Abstract
Purpose
To assess the tolerability, pharmacokinetics (PKs), and pharmacodynamics (PDs) of the mitogen-activated protein kinase kinase (MEK) 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancer.
Patients and Methods
In part A, patients received escalating doses to determine the maximum-tolerated dose (MTD). In both parts, blood samples were collected to assess PK and PD parameters. In part B, patients were stratified by cancer type (melanoma v other) and randomly assigned to receive the MTD or 50% MTD. Biopsies were collected to determine inhibition of ERK phosphorylation, Ki-67 expression, and BRAF, KRAS, and NRAS mutations.
Results
Fifty-seven patients were enrolled. MTD in part A was 200 mg bid, but this dose was discontinued in part B because of toxicity. The 50% MTD (100 mg bid) was well tolerated. Rash was the most frequent and dose-limiting toxicity. Most other adverse events were grade 1 or 2. The PKs were less than dose proportional, with a median half-life of approximately 8 hours and inhibition of ERK phosphorylation in peripheral-blood mononuclear cells at all dose levels. Paired tumor biopsies demonstrated reduced ERK phosphorylation (geometric mean, 79%). Five of 20 patients demonstrated ≥ 50% inhibition of Ki-67 expression, and RAF or RAS mutations were detected in 10 of 26 assessable tumor samples. Nine patients had stable disease (SD) for ≥ 5 months, including two patients with SD for 19 (thyroid cancer) and 22 (uveal melanoma plus renal cancer) 28-day cycles.
Conclusion
AZD6244 was well tolerated with target inhibition demonstrated at the recommended phase II dose. PK analyses supported twice-daily dosing. Prolonged SD was seen in a variety of advanced cancers. Phase II studies are ongoing.
INTRODUCTION
Mitogen-activated protein kinase kinase (MEK or MAPK/ERK kinase) is a critical enzyme in the RAS/RAF/MEK/ERK pathway that regulates key cellular activities including proliferation, survival, and cell cycle regulation. This pathway is composed of a protein kinase cascade in which RAF, MEK, and ERK are in a sequential order.
MEK1/2 are attractive therapeutic targets because their only known substrates are ERK1/2. MEK inhibitors inhibit growth of human tumors in mouse xenografts1–7 and leukemia cells in vitro.8 Two other MEK inhibitors have been tested in clinical trials. CI-1040 showed insufficient antitumor activity to warrant further development,9 and development of a second-generation MEK inhibitor, PD0325901,10 has recently been discontinued.11 AZD6244 is a potent, selective, adenosine triphosphate–uncompetitive inhibitor of MEK1/2, with an in vitro half maximal inhibitory concentration of 10 to 14 nmol/L against purified enzyme and no inhibition up to 10 µmol/L against numerous other serine/threonine and tyrosine kinases.4 AZD6244 has excellent preclinical activity against many different tumors in cell-based growth assays and in human tumor mouse xenograft models, including colorectal,4,6 pancreatic,4 non–small-cell lung,6 and hepatocellular cancer5 and melanoma.7
Given this spectrum of preclinical activity4 and the acceptable toxicology profile, a phase I study was undertaken to evaluate the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of AZD6244 in patients with advanced malignancies.
PATIENTS AND METHODS
Patient Selection
Eligibility criteria included patients aged ≥ 18 years with histologic or cytologic evidence of advanced cancer for which there was no curative or life-prolonging therapy; Eastern Cooperative Oncology Group performance status ≤ 2; prior radiation completed ≥ 3 weeks before study enrollment; life expectancy of ≥ 12 weeks; and adequate bone marrow (platelets ≥ 100,000/µL, absolute neutrophil count > 1,500/µL, and hemoglobin ≥ 9 g/dL), hepatic (total bilirubin ≤ 2.5× the upper limit of normal and AST ≤ 2.5× normal), and renal (serum creatinine ≤ 1.5× the upper limit of normal) function. In part B, patients were required to have a tumor that was safely accessible for biopsy. All patients gave written informed consent.
Experimental Treatment
This phase I, open-label, multiple-dose study assessed the safety, tolerability, PK, and PD of AZD6244 in patients with advanced solid malignancies. AZD6244 was formulated as an oral powder for reconstitution and supplied in dosing kits in 30-mL amber bottles. Antiemetic prophylaxis was not administered.
Part A was conducted to determine the maximum-tolerated dose (MTD) and used a standard three- to six-patient cohort design12 evaluating doses of 50, 100, 200, and 300 mg bid. The incidence and severity of adverse events were evaluated and coded according to National Cancer Institute Common Terminology Criteria of Adverse Events (version 3). Response to therapy was monitored by modified Response Evaluation Criteria in Solid Tumors.13 AZD6244-related dose-limiting toxicity (DLT) was defined as follows: any grade 4 toxicity (grade 4 neutropenia for > 7 days), grade 3 or 4 neutropenia with fever, grade 3 or 4 thrombocytopenia associated with bleeding (excluding patients receiving systemic anticoagulation), or any grade 3 or 4 nonhematologic toxicity. Grade 2 vomiting on 2 consecutive days despite optimal antiemetic therapy was considered dose limiting, as was any grade 2 toxicity lasting for more than 2 weeks or dosing interruption of more than 2 weeks for drug-related toxicity. The MTD was defined as one dose level below that which induced DLT in more than one third of patients (at least two of a maximum of six patients). Each patient began the study with a single dose of AZD6244 on day 1, with assessment of adverse events on days 1, 2, and 3. If there were no DLTs through day 8, continuous bid dosing commenced. A cycle was defined as 28 days of twice-daily therapy.
In part B, patients were stratified by cancer type (melanoma v other) and randomly assigned to receive the MTD (200 mg bid) or 50% of the MTD dose (100 mg bid) to evaluate the dose that provided the best balance of safety/tolerability and PD effect for future clinical development. Tissue samples (tumor and normal skin) were obtained for PD assessments before dose and after 7 to 21 days of AZD6244 (day 15 ± 7 days). Patients must have taken the assigned dose uninterrupted for ≥ 7 days before the postdose biopsy.
Clinical Care of Patients
In the single-dose phase of part A, physical examinations, toxicity assessments, and laboratory analyses were conducted on days 1, 2, and 3. In the bid dosing phase, weekly assessments commenced on day 8 of the first 28-day cycle. ECG and PK assessments were conducted on day 22. In part B, assessments were conducted weekly in cycle 1 and every 28 days in subsequent cycles. Patients could continue on uninterrupted 28-day cycles of AZD6244 provided that there was no disease progression or unacceptable toxicity.
PD Analysis
Blood samples were collected on days 1 and 22 in part A and days 1 and 15 in part B before dose and 1 hour after dose for measurement of pERK levels by fluorescence-activated cell sorting analysis. Samples were treated ex vivo with 12-O-tetradecanoylphorbol-13-acetate for 10 minutes at 37°C within 1 hour of being drawn. ERK phosphorylation was preserved by immediate fixation of the cells with 1.2% methanol-free formaldehyde. Peripheral-blood mononuclear cells (PBMCs) were isolated, washed, and stored at −20°C. For analysis of ERK phosphorylation, cells were treated with an antibody to pERK, followed by a fluorescein isothiocyanate–conjugated secondary detection antibody and pERK quantitation by fluorescence-activated cell sorting analysis.
PK Analysis
Maximum observed plasma concentration (Cmax) and median observed time to maximum plasma concentration values for each patient were derived from the plasma concentration-time profile, and the area under the time-concentration curve (AUC0–24 hours) was calculated using the linear trapezoidal rule (for details, see Appendix, online only).
Skin and Tumor Biopsy Sample Collection
Tissue samples (tumor and normal skin) were obtained for PD assessments before dose and after 7 to 21 days of AZD6244 (day 15 ± 7 days). The day 15 (± 7 days) postdose tumor and normal skin biopsies were collected 2 to 4 hours after dose on the same day as PK and PD assessments. Tumor biopsies (18-guage core needle) were taken using computed tomography or ultrasound scan guidance. Samples were fixed and stained with hematoxylin and eosin to confirm the diagnosis and the quality of the biopsy tissue. For optimal comparative biomarker studies, subsequent biopsies were taken from the same site as the screening biopsy. Skin biopsies were taken from the upper arm or buttocks using a 3- to 4-mm punch, using the same fixation method.
Immunohistochemistry
An indirect immunoperoxidase method, with antibodies against pERK1/2 or Ki-67, was used to evaluate pERK status and growth fraction (Ki-67) in situ. Negative and positive controls were included in each immunostained batch of slides. In all cases, these controls stained appropriately. Slides were scored, and representative microscopic fields were photographed. Nuclei and cytoplasm were scored for pERK by estimating the proportion of positive viable tumor cells multiplied by intensity of staining quantified on a 0 to 4+ scale. The proportion of tumor cell nuclei staining for Ki-67 was estimated by microscopic inspection in 10% increments. Only viable tumor was scored, with care taken to avoid necrotic areas of tumor.
Tumor DNA Mutation Analysis
Tumor tissue sections were isolated from paraffin-embedded sample blocks using a 1-mm array punch. Samples were washed and air dried, and DNA was extracted from fixed tissue. Analyses for KRAS, NRAS, and BRAF mutations were performed by established methods (see Appendix).
Statistical Evaluation
Safety data were summarized using appropriate descriptive statistics. Baseline scaled ratios were calculated for each assessable pair of biopsies (corresponding to the postdose/predose value). Because the data were treated as being multiplicative, geometric means (gmean) were calculated to give an overall mean level of inhibition, and corresponding CIs were calculated for these mean levels of inhibition.
Correlations of markers between tumor and skin samples were assessed using the Spearman rank correlation coefficient. Differences in time on study between patients who had an oncogene mutation at baseline and those who did not were assessed using a Wilcoxon signed rank test, as were differences in biomarker inhibition between patients with and without the mutation.
RESULTS
Fifty-seven patients (35% malignant melanoma; Table 1) received a total of 184 assessable cycles of therapy across four dose levels. The median number of cycles administered per patient was two (range, one to 22 cycles). Other baseline patient characteristics are listed in Table 1.
Table 1.
Patient Characteristics
| All Patients in Safety Population | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part A (n = 23) | Part B (n = 34) | Total (N = 57) | |||||||
| Characteristic | No. | % | No. | % | No. | % | |||
| Sex | |||||||||
| Male | 13 | 56.5 | 20 | 58.8 | 33 | 57.9 | |||
| Female | 10 | 43.5 | 14 | 41.2 | 24 | 42.1 | |||
| Age, years | |||||||||
| Median | 58 | 60 | 59 | ||||||
| Range | 29–78 | 34–84 | 29–84 | ||||||
| Race | |||||||||
| White | 21 | 91.3 | 33 | 97.1 | 54 | 94.7 | |||
| African American | 1 | 4.3 | 1 | 2.9 | 2 | 3.5 | |||
| Asian | 1 | 4.3 | 0 | 0 | 1 | 1.8 | |||
| ECOG performance status at screening | |||||||||
| 0 | 9 | 39.1 | 13 | 38.2 | 22 | 38.6 | |||
| 1 | 14 | 60.9 | 20 | 58.8 | 34 | 59.6 | |||
| 2 | 0 | 0 | 1 | 2.9 | 1 | 1.8 | |||
| No. of metastatic sites | |||||||||
| 1 | 6 | 26.1 | 6 | 17.6 | 12 | 21.1 | |||
| 2 | 39.1 | 26.5 | 18 | 31.6 | |||||
| ≥ 3 | 8 | 34.8 | 19 | 55.9 | 27 | 47.3 | |||
| Prior anticancer treatments | |||||||||
| Surgery | 21 | 91.3 | 32 | 94.1 | 53 | 93.0 | |||
| Radiation | 13 | 56.5 | 25 | 73.5 | 38 | 66.7 | |||
| Chemotherapy regimens | 22 | 95.7 | 30 | 88.2 | 52 | 91.2 | |||
| 0 | 1 | 4.3 | 4 | 11.8 | 5 | 8.8 | |||
| 1 | 7 | 30.4 | 6 | 17.6 | 13 | 22.8 | |||
| ≥ 2 | 15 | 65.2 | 24 | 70.6 | 39 | 68.4 | |||
| Cancer types | |||||||||
| Melanoma | 20 | 35.1 | |||||||
| Breast | 10 | 17.5 | |||||||
| Colorectal | 5 | 8.8 | |||||||
| Other* | 22 | 38.6 | |||||||
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Two patients each: non–small-cell lung cancer, hepatocellular, head and neck, sarcoma, thyroid, gastroesophageal junction; one each: lung squamous cell carcinoma, adenoid cystic, mesothelioma, renal cell, pancreatic, ovarian, bronchoalveolar, bladder, thymus, and leiomyosarcoma.
Toxicity
The toxic effects of AZD6244 are listed in Table 2 and Table 3.
Table 2.
Treatment-Related Adverse Events*
| AZD6244 bid Dose (No. of patients) | Total No. of Patients (N = 57) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 mg (n = 3) | 100 mg (n = 31) | 200 mg (n = 15) | 300 mg (n = 8) | ||||||||||
| Adverse Event | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |||||
| Any event | 3 | 30 | 14 | 7 | 54 | ||||||||
| Rash† | 2 | 0 | 21 | 3 | 8 | 4 | 3 | 2 | 43 | ||||
| Diarrhea | 2 | 0 | 16 | 0 | 12 | 0 | 2 | 1 | 33 | ||||
| Nausea | 0 | 0 | 13 | 1 | 8 | 0 | 3 | 0 | 25 | ||||
| Fatigue | 2 | 0 | 8 | 1 | 8 | 1 | 2 | 0 | 22 | ||||
| Peripheral edema | 1 | 0 | 9 | 1 | 6 | 0 | 2 | 0 | 19 | ||||
| Vomiting | 0 | 0 | 7 | 0 | 5 | 0 | 2 | 0 | 14 | ||||
| ALT elevation | 0 | 1 | 4 | 0 | 4 | 0 | 0 | 0 | 9 | ||||
| AST elevation | 1 | 0 | 2 | 1 | 5 | 0 | 0 | 0 | 9 | ||||
| Blurred vision | 0 | 0 | 2 | 0 | 2 | 0 | 3 | 0 | 7 | ||||
Data include treatment-related adverse events with an onset date on or after the day 1 single dose in part A or the first dose in Part B.
Rash includes the following Medical Dictionary for Regulatory Activities preferred terms: Dermatitis Acneiform, Rash, Rash Erythematous, Rash Maculo-papular, and Rash Pruritic.
Table 3.
Dose-Limiting Toxicities in Cycle 1
| Dose-Limiting Toxicity |
AZD6244 Dose (No. of patients) | Total No. of Patients |
||
|---|---|---|---|---|
| 100mg | 200mg | 300 mg | ||
| Total | 31 | 15 | 8 | 54 |
| Rash | 2 | 3 | 2 | 7 |
| Hypoxia | 1* | 1 | — | 2 |
| Diarrhea | — | — | 1 | 1 |
| T-wave inversion | 1* | — | — | 1 |
Occurred in same patient.
Hematologic toxicity
Minimal hematologic toxicity was seen with AZD6244.
Rash
Rash was the most frequent toxicity and DLT, occurring in 74% of all patients, and precluded dose escalation greater than 300 mg bid. The rash was dose dependent, erythematous, and maculopapular, occurring predominantly on the torso. Resolution typically occurred with dosing interruption and/or dose reduction. In part B, an increase in frequency and severity of this rash led to selection of 100 mg bid as the tolerable phase II dose. Of the 43 episodes of skin rash, 34 were of maximum grade 1 or 2, and nine were grade 3 or 4.
GI toxicity
Mild to moderate diarrhea was the principal GI toxicity (56% of patients). Abdominal examination during the diarrhea episodes was benign. Diarrhea resolved promptly with loperamide therapy and/or drug discontinuation. In addition to diarrhea, nausea (n = 25) and vomiting (n = 14) were observed, which resolved quickly and completely with antiemetic therapy.
Edema
Mild to moderate edema occurred in 19 of 57 patients, whereas severe edema occurred in one patient with pre-existing abdominal distension from ascites.
Fatigue
Fatigue was dependent on dose and duration of treatment and mild to moderate in 20 of 22 patients. It was reversible with dose reduction and/or interruption.
Other toxicities
Mild to moderate reversible ALT and AST elevation occurred in 14% and 14% of patients, respectively. Blurred vision, which was transient and reversible, occurred in 12% of patients. These events were all grade 1 or 2. Eight patients (14%) experienced serious adverse events, including hypoxia, pneumonitis, bradycardia, renal insufficiency, and exfoliative dermatitis.
Dose reductions and study discontinuation
Seven patients (12%) required dose reductions for treatment-related toxicity, 24 patients (42%) required drug holidays of up to 2 weeks, and eight patients (14%) discontinued treatment for drug-related toxicity. On the basis of these results, the MTD and recommended dose of AZD6244 as an oral powder for reconstitution formulation for subsequent clinical testing is 100 mg bid.
PK
After a single dose of AZD6244, the median terminal half-life was 8.3 hours. Cmax increased with increasing dose (Table 4 and Table 5). The mean area under the plasma concentration-time curve (AUCinf) after single doses of AZD6244 also increased with increasing dose. Similarly, the steady-state AUC over the 12-hour dosing interval (AUC0–12 hours) increased to a maximum at 200 mg bid. In part B, the median observed time to maximum plasma concentration was 1 hour after dose. The mean single-dose Cmax values for the 100-mg and 200-mg cohorts were similar to the respective steady-state (day 15) Cmax values. In both parts, the single-dose and steady-state AUC values increased with increasing dose in a less than dose-proportional manner (Table 4 and Table 5). In part B, however, it is likely that the median terminal half-life (4.7 hours) is an underestimate because of the shorter PK sampling schedule, which ended at 12 hours after dose (before the evening dose).
Table 4.
Summary of Pharmacokinetic Parameters for AZD6244 on Days 1 (single dose) and 22 (bid dosing)
| No. of Patients |
Cmax (ng/mL) | Tmax (h) | AUC* (ng • h/mL) | t1/2 (h) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 22 | Day 1 | Day 22 | Day 1 | Day 22 | Day 1 | Day 22 | |||||||||||
| Dose (mg) |
Day 1 |
Day 22 |
Geometric Mean |
CV | Geometric Mean |
CV | Median | Range | Median | Range | Geometric Mean |
CV | Geometric Mean |
CV | Median | Range | Median | Range |
| 50 | 3 | 3 | 528 | 64 | 528 | 40 | 3 | 1–3 | 1 | 1–1 | 3,581 | 18 | 2,193 | 57 | 6.7 | 6.6–8.7 | — | — |
| 100 | 4 | 5 | 486 | 75 | 718 | 63 | 1 | 1–4 | 1 | 1–1 | 2,929 | 23 | 2,365 | 78 | 11.1 | 7.6–24.0 | — | — |
| 200 | 7 | 6 | 781 | 93 | 1,010 | 116 | 1 | 1–4 | 1 | 1–1 | 4,900 | 112 | 2,960 | 145 | 6.1 | 4.5–28.3 | — | — |
| 300 | 8 | — | 952 | 74 | — | — | 1 | 1–4 | — | — | 6,488 | 63 | — | — | 14.5 | 7.1–19.4 | — | — |
Abbreviations: Cmax, maximum observed plasma concentration; Tmax, time to maximum plasma concentration; AUC, area under the concentration-time curve; t1/2, terminal half-life; CV, coefficient of variation.
All AUC values are from time 0 to 12 hours after dose, except for the day 1, which are AUCinf values.
Table 5.
Summary of Pharmacokinetic Parameters for AZD6244 on Days 1 (single dose) and 15 (bid dosing)
| No. of Patients |
Cmax (ng/mL) | Tmax (h) | AUC* (ng • h/mL) | t1/2 (h) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 15 | Day 1 | Day 15 | Day 1 | Day 15 | Day 1 | Day 15 | |||||||||||
| Dose (mg) |
Day 1 |
Day 15 |
Geometric Mean |
CV | Geometric Mean |
CV | Median | Range | Median | Range | Geometric Mean |
CV | Geometric Mean |
CV | Median | Range | Median | Range |
| 100 | 22 | 18 | 807 | 68 | 895 | 52 | 1 | 1–8 | 1 | 1–4 | 3,124 | 43 | 5,006 | 47 | 4.5 | 2.2–10.5 | — | — |
| 200 | 8 | 3 | 933 | 61 | 952 | 38 | 2 | 1–4 | 1 | 1–2 | 5,234 | 42 | 6,944 | 27 | 5.4 | 3.5–6.6 | — | — |
Abbreviations: Cmax, maximum observed plasma concentration; Tmax, time to maximum plasma concentration; AUC, area under the concentration-time curve; t1/2, terminal half-life; CV, coefficient of variation.
All AUC values are from time 0 to 12 hours after dose.
PD
Inhibition of ERK phosphorylation in PBMCs
Inhibition of ERK phosphorylation has been proposed as a PD biomarker of MEK inhibitor activity.14 We initially measured inhibition of ERK phosphorylation in lymphocytes from 12-O-tetradecanoylphorbol-13-acetate-treated whole blood as a surrogate for tumor tissue (Appendix Table A1, online only). Up to 100% inhibition of ERK phosphorylation was seen 1 hour after the first dose, indicating rapid distribution and activity of AZD6244 in the bloodstream. Importantly, up to 90% inhibition of ERK phosphorylation (gmean = 51%) was seen in the trough samples on day 15 or 22, indicating that target inhibition was maintained throughout the bid dosing regimen.
Inhibition of ERK phosphorylation and Ki-67 labeling index in tumor biopsies
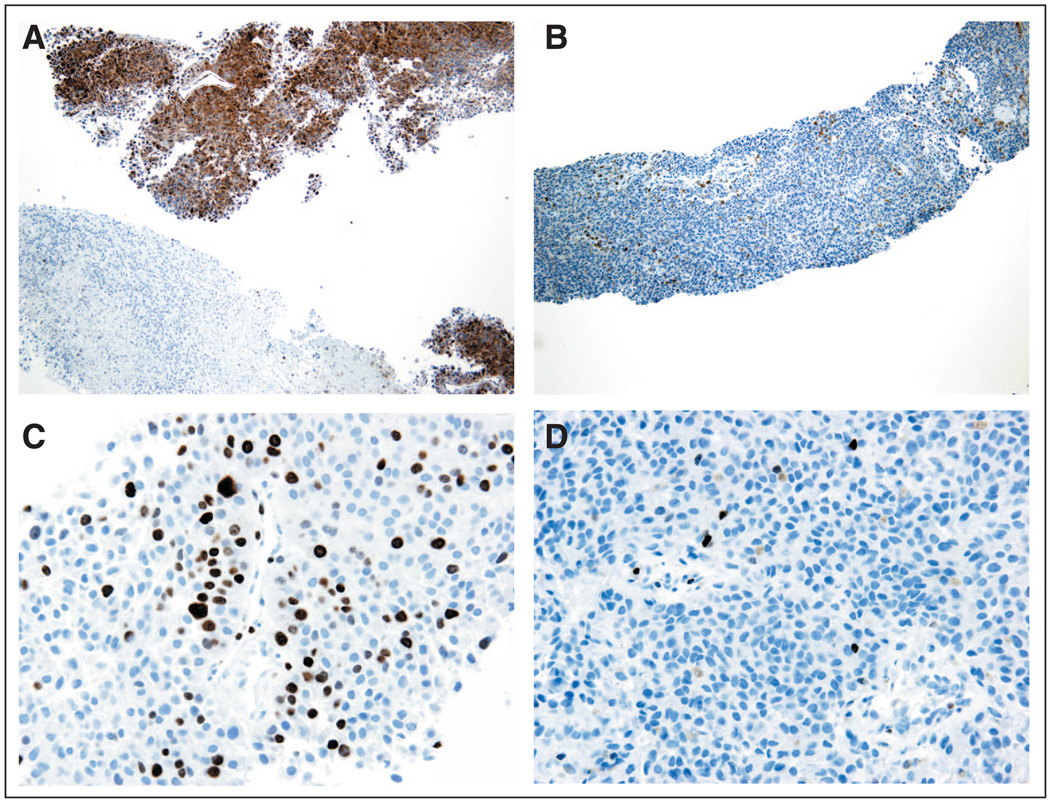
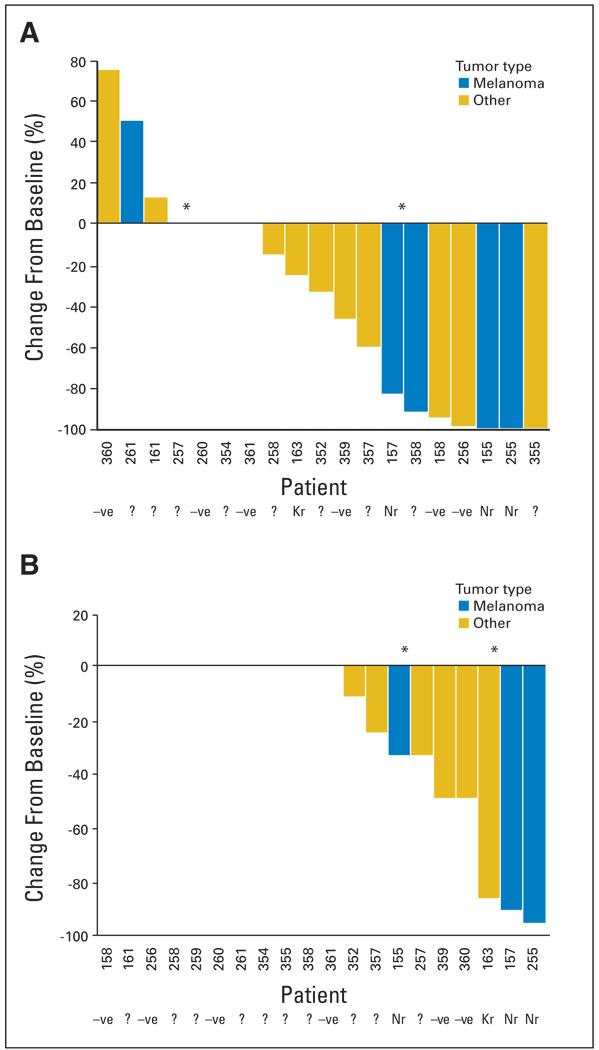
After documenting target inhibition in a surrogate tissue in part A, paired tumor samples were collected before treatment and after at least 7 continuous days of treatment and evaluated for inhibition of ERK phosphorylation by immunohistochemistry. We also evaluated drug effects on downstream signaling events by examining the reduction in the Ki-67 labeling index, a marker of cell proliferation. Figure 1 shows representative immunohistochemistry photomicrographs. Twenty of the 24 paired biopsies were assessable, with 19 having detectable pretreatment pERK expression and all 20 having detectable pretreatment Ki-67 expression. Strong inhibition of ERK phosphorylation was seen with a gmean inhibition of 79% (90% CI, 50% to 91%; Fig 2A). Ki-67 labeling was reduced in post-treatment tumor samples but not as consistently as pERK, the primary proof-of-mechanism biomarker. Nine of 20 samples showed some reduction, with ≥ 50% reduction in five samples (Fig 2B). The skin biopsies were generally uninformative because of variable and minimal baseline levels of pERK.
Fig 1.
Immunostains of pre- and post-treatment melanoma specimens from the same patient. (A) Before dose, tumor cells are reactive to anti-pERK antibody (brown staining; magnification, ×100). (B) After dose, cells are unreactive to same anti-pERK antibody (magnification, ×100). (C) Before dose, variable nuclear Ki-67 labeling (approximately 30% positive nuclei; magnification, ×400). (D) After dose, marked reduction in nuclear Ki-67 labeling (< 1% positive nuclei; magnification, ×400)
Fig 2.
Percent change from baseline at day 15 (± 7 days) in (A) tumor cell nuclei H-score for pERK and (B) proportion of tumor cell nuclei staining for Ki-67. Patients received 100 mg bid or 200 mg bid (denoted by *).
DNA Mutation Analysis
Activating mutations in the RAS genes (KRAS and NRAS) and in the BRAF gene have been reported to identify tumors that may be sensitive to MEK inhibition.6,15 Therefore, the presence of specific mutations in these genes was evaluated in tumor samples from this study. Appendix Table A2 (online only) lists these data. Of the 26 patients with samples assessable for mutational status, 10 had a single mutation in KRAS (n = 5), NRAS (n = 4), or BRAF (n = 1).
The average length of time on study for patients carrying mutations (median, 3.5 months; range, 1 to 6 months) was greater than for those without a mutation (median, 2 months; range, 1 to 4 months). There is no statistical evidence of effect (P = .30 by Wilcoxon signed rank test) in this small sample. Four of the 10 patients with a mutation had tumor biopsies assessable for the pERK assay, which showed strong inhibition of ERK phosphorylation (100%, 100%, 83%, and 25%). These four patients, plus one other patient with a mutation, had tissue assessable for Ki-67 labeling and showed a strong labeling index (100%, 97%, 92%, 88%, and 33%). Possibly because of the small numbers in the study, there was no significant difference between biomarker knockdown for those patients with mutation versus those without mutation or with unknown mutation status (pERK: P = .13; Ki-67: P = .13). Of note, three patients showing the strongest reduction in Ki-67 labeling were all mutation positive.
Antitumor Activity
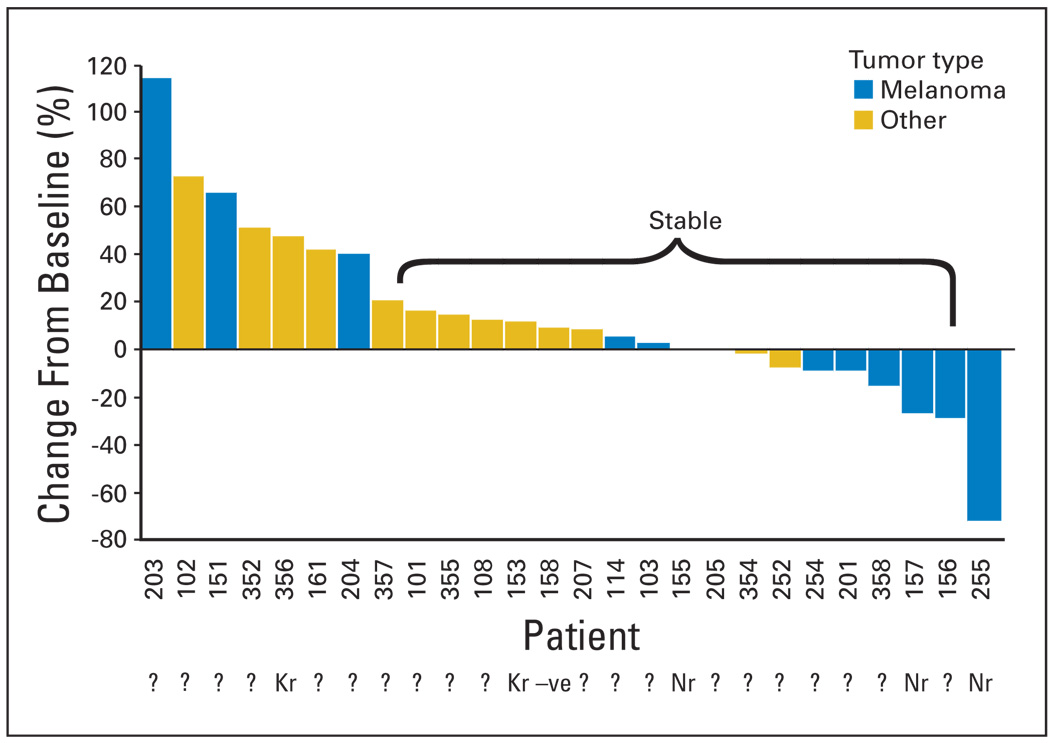
Figure 3 summarizes tumor responses by Response Evaluation Criteria in Solid Tumors. Nineteen patients (33%) had stable disease (SD) at the end of cycle 2, and nine patients (16%) had SD for ≥ 5 months. One patient with medullary thyroid cancer experienced SD for 19 cycles, whereas one patient with both uveal melanoma and renal cell carcinoma had SD for 22 cycles.
Fig 3.
Best percent change from baseline in target lesion size for patients who have at least postbaseline efficacy assessment.
DISCUSSION
AZD6244 is a potent and selective MEK1/2 inhibitor that has shown excellent preclinical activity in a range of tumor models4 with an acceptable toxicology profile, and this phase I study demonstrates that AZD6244 is well tolerated up to 100 mg bid. In part A, the MTD was 200 mg bid, but because of an increase in the frequency and severity of rash in part B, the lower dose level (50% of the MTD; 100 mg bid) was recommended as the tolerable phase II dose. The most common treatment-related toxicities observed with AZD6244 were rash, diarrhea, nausea, and fatigue, which are consistent with those observed for PD0325901 and CI-1040.9,10,16 Seven patients developed transient and reversible blurred vision while receiving AZD6244, an adverse effect also observed with PD0325901 and CI-1040.9,10,16 Five of these ocular events were observed at doses greater than the recommended phase II dose. When conducted, ophthalmologic examinations were unrevealing in regard to etiology. Rigorous physical examination and laboratory tests did not identify any other significant toxicities observed with other MEK inhibitors, including syncope and neurotoxicity.16,17
Despite a growing clinical literature on MEK inhibitors, there is only limited evidence to date that MEK can be inhibited consistently in patient tumors at tolerable inhibitor doses. In addition, it is unclear whether such inhibition correlates with clinical outcome and whether MEK inhibition in surrogate tissues corresponds to MEK inhibition in tumors. Accordingly, we determined whether tolerable doses of AZD6244 would inhibit MEK in PBMCs, skin, and patient tumors. Skin biopsies were generally uninformative because of the variable and minimal baseline levels of pERK. We observed a dose-dependent inhibition of ERK phosphorylation in PBMCs, as well as consistent inhibition of ERK phosphorylation when comparing pre- and post-treatment tumor biopsies, but there were insufficient data to suggest a correlation between surrogate tumor tissue PD. We also demonstrated inhibition of Ki-67 in patient tumors, but again, there were insufficient data to conclude whether PBMC samples are suitable surrogate tissues for tumor samples. Because activating mutations in NRAS, KRAS, and BRAF genes correlate in preclinical studies with sensitivity to MEK inhibitors, mutational analysis of these genes was performed in 26 available tumors. In this small sample size, there was a nonsignificant trend towards delayed progression on study in patients with mutations compared with wild-type tumors.
AZD6244 displayed less than dose-proportional PK with increasing Cmax and AUC as doses increased from 50 to 300 mg bid. There was a high degree of interpatient variability, which is not surprising for an oral agent. No food effect study was performed, and no guidance for food intake was given except for PK assessments that were performed in the fasting state (1 hour before and 2 hours after dosing). The PK profile supports a bid dosing scheme that results in exposures that adequately inhibit the drug target.
The best clinical response was SD that lasted for 5 or more months in nine patients. Two patients maintained SD for 19 and 22 cycles. One patient with malignant melanoma had a 70% tumor shrinkage after three cycles of AZD6244 but developed symptomatic brain metastases before confirmatory scans could be performed. This patient had an NRAS mutation and showed 100% inhibition of ERK phosphorylation and 97% inhibition of Ki-67. Thus, the present phase I study provides preliminary evidence of antineoplastic activity in humans.
In summary, this study establishes that the MEK inhibitor AZD6244 has a manageable safety and tolerability profile and identifies a suitable dose for subsequent clinical trials (100 mg orally, twice daily continuously) that results in target inhibition. Although this study demonstrates that the MEK1/2 target can be safely inhibited in vivo in humans, our data also suggest that target inhibition may be necessary but not sufficient for antineoplastic activity. These findings support future clinical development of AZD6244, and phase II studies are in progress.
Acknowledgment
We acknowledge the contributions of Brenda Hippert, R. Kathryn Alpaugh, Patricia Murphy, Edward Temmer, Jackie Heim, Bradley Christensen, Richard Needleman, Colette Toavs, Norma Aumen, Pete Laud, Mireille Cantarini, Lee Booras, Sue Yancik, Tammie Yeh, Michael Doyle, Andrew Hughes, David Whitcombe, Nicola Thelwell, and Harminder Singh.
Supported by Array BioPharma Inc (Boulder, CO), and National Cancer Institute Grants No. NCI P30 CA46934 (University of Colorado Health Sciences Center), NCI K24 CA106349 (S.G.E.), and 5P30CA006927 (Fox Chase Cancer Center).
Appendix
The Appendix is included in the full-text version of this article, available online at www.jco.org. It is not included in the PDF version (via Adobe® Reader®).
Footnotes
Presented at the American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics, November 14-18, 2005, Philadelphia, PA.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Clive Morris, AstraZeneca (C); David Wilson, AstraZeneca (C); Lara Maloney, Array BioPharma Inc (C); Heidi Simmons, Array BioPharma Inc (C); Allison Marlow, Array BioPharma Inc (C); Kevin Litwiler, Array BioPharma Inc (C); Suzy Brown, Array BioPharma Inc (C); Gregory Poch, Array BioPharma Inc (C); Katie Kane, Array BioPharma Inc (C) Consultant or Advisory Role: Alex A. Adjei, Array BioPharma Inc (C); Roger B. Cohen, Array BioPharma Inc (C); Gilad Gordon, Array BioPharma Inc (C); S. Gail Eckhardt, AstraZeneca (C), Array BioPharma Inc (C) Stock Ownership: Clive Morris, AstraZeneca; David Wilson, AstraZeneca; Lara Maloney, Array BioPharma Inc; Kevin Litwiler, Array BioPharma Inc; Gregory Poch, Array BioPharma Inc Honoraria: None Research Funding: Roger B. Cohen, Array BioPharma Inc, AstraZeneca; S. Gail Eckhardt, AstraZeneca Expert Testimony: None Other Remuneration: S. Gail Eckhardt, AstraZeneca, fellowship funding
REFERENCES
- 1.Sebolt-Leopold JS, Dudley DT, Herrera R, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999;5:810–816. doi: 10.1038/10533. [DOI] [PubMed] [Google Scholar]
- 2.Tecle H. The development of kinase inhibitors with unusual mechanism of action: CI-1040, a selective, non-ATP competitive MEK inhibitor in the clinic. Presented at the IBC 2nd International Conference of Protein Kinases: Target Validation, Drug Discovery and Clinical Development of Kinase Therapeutics; September 9–10, 2002; Boston, MA. [Google Scholar]
- 3.Trachet E, Przybranowski S, Howard C. In vivo evaluation of MEK inhibitor, CI-1040 (PD 0184352), against a panel of human pancreatic tumor xenografts. Proc Am Assoc Cancer Res. 2002;43:2096. (abstr 5426) [Google Scholar]
- 4.Yeh TC, Marsh V, Bernat BA, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–1583. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 5.Huynh H, Soo KC, Chow PK, et al. Targeted inhibition of the extracellular signal-regulated kinase kinase pathway with AZD6244 (ARRY-142886) in the treatment of hepatocellular carcinoma. Mol Cancer Ther. 2007;6:138–146. doi: 10.1158/1535-7163.MCT-06-0436. [DOI] [PubMed] [Google Scholar]
- 6.Davies B, Logie A, McKay J, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signalregulated kinase kinase 1/2 kinases: Mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–2219. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 7.Haass NK, Sproessor K, Nguyen TK, et al. The mitogen-activated protein/extracellular signalregulated kinase kinase inhibitor AZD6244 (ARRY-142886) induces growth arrest in melanoma cells and tumor regression when combined with docetaxel. Clin Cancer Res. 2008;14:230–239. doi: 10.1158/1078-0432.CCR-07-1440. [DOI] [PubMed] [Google Scholar]
- 8.Milella M, Kornblau SM, Estrov Z, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–4462. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 10.Lorusso P, Krishnamurthi S, Rinehart JR, et al. A phase 1–2 clinical study of a second generation oral MEK inhibitor, PD 0325901, in patients with advanced cancer. J Clin Oncol. 2005;23:194S. (suppl, abstr 3011) [Google Scholar]
- 11.Pfizer Inc. Pfizer pipeline as of July 31, 2007. http://www.pfizer.com/files/research/pipeline/2007_0731/pipeline_2007_0731.pdf.
- 12.Ratain MJ, Mick R, Schilsky RL, et al. Statistical and ethical issues in the design and conduct of phase I and II clinical trials of new anticancer agents. J Natl Cancer Inst. 1993;85:1637–1643. doi: 10.1093/jnci/85.20.1637. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Waterhouse D, Rinehart J, Adjei AA, et al. A phase 2 study of an oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, or pancreatic cancer. Proc Am Soc Clin Oncol. 2003;22:204. doi: 10.1200/JCO.2004.01.185. (abstr 816) [DOI] [PubMed] [Google Scholar]
- 15.Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006;439:358–362. doi: 10.1038/nature04304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LoRusso P, Krishnamurthi S, Rinehart J, et al. Clinical aspects of a phase I study of PD0325901, a selective oral MEK inhibitor in patients with advanced cancer. American Association for Cancer Research–National Cancer Institute–European Organisation for Research and Treatment of Cancer Molecular Targets and Cancer Therapeutics Meeting; October 22–26, 2007; San Francisco, CA. (abstr B113) [Google Scholar]
- 17.Wang D, Boerner SA, Winkler JD, et al. Clinical experience of MEK inhibitors in cancer therapy. Biochim Biophys Acta. 2007;1773:1248–1255. doi: 10.1016/j.bbamcr.2006.11.009. [DOI] [PubMed] [Google Scholar]