Abstract
Recent studies have demonstrated that kynurenic acid (KYNA), a compound produced endogenously by the interferon-γ-induced degradation of tryptophan by indoleamine 2,3-dioxygenase, activates the previously orphaned G protein-coupled receptor, GPR35. This receptor is expressed in immune tissues, although its potential function in immunomodulation remains to be explored. We determined that GPR35 was most highly expressed on human peripheral monocytes. In an in vitro vascular flow model, KYNA triggered the firm arrest of monocytes to both fibronectin and ICAM-1, via β1 integrin- and β2 integrin-mediated mechanisms, respectively. Incubation of monocytes with pertussis toxin prior to use in flow experiments significantly reduced the KYNA-induced monocyte adhesion, suggesting that adhesion is triggered by a Gi-mediated process. Furthermore, KYNA-triggered adhesion of monocytic cells was reduced by short hairpin RNA-mediated silencing of GPR35. Although GPR35 is expressed at slightly lower levels on neutrophils, KYNA induced firm adhesion of these cells to an ICAM-1-expressing monolayer as well. KYNA also elicited neutrophil shedding of surface L-selectin, another indicator of leukocyte activation. Taken together, these data suggest that KYNA could be an important early mediator of leukocyte recruitment.
Leukocyte recruitment into tissue compartments is a tightly regulated process orchestrated by chemokines (1). Chemokines convert leukocyte rolling or tethering on the vascular endothelium to firm arrest via the activation of leukocyte surface integrins (2, 3). As chemoattractants, chemokines subsequently play an important role in the directional migration of leukocytes through tissues.
Chemoattractant receptors are a subtype of G protein-coupled receptors (GPCRs),3 one of the largest known families of human proteins. Chemoattractant receptors bind a variety of agonists, including proteins such as interleukin-8 (IL-8, CXCL8) (4) and monocyte chemoattractant protein-1 (MCP-1, CCL2) (5), small peptides such as fMLP (6), as well as bioactive lipids including leukotriene B4 (7). As such, chemoattractant receptors mirror the entire family of GPCRs, which can be activated by ligands ranging in size from metabolites to large proteins (8).
Because GPCRs serve as targets for therapeutic intervention, considerable activity has gone into the identification of both putative GPCR genes and the ligands for the resulting receptors (8). Recently, the tryptophan metabolite kynurenic acid (KYNA) was identified as an agonist for the previously “orphaned” receptor GPR35. KYNA was shown to elicit intracellular release of Ca2+ in Chinese hamster ovary cells in which GPR35 was co-expressed in the context of a chimeric G protein signaling apparatus. HEK93 cells transfected with GPR35 and Gqo proteins accumulated inositol phosphate upon exposure to KYNA. KYNA also induced internalization of GPR35 on HeLa cells, which is commonly seen following the activation of GPRs with agonists such as chemokines (9).
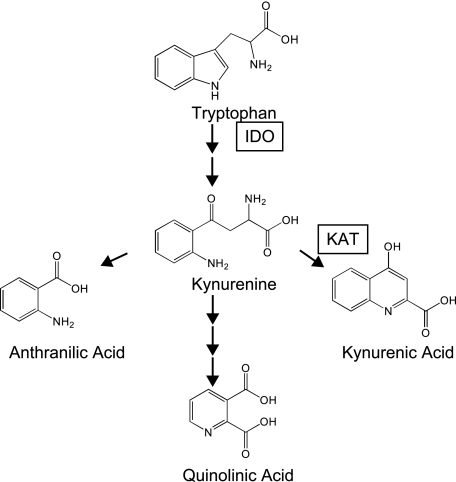
KYNA is produced endogenously as a result of the degradation of tryptophan (Fig. 1). In most tissues, the rate-limiting step in this degradation is the conversion of tryptophan to N-formylkynurenine, a reaction that can be catalyzed by the inducible enzyme indolemine 2,3-dioxygenase (IDO). IDO is induced by interferon-γ, which leads to a substantial increase in the concentration of KYNA and other tryptophan catabolites during inflammatory processes.
FIGURE 1.
Tryptophan catabolism pathway. Schematic diagram of tryptophan catabolism. Tryptophan is converted to N-formylkynurenine by IDO, and is then deformylated to form kynurenine. Kynurenine can be converted to kynurenic acid via kynurenine aminotransferases I and II (KAT), or converted via alternative pathways to anthranilic acid or quinolinic acid, a NAD precursor.
Previous work has demonstrated that KYNA acts as a neuroprotective agent by antagonizing both N-methyl-d-aspartate and α7-nicotinic receptors (10–12). Many peripheral tissues, including the heart and vasculature, are also capable of generating KYNA (13, 14), although its role in these tissues has not been well defined. Expression analysis indicates that GPR35 is selectively present in immune and intestinal tissues. From a functional perspective, KYNA treatment inhibited the secretion of tumor necrosis factor-α by mononuclear cells treated with lipopolysaccharide (9). This finding is in general agreement with the prevailing literature suggesting that IDO-mediated tryptophan catabolism appears to play a significant counter-regulatory role in dampening down the activation of the immune system (15). However, the potential spectrum of physiological roles for KYNA in immune modulation remains incompletely characterized.
Given the reported high level of GPR35 expression on circulating leukocytes, here we tested the hypothesis that KYNA may play a chemokine-like role in modulating leukocyte-endothelial interactions under physiologically relevant flow conditions as seen in the vasculature. We explored the intracellular signaling pathways by which KYNA may be activating leukocytes, as well as the surface integrins modulating these effects. We report the unanticipated finding that KYNA is sufficient to drive early leukocyte adhesion.
EXPERIMENTAL PROCEDURES
Materials
RPMI 1640 and Dulbecco's phosphate-buffered saline with or without Ca2+ and Mg2+ were obtained from Invitrogen. KYNA, tryptophan, anthranilic acid (AA), and pertussis toxin (PTX) were obtained from Sigma. Human chemokines MCP-1 and IL-8 were obtained from Peprotech (Rocky Hill, NJ).
Cell Culture
Human umbilical vein endothelial cells (HUVECs), endothelial growth medium-2, and endothelial basal medium-2 were obtained from Lonza (Basel, Switzerland); HUVECs were cultured according to the supplier's directions. To prepare slides for flow adhesion studies, 0.8-cm2 chambers were attached to Permanox slides (Nunc, Rochester, NY) using 2% agarose in phosphate-buffered saline. The interior surface of the resulting well was coated with 0.05% fibronectin (Sigma) in phosphate-buffered saline (with Ca2+ and Mg2+) for 1 h at 37 °C. For monolayers, ∼20,000 freshly trypsinized HUVECs (passages 2–4) were seeded in the fibronectin-coated wells and grown at 37 °C for 18 h prior to transfection with adenoviral ICAM-1 vector as previously described (16) using endothelial basal medium-2 as a transfection medium and endothelial growth medium-2 for growth conditions. HUVEC monolayers were used in flow experiments 48 h after transfection.
Antibodies
HP2/1, a monoclonal antibody to α4 integrin (CD49d) was obtained from Immunotech (Marseille, France). TS1/18, a monoclonal antibody to human β2 integrin (CD18) was obtained from Endogen (Rockford, IL). Mouse IgG1 isotype control antibody was obtained from Pharmingen. LM609, a blocking antibody to αvβ3 integrin was obtained from Millipore (Billerica, MA).
Leukocyte Isolation
Leukocytes were freshly prepared from healthy human donors. Mononuclear cells were isolated via Ficoll-Hypaque (LSM, Fisher Scientific, Fair Lawn, NJ) density gradient centrifugation and washed in RPMI 1640. CD14+ monocytes were further purified from this subset of cells by depletion of other mononuclear cell types with magnetic-activated cell separation (Monocyte Isolation Kit II, Miltenyi Biotec, Auburn, CA). Neutrophils were isolated by Ficoll-Hypaque gradient, followed by dextran sedimentation and hypotonic lysis of contaminating red blood cells (17).
Quantitative PCR Gene Expression Analysis
Quantitative PCR (QPCR) analysis of transcript levels of GPR35 and other chemokine receptors was performed on freshly isolated leukocytes. Total cellular RNA was isolated using RNeasy columns (Qiagen, Valencia, CA), treated with DNase I (Invitrogen), and reverse transcribed to cDNA using TaqMan reagents (Applied Biosystems, Foster, CA). QPCR was performed on an Mx4000 quantitative PCR instrument (Stratagene, La Jolla, CA) using Power SYBR Green PCR reagents (Applied Biosystems). Primers for GPR35 were as previously described (9); primers for chemokine receptors were the generous gift of Dr. Terry Means, and were designed as previously described (18).
Measurement of L-selectin Shedding
To measure shedding of L-selectin, 3 × 106 neutrophils in a total volume of 200 μl in RPMI 1640 were incubated with test compounds for 30 min at room temperature. Cleaved L-selectin in the supernatant was measured using the Human sL-Selectin ELISA kit (BIOSOURCE, Nivelles, Belgium).
Adhesion under Flow Conditions
Flow adhesion studies were carried out in a parallel plate laminar flow chamber (Immunetics, Cambridge, MA), using either phosphate-buffered saline with Ca2+ and Mg2+ (for fibronectin-coated slides) or RPMI + 1% autologous serum (for ICAM-1-transfected monolayers) as perfusion medium. Estimated shear stress ranged from 1 dyne/cm2 (0.2 ml/min) for fibronectin-coated surfaces to 1.75 dyne/cm2 (0.35 ml/min) for the ICAM-1-expressing monolayers. Compounds to be tested were added to the leukocyte reservoir, and the adherent cells on the test surface were counted at the indicated time in at least three fields. For inhibitor studies, leukocytes were incubated with the appropriate antibody for 10 min on ice, or with PTX for 60 min at 37 °C, prior to introduction into the flow chamber.
shRNA Knockdown of GPR35
Plasmids encoding lentiviruses expressing shRNAs were obtained from the library of The RNAi Consortium (TRC) at the Broad Institute (19). Plasmids were purified using the QiaPrep miniprep kit (Qiagen), then transfected into HEK 293T cells with a three-plasmid system to produce lentivirus (19, 20). THP-1 cells at 2 × 105 cells/ml were infected with the virus in media containing 7.5 μg/ml Polybrene, and centrifuged for 30 min at 37 °C and 2000 × g. Three days post-infection, infected cells were selected by adjusting the media to 3 μg/ml puromycin. Degree of knockdown was determined by QPCR analysis as described above; the cell strain with the highest degree of GPR35 knockdown (Table S1) was selected for further study of adhesion under flow conditions.
Statistical Analysis
All data are presented as mean ± S.E. For significance, an unpaired two-tailed Student's t test was performed. The null hypothesis was rejected at p < 0.05.
RESULTS
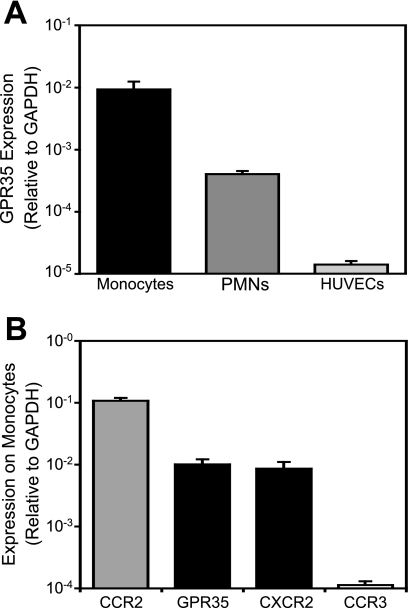
To assess the potential role of KYNA in leukocyte adhesion processes, we first measured the level of GPR35 mRNA in several leukocyte cell types, as well as in endothelial cells. GPR35 was most highly expressed in monocytes, with significant expression in neutrophils as well. By contrast, QPCR experiments suggested virtually no expression in endothelial cells (Fig. 2A). The level of GPR35 expression on monocytes was less than that of the prototypical monocyte chemokine receptor, CCR2, although comparable with that of CXCR2 (Fig. 2B). Activation of both of these receptors results in robust monocyte adhesion (3) and also contributes to monocyte trafficking in atherosclerosis in vivo (21, 22). GPR35 expression greatly exceeded that of CCR3, a chemokine receptor that is highly expressed on basophils and eosinophils but present only at very low levels on monocytes (23).
FIGURE 2.
GPR35 expression in human cells measured by QPCR. RNA was harvested from freshly isolated leukocyte subtypes and HUVECs, and receptor expression was determined with real-time QPCR using SYBR Green fluorescence relative to that of ROX reference dye to determine the transcript copy number; this figure was then normalized to the copy number of the housekeeping gene, glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) as previously performed (40, 41). Data shown are mean ± S.E. from at least 3 donors. A, GPR35 levels in leukocyte subtypes and HUVECs. B, GPR35 expression in monocytes is comparable with that of the chemokine receptor CXCR2, and substantially greater than CCR3.
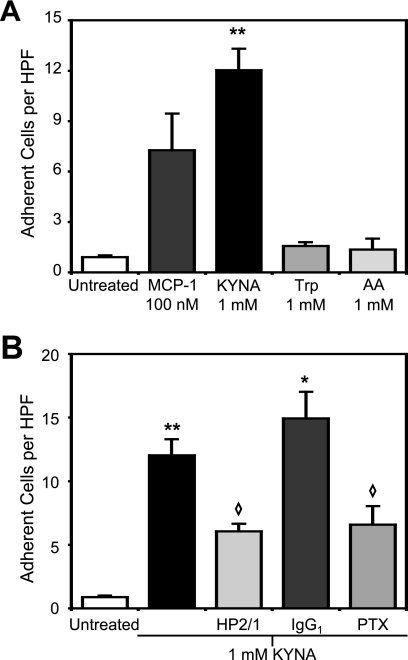
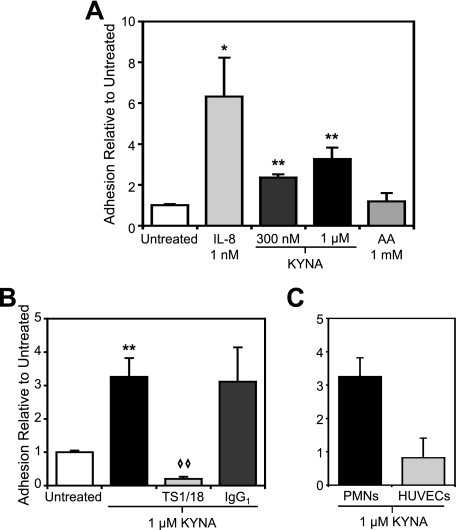
To test whether KYNA was sufficient to induce β1 integrin-mediated firm arrest under physiological flow conditions, we employed a flow chamber to pass monocytes over a fibronectin-coated surface. At a shear stress of 1.0 dyne/cm2, KYNA treatment resulted in a 13-fold increase in the number of adherent cells as compared with baseline, slightly higher than adhesion induced by the CCR2 agonist MCP-1. Tryptophan and AA, two metabolites closely related to KYNA that do not bind GPR35 (9), had no effect on the adhesion of monocytes (Fig. 3A). Pretreatment of the monocytes with HP2/1, an α4 integrin-blocking antibody that interferes with α4β1-mediated arrest, significantly reduced the number of adherent cells induced by KYNA exposure. By contrast, pretreatment with an isotype-matched control antibody did not result in any reduction in KYNA-induced firm arrest (Fig. 3B).
FIGURE 3.
Kynurenic acid induces firm arrest of monocytes on fibronectin through a mechanism that is β1 integrin-mediated and Gi-coupled. Interaction of monocytes with a fibronectin-coated surface was studied at 1.0 dyne/cm2. Adhesion was quantified 8 min after the introduction of the indicated compound. A, KYNA induces firm arrest of monocytes on fibronectin (**, p < 0.005), whereas tryptophan (Trp) and AA treatment does not differ from background. B, pretreatment with the α4 integrin-blocking antibody HP2/1 interferes with KYNA-induced adhesion (◇, p < 0.05 when compared with KYNA adhesion), whereas pretreatment with isotype-matched control antibody has no effect (*, p < 0.05 relative to untreated). Pretreatment of monocytes with PTX also significantly reduced KYNA-induced arrest. n ≥ 2 for all conditions shown.
Many chemokine-triggered effector functions in leukocytes are dependent on the G protein αi subunit of the G protein heterotrimer (24). To assess whether the effects of KYNA on monocyte adhesion involved this pathway, we pretreated cells with PTX prior to introducing them into the flow chamber. PTX exerts its effects by ADP-ribosylating the α subunit of the G protein heterotrimer, thus preventing the complex from coupling with receptors on the surface of the cell (25). Incubation of monocytes with PTX prior to use in flow experiments significantly reduced the KYNA-induced adhesion of monocytes to fibronectin (Fig. 3B), confirming that this adhesion is triggered by a Gi-mediated process.
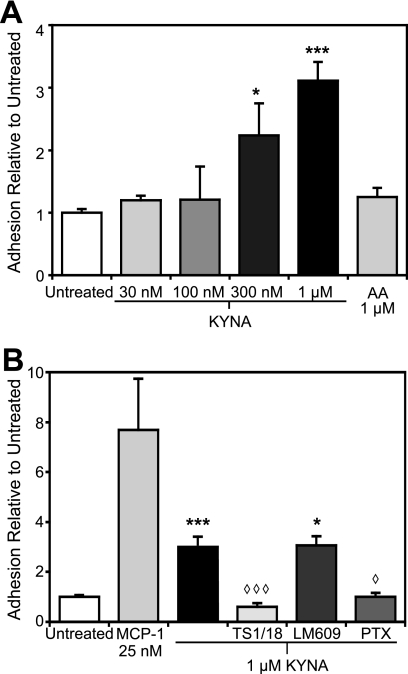
Interactions between activated β2 integrins and intercellular adhesion molecule (ICAM)-1 are also sufficient to confer monocyte arrest under flow conditions (3). We therefore examined the adhesion of monocytes to endothelial monolayers expressing ICAM-1 using an adenoviral expression system. We have previously demonstrated that this system is extremely sensitive to adhesive triggers (3). KYNA treatment of monocytes in this system increased adhesion in a dose-dependent fashion, with significant arrest observed as low as 300 nm, while treatment with 1 μm anthranilic acid elicited no effect (Fig. 4A). This is significantly lower than the concentration necessary to induce adhesion to fibronectin surfaces, consistent with previous reports that firm arrest of leukocytes on adhesion molecule-expressing endothelial monolayers (as opposed to purified protein immobilized on coverslip) is highly sensitive to chemoattractant agonists (3). To verify the specificity of the observed interactions, we pretreated the leukocytes with the β2 integrin-blocking antibody, TS1/18. Pretreatment with this antibody, but not an αvβ3-blocking antibody, LM609, abolished the KYNA-induced response (Fig. 4B). As with adhesion to fibronectin, pretreatment of monocytes with PTX significantly reduced adhesion to ICAM-1-expressing monolayers, confirming Gi involvement (Fig. 4B). Although KYNA-triggered response of monocytes on fibronectin was greater than the MCP-1-triggered response, the reverse was observed with the ICAM-1 monolayers.
FIGURE 4.
Kynurenic acid induces firm arrest of monocytes on ICAM-1-expressing HUVECs in a dose-dependent fashion via a β2 integrin-mediated mechanism. HUVECs were transduced with adenoviral ICAM and cultured for 48 h. Interaction of monocytes with the ICAM-1-expressing monolayer was studied at 1.75 dyne/cm2. Adhesion was quantified 5 min after the introduction of the indicated compound. Arrested cell counts were normalized to the level of adhesion of untreated monocytes in each experiment. A, KYNA induces firm arrest of monocytes to ICAM-1-expressing HUVECs in a dose-dependent fashion (*, p < 0.05; ***, p < 0.0005), whereas AA treatment does not differ from background. B, pre-treatment of monocytes with the β2 integrin-blocking antibody TS1/18, but not the αvβ3-blocking antibody LM609, abolished KYNA-induced binding of monocytes, as did pretreatment with PTX (◇, p < 0.05; ◇ ◇ ◇, p < 0.005). n ≥ 3 for all conditions shown.
As KYNA and MCP-1 would both be expected to be present under certain inflammatory circumstances (and potentially other chemokines as well), we tested how the addition of both agonists modulated adhesion. Simultaneous treatment with both MCP-1 and KYNA resulted in an additive, but not synergistic effect on firm arrest (data not shown).
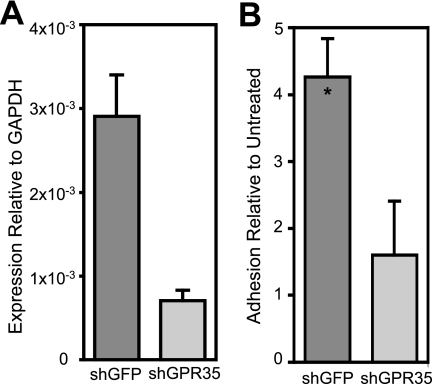
To confirm that the observed adhesion was indeed due to activation of GPR35 by KYNA, we used shRNA to generate THP-1 cells with reduced expression of GPR35. GPR35-silenced cells showed a marked reduction in GPR35 mRNA expression relative to control cells infected with an anti-green fluorescent protein lentivirus (Fig. 5A). Green fluorescent protein-silenced control THP-1 cells retained a level of KYNA-triggered firm arrest relative to baseline that is similar to that observed in freshly isolated human leukocytes. By contrast, cells with reduced GPR35 expression did not show a significant increase in firm arrest after KYNA treatment (Fig. 5B).
FIGURE 5.
Silencing of GPR35 reduces KYNA-induced adhesion to ICAM-1-expressing HUVECs. THP-1 cells were infected with lentivirus containing shRNA sequences to either green fluorescent protein or GPR35. Adhesion of puromycin-selected cells to ICAM-1-expressing HUVEC monolayers was studied at 1.75 dyne/cm2. A, mRNA expression of GPR35, measured by real-time QPCR as described above, is significantly reduced after silencing with GPR35-specific shRNA. B, THP-1 cells with reduced GPR35 expression show no significant firm arrest relative to baseline after KYNA treatment (*, p < 0.05 relative to baseline). The KYNA-treated adhesion was normalized to the baseline adhesion for each cell strain. n ≥ 3 for both untreated and KYNA-treated conditions in both cell strains.
Because GPR35 was also readily detected in human neutrophils, we tested whether KYNA was sufficient to trigger adhesion of this leukocyte subset as well. Because neutrophil adhesion under flow is predominantly dependent on β2 integrin-ICAM-1 interactions, we also flowed neutrophils over ICAM-1-expressing HUVECs. At a shear stress of 1.75 dyne/cm2, 300 nm KYNA was sufficient to trigger statistically significant increases in neutrophil adhesion (Fig. 6A), whereas treatment with 1 μm AA did not increase adhesion above background levels. In this system as well, pretreatment of the neutrophils with the β2 integrin-blocking antibody TS1/18 abrogated the effects of KYNA on leukocyte arrest.
FIGURE 6.
Kynurenic acid induces firm arrest of neutrophils on ICAM-1-expressing HUVECs in a β2 integrin-mediated process without induction of an endothelial cell response. HUVECs were transduced with adenoviral ICAM and cultured for 48 h. Interaction of neutrophils with the ICAM-1-expressing monolayer was studied at 1.75 dyne/cm2. Adhesion was quantified 5 min after the introduction of the indicated compound. Arrested cell counts were normalized to the level of adhesion of untreated neutrophils in each experiment. A, KYNA at 300 nm and 1 μm induces firm arrest of neutrophils on ICAM-1 (*, p < 0.05; **, p < 0.005), whereas 1 μm AA does not. B, pretreatment with the β2 integrin-blocking antibody TS1/18 abolishes KYNA-induced adhesion to the monolayer (◇ ◇, p < 0.005 compared with 1 μm KYNA adhesion), whereas pretreatment with an isotype-matched control antibody has no effect. C, treatment of the HUVEC monolayer with 1 μm KYNA followed by rigorous washing resulted in no increased adhesion of neutrophils relative to control. n ≥ 3 for all conditions shown.
To verify that KYNA-induced arrest is a result of leukocyte, and not endothelial activation, we treated the HUVEC monolayers for 15 min with KYNA in perfusion buffer and then washed the monolayers prior to placement in the flow chamber. As treatment of the monolayers resulted in no increase of neutrophil adhesion over background (Fig. 6C), KYNA appeared to be exerting its effects on the neutrophils.
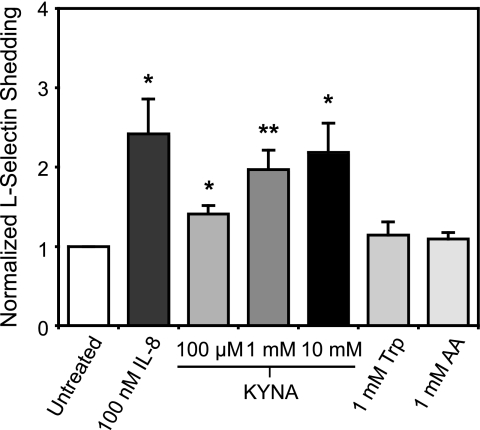
We also examined the cleavage of L-selectin from the surface of neutrophils as an indicator of the degree of cellular activation (26). After a 30-min incubation with 100 nm of the known activating agent IL-8, a significant increase in cleaved L-selectin over baseline was observed (Fig. 7), consistent with previous results (27). Treatment with KYNA resulted in shedding of L-selectin from neutrophils in a dose-dependent fashion, showing a greater than 2-fold increase in shed L-selectin over baseline at a concentration of 10 mm, and a significant increase in the cleaved molecule even at the lowest KYNA dose tested (100 μm) (Fig. 7). Additionally, we tested two other metabolites from the tryptophan metabolic pathway that are known not to activate GPR35 (9): tryptophan and anthranilic acid. At a concentration of 1 mm, neither compound elicited an increase in L-selectin shedding above the background level (Fig. 7).
FIGURE 7.
Kynurenic acid induces activation of neutrophils. Freshly isolated neutrophils were incubated with the indicated compounds for 30 min at room temperature, and the resulting cleaved L-selectin in the medium was measured with an enzyme-linked immunosorbent assay. In each experiment, data were normalized to the level of soluble L-selectin measured in the untreated sample. KYNA significantly increased soluble L-selectin at all concentrations tested (*, p < 0.05; **, p < 0.005), whereas tryptophan and anthranilic acid elicited no change. Data represent means from at least four separate experiments.
DISCUSSION
We found that KYNA is capable of activating multiple subsets of leukocytes, eliciting firm arrest in these cells through both β1 and β2 integrin-mediated adhesion. This adhesion was PTX-sensitive, which suggests that it occurs through a Gi-coupled pathway. By contrast, two related compounds that are known not to bind GPR35, tryptophan and anthranilic acid (9), did not evoke activation or adhesion of leukocytes, consistent with a GPR35-mediated mechanism. Silencing of GPR35 expression using shRNA significantly reduced KYNA-induced firm arrest of a monocytic cell line to ICAM-1-expressing surfaces, confirming GPR35 involvement in this process.
The ability of KYNA to trigger adhesion is consistent with other studies demonstrating unanticipated roles for small molecules in immune modulation. Medium chain fatty acids, particularly capric acid, undecanoic acid, and lauric acid, are able to activate GPR84, a receptor that is significantly up-regulated in lipopolysaccharide-stimulated monocytes. Treatment with these compounds amplifies the effect of lipopolysaccharide treatment on secretion of the cytokine IL-12p40 by RAW264.7 cells (28).
The baseline concentration of KYNA in human plasma is ∼25 nm (29–31), which is insufficient to evoke leukocyte adhesion in our studies. During inflammation, however, interferon-γ induction of IDO leads to the degradation of tryptophan and subsequent generation of catabolites including KYNA. As such, the concentration of KYNA could be expected to reach the micromolar range (32–34), a concentration sufficient to evoke significant adhesion of both monocytes and neutrophils to ICAM-1-expressing endothelial cells in our studies, particularly near the site of generation. Vascular endothelial cells have been demonstrated to convert kynurenine to KYNA, so localized regions with sufficient KYNA concentration to elicit a leukocyte response may be present in the vasculature.
Our studies indicate that KYNA can evoke firm adhesion at concentrations as low as 300 nm. This is significantly less than the EC50 of 39.2 μm previously reported for KYNA activation of human GPR35 overexpressed on Chinese hamster ovary cells (9). However, we would expect that the receptor expressed in its endogenous context would behave differently than when overexpressed in a transformed cell line. The coupling of GPRs to chimeric G proteins for Ca2+ mobilization assays may significantly increase the measured EC50 for some ligands, relative to assays utilizing native couplings (35, 36); differences up to 50-fold have been reported (37). Additionally, the arrest of leukocytes on ICAM-1-expressing endothelial cells is a highly sensitive assay (3), and significant adhesion may be seen with ligands at less than half-maximal activation concentration.
Reports have indicated that KYNA can reduce the lipopolysaccharide-stimulated secretion of the pro-inflammatory cytokines tumor necrosis factor-α and interferon-γ over long (18–72 h) time periods (9, 38). This would suggest a down-modulation of the immune response; however, other types of acute assays were not performed. Although immunosuppression is consistent with evidence that tryptophan metabolites generated after the induction of IDO act in an anti-inflammatory capacity (15), the data presented here suggest that the situation may in fact be more complex. Given the ability of KYNA to elicit early stage arrest of leukocytes, tryptophan catabolism by IDO may have a rapid pro-inflammatory phase, followed by a later anti-inflammatory period. Further investigation into the kinetic response of leukocytes to KYNA will be necessary to better understand both the pro- and anti-inflammatory actions of KYNA on specific leukocyte subsets.
shRNA-mediated decrease in GPR35 expression was accompanied by a concordant reduction in KYNA-triggered adhesion of monocytic cells. We were also interested to find that GPR35-deficient cells grew in clusters, although the cells could be easily dissociated by gentle pipetting for functional assays. The spectrum of chronic effects of GPR35 deletion in vitro and in vivo thus remains the subject of future investigation.
Also worth considering is the potential effect of KYNA on individuals with renal insufficiency. Because the primary method of excretion of tryptophan catabolites is through the kidneys, renal insufficient patients have chronically elevated levels of KYNA. A recent study has shown that uremic patients exhibit pre-dialysis serum levels of KYNA greater than 300 nm; this elevation is only partially reduced by hemodialysis, with KYNA remaining at ∼10 times the baseline level (39). As patients with renal insufficiency suffer from an increased rate of cardiovascular disease, further investigation into the consequences of chronically elevated KYNA in these patients may be merited.
In summary, our studies have shown that KYNA is capable of eliciting firm arrest of both neutrophils and monocytes under physiological flow conditions in an in vitro model. Clearly, multiple factors contribute to the adhesion process in vivo that are not present in this system, and additional studies in animal models are warranted.
Supplementary Material
Acknowledgment
We are grateful for the ICAM-1 adenovirus generated in the laboratory of Dr. Anthony Rosenzweig.
This work was supported, in whole or in part, by National Institutes of Health Grants HL65584 (to R. E. G.) and a T32 Training Grant (to M. B.). This work was also supported by an American Heart Association Grant-in-aid and Established Investigator Grant (to R. E. G.) and the Fondation Leducq (to R. E. G.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1.
- GPCR
- G protein-coupled receptor
- IL-8
- interleukin-8
- MCP-1
- monocyte chemoattractant protein-1
- fMLP
- formyl-methionyl-leucyl-phenylalanine
- KYNA
- kynurenic acid
- IDO
- indoleamine 2,3-dioxygenase
- AA
- anthranilic acid
- PTX
- pertussis toxin
- HUVEC
- human umbilical vein endothelial cell
- ICAM-1
- intercellular adhesion molecule-1
- QPCR
- quantitative polymerase chain reaction
- shRNA
- short hairpin RNA.
REFERENCES
- 1.Springer T. A. (1994) Cell 76, 301–314 [DOI] [PubMed] [Google Scholar]
- 2.Campbell J. J., Hedrick J., Zlotnik A., Siani M. A., Thompson D. A., Butcher E. C. (1998) Science 279, 381–384 [DOI] [PubMed] [Google Scholar]
- 3.Gerszten R. E., Garcia-Zepeda E. A., Lim Y. C., Yoshida M., Ding H. A., Gimbrone M. A., Jr., Luster A. D., Luscinskas F. W., Rosenzweig A. (1999) Nature 398, 718–723 [DOI] [PubMed] [Google Scholar]
- 4.Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. (1991) Science 253, 1278–12801840701 [Google Scholar]
- 5.Charo I. F., Myers S. J., Herman A., Franci C., Connolly A. J., Coughlin S. R. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 2752–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo C., Lefkowitz R. J., Snyderman R. (1983) J. Clin. Invest. 72, 748–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. (1997) Nature 387, 620–624 [DOI] [PubMed] [Google Scholar]
- 8.Lagerström M. C., Schiöth H. B. (2008) Nat. Rev. Drug Discov. 7, 339–357 [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Simonavicius N., Wu X., Swaminath G., Reagan J., Tian H., Ling L. (2006) J. Biol. Chem. 281, 22021–22028 [DOI] [PubMed] [Google Scholar]
- 10.Stone T. W. (1993) Pharmacol. Rev. 45, 309–379 [PubMed] [Google Scholar]
- 11.Parsons C. G., Danysz W., Quack G., Hartmann S., Lorenz B., Wollenburg C., Baran L., Przegalinski E., Kostowski W., Krzascik P., Chizh B., Headley P. M. (1997) J. Pharmacol. Exp. Ther. 283, 1264–1275 [PubMed] [Google Scholar]
- 12.Hilmas C., Pereira E. F., Alkondon M., Rassoulpour A., Schwarcz R., Albuquerque E. X. (2001) J. Neurosci. 21, 7463–7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baran H., Amann G., Lubec B., Lubec G. (1997) Pediatr. Res. 41, 404–410 [DOI] [PubMed] [Google Scholar]
- 14.Stazka J., Luchowski P., Wielosz M., Kleinrok Z., Urbańska E. M. (2002) Eur. J. Pharmacol. 448, 133–137 [DOI] [PubMed] [Google Scholar]
- 15.Mellor A. L., Munn D. H. (2004) Nat. Rev. Immunol. 4, 762–774 [DOI] [PubMed] [Google Scholar]
- 16.Gerszten R. E., Friedrich E. B., Matsui T., Hung R. R., Li L., Force T., Rosenzweig A. (2001) J. Biol. Chem. 276, 26846–26851 [DOI] [PubMed] [Google Scholar]
- 17.Clark R. A., Nauseef W. M. (1996) in Current Protocols in Immunology (Coligan J. E., Kruisbeek A. M., Margulies D. H., Shevach E. M., Strober W. eds) pp. 7.23.2–7.23.4, John Wiley and Sons, Inc., New York [Google Scholar]
- 18.Means T. K., Hayashi F., Smith K. D., Aderem A., Luster A. D. (2003) J. Immunol. 170, 5165–5175 [DOI] [PubMed] [Google Scholar]
- 19.Moffat J., Grueneberg D. A., Yang X., Kim S. Y., Kloepfer A. M., Hinkle G., Piqani B., Eisenhaure T. M., Luo B., Grenier J. K., Carpenter A. E., Foo S. Y., Stewart S. A., Stockwell B. R., Hacohen N., Hahn W. C., Lander E. S., Sabatini D. M., Root D. E. (2006) Cell 124, 1283–1298 [DOI] [PubMed] [Google Scholar]
- 20.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F. H., Verma I. M., Trono D. (1996) Science 272, 263–267 [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulou C., Corrigall V., Taylor P. R., Poston R. N. (2008) Cytokine 43, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boring L., Gosling J., Cleary M., Charo I. F. (1998) Nature 394, 894–897 [DOI] [PubMed] [Google Scholar]
- 23.Ponath P. D., Qin S., Post T. W., Wang J., Wu L., Gerard N. P., Newman W., Gerard C., Mackay C. R. (1996) J. Exp. Med. 183, 2437–2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuang Y., Wu Y., Jiang H., Wu D. (1996) J. Biol. Chem. 271, 3975–3978 [DOI] [PubMed] [Google Scholar]
- 25.Burns D. L. (1988) Microbiol. Sci. 5, 285–287 [PubMed] [Google Scholar]
- 26.Kishimoto T. K., Jutila M. A., Berg E. L., Butcher E. C. (1989) Science 245, 1238–1241 [DOI] [PubMed] [Google Scholar]
- 27.Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbassi O. (1991) J. Clin. Invest. 87, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Wu X., Simonavicius N., Tian H., Ling L. (2006) J. Biol. Chem. 281, 34457–34464 [DOI] [PubMed] [Google Scholar]
- 29.Forrest C. M., Mackay G. M., Oxford L., Stoy N., Stone T. W., Darlington L. G. (2006) Clin. Exp. Pharmacol. Physiol. 33, 1078–1087 [DOI] [PubMed] [Google Scholar]
- 30.Hartai Z., Juhász A., Rimanóczy A., Janáky T., Donkó T., Dux L., Penke B., Tóth G. K., Janka Z., Kálmán J. (2007) Neurochem. Int. 50, 308–313 [DOI] [PubMed] [Google Scholar]
- 31.Amirkhani A., Heldin E., Markides K. E., Bergquist J. (2002) J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 780, 381–387 [DOI] [PubMed] [Google Scholar]
- 32.Heyes M. P., Saito K., Crowley J. S., Davis L. E., Demitrack M. A., Der M., Dilling L. A., Elia J., Kruesi M. J., Lackner A., Larsen S. A., Lee K., Leonard H. L., Markey S. P., Martin A., Milstein S., Mouradian M. M., Pranzatelli M. R., Quearry B. J., Salazar A., Smith M., Strauss S. E., Sunderland T., Swedo S. W., Tourtellotte W. W. (1992) Brain 115, 1249–1273 [DOI] [PubMed] [Google Scholar]
- 33.Forrest C. M., Youd P., Kennedy A., Gould S. R., Darlington L. G., Stone T. W. (2002) J. Biomed. Sci. 9, 436–442 [DOI] [PubMed] [Google Scholar]
- 34.Forrest C. M., Gould S. R., Darlington L. G., Stone T. W. (2003) Adv. Exp. Med. Biol. 527, 395–400 [DOI] [PubMed] [Google Scholar]
- 35.Liu C., Wilson S. J., Kuei C., Lovenberg T. W. (2001) J. Pharmacol. Exp. Ther. 299, 121–130 [PubMed] [Google Scholar]
- 36.Chen J., Liu C., Lovenberg T. W. (2003) Eur. J. Pharmacol. 467, 57–65 [DOI] [PubMed] [Google Scholar]
- 37.Liu C., Chen J., Sutton S., Roland B., Kuei C., Farmer N., Sillard R., Lovenberg T. W. (2003) J. Biol. Chem. 278, 50765–50770 [DOI] [PubMed] [Google Scholar]
- 38.Maes M., Mihaylova I., Ruyter M. D., Kubera M., Bosmans E. (2007) Neuroendocrinol. Lett. 28, 826–831 [PubMed] [Google Scholar]
- 39.Pawlak D., Pawlak K., Malyszko J., Mysliwiec M., Buczko W. (2001) Int. Urol. Nephrol. 33, 399–404 [DOI] [PubMed] [Google Scholar]
- 40.Heller E. A., Liu E., Tager A. M., Sinha S., Roberts J. D., Koehn S. L., Libby P., Aikawa E. R., Chen J. Q., Huang P., Freeman M. W., Moore K. J., Luster A. D., Gerszten R. E. (2005) Circulation 112, 578–586 [DOI] [PubMed] [Google Scholar]
- 41.King V. L., Lin A. Y., Kristo F., Anderson T. J., Ahluwalia N., Hardy G. J., Owens A. P., 3rd, Howatt D. A., Shen D., Tager A. M., Luster A. D., Daugherty A., Gerszten R. E. (2009) Circulation 119, 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.