Abstract
The mechanisms underlying possible increased HIV-1 acquisition in adenovirus 5 (Ad5)-seropositive subjects vaccinated with Ad5-HIV-1 vectors in the Merck STEP trial remain unclear. We find Ad5 serostatus does not predict Ad5-specific CD4+ T-cell frequency, and no durable significant differences in Ad5-specific CD4+ T-cells between Ad5-seropositive and seronegative subjects were observed following vaccination. These findings indicate no causative role for Ad5-specific CD4+ T-cells in increasing HIV-1 susceptibility in the STEP trial.
Post-hoc analysis of the Merck STEP trial showed vaccination with an Ad5 vector-based HIV-1 vaccine was associated with increased HIV-1 acquisition rates in volunteers with baseline Ad5 neutralizing antibody (nAb) titers > 2001. It was proposed that vaccination of Ad5 seropositive subjects caused activation and expansion of pre-existing Ad5-specific CD4+ T-cells, potentially serving as targets for HIV infection2. However, neither the prevalence of Ad5-specific CD4+ T-cells in humans, nor their relationship with Ad5 nAb titer has been characterized. Moreover, it is unknown to what degree Ad5 vector administration stimulates pre-existing Ad5-specific CD4+ T-cells. Adequately addressing this from the STEP trial is impossible, as peripheral blood mononuclear cell (PBMC) samples were only obtained after vaccination (weeks 8, 30, 52, 104)3.
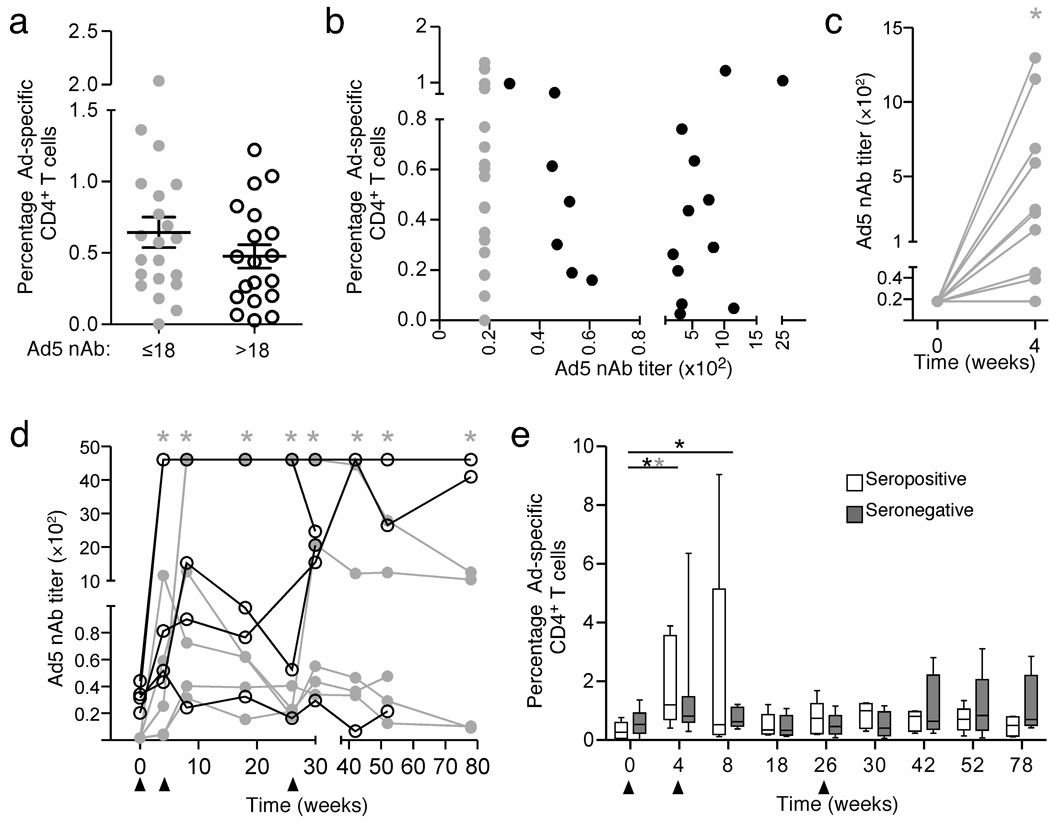
To characterize the relationship between Ad5 nAb titers and Ad5-specific CD4+ T-cell responses, we analyzed samples from 40 subjects with varying Ad5 nAb titers by intracellular cytokine staining using replication-defective Ad5 particles for stimulation4–7. (Supplementary Methods online, Supplementary Fig. 1, Supplementary Table 1). Of these subjects, 15 (five seronegative weeks 0–4, five seronegative weeks 0–78, and five seropositive weeks 0–78) were enrolled in the Merck 016 phase I HIV-1 vaccine safety trial and received Ad5 vectors used in the STEP trial at weeks 0, 4, and 268 (Supplementary Table 2). We detected similar frequencies of Ad5-specific CD4+ T-cells in > 80% of Ad5 seropositive and Ad5 seronegative subjects at baseline (Fig. 1a). Within Ad5 seropositive subjects, Ad5-specific CD4+ T-cell frequencies did not correlate with Ad5 nAb titers9 (Fig. 1b).
Figure 1. Ad5-specific CD4+ T-cell frequency does not correlate with Ad5 neutralizing antibody titer.
Forty total subjects with a range of Ad5 nAb titers were analyzed. Ten seronegative (five assessed weeks 0–4, five assessed weeks 0–78, gray symbols) and five seropositive subjects (black symbols) received Merck Ad5 gag/pol/nef as described in Supplementary Methods. a) Similar Ad5-specific CD4+ T-cell magnitude regardless of baseline Ad5 serostatus. IFN-γ IL-2, MIP-1α, TNF- α, and/or perforin production in response to Ad5 virus particles was measured by polychromatic flow cytometry. Frequencies reflect the total percent of cells responding by any of these functions. b) No correlation between total Ad5-specific CD4+ T-cell magnitude and Ad5 nAb titer. c) Ad5 nAbs titers increase in Ad5 seronegatives after one vaccination (P < 0.05). d) Ad5 nAb titers remain elevated in baseline seronegatives throughout the vaccine course (gray asterisk, P < 0.05). e) Ad5-specific CD4+ T-cell frequency increases after vaccination in Ad5 seropositives (open boxes, black asterisk) at weeks 4 (P < 0.002) and 8 (P < 0.03) and Ad5 seronegatives (grey boxes, gray asterisk) at week 4 (P < 0.02). Plots depict the median, 25th and 75th percentile (box plots) and the minimum and maximum values (whiskers). Triangles indicate vaccination time points.
Four weeks after the first Ad5-HIV-1 vector administration in the 15 vaccinated subjects (Supplementary Table 2), Ad5 nAb titers in baseline seronegative subjects (n = ten) increased (P < 0.05), becoming comparable to those seen in baseline Ad5 seropositive subjects (n = five) in all but one individual (Fig. 1c) who seroconverted by week 8 (Fig. 1d). Ad5-specific CD4+ T-cells increased in both groups (P < 0.002, baseline seropositive; P < 0.03, baseline seronegative) after the initial vector dose (Fig. 1e, Supplemental Fig. 2). Successive vaccinations further expanded Ad5-specific T-cells in some subjects, but these responses were transient in most individuals (Fig. 1e, Supplemental Fig. 3). At no point was there a statistical difference between the serogroups.
We next examined the relationship between Ad5 serostatus and potential functional differences in Ad5-specific CD4+ T-cells before and after vaccination. Ad5- specific CD4+ T-cells that produced IFN-γ, IL-2, MIP-1α, TNF- α, and/or perforin were present at baseline in most individuals at similar frequency regardless of Ad5 serostatus (Fig. 2a). There was no correlation between Ad5 nAb titer and % Ad5-specific CD4+ T-cells that produced any one or more functions (data not shown). IFN-γ dominated the response in both serogroups, but accounted for only ~50% of the total response (Fig. 2b).
Figure 2. CD4+ effector functions do not differ with baseline serostatus.
IL-2 (2, downward triangle), IFN-γ (G, circle), MIP-1α (M, diamond), perforin (P, square) and TNF-α (T, upward triangle) production in response to Ad5 virus were measured by intracellular cytokine staining. Subjects for all studies are as described previously. For all panels, gray symbols, lines, or box plots depict baseline Ad5 seronegative subjects, and open black symbols, lines, or box plots depict baseline Ad5 seropositive subjects. a) Percentage of baseline Ad5-specific CD4+ T-cells producing various responses separated by Ad5 seropositivity. Bars represent the mean ± SEM. b) Percent contribution of Ad5- specific CD4+ T-cells making each respective function to the total Ad5-specific CD4+ T-cell response at baseline. c) Fold change in each Ad-specific CD4+ T-cell function after a single vaccination. IL-2 fold change was significantly higher in Ad5 seropositives at week 4 (P < 0.02). d) Transient changes in Ad-specific CD4+ T-cell function after vaccination. In seropositive subjects IFN-γ increased from baseline (black asterisk) in the seropositive group at week 4 (P < 0.005), 8 (P < 0.05) and 30 (P < 0.5), IL-2 at week 4 (P < 0.03), MIP-1α at week 4 (P < 0.03), and TNF- α at weeks 4 (P < 0.0001) and 8 (P < 0.005); in seronegative subjects, IFN-γ increased (gray asterisk) above baseline at week 4 (P < 0.03), MIP-1α at weeks 4 (P < 0.005) and 42 (P < 0.001), and perforin at weeks 4 (P < 0.001), 42 (P < 0.0001), 52 (P < 0.05) and 78 (P < 0.05). Plots depict the median, 25th and 75th percentile (boxes) and the minimum and maximum values (whiskers). Triangles indicate vaccination time points. e) Ki67 expression on Ad-specific CD4+ T-cells does not change after vaccination. Plots depict the median, 25th and 75th percentile (boxes) and the minimum and maximum values (whiskers). Triangles indicate vaccination time points. f) Percentage of A5-specific CD4+ T memory (dashed lines) or effector memory (solid lines) that express both α4 and β7.g) α4 and β7 co-expression on memory (dashed line) or effector memory (solid lines) CD4+ T-cells. P value shown denotes a significant difference between the groups at week 8. h) Percentage of α4+ β7+ memory (dashed lines) or effector memory (solid lines) CD4+ T-cells that are Ad5-specific.
After the first vaccination Ad5-specific CD4+IFN-γ+ T-cells increased in both groups (P < 0.05), with the fold change in CD4+IFN-γ+ T-cells independent of serostatus (Fig. 2c, Supplemental Fig. 2–4). The frequency of Ad5-specific MIP-1 α+ CD4+ T-cells increased above baseline after vaccination (P<0.03 seropositives, P < 0.005 seronegatives), whereas IL-2 (P < 0.03) and TNF–α (P < 0.001) increased in seropositive subjects only and accounted for a higher proportion of the total response (P < 0.05) compared with seronegative subjects (Fig. 2c–d, Supplemental Fig. 2–3). Following three vector doses, perforin and MIP-1α were higher in seronegative subjects above baseline, but little differences in other functions were found in either serogroup after the second vaccine dose (Fig. 2d, Supplemental Fig. 3). Despite these transient increases in CD4+ T-cell functions within serogroups, there was never a significant difference between the groups for the percentage of Ad5-specific CD4+ T-cells producing IFN-γ, IL- 2, MIP-1α, TNF-α, or perforin. The degree of polyfunctionality of Ad5-specific CD4+ T-cells remained comparable between baseline Ad5 seronegative and seropositive subjects (Supplemental Fig. 4).
No difference was found in Ki-67 expression for total (data not shown) or Ad5- specific CD4+ T-cells (Fig. 2e, Supplemental Fig. 5) between the serogroups or compared to baseline. Expression of the mucosal trafficking-associated markers α4 and β7 did not differ significantly from baseline within either serogroup on total memory (TM: all CD45RO+ and CCR7−CD45RO−) and effector memory (TEM: CCR7−CD45RO+) CD4+ T-cells (Fig. 2f, Supplemental Fig. 5) or Ad5-specific TM or TEM CD4+ T-cells (Fig. 2g) after vaccination. Moreover, Ad5-specific CD4+ TM and TEM cells represented a small fraction of total circulating α4+β7+CD4+ T-cells, and did not change significantly after vaccination (Fig. 2h). Thus, while transient changes in the phenotype and magnitude of Ad5-specific CD4+ T-cell responses were detected within groups after vaccination, no significant differences between groups were observed.
Our results indicate that Ad5-specific CD4+ T-cells were unlikely to play a role in the possible increased susceptibility to HIV-1 infection observed in the STEP trial. Three findings support this conclusion: First, baseline Ad5 nAb titers are not predictive of Ad5-specific CD4+ T-cell responses. Second, Ad5-specific CD4+ T-cells within both baseline Ad5 seronegative and seropositive subjects expand in response to Ad vector administration. While slightly higher responses were observed early in baseline Ad5 seropositives, these differences were extremely transient, and would not account for increased acquisition primarily observed at later times (post-week 20) in STEP. Thus, vaccination does not appear to preferentially increase the pool of potentially infectable circulating Ad5-specific CD4+ T-cells in Ad5 seropositive individuals. Finally, Ad5 nAb seronegative subjects uniformly became Ad5 seropositive after a single vaccination, yet no enhanced susceptibility was noted in baseline Ad5 seronegative STEP participants after the first vaccine administration. Taken together, these data suggest that any linkage between Ad5 serostatus and Ad5-specific T-cell-related increase in HIV-1 acquisition should only have been observed early after the first vaccine dose, for afterwards Ad5-specific T-cell responses in baseline Ad5 seronegative subjects appear immunologically equivalent to baseline Ad5 seropositive subjects. With the caveat that our analyses are restricted to circulating CD4+ T-cells and do not address potential differences in activated Ad5-specific CD4+ T-cells within mucosal tissues after vaccination, our results do not support the hypothesis that Ad5-specific CD4+ T-cells contributed to the potential increased HIV-1 acquisition in the STEP trial.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health IPCAVD U19AI074078 to M.Betts and H.Ertl. R. Doms, F. Bushman, and D. Weiner provided critical evaluation of this manuscript. M. Robertson, D. Casimiro, S. Dubey, K. Cox, and L. Kierstead are paid employees of Merck, own Merck stock, and have Merck stock options.
References
- 1.Buchbinder SP, et al. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekaly RP. J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElrath JM, et al. The Lancet. 2008;6736:61592–61595. [Google Scholar]
- 4.Xiang ZQ, Yang Y, Wilson JM, Ertl HC. Virology. 1996;219:220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]
- 5.Grubb BR, et al. Nature. 1994;371:802–806. doi: 10.1038/371802a0. [DOI] [PubMed] [Google Scholar]
- 6.Burger SR, et al. J Gen Virol. 1991;72(Pt 2):359–367. doi: 10.1099/0022-1317-72-2-359. [DOI] [PubMed] [Google Scholar]
- 7.Graham FL, Smiley J, Russell WC, Nairn R. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 8.Priddy FH, et al. Clin Infect Dis. 2008;46:1769–1781. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- 9.Aste-Amezaga M, et al. Hum Gene Ther. 2004;15:293–304. doi: 10.1089/104303404322886147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.