatic tumour cells and macrophages migrate rapidly along collagen fibres 42. Highly metastatic tumour cells migrate preferentially along fibres. The reticular orientation of the collagen matrix surrounding mammary glands may anchor and/or restrain cells 43. However, the dense fibrous collagen characteristic of breast cancer stroma forms radial patterns extending away from tumours (FIG. 2C). In vitro experiments show that parallel collagen fibres radiating outward from tumour explants can promote tumour epithelial cell invasion, while non-linear matrix reduces invasive behaviour 44. Tumour cells remodel the matrix into these parallel fibres in order to migrate. These data suggest that oriented ECMs play a role in vivo in directional migration and invasion. Understanding these mechanisms may provide better models for cancer metastasis and developmental processes.
Connecting topography to directional migration
It will be important to determine how ECM topography links to intracellular signalling to promote directional cell migration. Integrin receptors and the physical arrangement of adhesions could trigger orientation of the cytoskeleton that favours directional cell migration. Alternatively, specific matrix topography could influence cell polarity or integrin trafficking (see below). Although matrix orientation can stabilize leading-edge protrusions to promote directionally persistent migration, the specific signalling pathways remain to be determined.
Polarity and directional migration
Cells contain polarity signalling machinery that can influence directional cell motility. This polarization influences the formation of the leading and trailing cell edges. The Par (partitioning defective) complex, consisting of Par3, Par6 and atypical protein kinase C (aPKC), connects Rho GTPase signalling, centrosome reorientation, microtubule stabilization and membrane trafficking to the regulation of directional persistence during intrinsic cell migration (FIG. 3). Par activation polarizes a broad spectrum of cellular processes, including the formation of the front–rear axis in moving cells, as well as asymmetric cell division and basal–apical polarity in epithelial cells 45. The stability of the front–rear axis correlates with the extent of persistent directional cell movement 46.
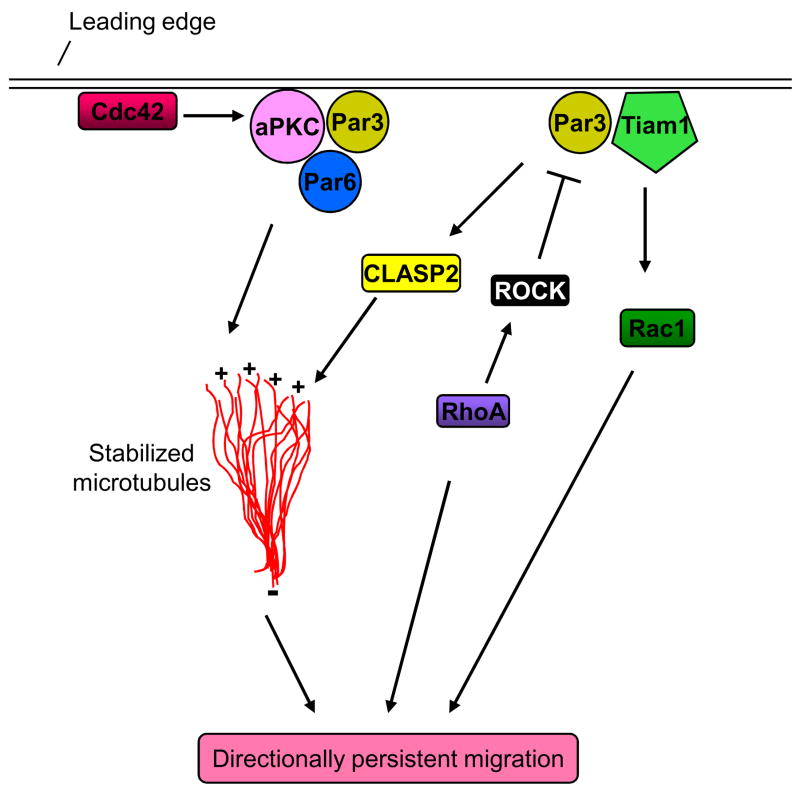
Figure 3. The Par polarity complex and directional migration.
Cdc42 targets and activates aPKC and Par6 at the leading edge to stabilize microtubules and promote directional migration. Par3 and the guanine nucleotide-exchange factor Tiam1 activate Rac1 and stabilize microtubules, possibly via the action of CLASP2, at the leading edge to promote front–rear polarity and directionally persistent migration. RhoA-activated ROCK phosphorylates Par3 and disrupts the formation of the Par3–Tiam1 complex, thereby preventing Rac activation. This pathway may coordinate mutual antagonism of Rac1 and RhoA locally within the cell to dictate directional migration.
The Rho GTPase family member Cdc42 is a master regulator of cell polarization that influences directional migration 47. Integrin engagement by components of the ECM can locally activate Cdc42 at subregions of the plasma membrane 48. Active Cdc42 then recruits the Par complex to the plasma membrane where aPKC is activated 48. Cdc42 activity can also be regulated at the leading edge of migrating cells via the phosphoprotein Nudel 49. Nudel is phosphorylated by the Ser/Thre kinase ERK (extracellular signal regulated kinase) at the leading edge to locally sequester the Cdc42 GTPase-activating protein Cdc42GAP. This sequestration can prevent Cdc42GAP from downregulating local Cdc42 activity and may contribute to Cdc42-dependent activation of the Par complex to trigger polarized protrusions and directionally persistent cell migration 49.
Cdc42 can promote directional cell motility in fibroblast scratch-wound healing assays in vitro as cells migrate into a region denuded of cells 50, 51. A caveat, however, is that such non-epithelial monolayers are seldom seen in vivo. Cdc42 activates p21 protein-activated kinase 1 (Pak1), which recruits the Rac guanine nucleotide-exchange factor (GEF) βPIX to the leading edge where it can locally activate Rac to initiate protrusions and directional migration 52. Simultaneously, Par6 and aPKC act downstream of Cdc42 to stabilize microtubules at the leading edge while the dynein motor acts to keep the centrosomes in position, resulting in the final arrangement of the nucleus, centrosome and leading edge along the front–rear axis 50 in 2D cell culture. Microtubule-binding proteins stabilize microtubules at the leading edge 53 to promote local protrusion and directional migration by regulating adhesion formation, and by facilitating the anterograde transport of material from the Golgi to the active leading edge to replenish material removed by a combination of protrusion and retrograde actin flow 54, 55. During this process, Cdc42 triggers actomyosin retrograde flow via its downstream effector, myotonic dystrophy kinase-related Cdc42 binding kinase (MRCK), in a complex with myosin 18A and the adaptor protein LRAP35a 50, 56. Cdc42-regulated actomyosin retrograde flow repositions the nucleus to the rear of the cell.
Wnt signalling and directional migration
Additional pathways can cooperate with Cdc42 and the Par complex to promote directional migration. Wnts are a family of secreted proteins that regulate cell fate and tissue patterning. Wnt signalling classically contributes to polarization of tissues within developing embryos and, more recently, has been shown to contribute to cell polarity and directional motility. Wnts regulate gene expression via a canonical pathway or cytoskeletal dynamics and cell polarization via a non-canonical pathway (BOX 3). Wnt5a triggers non-canonical Wnt signalling and cell motility by binding to the receptor Frizzled and the alternative or co-receptor Ror2, a Tyr kinase receptor 57. In scratch-wounded monolayers of fibroblasts, Wnt5a binding to these receptors causes the cytosolic mediator Disheveled to trigger Golgi and centrosome reorientation via the tumour-suppressor protein APC (adenomatous polyposis coli) and stabilizes microtubules toward the newly formed leading edge in cooperation with the Cdc42, Par–aPKC pathway 58. Importantly, engagement of Ror2 by Wnt5a is required for directional migration of fibroblasts during scratch-wound healing in the presence of Wnt5a and chemotaxis towards a source of Wnt5a 57, 59.
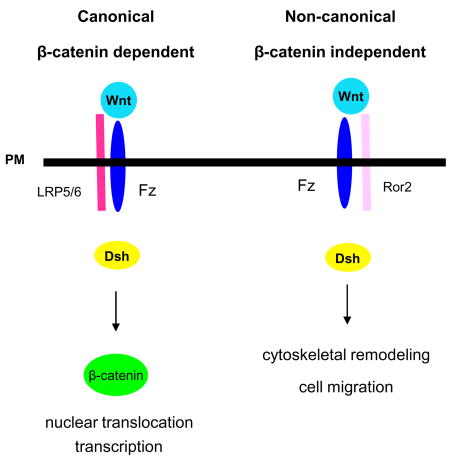
Box 3. Canonical and non-canonical Wnt signalling.
In higher vertebrates, the 19 members of the Wnt family of proteins induce intracellular signalling that is important for developmental processes such as cell migration, proliferation or differentiation 138. Historically, this complex signalling system has been categorized into canonical (part a) and non-canonical Wnt signalling pathways (part b). While these pathways share common elements, such as the receptor Frizzled and the cytoplasmic protein Disheveled (Dsh), they diverge in their biological effects. Canonical Wnt signalling can induce dorsal–ventral embryonic patterning 139, whereas non-canonical Wnt signalling can trigger convergent extension of tissues — a simultaneous narrowing and lengthening of a tissue that occurs during gastrulation and other formative processes 140. The type of receptor engaged by a particular Wnt determines the output of the signalling pathway. During canonical signalling (see the figure part a), Wnt (1, 3a, or 8) binding to Frizzled and its co-receptor LRP5/6 (low-density lipoprotein receptor-related protein 5/6) increases levels of the transcriptional co-activator β-catenin in the cytoplasm by inhibiting its phosphorylation-dependent ubiquitylation and degradation. This permits β-catenin to enter the nucleus and trigger transcription. Thus, an alternative name for canonical Wnt signalling is β-catenin-dependent Wnt signalling 140. Non-canonical Wnt signalling (see the figure part b) is triggered by Wnt (5a or 11) binding to Frizzled and the co-receptor Ror2 57. This complex activates JNK (c-Jun N-terminal kinase) and leads to Rho family GTPase activation, cell polarity, cytoskeletal remodelling and cell migration. An alternative name for this pathway is β-catenin-independent Wnt signalling 140.
Regulation of Rho GTPases by the Par complex
In addition to forming the front–rear axis that is important for directional cell migration, the Par complex is a focal point of crosstalk between the small GTPases Cdc42, Rac and RhoA. Rac and Cdc42 can promote RhoA activity at the back of the cell to aid in the formation of the leading and trailing edges that are required for efficient cell migration 60, 61. This crosstalk may also occur at the front of the cell to coordinate adhesion, protrusion and retraction of the leading edge 62. Thus, Par-mediated crosstalk between the Rho family of GTPases may be a crucial factor regulating cell morphology and migration.
In addition to recruiting βPIX, Cdc42 may activate Rac at the leading edge via the polarity complex. In neuroblastoma cells, active Cdc42 binds the Par complex and helps recruit the GEF Tiam1 to the leading edge, locally activating Rac 63. Similarly, Tiam1 is targeted to the leading edge by direct binding to Par3 in epithelial cells 64. Depletion of either Tiam1 or Par3 decreases front–rear polarization, increases random cell migration and reduces sensitivity of cells to a chemotactic cue 64, 65. Active Rho kinase (ROCK) downstream of GTP-bound RhoA can antagonize Rac activation at the leading edge by phosphorylating Par3 and disrupting the complex to prevent Rac activation by Tiam1 65. This phosphorylation also occurs at the leading edge, and these signalling circuits are required for cell polarization and directed cell migration 65. By targeting Tiam1 to the cell front, Par3 promotes microtubule stabilization and lamellipodium formation to generate directionally persistent migration for both intrinsic and directed cell motility. Although the precise link between Tiam1 and localized microtubule stabilization is not yet known, the microtubule plus-end binding protein CLASP2 mediates the stable association of microtubules with the cell cortex at the leading edge 66. As with Par3 and Tiam1, depletion of CLASP2 reduces the number of stable microtubules and increases random motility. While reduced Rac activity in fibroblasts leads to directionally persistent migration 14, loss of the Rac GEF Tiam1 in keratinocytes leads to a decrease in total Rac activity that increases random migration 64. Consequently, local restriction of active Rac to the leading edge by the combined action of Par3 and Tiam1 may be a key factor promoting directionally persistent motility. In fibroblasts, however, Tiam1-mediated activation of Rac is associated with an increase in cell–cell interactions and loss of cell motility. These discrepancies indicate that the function of specific GEFs may be context dependent 61.
Caveolin-1, the principal component of caveolae, may act in parallel with the Par complex and contribute to polarity and directional migration 67. Directional migration in both wound healing and chemotaxis assays requires phosphorylation of caveolin-1. Deficiency of caveolin-1 decreases Rho activity while increasing the levels of active Rac and Cdc42 68. These increased activity levels are associated with faster turnover of nascent adhesions and enhanced random protrusions in mouse embryonic fibroblasts 68. These cells show impaired directional cell migration during scratch-wound healing consistent with a global increase in Rac activity. Src activation of p190RhoGAP in these cells may contribute to inhibition of Rho activity and decreased directional migration. Alternatively, removal of caveolin-1 might promote Rac activity and increase random migration by reducing internalization of Rac binding sites from the plasma membrane 69. Together, these findings are consistent with the notion that the mutual antagonism between Rac and RhoA activity coordinated by the Par complex and caveolin-1 can be important for directionally persistent cell migration.
Protrusion and directional migration
The main factors that determine the orientation of cell migration are the frequency and direction of local lamellipodial protrusions extending laterally from the main longitudinal axis of the cell 5, 15. Intracellular signalling pathways at the leading edge 2 that regulate actin cytoskeleton remodelling or adhesion formation to create or stabilize local protrusions therefore likely contribute to directional migration 6.
Calcium regulation of the leading edge
Local changes in the concentration of intracellular calcium regulate directional cell migration. Transient, spatially restricted increases of intracellular calcium guide growth cone migration during haptotaxis 70 and chemotaxis 71. Local fluxes of intracellular calcium can activate Rac and Cdc42 and inactivate RhoA, regulating growth cone motility 72. In migrating fibroblasts undergoing chemokinesis, TRPM7 (transient receptor potential) calcium channels intermittently open and trigger intense local bursts of intracellular calcium at the leading edge 73. Symmetric application of PDGF increases random fibroblast migration along with the number and amplitude of the local calcium bursts, whereas inhibition of TRPM7 channels prevents fibroblast chemotaxis towards PDGF. Whether calcium is an upstream mediator of Rho family GTPase function or regulates additional signalling pathways during directional fibroblast migration 74 is currently unresolved.
PI3K and Rac signalling at the leading edge
The non-overlapping distribution and combined action of PI3K and the lipid phosphatase PTEN (PI3K phosphatase and tensin homolog) produces PtdInsP3 at the leading edge during intrinsic and directed migration 75. In D. discoideum, PI3K controls the rate of pseudopod generation during chemotaxis 15, where it may cooperate with other pathways such as PLA2 to trigger efficient chemotaxis of these cells 12, 76. In fibroblasts, PtdInsP3 is localized to the leading edge of fibroblasts during intrinsic and directed cell migration 77, 78. During chemotaxis, local PIP3 generation within lamellipodia may trigger actin polymerization and protrusion of the lamellipodia towards the source of guidance cue 5.
Rac may be a key target of PI3K signalling at the leading edge during cell migration 79. Inhibiting PI3K during intrinsic fibroblast motility partially reduces Rac activity and random migration compared with directly reducing active Rac by siRNA 14, indicating that other pathways may cooperate to regulate Rac at the leading edge during cell migration. Phospholipase D (PLD) hydrolyses the phospholipid phosphatidylcholine (PC) to generate phosphatidic acid (PA) in response to growth factor or integrin engagement 80. PA binds directly to the membrane targeting motif of active Rac to recruit it to the plasma membrane to promote fibroblast migration 81, 82. Interestingly, PLD cooperates with PI3K signalling to mediate Rac activation during neutrophil chemotaxis 83; a similar interplay may occur in other cell types during intrinsic cell motility.
The level and localization of Rac activity plays a central role in determining the choice between random and directionally persistent motility, though this relationship may not be universal 84. Rac is highly active at the leading edge during intrinsic migration 85 and the level of Rac activity determines whether the intrinsic migration of a cell is random or directional 14, 86. Relatively high levels of Rac activity induce formation of multiple lamellae, leading to more non-directional, random cell migration. Moderate levels of Rac activity support fewer lateral lamellae, thereby promoting directional cell migration and chemotaxis. Compared with cells migrating on 2D surfaces, cells migrating in complex 3D environments have lower levels of Rac activity with an elongated morphology, fewer lateral lamellae, and more rapid and directional migration 14, 35, 87. Increased levels of Rac activity likely increase the targeting of active Rac to the plasma membrane and formation of lateral lamellipodia to promote random intrinsic migration 14, 88.
Mechanistically, Rac activation at the leading edge may promote directional cell migration by triggering local actin polymerization or adhesion formation. Rac is known to trigger formation of adhesive structures in the lamellipodium 89 and this contributes to epidermal growth factor (EGF)-triggered motility in carcinoma cells 90. The WAVE (WASP family verprolin-homologous protein) family (WAVE1, 2, and 3) and Pak1 link Rac signalling to membrane ruffling and lamellipodia formation 91, 92. However, fibroblasts deficient in WAVE2 migrate randomly during wound healing and chemotax less effectively 93, contrary to the affects of diminished Rac signalling 14. By contrast, expression of kinase-inactive Pak1 in fibroblasts increases random migration 94. Independent of its kinase activity, Pak1 can recruit the protein kinase Akt to the plasma membrane where it is activated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) 95, an important pathway for endothelial cell migration 96. Consistent with the scaffolding function of Pak1 acting downstream of Rac, Akt activity can compensate for changes in the directionality of migration caused by diminished Rac activation 14.
The cofilin pathway and directional migration
Localized activity of the actin-severing protein cofilin is important in directional migration. Cofilin functions at the leading edge by severing F-actin filaments at the minus end to provide more free-barbed ends for actin polymerization 97 When cofilin activity is decreased in fibroblasts either via siRNA treatment 98 or α5β1 integrin-triggered phosphorylation of cofilin on Ser14 by ROCK (Rho-associated kinase), cells undergo increased random migration 99, 100. However, in metastatic cancer cells, depletion of cofilin leads to more directionally persistent intrinsic migration in response to EGF 101. This increase in directional migration is associated with stable and persistent lamellipodial protrusions and a decrease in sensitivity to a point source of the chemoattractant EGF at the posterior of the cell 101. Thus, lamellipodial dynamics controlled by cofilin and Arp2/3 are a critical factor in dictating the directional migration of these cells.
Cofilin activity at the leading edge is also sensitive to local changes in intracellular pH (pHi). The ubiquitously expressed NHE1 Na-H exchanger is targeted to lamellipodia, and locally modulates intracellular pHi to promote directional migration of fibroblasts in a scratch-wound assay 102. NHE1-mediated deprotonation of His133 of cofilin prevents cofilin binding to its negative regulator phosphatidylinositol-4,5-bisphosphate (PIP2) 103. This process may activate cofilin at the leading edge to promote directional migration 104.
ECM receptors, trafficking and motility
Integrin trafficking and co-receptors contribute to integrin function and adhesion formation during cell migration 105. Integrin trafficking may contribute to directional migration by facilitating the formation of new adhesions at the leading edge 105. Recent work shows that integrin trafficking and the co-receptor syndecan-4 can contribute to directional migration, in part, by modulating Rho GTPase signalling to control protrusion formation.
Integrin trafficking and persistent migration
The Par complex can contribute to polarized integrin trafficking and adhesion formation at the leading edge of migrating cells 106 (FIG. 4). Par3 cooperates with the endocytic machinery by regulating Numb, an adaptor that couples specific cargo to clathrin-coated pits 107. Numb directs internalization of integrin β1 or β3 subunits behind the leading edge. Par3 binds directly to Numb and promotes its Ser phosphorylation by aPKC 106 (FIG. 4A). Phosphorylation of Numb prevents its interaction with the integrin β subunits and inhibits their internalization. Inhibiting either phosphorylation or dephosphorylation of Numb blocks the directed migration that occurs during wound healing of fibroblast monolayers in the presence of serum. This mechanism links the trafficking of integrins on the cell surface to the polarization machinery at the leading edge.
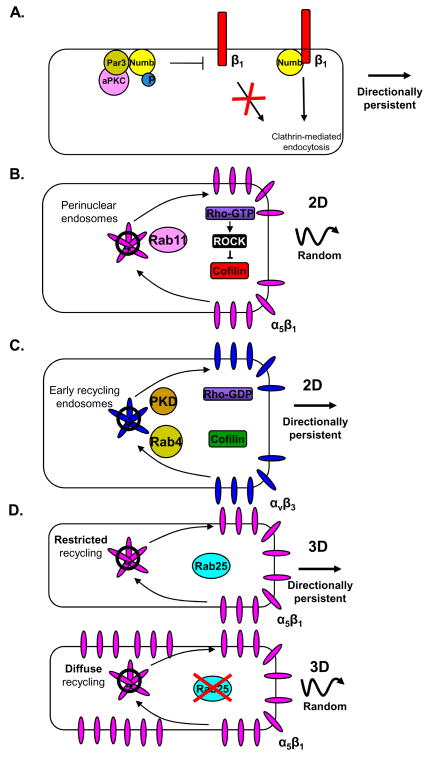
Figure 4. Integrin trafficking and directional migration.
Trafficking of specific integrin heterodimers contributes to the directional persistence of cell migration. A. The endocytic cargo adaptor protein Numb binds to the cytoplasmic tail of integrin β1 to trigger its clathrin-mediated endocytosis. Numb binds to Par3 and is phosphorylated by aPKC. Phosphorylation of Numb prevents its binding and initiating the internalization of integrin β1 at the leading edge. This spatial regulation of integrin β1 endocytosis leads to directional migration. B. Integrin α5β1 is trafficked from the cell surface to a perinuclear endosomal compartment and is recycled back to the plasma membrane via a Rab11-dependent trafficking pathway. Trafficking of integrin α5β1 via this pathway increases Rho activity, which triggers ROCK phosphorylation and inactivation of cofilin, which stimulates random cell migration. C. Integrin αVβ3 traffics from the leading edge to an early endosome compartment and is recycled to the cell surface via a pathway that depends on the activity of PKD and Rab4. This pathway leads to cofilin activation and directionally persistent migration. D. During epithelial cell migration in 3D environments, Rab25 trafficking restricts integrin α5β1 recycling to protrusions at the leading edge of the cell, which in turn results in fewer lateral protrusions and more directionally persistent migration. Upon inhibition of this recycling pathway, trafficking of α5β1 is no longer restricted to the leading-edge pseudopodia, thereby increasing random protrusions and decreasing directional migration.
Recycling of specific integrins is another process that contributes to directional migration in both 2D and 3D contexts (FIG. 4B and C). Integrin αVβ3 expression in wounded epithelial cell monolayers promotes stable centrosome re-orientation and directional migration compared to cells expressing integrin α5β1 99. Integrin αVβ3 suppresses RhoA–ROCK-mediated phosphorylation and inhibition of the actin-severing protein cofilin, thereby triggering broad lamellipodia, stable adhesions and increased directional migration. Cofilin functions at the leading edge by severing F-actin filaments at the minus end to provide more free-barbed ends for actin polymerization 97. In fibroblasts, PKD1 and Rab4 (a small GTPase of the Rab family) drive the rapid recycling of integrin αVβ3 from early endosomes to the cell surface 108, whereas Rab11 controls integrin α5β1 recycling via a longer pathway from a perinuclear endosomal compartment 109. Perturbation of the rapid Rab4-dependent recycling of integrin αVβ3 increases the rate of integrin α5β1 recycling and promotes random fibroblast migration 100. Consistent with epithelial cells, integrin α5β1-mediated random migration in fibroblasts is triggered by the ROCK-dependent phosphorylation and inactivation of cofilin 99, 100.
3D matrix and integrin trafficking
Matrix dimensionality influences Rab11-dependent recycling of integrin α5β1 and directional cell migration. Inhibition of integrin αVβ3 in an epithelial cancer cell line increases integrin α5β1 recycling by stimulating the Rab11-mediated return of integrin α5β1 to the plasma membrane 110. This switch of integrin trafficking correlates with an increase in random migration of fibroblasts on a 2D surface, but it promotes directional cell migration in 3D fibronectin-containing Matrigel and cell-derived matrices. Integrin α5β1 recycling in epithelial cells driven by the epithelial-specific Rab11 family member Rab25 promotes the directional migration of these cells within 3D environments without affecting their mode of migration on 2D surfaces 111. Cell-derived matrix increases the association of Rab25 with β1 integrin and restricts its recycling to the tips of leading-edge protrusions in cell-derived matrix to promote directionally persistent migration 111 (FIG. 4D).
It is not yet known how matrix dimensionality regulates Rab25 activity or its association with integrin β1, but the Rab-coupling protein (RCP) mediates the formation of a tripartite complex between the EGF receptor 1 (EGFR1) and integrin α5β1 in recycling endosomes to increase integrin α5β1 and EGFR1 recycling to the cell surface 110. Thus, the trafficking of integrin α5β1 and an EGFR1 co-receptor can initiate intracellular signalling leading to cytoskeletal rearrangements that promote directional cell migration. How these integrin trafficking pathways guide cell migration within tissues is uncertain, but different components of the extracellular matrix can modulate recycling of specific integrins to promote cell motility in vitro 112.
Syndecan-4 and directional cell migration
The transmembrane proteoglycan syndecan-4 may sense ECM topography to control directional migration in 3D environments. Syndecan-4 cooperates with integrin α5β1 to bind fibronectin, form focal adhesions and support cell migration 113, 114 by activating Rac downstream of protein kinase C (PKC) 88. Syndecan-4, via PKC, restricts Rac activity to the leading edge of fibroblasts migrating on cell-derived matrices. Correspondingly, deletion of syndecan-4 leads to an increase in active Rac around the cell periphery and more random migration on cell-derived 3D matrix 88. Thus, syndecan-4 restricts Rac activation to generate a dominant lamella to drive directionally persistent fibroblast migration in response to linear fibrils in the extracellular matrix. Unlike Par3 targeting of the Rac GEF Tiam1 to the leading edge to locally activate Rac 64, syndecan-4 suppresses Rac activation except at the leading edge of the cell to promote directionally persistent cell migration. The mechanism by which syndecan-4 limits Rac activation resembles a mechanism used by the integrin α4–paxillin–Arf GAP (GTPase-activating protein) complex to inhibit Rac activation around the periphery of migrating epithelial-like CHO cells 115.
Syndecan-4 also cooperates with non-canonical Wnt signalling to control directional migration of neural crest cells in 3D environments during development 116, 117. Migration of neural crest cells to specific locations is important for their differentiation 118. Ablation of syndecan-4 expression by morpholino injection blocks neural crest differentiation by diminishing neural crest migration from the dorsal neural tube. In vitro cultures of neural crest cells lacking syndecan-4 undergo increased random migration resulting from larger numbers of random membrane protrusions. As in mouse embryonic fibroblasts migrating on cell-derived matrix 88, syndecan-4 via PKC restricts Rac activation to the leading edge for directionally persistent neural crest cell migration in vitro and in vivo 117. Perturbing Disheveled function decreases RhoA activity and prevents neural crest cell emigration from the dorsal neural tube. This pathway mediates contact inhibition 119 that is partially responsible for the directional migration of neural crest cells 120. In contact-inhibited cells, Wnt11 and Disheveled cooperate to trigger RhoA-dependent collapse of protrusions that contact neighbouring neural crest cells. Thus, mutually exclusive zones of Rac and RhoA activity, controlled by syndecan-4 contacting extracellular matrix and the non-canonical Wnt receptor, respectively, drive directional cell migration in response to contact inhibition 117, 120.
Steering from the back
The trailing edge of a migrating cell contributes to the maintenance of directional migration by generating contraction forces to pull the cell rear forward and limiting the formation of protrusions to maintain the orientation of migration.
RhoA activates ROCK, which phosphorylates myosin phosphatase and the regulatory light chain on myosin II to increase actin–myosin contractility121 and trigger tail retraction and disassemble focal adhesions 122. Inhibition of RhoA by active Rac contributes to the formation of myosin-mediated contractility at the rear of migrating neutrophils during chemotaxis 60, in part by limiting the formation of protrusions at the rear of the cell 123. An analogous pathway may be mediated by PTEN in D. discoideum. PTEN localized to the cell rear promotes intrinsic and directed migration by suppressing pseudopod formation 124. Fibroblasts commonly express two isoforms of myosin II, A and B 125. Myosin IIA (MIIA) deficiency leads to formation of broad lamellipodia, increased Rac activation and random migration, and a defect in tail retraction 125, 126. By contrast, myosin IIB (MIIB) depletion causes unstable protrusions, increases random intrinsic migration, and inhibits haptotaxis 127. MIIB promotes directional migration by forming contractile actomyosin bundles at the cell rear, which prevent protrusion formation and thereby promote directional migration 128. Similarly, during migration of EC tip cells in 3D collagen matrix, MIIB activity prevents protrusion initiation away from the leading edge to maintain directional migration 21.
Intracellular membrane trafficking in response to Wnt5a may be a novel mechanism to direct MIIB-mediated retraction at the cell rear. Increased Wnt5a expression in metastatic melanoma cells is associated with increased migration and invasiveness 129. Cultured melanoma cells require Wnt5a plus a chemokine gradient in order to polarize and migrate effectively 130. Under these conditions, Wnt5a polarizes the cell by promoting the recycling of specific membrane components, such as the melanoma cell adhesion molecule (MCAM), to the rear of the cell. In these cells, the coupling of adhesion with MIIB-mediated retraction may establish the polarity of cell migration. Whether Wnt5a is required more generally for directed cell migration remains to be determined.
Conclusions
Specific molecular mechanisms operate at each step of cell motility to control directional cell migration. These mechanisms are used by the cell to integrate information provided the topography of the ECM, constituents of the matrix, distribution of soluble or substrate-bound guidance cues and/or other factors. The cell distils this array of guidance information to select a direction of migration. While not all steps of this process are known, it is clear that Rho GTPase signalling and control of directional protrusions are critical for directional cell migration. A morphological view of directional cell migration highlights the frequency and direction of local protrusions extending laterally away from the front–rear axis of migration as being important in determining directionality 5, 15. In other words, if the protrusions and subsequent new adhesions formed by a polarized cell are themselves directionally persistent, the cell will move in a directionally persistent manner. Processes occurring at each step of the cell motility cycle can act to regulate Rho GTPase signalling in order to promote stable and directionally persistent protrusions, which in turn promote directional migration.
The diverse array of mechanisms that contribute to directional migration may be a reflection of the complex environments cells must navigate. For example, axonal growth cones integrate guidance information provided by matrix components, soluble and matrix-bound guidance molecules, and cell–cell contacts, in order to arrive at the correct position in the body 131. Similar complexity is illustrated in fibroblast-mediated wound-healing 132 and in the immune system, where cells must often prioritize between competing guidance cues 133. In addition, the conceptual model of a distinct steering mechanism coupled to the cell motility machinery may be oversimplified in some cases. For example, roles of α5β1 and αVβ3 in directional migration may suggest that the steering mechanism is embedded within the underlying motile apparatus to respond to environmental cues and trigger directional migration.
Future efforts to understand the processes driving cell migration will need to use models that recapitulate the competing guidance cues and the physically and biochemically complex environments found in vivo. Doing so should clarify whether the many molecular mechanisms controlling directional migration operate within a hierarchical framework or whether they are functionally redundant or even synergistic.
Acknowledgments
We thank Y. Endo, A. Green, J. Harunaga, and E. Joo for helpful comments on the manuscript. Support provided by the Intramural Research Program of the NIDCR, NIH.
Glossary
- Motogenic signal
A signal, such as a growth factor, which activates the cell motility machinery without providing directional information to trigger intrinsic cell migration
- Extracellular matrix
A network of proteins and polysaccharides secreted by cells; provides structural support for cells within tissues
- Matrigel
Commercially available basement membrane matri composed primarily of laminin and collagen, which can be used as a 3D tissue culture model for studying cell migration and differentiation
- Focal adhesion
A large protein complex that mediates the attachment of the extracellular matrix to the actin cytoskeleton through an integrin heterodimer
- Morpholino
A synthetic molecule that binds to specific mRNAs and blocks their translation; used to assay protein function
- Gastrulation
The process during embryogenesis when the embryo is transformed from a hollow sphere of cells to a structure with three germ layers: ectoderm, mesoderm, and endoderm
- Metastasis
The spreading of cancer cells from a site of origin to distant parts of the body that often involves cell motility
- Contact guidance
he process by which cells are guided by topographical structures often associated with the extracellular matrix
- Glu-tubulin
A posttranslational modification of tubulin associated with MT stabilization. Also known as detyrosinated tubulin
- Lamellipodium
A flattened, actin-rich protrusion found at the leading edge of a migrating cell
- Scratch-wound healing assay
An in vitro cell motility assay. When an area of cells is cleared (scratched) in a monolayer of cells, cells will directionally migrate into and close the wound
- Anterograde transport
Transport of material from the Golgi to the cell surface through the secretory pathway
- Retrograde flow
Net movement of filamentous actin away from the cell edge
- Cell cortex
An actin-rich layer near the inner surface of the plasma membrane
- Caveolae
Flask-shaped invaginations of the plasma membrane that contribute to cell polarity and directional cell migration
- GEF
Guanine nucleotide exchange factor; activates small G proteins by catalyzing the exchange of GDP for GTP
- GAP
GTPase activating protein; accelerates the intrinsic GTPase activity of small G proteins to inactivate them
- Integrins
A large family of transmembrane proteins that exists in the plasma membrane as heterodimers of α and β subunits; they frequently mediate the interaction of cells with the extracellular matrix
- Lamellae
Flattened region immediately behind the lamellipodium
- Clathrin coated pits
Invaginations in the plasma membrane coated by lattices made up of the protein clathrin which are precursors to endocytic vesicles
- Disheveled
A cytoplasmic protein which participates in Wnt signalling immediately downstream of frizzled receptors
- Neural crest
A group of cells that migrates to various parts of the embryo and form, in part, the bones of the skull, teeth, and portions of the peripheral nervous system
- Proteoglycan
A protein core linked to one or more long, linear, and highly charged polysaccharide chains
- Contact inhibition
Response when a migrating cell contacts another and changes direction to move away from the point of contact
- Rab proteins
Large family of small GTPases found on organelles and the plasma membrane that confer specificity on vesicle docking and membrane trafficking
- Arp2/3
A protein complex that nucleates actin filament growth from the sides of pre-existing actin filaments to form branched actin networks
References
- 1.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Stoker M, Gherardi E. Regulation of cell movement: the motogenic cytokines. Biochim Biophys Acta. 1991;1072:81–102. doi: 10.1016/0304-419x(91)90008-9. [DOI] [PubMed] [Google Scholar]
- 4.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR. Platelet-derived growth factor is chemotactic for fibroblasts. J Cell Biol. 1982;92:584–8. doi: 10.1083/jcb.92.2.584. Besides identifying PDGF as a chemotactic factor for fibroblasts, this paper is an excellent resource for understanding how to distinguish between haptotactic, chemotactic, chemokinetic, and mitogenic responses when studying cell motility, as well as why it is important to do so. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrieumerlou C, Meyer T. A local coupling model and compass parameter for eukaryotic chemotaxis. Dev Cell. 2005;8:215–27. doi: 10.1016/j.devcel.2004.12.007. Challenged fundamental assumptions underlying directed cell migration by showing that local signalling within lamellipodia generates small protrusions towards the source of the guidance cue as the basis of chemotaxis in vitro, rather than global integration of competing signals. [DOI] [PubMed] [Google Scholar]
- 6.Bourne HR, Weiner O. A chemical compass. Nature. 2002;419:21. doi: 10.1038/419021a. [DOI] [PubMed] [Google Scholar]
- 7.Carter SB. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965;208:1183–7. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin Cell Dev Biol. 2008 doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gail MH, Boone CW. The locomotion of mouse fibroblasts in tissue culture. Biophys J. 1970;10:980–93. doi: 10.1016/S0006-3495(70)86347-0. One of the first studies to examine fibroblast migration in culture by combining time-lapse imaging and quantitative analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bear JE, et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–21. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, et al. PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell. 2007;12:603–14. doi: 10.1016/j.devcel.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosgraaf L, et al. RasGEF-containing proteins GbpC and GbpD have differential effects on cell polarity and chemotaxis in Dictyostelium. J Cell Sci. 2005;118:1899–910. doi: 10.1242/jcs.02317. [DOI] [PubMed] [Google Scholar]
- 14.Pankov R, et al. A Rac switch regulates random versus directionally persistent cell migration. J Cell Biol. 2005;170:793–802. doi: 10.1083/jcb.200503152. This paper determined that Rac activity can control the pattern of cell migration during both intrinsic and directed motility by regulating the formation of lateral protrusions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. doi: 10.1038/ncb1536. [DOI] [PubMed] [Google Scholar]
- 16.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–84. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–47. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol. 2008;9:455–63. doi: 10.1038/nrm2419. [DOI] [PubMed] [Google Scholar]
- 19.Petri B, Phillipson M, Kubes P. The physiology of leukocyte recruitment: an in vivo perspective. J Immunol. 2008;180:6439–46. doi: 10.4049/jimmunol.180.10.6439. [DOI] [PubMed] [Google Scholar]
- 20.Martini FJ, et al. Biased selection of leading process branches mediates chemotaxis during tangential neuronal migration. Development. 2009;136:41–50. doi: 10.1242/dev.025502. [DOI] [PubMed] [Google Scholar]
- 21.Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr Biol. 2009;19:260–5. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailly M, Yan L, Whitesides GM, Condeelis JS, Segall JE. Regulation of protrusion shape and adhesion to the substratum during chemotactic responses of mammalian carcinoma cells. Exp Cell Res. 1998;241:285–99. doi: 10.1006/excr.1998.4031. [DOI] [PubMed] [Google Scholar]
- 23.Harms BD, Bassi GM, Horwitz AR, Lauffenburger DA. Directional persistence of EGF-induced cell migration is associated with stabilization of lamellipodial protrusions. Biophys J. 2005;88:1479–88. doi: 10.1529/biophysj.104.047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garber B. Quantitative studies on the dependence of cell morphology and motility upon the fine structure of the medium in tissue culture. Exp Cell Res. 1953;5:132–46. doi: 10.1016/0014-4827(53)90099-8. [DOI] [PubMed] [Google Scholar]
- 25.Weiss P, Garber B. Shape and Movement of Mesenchyme Cells as Functions of the Physical Structure of the Medium: Contributions to a Quantitative Morphology. Proc Natl Acad Sci U S A. 1952;38:264–80. doi: 10.1073/pnas.38.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunn GA, Heath JP. A new hypothesis of contact guidance in tissue cells. Exp Cell Res. 1976;101:1–14. doi: 10.1016/0014-4827(76)90405-5. [DOI] [PubMed] [Google Scholar]
- 27.Nakatsuji N, Johnson KE. Cell locomotion in vitro by Xenopus laevis gastrula mesodermal cells. Cell Motil. 1982;2:149–61. doi: 10.1002/cm.970020206. [DOI] [PubMed] [Google Scholar]
- 28.Nakatsuji N, Johnson KE. Ectodermal fragments from normal frog gastrulae condition substrata to support normal and hybrid mesodermal cell migration in vitro. J Cell Sci. 1984;68:49–67. doi: 10.1242/jcs.68.1.49. [DOI] [PubMed] [Google Scholar]
- 29.Nakatsuji N, Johnson KE. Experimental manipulation of a contact guidance system in amphibian gastrulation by mechanical tension. Nature. 1984;307:453–5. doi: 10.1038/307453a0. [DOI] [PubMed] [Google Scholar]
- 30.Wood A. Contact guidance on microfabricated substrata: the response of teleost fin mesenchyme cells to repeating topographical patterns. J Cell Sci. 1988;90 (Pt 4):667–81. doi: 10.1242/jcs.90.4.667. [DOI] [PubMed] [Google Scholar]
- 31.Webb A, Clark P, Skepper J, Compston A, Wood A. Guidance of oligodendrocytes and their progenitors by substratum topography. J Cell Sci. 1995;108 (Pt 8):2747–60. doi: 10.1242/jcs.108.8.2747. [DOI] [PubMed] [Google Scholar]
- 32.Gomez N, Chen S, Schmidt CE. Polarization of hippocampal neurons with competitive surface stimuli: contact guidance cues are preferred over chemical ligands. J R Soc Interface. 2007;4:223–33. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–92. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loesberg WA, et al. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials. 2007;28:3944–51. doi: 10.1016/j.biomaterials.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 36.Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci U S A. 2004;101:18024–9. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 38.Amatangelo MD, Bassi DE, Klein-Szanto AJ, Cukierman E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am J Pathol. 2005;167:475–88. doi: 10.1016/S0002-9440(10)62991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle AD, Wang FW, Matsumoto K, Yamada KM. One-dimensional topography underlies three-dimensional fibrillar cell migration. J Cell Biol. 2009;184:481–90. doi: 10.1083/jcb.200810041. Establishes that aligned fibrillar matrices can be functionally mimicked by simple one-dimensional lines, but not two-dimensional surfaces, to promote directional cell migration; also introduces a novel micropatterning technique for generating matrix patterns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnell E, et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28:3012–25. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Tysseling-Mattiace VM, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–23. doi: 10.1523/JNEUROSCI.0143-08.2008. Demonstrates the in vivo use of self-assembling peptide amphiphile molecules to generate nanofibres that inhibit glial cell differentiation and scar tissue formation while promoting motor and sensory neuron regeneration at the site of a spinal cord injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sidani M, Wyckoff J, Xue C, Segall JE, Condeelis J. Probing the microenvironment of mammary tumors using multiphoton microscopy. J Mammary Gland Biol Neoplasia. 2006;11:151–63. doi: 10.1007/s10911-006-9021-5. [DOI] [PubMed] [Google Scholar]
- 43.Provenzano PP, et al. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Provenzano PP, Inman DR, Eliceiri KW, Trier SM, Keely PJ. Contact Guidance Mediated Three-Dimensional Cell Migration is Regulated by Rho/ROCK-Dependent Matrix Reorganization. Biophys J. 2008;95:5374–5384. doi: 10.1529/biophysj.108.133116. This article uses multiple technical methods to demonstrate that cancer cells reorganize the extracellular matrix perpendicular to tumor explants, a process that depends on Rho kinase and precedes cell migration and invasion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–80. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 46.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol. 2008;9:846–59. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 47.Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci. 2004;117:1291–300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 48.Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–98. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- 49.Shen Y, et al. Nudel binds Cdc42GAP to modulate Cdc42 activity at the leading edge of migrating cells. Dev Cell. 2008;14:342–53. doi: 10.1016/j.devcel.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–63. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 51.Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A. 1982;79:2603–7. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–87. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- 53.Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–96. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- 54.Bergmann JE, Kupfer A, Singer SJ. Membrane insertion at the leading- edge of motile fibroblasts. Proc Natl Acad Sci U S A. 1983;80:1367–1371. doi: 10.1073/pnas.80.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prigozhina NL, Waterman-Storer CM. Protein kinase D-mediated anterograde membrane trafficking is required for fibroblast motility. Current Biology. 2004;14:88–98. doi: 10.1016/j.cub.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Tan I, Yong J, Dong JM, Lim L, Leung T. A tripartite complex containing MRCK modulates lamellar actomyosin retrograde flow. Cell. 2008;135:123–36. doi: 10.1016/j.cell.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Nishita M, et al. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol. 2006;175:555–62. doi: 10.1083/jcb.200607127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nomachi A, et al. Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migration by activating c-Jun N-terminal kinase via actin-binding protein filamin A. J Biol Chem. 2008;283:27973–81. doi: 10.1074/jbc.M802325200. [DOI] [PubMed] [Google Scholar]
- 60.Pestonjamasp KN, et al. Rac1 links leading edge and uropod events through Rho and myosin activation during chemotaxis. Blood. 2006;108:2814–20. doi: 10.1182/blood-2006-01-010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–22. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–72. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- 63.Nishimura T, et al. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–7. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- 64.Pegtel DM, et al. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol. 2007;17:1623–34. doi: 10.1016/j.cub.2007.08.035. This paper shows that the Par polarity complex, previously known to establish apical-basal polarity, drives front-rear polarization in migrating keratinocytes; blocking Par complex function increases random migration and inhibits chemotaxis, probably by interfering with microtubule stabilization at the leading edge downstream of Rac signalling. [DOI] [PubMed] [Google Scholar]
- 65.Nakayama M, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–15. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 66.Drabek K, et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol. 2006;16:2259–64. doi: 10.1016/j.cub.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 67.Beardsley A, et al. Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J Biol Chem. 2005;280:3541–7. doi: 10.1074/jbc.M409040200. [DOI] [PubMed] [Google Scholar]
- 68.Grande-Garcia A, et al. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol. 2007;177:683–94. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.del Pozo MA, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7:901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–7. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 71.Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–25. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- 72.Jin M, et al. Ca2+-dependent regulation of rho GTPases triggers turning of nerve growth cones. J Neurosci. 2005;25:2338–47. doi: 10.1523/JNEUROSCI.4889-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei C, et al. Calcium flickers steer cell migration. Nature. 2009;457:901–5. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- 75.Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–9. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Haastert PJ, Keizer-Gunnink I, Kortholt A. Essential role of PI3-kinase and phospholipase A2 in Dictyostelium discoideum chemotaxis. J Cell Biol. 2007;177:809–16. doi: 10.1083/jcb.200701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haugh JM, Codazzi F, Teruel M, Meyer T. Spatial sensing in fibroblasts mediated by 3′ phosphoinositides. J Cell Biol. 2000;151:1269–80. doi: 10.1083/jcb.151.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiger MC, et al. Spontaneous phosphoinositide 3-kinase signaling dynamics drive spreading and random migration of fibroblasts. J Cell Sci. 2009;122:313–23. doi: 10.1242/jcs.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108 ( Pt 1):225–33. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- 80.Oude Weernink PA, Han L, Jakobs KH, Schmidt M. Dynamic phospholipid signaling by G protein-coupled receptors. Biochim Biophys Acta. 2007;1768:888–900. doi: 10.1016/j.bbamem.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Chae YC, et al. Phospholipase D activity regulates integrin-mediated cell spreading and migration by inducing GTP-Rac translocation to the plasma membrane. Mol Biol Cell. 2008;19:3111–23. doi: 10.1091/mbc.E07-04-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JH, Kim HW, Jeon H, Suh PG, Ryu SH. Phospholipase D1 regulates cell migration in a lipase activity-independent manner. J Biol Chem. 2006;281:15747–56. doi: 10.1074/jbc.M509844200. [DOI] [PubMed] [Google Scholar]
- 83.Nishikimi A, et al. Sequential Regulation of DOCK2 Dynamics by Two Phospholipids during Neutrophil Chemotaxis. Science. 2009 doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monypenny J, et al. Cdc42 and Rac family GTPases regulate mode and speed but not direction of primary fibroblast migration during platelet-derived growth factor-dependent chemotaxis. Mol Cell Biol. 2009;29:2730–47. doi: 10.1128/MCB.01285-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kraynov VS, et al. Localized Rac activation dynamics visualized in living cells. Science. 2000;290:333–7. doi: 10.1126/science.290.5490.333. This paper highlights the importance of considering cell biological dynamics when studying signalling mechanisms driving cell migration; live cell imaging shows that Rac activity is targeted to the leading edge of migrating fibroblasts. [DOI] [PubMed] [Google Scholar]
- 86.Vidali L, Chen F, Cicchetti G, Ohta Y, Kwiatkowski DJ. Rac1-null mouse embryonic fibroblasts are motile and respond to platelet-derived growth factor. Mol Biol Cell. 2006;17:2377–90. doi: 10.1091/mbc.E05-10-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bard JB, Hay ED. The behavior of fibroblasts from the developing avian cornea. Morphology and movement in situ and in vitro. J Cell Biol. 1975;67:400–18. doi: 10.1083/jcb.67.2.400. This comprehensive study of the comparative morphology and behaviour of the same population of fibroblasts migrating on glass, within 3D collagen gels, or in situ within the developing avian cornea provides a clear warning about the dangers of using 2D environments to understand cell migration normally occurring within 3D tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bass MD, et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–38. doi: 10.1083/jcb.200610076. This study brings together the themes of the extracellular environment, localized intracellular signalling, and random versus directionally persistent cell migration by showing that syndecan-4 senses external membrane topography to limit Rac activity to the leading edge and promote directionally persistent cell migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 90.Yip SC, et al. The distinct roles of Ras and Rac in PI 3-kinase-dependent protrusion during EGF-stimulated cell migration. J Cell Sci. 2007;120:3138–46. doi: 10.1242/jcs.005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 1998;17:6932–41. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sells MA, et al. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–10. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- 93.Suetsugu S, Yamazaki D, Kurisu S, Takenawa T. Differential roles of WAVE1 and WAVE2 in dorsal and peripheral ruffle formation for fibroblast cell migration. Dev Cell. 2003;5:595–609. doi: 10.1016/s1534-5807(03)00297-1. [DOI] [PubMed] [Google Scholar]
- 94.Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–49. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–64. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- 96.Primo L, et al. Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol. 2007;176:1035–47. doi: 10.1083/jcb.200607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carlier MF, et al. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–22. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell. 2005;16:649–64. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danen EH, et al. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–26. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–25. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sidani M, et al. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J Cell Biol. 2007;179:777–91. doi: 10.1083/jcb.200707009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–96. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Rheenen J, et al. EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol. 2007;179:1247–59. doi: 10.1083/jcb.200706206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frantz C, et al. Cofilin is a pH sensor for actin free barbed end formation: role of phosphoinositide binding. J Cell Biol. 2008;183:865–79. doi: 10.1083/jcb.200804161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 106.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Santolini E, et al. Numb is an endocytic protein. J Cell Biol. 2000;151:1345–52. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–43. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roberts M, Barry S, Woods A, van der Sluijs P, Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol. 2001;11:1392–402. doi: 10.1016/s0960-9822(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 110.Caswell PT, et al. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–55. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caswell PT, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. doi: 10.1016/j.devcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 112.Strachan LR, Condic ML. Cranial neural crest recycle surface integrins in a substratum-dependent manner to promote rapid motility. J Cell Biol. 2004;167:545–54. doi: 10.1083/jcb.200405024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mostafavi-Pour Z, et al. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol. 2003;161:155–67. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saoncella S, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci U S A. 1999;96:2805–10. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An alpha4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005;7:343–52. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- 116.De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–97. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- 117.Matthews HK, et al. Directional migration of neural crest cells in vivo is regulated by Syndecan-4/Rac1 and non-canonical Wnt signaling/RhoA. Development. 2008;135:1771–80. doi: 10.1242/dev.017350. [DOI] [PubMed] [Google Scholar]
- 118.Trainor PA. Specification of neural crest cell formation and migration in mouse embryos. Semin Cell Dev Biol. 2005;16:683–93. doi: 10.1016/j.semcdb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 119.Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954;6:293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- 120.Carmona-Fontaine C, et al. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456:957–61. doi: 10.1038/nature07441. An elegant study showing how the basic cell biological processes of contact inhibition of movement, non-canonical Wnt signalling, and RhoA-mediated actin-myosin contraction combine to trigger directional migration of neural crest cells in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Totsukawa G, et al. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J Cell Biol. 2000;150:797–806. doi: 10.1083/jcb.150.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Keymeulen A, et al. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J Cell Biol. 2006;174:437–45. doi: 10.1083/jcb.200604113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wessels D, Lusche DF, Kuhl S, Heid P, Soll DR. PTEN plays a role in the suppression of lateral pseudopod formation during Dictyostelium motility and chemotaxis. J Cell Sci. 2007;120:2517–31. doi: 10.1242/jcs.010876. [DOI] [PubMed] [Google Scholar]
- 125.Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176:573–80. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Even-Ram S, et al. Myosin IIA regulates cell motility and actomyosin- microtubule crosstalk. Nat Cell Biol. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 127.Lo CM, et al. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15:982–9. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183:543–54. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Weeraratna AT, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–88. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 130.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–9. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dodd J, Jessell TM. Axon guidance and the patterning of neuronal projections in vertebrates. Science. 1988;242:692–9. doi: 10.1126/science.3055291. [DOI] [PubMed] [Google Scholar]
- 132.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 133.Heit B, et al. PTEN functions to ‘prioritize’ chemotactic cues and prevent ‘distraction’ in migrating neutrophils. Nat Immunol. 2008;9:743–52. doi: 10.1038/ni.1623. This paper combines in vitro and in vivo approaches to model the complex environment found during the inflammatory neutrophil response, where cells are often forced to choose between competing guidance cues; it exemplifies the innovative experimentation needed to decipher the complex mechanisms underlying directional cell migration. [DOI] [PubMed] [Google Scholar]
- 134.Gail M. Locomotion of Tissue Cells. Elsevier Science Publishing Co., Inc; North Holland, Amsterdam: 1973. pp. 287–302. [Google Scholar]
- 135.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 136.Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 137.Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–77. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 138.Kestler HA, Kuhl M. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci. 2008;363:1333–47. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Croce JC, McClay DR. The canonical Wnt pathway in embryonic axis polarity. Semin Cell Dev Biol. 2006;17:168–74. doi: 10.1016/j.semcdb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 140.van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal. 2008;1:re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]