Abstract
Dyskeratosis congenita (DC) is a rare syndrome, characterized by cutaneous abnormalities and premature death caused by bone marrow failure. In this issue of Genes & Development, Hockemeyer and colleagues (pp. 1773–1785) report a new mouse model that reconstitutes key features of DC. Disease phenotypes are generated by a POT1b deletion in a telomerase-deficient background that accelerates the shortening of telomeres by degradation.
Keywords: Bone marrow failure, dyskeratosis congenita, POT1, shelterin, telomere, telomerase
Accurately modeling human disease is a major goal of contemporary medical research. In addition to species differences, the multifactorial regulation of disease leads to formidable challenges in the development of valid disease models. In this issue of Genes & Development, Hockemeyer et al. (2008) describe a new mouse model that reconstitutes many aspects of the human disease dyskeratosis congenita (DC).
Features and genetics of dyskeratosis congenita
Dyskeratosis congenita is a rare syndrome, characterized by a triad of mucocutaneous abnormalities including aberrant skin pigmentation, nail dystrophy, and mucosal leukoplakia (Kirwan and Dokal 2008; Walne and Dokal 2008). Mean age of death in the third decade is caused by bone marrow failure with predisposition to malignancy and pulmonary complications. Other features of this clinically and genetically heterogeneous disease that warrant its description as a premature aging syndrome include early hair loss or graying and osteoporosis.
There are three genetically distinct forms of DC: X-linked recessive, autosomal dominant, and autosomal recessive. The affected gene in the X-linked form, DKC1, encodes dyskerin, a nucleolar protein associated with small nucleolar RNAs (snoRNAs) of the H/ACA class (Heiss et al. 1998; Meier 2005). Based on extensive conservation of dyskerin orthologs in other organisms (Cbf5p in yeast, minifly in Drosophila, and NAP57 in rat) and their function in pseudouridylation of rRNAs and ribosome biogenesis, a reasonable hypothesis was proposed that X-linked DC disease phenotypes may be the result of defective rRNA processing (Heiss et al. 1998; Meier 2005). Consistent with the ubiquitous expression and housekeeping functions of dyskerin, mice deleted for dyskerin are embryonic lethal (He et al. 2002). Transgenic mice expressing reduced levels of dyskerin due to a hypomorphic mutation, or mouse stem cell lines expressing dyskerin variants found in patients, display alterations in pseudouridylation and rRNA maturation, in support of X-linked DC as a defective ribosome biogenesis syndrome (Ruggero et al. 2003; Mochizuki et al. 2004). Although earlier analyses of human X-linked DC patient lymphoblasts hadn’t noted defects in ribosomal RNA maturation, associated changes were identified in the activity of a key ribonucleoprotein, the telomerase enzyme (Mitchell et al. 1999b). Cells from these patients have reduced levels of the H/ACA motif-containing telomerase RNA (TR) and telomerase activity, and consequentially, shorter telomeres relative to age-matched controls. Dyskerin has a direct role in telomerase RNA biogenesis as well as telomerase assembly and function through its association with TR and a catalytically competent enzyme (Mitchell et al. 1999a; Cohen et al. 2007).
Dyskeratosis congenita and telomere maintenance
The concept that DC represents a telomere maintenance syndrome rapidly gained acceptance with the subsequent identification, in autosomal dominant DC and related syndromes, of distinct mutations in genes encoding the two essential telomerase subunits, the TR and the telomerase reverse transcriptase (TERT) (Vulliamy et al. 2001, 2005, 2006; Armanios et al. 2005; Yamaguchi et al. 2005). Notably, in autosomal recessive DC, the genes encoding NOP10 or NHP2, two H/ACA ribonucleoproteins implicated in telomerase assembly, are mutated (Dez et al. 2001; Walne et al. 2007; Villiamy et al. 2008).
Telomere length and structure maintenance is directly regulated by telomerase and a complex of proteins known as shelterin (de Lange 2005; Autexier and Lue 2006). The synthesis of short G-rich telomere sequences (T2AG3 in human and mice) is mediated by the enzyme telomerase. Formation of the “capped” T-loop structure of the telomere that prevents it from being recognized as a double-strand break is coordinated by shelterin. Shelterin is composed of six proteins including TRF1, TRF2, Rap1, TPP1, TIN2, and POT1. TRF1 and TRF2 are sequence-specific double-stranded telomere-binding proteins, POT1 binds the single-stranded G-rich overhang typically found at telomeres, and Rap1, TPP1, and TIN2 mediate protein–protein interactions within the shelterin complex. The T-loop is generated by the shelterin-mediated invasion of the G-rich single strand into the telomere duplex region. Establishment of the G-rich overhang occurs in the absence of telomerase, suggesting that it is formed by nuclease processing of the C-strand (Dionne and Wellinger 1996; Hemann and Greider 1999; Nikaido et al. 1999). In yeast, processing of the C-strand is regulated by the cyclin-dependent kinase, Cdk1, and the exonuclease, ExoI (Maringele and Lydall 2002; Frank et al. 2006; Vodenicharov and Wellinger 2006).
The POT1 shelterin telomere component
The function of POT1 in the “protection of telomeres” is complex, as revealed by studies in various organisms and the identification of multiple genes and spliced variants encoding POT1-related proteins in mice, plants, and Caenorhabditis elegans (Baumann and Cech 2001; Colgin et al. 2003; Baumann 2006; Hockemeyer et al. 2006; Price 2006; Wu et al. 2006; Surovtseva et al. 2007; Raices et al. 2008). Humans possess a single POT1 gene that controls telomerase-mediated telomere elongation and protects telomeres from a transient DNA damage response (Colgin et al. 2003; Loayza and de Lange 2003; Liu et al. 2004; Veldman et al. 2004; Ye et al. 2004; Hockemeyer et al. 2005; Yang et al. 2005). The mouse genome contains two POT1 orthologs, POT1a and POT1b (Hockemeyer et al. 2006; Wu et al. 2006). Conditional deletions and complementation experiments identified a role for POT1a in repressing a DNA damage response at telomeres, whereas POT1b negatively regulates the amount of ssDNA at the telomere end. A lack of POT1a results in embryonic lethality, whereas a POT1b deficiency leads to unusually long 3′ G-rich overhangs suggestive of excessive 5′ C-strand degradation resulting from aberrant nuclease regulation (Hockemeyer et al. 2006).
Mouse models of dyskeratosis congenita
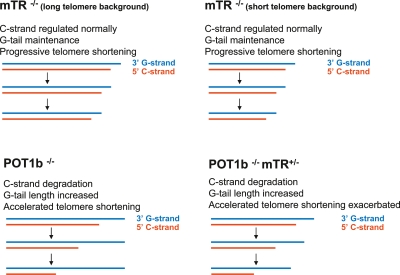
Despite our increased understanding of DC as a telomere maintenance syndrome, there are unresolved issues. One outstanding question is why early mouse models have not recapitulated the link found between telomere dysfunction and associated phenotypes observed in DC (Mitchell et al. 1999b; Montanaro et al. 2002)? Mice with the hypomorphic dyskerin mutation have reduced levels of pseudouridylated rRNA, yet normal telomere lengths in the first generation, and reveal dyskeratosis of the skin and anemia (Ruggero et al. 2003). Other features of these mice may be described as DC-like. Although the mice develop tumors, they form in the lungs and mammary glands, rather than in the gut and skin as predominantly occurs in X-linked DC patients (Kirwan and Dokal 2008). Only the late-generation hypomorphic dyskerin mice experience telomere dysfunction; apparently this event occurs after, and is thus not a prerequisite for, the appearance of skin and hematopoietic changes. In another mouse model based on a telomerase TR knockout (mTR−/− in a C57BL/6J genetic background with long telomeres), telomeres undergo progressive shortening (Fig. 1), and these mice display some features common to DC, including reduced life span (though moderate), premature aging, and increased cancer incidence (Blasco et al. 1997; Lee et al. 1998; Rudolph et al. 1999). However, these mice fail to reconstitute the progressive bone marrow failure or the three key mucocutaneous abnormalities of DC. The temporal characteristic of human DC phenotypes, which are more severe and are observed at an earlier age with successive generations, is associated with the inheritance of shorter telomeres (Vulliamy et al. 2004; Armanios et al. 2005). The differences between the human disease and mouse models suggest that progressive telomere shortening of typically longer mouse telomeres is insufficient to elicit all of the human DC phenotypes (Wright and Shay 2000).
Figure 1.
Telomere-shortening mouse models. (Top left panel) Telomerase deficiency (mTR−/− in a C57BL/6J genetic background with long telomeres) leads to progressive shortening. Telomerase deficiency (mTR−/− in a CAST/EiJ genetic background with short telomeres) also leads to progressive telomere shortening, though the final telomere lengths are shorter (cf. top panels). A POT1b deletion leads to accelerated telomere shortening due to excessive 5′ C-strand degradation. (Bottom left panel) Excessive C-strand degradation results in longer G-tails. (Bottom right panel) Telomeres are further shortened in the context of mTR heterozygosity.
Notably, when telomere shortening is induced by a telomerase RNA deletion in a strain of mice (CAST/EiJ) with short telomeres (comparable with the length of human telomeres) (Fig. 1), the phenotypes are similar to those observed in DC (Hao et al. 2005). Homozygous mTR−/− CAST/EiJ mice and late generation heterozygous mTR+/− CAST/EiJ mice experience a decrease in tissue renewal capacity in the bone marrow, intestines, and testes. As a result of germ cell apoptosis in the testes, G2 mTR−/− mice are sterile. G1 and G2 mTR−/− CAST/EiJ mice also suffer from severe depletion of the intestinal epithelial crypts and villus atrophy in the small intestine. These mice develop microadenomas of the intestinal epithelium reminiscent of the leukoplakia that occurs in DC patients. Consistent with premature death in DC, G2 mTR−/− CAST/EiJ mice have a reduced median survival rate of 129 d. CAST/EiJ mTR−/−-null animals derived from late-generation heterozygotes have a reduced life span relative to genetically similar animals derived from early generation heterozygotes, suggestive of a genetic anticipation characteristic of DC.
Modeling short telomeres in POT1b-deficient mice
Using an alternative approach to model short telomeres, Hockemeyer et al. (2008) created a mouse that exhibits enhanced telomere degradation rather than progressive telomere shortening. Enhanced degradation was effected by ablating POT1b function. They reasoned that POT1b deficiency would alter the regulation of the 5′ C-rich telomere ends and enhance telomere degradation. Due to the distinction between POT1a and POT1b functions in mice, POT1b deletion does not elicit an immediate telomere dysfunction-induced DNA damage response, and thus the effects of 5′ C-strand deregulation can be analyzed (Hockemeyer et al. 2006; Wu et al. 2006). Hockemeyer et al. (2008) examined telomere dynamics in SV40-Large T antigen immortalized mouse embryonic fibroblasts (MEFs) carrying conditional alleles of POT1b. As hypothesized, a POT1b deletion led to accelerated telomere shortening due to excessive 5′ C-strand degradation (Fig. 1). Telomere shortening occurred independently of telomerase status, but the telomeres were further shortened upon telomerase inactivation (Fig. 1). The precise mechanism of C-strand degradation in these mice remains to be characterized, but is apparently not regulated by the exonuclease EXO1 as observed previously in yeast (Maringele and Lydall 2002).
Although mice lacking POT1b are viable, initially fertile, and have a normal life span (Hockemeyer et al. 2006), they have reduced body weight and testis size, the latter due to a reduction in spermatids. Consequently, POT1b−/− male mice become prematurely infertile. Apoptosis in intestinal crypts is also observed in these mice. By 3–4 mo and progressing with age, the mice display hyperpigmentation, a feature of DC, localized to the paws, snout, ears, and tails. The hyperpigmentation is the result of an increase in melanin content and melanosome density in a “microparasol” over nuclei in keratinocytes, suggestive of a p53-dependent DNA damage response (Cui et al. 2007). Ten-month-old fifth generation mice also develop an additional feature of DC, nail dystrophy. However, other features of DC, including fatal bone marrow failure and premature death are not observed in POT1b−/− mice.
A new mouse model of dyskeratosis congenita
The accelerated telomere shortening observed in POT1b−/−mTR−/− relative to POT1b−/− immortalized MEFs led Hockemeyer et al. (2008) to reason that the lack of telomerase may exacerbate POT1b−/− phenotypes. Indeed, the numbers of doubly deficient mice resulting from intercrossing POT1b mTR heterozygous mice were 15-fold less frequent than expected, and the single POT1b−/−mTR−/− pup born was runted, hairless, and died 3 wk after birth. Heterozygosity for mTR exacerbated the phenotypes observed in POT1b−/− mice, including further reduction in body weight, more frequent and severe testicular atrophy, and increased apoptosis in intestinal villi. The onset of hyperpigmentation was more evident and occurred in younger POT1b−/−mTR+/− mice. Importantly, key features of DC, such as bone marrow failure and premature death, were reproduced in the POT1b−/−mTR+/− mice. Peripheral blood cell counts indicated significant pancytopenia with severe leukocytopenia, thrombocytopenia, and mild anemia. Bone marrow analyses showed replacement of hematopoietic precursor cells by stromal adipose tissue. The POT1b−/−mTR+/− mice had a significantly reduced median life span (135 d) relative to POT1b−/− mice (24 mo in the C57BL/6J genetic background). The life span of the POT1b−/−mTR+/− mice is similar to that of the G2 mTR−/− CAST/EiJ mice (Hao et al. 2005). The accelerated telomere shortening phenotype observed in POT1b−/−mTR−/− immortalized MEFs is reproduced in the bone marrow of POT1b−/−mTR+/− mice and correlates with increased telomere signal-free chromosome ends and a higher frequency of chromosome end fusions.
Several DC phenotypes, however, were not observed in POT1b−/−mTR+/− mice, including cancer predisposition, oral leukoplakia, nail dystrophy, and lung fibrosis. The investigators suggest that the failure to observe certain of these phenotypes may be due to their late onset and the premature death of these mice due to bone marrow failure. They propose to rescue these mice from premature death by bone marrow transplantation in order to investigate some of the typically late onset symptoms such as tumor development.
One interesting aspect of the model is the spectrum of tissues that are affected by the accelerated telomere shortening in contrast to the tissues that are affected in the telomerase knockout mouse. For example, POT1b−/−mTR+/− mice develop progressive bone marrow failure, but no alopecia or hair graying, whereas mTR−/− mice develop alopecia and hair graying, but no bone marrow failure. The investigators account for the distinctive tissue effects by proposing that the putative C-strand processing nuclease in the POT1b−/−mTR+/− context is differentially expressed in these tissues. Higher levels of the nuclease in hematopoietic cells are hypothesized to elicit enhanced telomere degradation with a more pronounced phenotype relative to tissues with lower levels of nuclease activity or higher telomerase activity (such as skin) that will be protected from telomere dysfunction. They contemplate that nuclease levels are properly regulated by POT1b in the mTR−/− mice so that the first phenotypic changes observed in these mice emerge in tissues with the largest number of cell divisions. In other tissues, such as bone marrow, the telomere reserve in the last viable generation of mTR−/− mice would be sufficient to avert the development of progressive bone marrow failure. Clearly, the identification and characterization of the putative nuclease is critical to test this hypothesis and its implication on the distinct tissue effects in the different knockout mice.
The investigators note that a defective POT1b gene has yet to be reported in DC patients, although there is a precedent for a defective telomere binding protein, TIN2, in several cases of DC (Savage et al. 2008). Furthermore, unpublished observations from Titia de Lange’s group indicate that POT1b domains that prevent telomere degradation are functionally conserved in human POT1, and thereby support a potential role for human POT1 in negatively regulating telomere degradation. As discussed by Hockemeyer et al. (2008), the genetic defect has not been identified in all DC patients, and it may be informative to screen this cohort for alterations in POT1. Lastly, TPP1 and POT1, as part of the shelterin complex, are implicated in the recruitment of telomerase to telomeres, as well as the modulation of telomerase processivity (Colgin et al. 2003; Lei et al. 2005; Wang et al. 2007; Xin et al. 2007). Thus, it will be interesting to investigate the effect of a POT1b deletion on the regulation of telomerase recruitment and activity at the mouse telomere.
Final remarks
The recognition of DC as a telomere maintenance syndrome has led to the generation of mouse disease models that recapitulate the telomere dysfunction and key features observed in DC (Hao et al. 2005; Hockemeyer et al. 2008). Telomere dysfunction can be elicited by progressive telomere shortening in a strain of mice with initially short telomeres, or by accelerating telomere shortening via C-strand degradation in the mTR−/− CAST/EiJ and the POT1b−/− mTR+/− mice, respectively. Both mice exhibit significantly reduced life span and bone marrow defects. Additionally, two features of DC, the nail dystrophy and abnormal skin pigmentation, are reproduced in the POT1b−/− mouse model described by Hockemeyer et al. (2008). Does the POT1b deletion additionally activate telomere deprotection signaling responses? Deletion of POT1b in the context of a telomerase-deficient mouse with telomere lengths comparable to human may provide insight on the mechanisms that are engaged when telomeres are shortened by different triggers. The “POT of gold” apparently contains additionally rich questions with testable hypotheses.
Acknowledgments
Ongoing research in the group of C.A. is funded by the Canadian Institutes of Health Research (CIHR) MOP-81215 and MOP-86672. C.A. is a recipient of the Fonds de la Recherche en Santé du Québec (FRSQ) Chercheur-Boursier Award.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1695808.
References
- Armanios M., Chen J.-L., Chang Y.-P.C., Brodsky R.A., Hawkins A., Griffin C.A., Eshleman J.R., Cohen A.R., Chakravarti A., Hamosh A., et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc. Natl. Acad. Sci. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autexier C., Lue N. The structure and function of telomerase reverse transcriptase. Annu. Rev. Biochem. 2006;75:493–517. doi: 10.1146/annurev.biochem.75.103004.142412. [DOI] [PubMed] [Google Scholar]
- Baumann P. Are mouse telomeres going to pot? Cell. 2006;126:33–36. doi: 10.1016/j.cell.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Baumann P., Cech T.R. Pot1, the putative telomere end-binding protein in fission yeast and humans. Science. 2001;292:1171–1175. doi: 10.1126/science.1060036. [DOI] [PubMed] [Google Scholar]
- Blasco M.A., Lee H.-W., Hande M.P., Samper E., Lansdorp P.M., DePinho R.A., Greider C.W. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Cohen S.B., Graham M.E., Lovrecz G.O., Bache N., Robinson P.J., Reddel R.R. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- Colgin L.M., Baran K., Baumann P., Cech T.R., Reddel R.R. Human Pot1 facilitates telomere elongation by telomerase. Curr. Biol. 2003;13:942–946. doi: 10.1016/s0960-9822(03)00339-7. [DOI] [PubMed] [Google Scholar]
- Cui R., Widlund H.R., Feige E., Lin J.Y., Wilensky D.L., Igras V.E., d'Orazio J., Fung C.Y., Schanbacher C.F., Granter S.R., et al. Central role of p53 in the suntan response and pathological hyperpigmentation. Cell. 2007;128:853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- de Lange T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes & Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Dez C., Henras A., Faucon B., Lafontaine D.L.J., Caizergues-Ferrer M., Henry Y. Stable expression in yeast of the mature form of human telomerase RNA depends on its association with the box H/ACA small nucleolar RNP proteins Cbf5p, Nhp2p and Nop10p. Nucleic Acids Res. 2001;29:598–603. doi: 10.1093/nar/29.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne I., Wellinger R.J. Cell cycle-regulated generation of single-stranded G-rich DNA in the absence of telomerase. Proc. Natl. Acad. Sci. 1996;93:13902–13907. doi: 10.1073/pnas.93.24.13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C.J., Hyde M., Greider C.W. Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol. Cell. 2006;24:423–432. doi: 10.1016/j.molcel.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Hao L.-Y., Armanios M., Strong M.A., Karim B., Feldser D.M., Huso D., Greider C.W. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- He J., Navarrete S., Jasinski M., Vulliamy T., Dokal I., Bessler M., Mason P.J. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene. 2002;21:7740–7744. doi: 10.1038/sj.onc.1205969. [DOI] [PubMed] [Google Scholar]
- Heiss N.S., Knight S.W., Vulliamy T., Klauck S.M., Wiemann S., Mason P.J., Poustka A., Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nature. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- Hemann M.T., Greider C.W. G-strand overhangs on telomeres in telomerase-deficient mouse cells. Nucleic Acids Res. 1999;27:3964–3969. doi: 10.1093/nar/27.20.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D., Sfeir A.J., Shay J.W., Wright W.E., de Lange T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosome end. EMBO J. 2005;24:2667–2678. doi: 10.1038/sj.emboj.7600733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockemeyer D., Daniels J.-P., Takai H., de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006;126:63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- Hockemeyer D., Palm W., Wang R.C., Couto S.S., de Lange T. Engineered telomere degradation models dyskeratosis congenita. Genes & Dev. 2008 doi: 10.1101/gad.1679208. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan M., Dokal I. Dyskeratosis congenita: A genetic disorder of many faces. Clin. Genet. 2008;73:103–112. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Lee H.-W., Blasco M.A., Gottlieb G.J., Horner J.W., Greider C.W., DePinho R.A. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- Lei M., Zaug A.J., Podell E.R., Cech T.R. Switching human telomerase on and off with hPot1 protein in vitro. J. Biol. Chem. 2005;280:20449–20456. doi: 10.1074/jbc.M502212200. [DOI] [PubMed] [Google Scholar]
- Liu D., Safari A., O'Connor M.S., Chan D.W., Laegeler A., Qin J., Songyang Z. PTOP interacts with POT1 and regulates its localization to telomeres. Nat. Cell Biol. 2004;6:673–680. doi: 10.1038/ncb1142. [DOI] [PubMed] [Google Scholar]
- Loayza D., de Lange T. POT1 as a terminal transducer of TRF1 telomere length control. Nature. 2003;423:1013–1018. doi: 10.1038/nature01688. [DOI] [PubMed] [Google Scholar]
- Maringele L., Lydall D. EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Δ mutant. Genes & Dev. 2002;16:1919–1933. doi: 10.1101/gad.225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier U.T. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.R., Cheng J., Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol. Cell. Biol. 1999a;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.R., Wood E., Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999b;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y., He J., Kulkarni S., Bessler M., Mason P.J. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity and ribosomal RNA processing. Proc. Natl. Acad. Sci. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro L., Chilla A., Trere D., Pession A., Govoni M., Tazzari P.L., Derenzini M. Increased mortality rate and not impaired ribosomal biogenesis is responsible for proliferative defect in dyskeratosis congenita cell lines. J. Invest. Dermatol. 2002;118:193–198. doi: 10.1046/j.0022-202x.2001.01634.x. [DOI] [PubMed] [Google Scholar]
- Nikaido R., Haruyama T., Watanabe Y., Iwata H., Iida M., Sugimura H., Yamada N., Ishikawa F. Presence of telomeric G-strand tails in the telomerase catalytic subunit TERT knockout mice. Genes Cells. 1999;4:563–572. doi: 10.1046/j.1365-2443.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Price C.M. Stirring the POT1: Surprises in telomere protection. Nat. Struct. Mol. Biol. 2006;13:673–674. doi: 10.1038/nsmb0806-673. [DOI] [PubMed] [Google Scholar]
- Raices M., Verdun R.E., Compton S.A., Haggblom C.I., Griffith J.D., Dillin A., Karlseder J. C. elegans telomeres contain G-strand and C-strand overhangs that are bound by distinct proteins. Cell. 2008;132:745–757. doi: 10.1016/j.cell.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Rudolph K.L., Chang S., Lee H.-W., Blasco M., Gottlieb G.J., Greider C., DePinho R.A. Longevity, stress response and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Ruggero D., Grisendi S., Piazza F., Rego E., Mari F., Rao P.H., Cordon-Cardo C., Pandolfi P.P. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- Savage S.A., Giri N., Baerlocher G.M., Orr N., Lansdorp P.M., Alter B.P. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am. J. Hum. Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surovtseva Y.V., Shakirov E.V., Vespa L., Osbun N., Song X., Shippen D.E. Arabidopsis POT1 associates with the telomerase RNP and is required for telomere maintenance. EMBO J. 2007;26:3653–3661. doi: 10.1038/sj.emboj.7601792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman T., Etheridge K.T., Counter C.M. Loss of hPot1 function leads to telomere instability and cut-like phenoptype. Curr. Biol. 2004;14:2264–2270. doi: 10.1016/j.cub.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Vodenicharov M.D., Wellinger R.J. DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (Cdc28/clb) cell cycle kinase. Mol. Cell. 2006;24:127–137. doi: 10.1016/j.molcel.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Marrone A., Goldman F., Dearlove A., Bessler M., Mason P.J., Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Marrone A., Szydlo R., Walne A., Mason P.J., Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat. Genet. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- Vulliamy T.J., Walne A., Baskaradas A., Mason P.J., Marrone A., Dokal I. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood Cells Mol. Dis. 2005;34:257–263. doi: 10.1016/j.bcmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vulliamy T.J., Marrone A., Knight S.W., Walne A., Mason P.J., Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- Vulliamy T., Beswick R., Kirwan M., Marrone A., Digweed M., Walne A., Dokal I. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita . Proc. Natl. Acad. Sci. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walne A.J., Dokal I. Dyskeratosis congenita: A historical perspective. Mech. Ageing Dev. 2008;129:48–59. doi: 10.1016/j.mad.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Walne A.J., Vulliamy T., Marrone A., Beswick R., Kirwan M., Masunari Y., Al-Qurashi F.H., Aljurf M., Dokal I. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations inthe telomerase-associated protein NOP10. Hum. Mol. Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Podell E.R., Zaug A.J., Yang Y., Baciu P., Cech T.R., Lei M. The Pot1–TPP1 telomere complex is a telomerase processivity factor. Nature. 2007;445:506–510. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- Wright W.E., Shay J.W. Telomere dynamics in cancer progression and prevention: Fundamental differences in human and mouse biology. Nat. Med. 2000;6:849–851. doi: 10.1038/78592. [DOI] [PubMed] [Google Scholar]
- Wu L., Multani A.S., He H., Cosme-Blanco W., Deng Y., Deng J.M., Bachilo O., Pathak S., Tahara H., Bailey S.M., et al. Pot 1 deficiency initiates DNA damage checkpoint activation and aberrant homologous recombination at telomeres. Cell. 2006;126:49–62. doi: 10.1016/j.cell.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Xin H., Liu D., Wan M., Safari A., Kim H., Sun W., O'Connor M.S., Songyang Z. TPP1 is a homologue of ciliate TEBP-β and interacts with Pot1 to recruit telomerase. Nature. 2007;445:559–562. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Calado R.T., Ly H., Kajigaya S., Baerlocher G.M., Chanock S.J., Lansdorp P.M., Young N.S. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N. Engl. J. Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- Yang Q., Zheng Y.L., Harris C.C. POT1 and TRF2 cooperate to maintain telomere integrity. Mol. Cell. Biol. 2005;25:1070–1080. doi: 10.1128/MCB.25.3.1070-1080.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.Z.-S., Hockemeyer D., Krutchinsky A.N., Loayza D., Hooper S.M., Chait B.T., de Lange T. POT1-interacting protein PIP1: A telomere length regulator that recruits POT1 to the TIN2/TRF1 complex. Genes & Dev. 2004;18:1649–1654. doi: 10.1101/gad.1215404. [DOI] [PMC free article] [PubMed] [Google Scholar]