Abstract
A number of distinct β-amyloid (Aβ) variants or multimers have been implicated in Alzheimer's disease (AD), and antibodies recognizing such peptides are in clinical trials. Humans have natural Aβ-specific antibodies, but their diversity, abundance, and function in the general population remain largely unknown. Here, we demonstrate with peptide microarrays the presence of natural antibodies against known toxic Aβ and amyloidogenic non-Aβ species in plasma samples and cerebrospinal fluid of AD patients and healthy controls aged 21–89 years. Antibody reactivity was most prominent against oligomeric assemblies of Aβ and pyroglutamate or oxidized residues, and IgGs specific for oligomeric preparations of Aβ1-42 in particular declined with age and advancing AD. Most individuals showed unexpected antibody reactivities against peptides unique to autosomal dominant forms of dementia (mutant Aβ, ABri, ADan) and IgGs isolated from plasma of AD patients or healthy controls protected primary neurons from Aβ toxicity. Aged vervets showed similar patterns of plasma IgG antibodies against amyloid peptides, and after immunization with Aβ the monkeys developed high titers not only against Aβ peptides but also against ABri and ADan peptides. Our findings support the concept of conformation-specific, cross-reactive antibodies that may protect against amyloidogenic toxic peptides. If a therapeutic benefit of Aβ antibodies can be confirmed in AD patients, stimulating the production of such neuroprotective antibodies or passively administering them to the elderly population may provide a preventive measure toward AD.
Alzheimer's disease (AD) is the most common cause of dementia, affecting an estimated 5.3 million individuals in the United States alone. Deposits of β-amyloid peptide (Aβ) in extracellular plaques characterize the AD brain, but soluble oligomeric Aβ species appear to be more neurotoxic than plaques and interfere with synaptic function (reviewed in ref. 1). Notably, most Aβ peptides isolated from AD brains are posttranslationally modified and truncated (2–6), and some are proposed to be oxidized (7, 8) or cross-linked at Tyr-10 (9). Although the pathogenic consequences of these modifications need to be resolved, most of them can stabilize Aβ assemblies, interfere with proteolytic degradation, and increase Aβ toxicity in vitro (7, 8, 10).
One line of defense against toxic Aβ species could be neutralizing antibodies. Stimulating the production of Aβ antibodies by active immunization with synthetic Aβ (11) or administering monoclonal Aβ antibodies (12, 13) reduced amyloid pathology and inflammation and improved cognitive function in mouse models of AD (14). In patients with mild to moderate AD active immunization appears to reduce plaque load (15), and in some patients production of Aβ antibodies correlated with attenuated cognitive decline (16). It has also been suggested that antibodies recognizing different domains (12, 13, 17) or conformations (18, 19) of Aβ may have different efficacy in humans.
Interestingly, antibodies against Aβ occur naturally in blood and cerebrospinal fluid (CSF) in free form or in complex with Aβ, both in AD patients and healthy individuals (20–26). The titers of these antibodies are low, and their origin and physiological or pathological role is unknown. Using crude methods Aβ antibody titers were found to be increased (22), decreased (23, 27, 28), or unchanged (21) in AD compared with healthy controls, but a large-scale analysis of Aβ antibody subspecificities or changes in antibody repertoire with age or in response to Aβ vaccination has not been described. Based on the presence of Aβ antibodies in normal plasma and the promise of therapeutic Aβ vaccines polyclonal i.v. immune globulins (IVIg) are being tested in clinical trials for the treatment of AD (25, 29).
Results
Plasma Aβ Antibodies Predominantly Recognize High Molecular Mass Assemblies in Oligomeric Preparations of Aβ1-42.
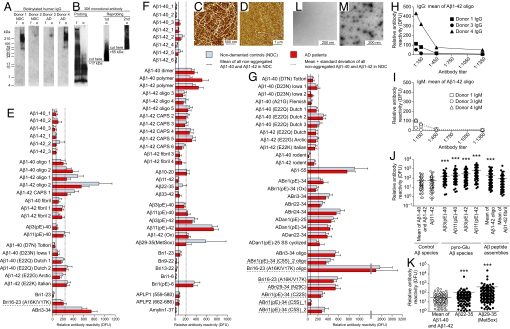
To characterize the specificity of human plasma Aβ antibodies we isolated IgGs, and analyzed their reactivity to assemblies of Aβ1-42 peptides on Western blots. Some IgG samples reacted distinctively with a nonfibrillar Aβ assembly between 55 and 78 kDa, reminiscent of the previously described 56-kDa Aβ*56 (30) but the main reactivity in all samples was against entities >210 kDa (Fig. 1A). The lack of binding to fibrillar Aβ preparations (Fig. 1A) indicates that most of the IgG antibodies are specific to smaller, potentially oligomeric conformations. The Aβ1-5 specific antibody 3D6 detected various assemblies in the oligomeric and fibrillar Aβ preparations (Fig. 1B). Atomic force microscopy confirmed the presence of predominantly fibrillar structures in fibrillar preparations (Fig. 1C) or nonfibrillar Aβ species in oligomeric preparations of Aβ1-42 (Fig. 1D). Together these data demonstrate the existence of highly specific IgG antibodies in human plasma, recognizing conformational epitopes in high molecular mass Aβ1-42 assemblies.
Fig. 1.
Human plasma IgGs preferentially recognize oligomeric and postranslationally modified Aβ. (A) Membranes with fibrillar (f) and oligomeric (o) preparations of 2 μg of Aβ1-42 were probed with biotinylated human IgG fractions isolated from plasma of 2 NDCs and 2 AD patients. Bound IgGs were detected by HRP-tagged avidin. (B) In parallel experiments membranes were probed with Aβ1–5-specific mAb 3D6 (0.5 μg/mL). Because 3D6 did not detect the high molecular mass species of Aβ1-42 in the oligomeric preparation after the first probing, the lane with the fibrillar preparation was cut off and the truncated membrane strip containing oligomeric Aβ1-42 >17 kDa was reprobed with fresh antibody. This was repeated 1 more time after cutting the strip >55 kDa. (C and D) Fibrillar (C) and oligomeric (D) preparations of Aβ1-42 analyzed by atomic force microscopy (5-nm total z-range). (E–G) Human IgG reactivities against peptide preparations were measured with antigen microarrays in plasma from AD patients and NDC in sample set 1 (E) and sample set 2 (F and G). Bars represent mean ± SEM. Names underlined with dashed lines are synthetic mutant peptides not present in vivo. No significant differences in antibody reactivities were observed between samples from AD patients and NDC for any antigen in both sample sets (SAM). (H and I) Titration of plasma IgG (H) and IgM (I) reactivity to oligomeric Aβ1-42 in 3 samples. (J and K) Antibody reactivities against groups of peptides (closed symbols) compared with reactivities against control antigens (open symbols) in sample set 2. Each dot represents median measurement for 1 plasma sample (log scale; mean reactivity; ***, P ≤ 0.001; Mann–Whitney U test or Kruskal-Wallis 1-way ANOVA followed by Dunn's post hoc test.) (L and M) Electron microscopy analysis of assembly status of nonaggregated (L) and oligomeric (M) preparation of Bri16–23(A16K/V17K). DFU, digital fluorescent units.
Plasma Aβ IgG Antibodies Are Abundant and Directed Preferentially Against Oligomers and Posttranslationally Modified Aβ.
To characterize and quantify the repertoire of natural antibodies against Aβ in human plasma on a large scale, we developed peptide microarrays containing various synthetic Aβ peptide variants, either nonaggregated or in different assembly states, and non-Aβ amyloidogenic peptides and controls (Table S1 and Fig. S1). To mimic some effects of oxidative stress in vitro we generated di-tyrosine cross-linked Aβ peptide species (CAPS) (9, 23). In a first experiment we measured relative Aβ antibody reactivities of the IgG class against 25 different peptide preparations (Table S1) in plasma from 75 AD patients at various stages of disease and 36 healthy nondemented controls (NDC) (Table S2). Strikingly, antibodies recognizing higher-order Aβ assemblies, including oligomer and fibrillar preparations, were more prevalent and up to 12-fold higher than the average reactivity against presumably nonaggregated preparations of Aβ1-40 and Aβ1-42 (Fig. 1E). No significant differences were observed between plasma from this mixed group of AD patients and NDC. In addition to antibodies against conformational Aβ species we discovered antibody reactivities against pyroglutamate Aβ variants, and curiously, against the peptide residue ABri3–34 (Fig. 1E).
To replicate these findings in an independent sample set and extend the panel of peptides, we screened plasma samples from an additional sample set of 55 AD patients and 62 NDC subjects (Table S2) for antibodies against 74 different peptide preparations and controls (Table S1 and Fig. 1 F and G). We confirmed that IgG reactivities against Aβ assemblies were generally higher than reactivities against nonaggregated Aβ (Fig. 1F). Although IgG titers to oligomeric preparations of Aβ1-42 (Fig. 1H) and other peptides (Fig. S2A) are relatively low, they were 2- to 4-fold higher than IgM reactivities within the same plasma sample (Fig. 1I) and for certain peptides up to 86-fold higher (Fig. S2 A and B). These antibody titers are also consistent with peptide array-based measurements of IgGs reactive to Aβ and myelin proteins in patients with multiple sclerosis (31) and support the concept of a circulating pool of self-reactive IgG antibodies in the general population (32, 33).
Interestingly, most plasma samples had relatively high IgG antibody reactivity against posttranslationally modified peptides. For example, IgGs against pyroglutamate variants Aβ3(pE)-42 or Aβ11(pE)-42 were up to 12-fold higher than those against Aβ11-42 or the average reactivities against Aβ1-40 and Aβ1-42 (Fig. 1J). Similarly, the pyroglutamate form of Bri1(pE)-6 elicited a 26-fold higher reactivity than Bri1–6 (Fig. S2C). Oxidative modification of Aβ peptide as present in Aβ29-M35(MetSox) (Fig. 1K) or Aβ1-42 (Ox) (Fig. S2D) led to a prominent increase in IgG binding compared with the median IgG reactivity to nonaggregated Aβ1-40 and Aβ1-42. In contrast, antibody reactivities against control peptides APLP1 (residues 559–580), APLP2 (residues 662–686), or human Amylin1–37 were similar to reactivities against nonaggregated Aβ1-40 or Aβ1-42 peptides (Fig. 1F).
General Elder Population Carries Relatively High Antibody Reactivity Against Amyloidogenic Peptides Unique to Autosomal Dominant Dementias.
Of particular interest in the first experiment was the increased antibody reactivity against mutant peptides that are found exclusively in different familial dementias. Consistent with this finding, relative antibody reactivities to mutant forms of Aβ1-40, Aβ1-42 or Aβ1-55 were severalfold increased compared with median reactivity for nonaggregated preparations of Aβ1-40 or Aβ1-42 in the second experiment (Fig. 1G). We observed relatively high antibody reactivities against full-length ABri and measured similar IgG titers for ADan (Fig. 1G), both peptides being mutant amyloidogenic non-Aβ peptides present exclusively either in British (34) or Danish dementia (35). Oligomer preparations and posttranslationally modified residues or artificial variants of the same peptides typically elicited even higher reactivities, e.g., relative reactivity against an oligomeric preparation of artificial mutant Bri16–23(A16K/V17K) was the strongest observed in our study (Fig. 1G). Electron microscopy confirmed in this preparation the presence of structures reminiscent of previously described globular or annular assemblies of Aβ and other amyloidogenic peptides (36, 37) but no assemblies were detected in freshly dissolved peptide (Fig. 1 L and M). Together, these data from 2 independent sample sets demonstrate the abundance of a diverse IgG repertoire in human plasma recognizing higher-order assemblies of Aβ peptides, pyroglutamate Aβ, oxidized Aβ, and mutant Bri peptide residues. The presence of antibodies in individuals who are not carriers of a mutation for familial dementia supports the existence of cross-reactive conformation-specific antibodies.
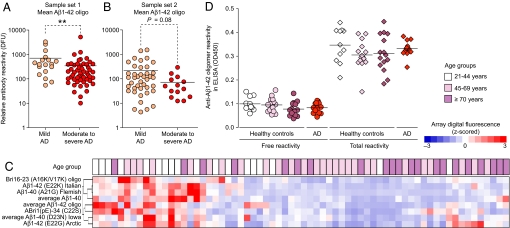
Reduced Antibody Reactivities Against Oligomeric Aβ1-42 Assemblies in Advanced AD and in Normal Aging.
Although no overall differences in Aβ or other peptide antibody reactivities were observed between a diverse group of AD patients and NDC, patients in sample set 1 with moderate to severe AD [minimental state examination (MMSE) scores ≤19] had significantly lower levels of antibody reactivities against oligomeric preparations of Aβ1-42 than patients with mild disease (MMSE scores ≥20) (Fig. 2A). A similar group difference was found in sample set 2, although it did not reach significance (Fig. 2B), possibly because of the low number of patients with severe disease (Table S2). No group difference was detected for antibodies recognizing nonaggregated Aβ, control antigens “pneumococcal vaccine polyvalent” and Candida albicans extract, arguing against a general age-related decline in antibody titers in our sample sets. Also, ApoE4 carriers did not differ from noncarriers in their immunoreactivity with all tested antigens.
Fig. 2.
Reactivity to oligomeric assemblies of Aβ peptides decline with progression of AD and together with other reactivities also decrease with age. (A and B) Average antibody reactivity for Aβ1-42 oligomer preparations in AD patients with mild (MMSE score ≥20, orange dots) or moderate to severe stages (MMSE score ≤19, red dots) of the disease in sample set 1 (A) and sample set 2 (B) (mean reactivity; **, P ≤ 0.01, Mann–Whitney U test). Each dot represents median measurement for 1 plasma sample. (C) Unsupervised clustering of antibody reactivities that are significantly associated with age in healthy females (n = 66) ages 21–85 years (SAM). Age of donors is indicated on top of the node map as boxes with increasing intensities of purple for higher age. Color shades in node map indicate higher (red) or lower (blue) antibody reactivity. (D) ELISA measurements for free (untreated) and total (immune complex dissociation at pH 3.5) antibody reactivity against oligomeric species of Aβ1-42 in randomly selected plasma samples of healthy individuals from different age groups (n = 11–15 samples per group) of sample set 3 and AD patients (n = 13) of sample set 2 (mean; 1-way ANOVA). DFU, digital fluorescent units; OD450, optical density at 450 nm.
To determine antibody reactivities at different ages we screened plasma samples from a third set of samples collected from 66 healthy, nondemented females ages 21–85 years old (Table S2 and Fig. S3) and found 6 antibody reactivities that were on average 45–65% higher in plasma from the youngest (21–44 years) compared with the oldest (≥70 years) age groups (Fig. S4 A--F). Total IgG levels and other reactivities did not change (Fig. S4 G–L), and only reactivity to pneumococcal vaccine increased with age (Fig. S4M), again arguing against a general loss in overall immunity in the elderly studied here. Using linear regression methods we found 8 antibody reactivities that were strongest associated with age as illustrated in a node map generated by an unsupervised cluster algorithm to arrange all samples based on similarity of antibody reactivities (Fig. 2C). Antibody reactivities against some of these peptides showed significant inverse correlations with age (Fig. S4 N and O). Low pH treatment (38) increased antibody reactivities against oligomeric Aβ in all tested plasma samples 2- to 7.4-fold (Fig. 2D) compared with untreated samples. This finding is similar to reports in human serum and IvIg preparations (25, 26). The percentage of free antibodies out of the total pool recognizing Aβ1-42 oligomers ranged from 13% to 50% but was not associated with age or AD (Fig. S5). Together, these data indicate that relative plasma antibody reactivities against several amyloidogenic Aβ mutant and non-Aβ peptides and in particular to oligomeric Aβ1-42 are reduced with age.
Aβ IgG Antibodies Are Present in CSF and Plasma IgGs Protect Primary Neurons from Aβ Toxicity.
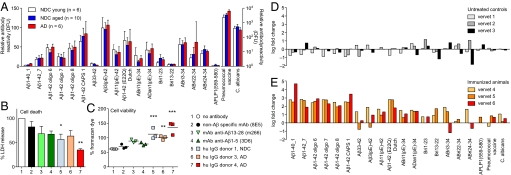
To investigate whether any of the antibodies detected in the periphery may have access to the CNS we measured antibody titers in CSF samples from 16 healthy aged normal controls and 6 patients with mild AD using peptide arrays. Average reactivities in CSF were ≈30–230 times lower in CSF than in plasma but a similar repertoire of IgG antibodies was seen. Highest titers were observed for oligomeric preparations of Aβ1-42, pyroglutamate-modified Aβ peptides, and mutant variants of Aβ, ABri, and ADan (Fig. 3A). There was no significant difference in reactivities in the analyzed sample groups and IgM was not detectable.
Fig. 3.
IgGs to Aβ are present in human CSF, protect neurons from Aβ toxicity in vitro, and are amplified together with ABri and ADan cross-reactive antibodies in an Aβ immunization paradigm. (A) IgG reactivity pattern in CSF of AD patients (≥70 years) and NDC young (24–48 years) and aged individuals (58–83 years). Bars represent means of median reactivities ± SEM. (B and C) Protective effect of human IgGs on primary mouse E16 neurons incubated with oligomeric preparation of Aβ1-42 was measured by percentage LDH release in dying cells (B) and percentage generation of formazan (C). Amount of water-soluble formazan produced by surviving cells in culture medium alone was considered 100% survival. Bars represent mean ± SEM of 3 experiments done in triplicates. Symbols in scatter plot represent mean of 1 triplicate experiment. *, P ≤ 0.05, 1-way ANOVA and Dunnett's multiple comparison test with “no antibody” as control. (D and E) Vervet monkeys were left untreated (D) or immunized with a mixture of Aβ1-40 and Aβ1-42 (E) (39). Immunoreactivity at the end of the study (day 301) was divided by the baseline reactivity to individual peptides, and the result is presented as log fold change. Bars represent median fold change of reactivity to a peptide for each individual animal. As expected, immunoreactivity to Aβ1-40 and Aβ1-42 peptides and oligomeric assemblies increase over several log ranges. Note the increase in reactivity to Aβ11(pE)-42, ABri, and ADan but the lack of increase to Aβ33–42. DFU, digital fluorescent units.
To assess the potential physiological relevance of natural Aβ antibodies we tested the protective potential of IgGs against Aβ toxicity. IgGs purified from 3 plasma samples and acidified to release bound peptides (38) were preincubated with oligomeric preparations of Aβ1-42 and this mix was added to primary hippocampal neurons from embryonic day (E) 16 mice. All 3 IgG preparations significantly reduced cell death (Fig. 3B) and increased cell viability (Fig. 3C) to an equal or greater extent than monoclonal antibodies to Aβ, consistent with similar experiments done with IvIg (25).
Immunization with Aβ Peptides Amplifies Immune Response to Aβ, ABri, and ADan Peptides in Nonhuman Primates.
To further study the in vivo relevance of natural antibodies to amyloidogenic peptides, we measured immunoreactivity in plasma of a subcohort of vervet monkeys from a previously published study (39). Aged vervets can develop features of AD including cerebral amyloid plaques that stain positive with anti-human Aβ antibodies (39), indicating high homology between human and vervet Aβ similar to other nonhuman primates (40). Immunization with a mixture of human Aβ1-40 and Aβ1-42 almost entirely cleared cerebral Aβ plaques (39). Although standard ELISA methods detected miniscule levels of Aβ1-40 antibodies in nonimmunized vervets (39), using our peptide arrays, we were able to measure reactivities to nonaggregated and oligomeric Aβ preparations and to Aβ3(pE)-42 species comparable to what we detected in human plasma (Fig. S6 A–C). At the end of the study, at 301 days, control animals had unchanged or decreased titers to most of the tested peptides (Fig. 3D and Fig. S6B), whereas immunized vervets developed a 370-fold median increase (range of 80- to 50,000-fold) in IgG antibodies to full-length Aβ preparations (Fig. 3E and Fig. S6D). In support of earlier findings that immunization with Aβ was mainly targeting the peptide's N terminus (39), immunized vervets failed to develop a comparable immune response to Aβ33–42 (0.2- to 22-fold). Most importantly, and in likely support of their physiological relevance, immunized vervets significantly increased antibody levels to pyroglutamate-modified Aβ [e.g., Aβ11(pE)-42], mutant Aβ [e.g., Aβ1-42(E22Q) Dutch], and the foreign peptides ABri and ADan (Fig. 3E and Fig. S6D). These data show that immunization with full-length Aβ not only increases immunity to select Aβ peptide species but also triggers the expansion of cross-reactive antibodies against other known toxic amyloidogenic peptides.
Discussion
This study of naturally occurring antibodies in humans and nonhuman primates demonstrates that plasma and CSF contain IgGs that are reactive against a broad range of amyloidogenic peptides and that these IgGs can protect primary neurons from Aβ toxicity in vitro. Studies in mice (22, 38, 41) and humans (20–22, 24–26) described natural antibodies against Aβ1-40 or Aβ1-42 before, without assessing their fine specificity or preference for specific assembly states in detail. Because the typical overnight coating of ELISA plates with Aβ resuspended in PBS is similar to the protocol for generating oligomeric Aβ species (42) it is likely that some of these previous studies measured antibodies against various mixtures of oligomeric and monomeric Aβ. The first study to specifically measure circulating natural human antibodies against Aβ oligomers used redox-modified cross-linked oligomeric species of Aβ (CAPS) and reported ≈25% less reactivity in AD patients compared with healthy controls (23). Others described increased titers of antibodies specific for Aβ25-35 oligomers in mild to moderate stages of AD (43). Our large-scale survey of antibody reactivities confirmed the existence of antibodies recognizing CAPS described by Moir et al. (23), although we could not find a significant decrease in AD patients. We also detected in general only low titers to nonaggregated Aβ1-40 or Aβ1-42 and unmodified Aβ (Fig. 1) but higher reactivities for antibodies recognizing other oxidized or oligomeric assemblies of Aβ (Fig. 1 J and K).
Why would many of these antibodies, particularly those against oligomeric Aβ1-42, decrease with normal aging and more so with progression from mild to severe AD (Fig. 2 A–C)? Cerebral Aβ appears to be in equilibrium with plasma Aβ (44) and it was proposed that CNS Aβ levels could be reduced by trapping the peptide in a “peripheral sink” (12, 45). Once Aβ is in the periphery, it is possible that Aβ-specific IgGs form immune complexes more readily in plasma of certain AD patients compared with NDC (26). This could be because of higher levels of Aβ available for binding to antibodies or because of increased complement-mediated clearance of immune complexes (46, 47). Our data confirm that certain peripheral plasma Aβ antibodies are complexed (Fig. 2D). Alternatively, the reduction of certain Aβ antibodies with AD may represent a “failed” immune response in AD, in line with recently described dysfunctional immune signaling pathways in the blood of AD patients (48).
The function of natural plasma Aβ antibodies is unknown, but immunization trials in AD mouse models, vervet monkeys, and AD patients suggest that Aβ sequence- or conformation-specific antibodies are sufficient to induce the clearance of Aβ from the brain through a number of different pathways and that the fine specificity of these antibodies may affect their therapeutic efficacy (12, 13, 17–19, 39, 49). For example, Aβ antibodies isolated from human plasma are capable of binding to plaques and neurons in brain sections of AD patients (20) and AD mouse models (16), and such antibodies appear to facilitate the phagocytosis of Aβ by microglia in vivo (17, 50) or solubilize fibrillar amyloid directly (51). Here, we show that unmasked IgGs are capable of neutralizing some of the toxic properties of Aβ1-42 in vitro (Fig. 3 B and C). We also detected low levels of IgGs but not IgMs reactive to Aβ and other peptides in CSF (Fig. 3A), consistent with findings in mice that a small fraction of peripheral IgGs reaches the CNS (44).
What is the origin of these Aβ- and amyloidogenic peptide-specific antibodies? Without getting too much into the complexity of natural autoantibodies (32, 33) there are 2 main possibilities: the specific antigen triggers the expansion of antibodies, or cross-reactive epitopes, endogenous or foreign, provide the antigenic stimulus. In favor of the first possibility, no Aβ-specific antibodies could be detected in wild-type mice (22, 38), but they are present in human mutant amyloid precursor protein (APP) transgenic mice where Aβ antibody titers correlated inversely with plaque pathology (41). It is also possible that posttranslationally modified and toxic Aβ triggers the production and maturation of at least some antibodies. Pyroglutamate variant Aβ3(pE)-42 and to a lesser extent Aβ11(pE)-42 are a predominant Aβ species in AD plaques (2–4, 10, 52), and together with other N- and C-terminally truncated Aβ species, they are also formed in plaque-free brains of healthy individuals aged at least 70 years (6). Moreover, because Aβ3(pE)-42 forms aggregation seeds 250-fold faster than unmodified Aβ (53) it may trigger antibody responses at very low concentrations long before pathological changes are observed in the brain. Indeed, human plasma in general showed high reactivity against pyroglutamate-modified peptides such as Aβ3(pE)-42, Aβ11(pE)-42, but only low reactivity against unmodified Aβ11-42 (Fig. 1J and Fig. S2C).
More likely, however, unknown epitopes trigger the formation of cross-reactive IgG antibodies with reactivities against Aβ and related amyloidogenic peptides. The strongest support for this idea comes from the observation that healthy humans have antibodies against ABri1–34 and ADan1–34 (Figs. 1 E and G and 3A and Fig. S3B), which are exclusively generated as a result of 2 separate mutations in the ITM2B gene in familial British (34) or Danish (35) dementia, respectively. We also found in plasma and CSF increased IgG immunoreactivity to familial AD-associated mutant forms of Aβ peptides that are not present in the general population (Figs. 1 E and G and 3A and Fig. S3B). In addition, the observation that some of the antibodies against amyloidogenic peptides are at their highest levels in young, presumably pathology-free, individuals (Fig. 2C and Fig. S4), seems counterintuitive to an immune response triggered by disease-associated Aβ peptides. In fact, our findings do not support studies describing a general increase in autoantibody titers to endogenous antigens with age in humans (54), but may be more consistent with an age-related decrease of immune response to exogenous antigens (54) that mimic amyloidogenic peptides. In support of this, new evidence in mice indicates that at least some Aβ antibodies may be induced by a peptide sequence in the common potato virus Y (PVY) homologous to the N terminus of Aβ (55). Antibodies against PVY were also isolated in a screen of a human synthetic phage-antibody library (56), but it remains unknown whether the general population develops a specific immune response to PVY with cross-reactivity to Aβ.
No matter what their origin, antibodies against many of the amyloid peptides we measured can be dramatically expanded upon immunization with Aβ in vervets (Fig. 3E). What's more, even though unmodified Aβ1-40 and Aβ1-42 was used in the immunization paradigm, antibodies against pyroglutamate-modified Aβ3(pE)-42 and Aβ11(pE)-42 or against ABri or ADan peptides were also strongly expanded, again supporting the concept of cross-reactive tertiary epitopes shared among amyloidogenic peptides (57). Because immunization of the vervets led to an almost complete clearance of cerebral Aβ deposits (39) it becomes interesting to determine which types of antibodies are most potent in clearing Aβ. Although we have no comparable data from human clinical trials, it is reasonable to assume that active immunization with Aβ peptides would trigger a similar expansion of a repertoire of amyloidogenic peptide-specific antibodies in humans, possibly leading to cognitive improvement as well. If individual or groups of antibodies against e.g., pyroglutamate-modified or tertiary structures of Aβ turn out to have better therapeutic benefits, active immunization with such structures may provide a better and more economic approach than treatment with a single, potentially mono-specific antibody.
In summary, our findings demonstrate that naturally occurring antibodies specific for known toxic Aβ and amyloidogenic non-Aβ species are abundant in healthy humans and some decrease with age and advancing AD. Plasma IgGs are capable of reducing part of Aβ's neurotoxicity but more studies are necessary to understand the role of these antibodies in vivo. If a therapeutic benefit of Aβ antibodies can be confirmed, stimulating production of conformation-specific Aβ antibodies or passively administering the most efficacious ones to the elderly population may provide a preventive measure toward AD. We speculate that certain nonself amyloidogenic peptides might be more powerful than Aβ preparations in inducing therapeutic immune responses in AD and carry potentially a lower risk to trigger autoimmune inflammation.
Materials and Methods
Detailed technical descriptions are provided in SI Text.
Plasma and CSF Samples.
Archived human EDTA plasma and CSF samples were obtained from academic centers specialized in neurological or neurodegenerative diseases (Table S2). Informed consent was obtained from all human subjects in accordance with Institutional Review Board-approved protocols. Vervet plasma samples came from a previously published Aβ immunization study (39) (Table S2). One control animal was not part of the original study.
Peptides and Arrays.
We obtained RP-HPLC-purified peptides from different sources (Table S1). Aβ assemblies were made as described (23, 42). Some preparations were analyzed by atomic force or electron microscopy and immunoblots. Antigen microarrays (Table S1 and Fig. S1) were printed in batches of up to 200 slides with a custom-built robotic microarrayer by contact printing onto reactive epoxide-coated glass slides. Glass slides were probed with diluted sample or specific antibodies and data were extracted as described (58).
Statistical Analyses.
Most statistical analyses were done in GraphPad Prism 5.0 with P ≤ 0.05 considered significant. Multivariate comparison analysis between the youngest and oldest age groups and linear regression analysis of age with antibody reactivities was done with the software package SAM (59) with 0% false discovery rate considered significant. Unsupervised cluster analysis was done in Cluster 3.0 (60), and a node map was generated in Java TreeView (61). Penalized linear regression models were calculated by the regularization and variable selection method elastic net (62).
ELISA and In Vitro Aβ Neurotoxicity Assay.
Dissociation of immune complexes in plasma at pH 3.5 and detection by indirect ELISA was done as described with modifications (25, 38). Hippocampal neuronal cultures from E15–16 CF1 mice were treated on day 6 with 2 μM Aβ oligomers in the presence or absence of antigen-dissociated and purified human IgGs or the indicated monoclonal antibodies. After 3 days, cell death or viability was measured with standard lactate dehydrogenase (LDH) release and tetrazolium salt-based assays.
Supplementary Material
Acknowledgments.
We thank the patients who participated in this study; M. BenBarak and P. Chandra for assistance with the peptide array; J. Frost for technical assistance with the vervet samples; R. Palmour and F. Ervin for housing the vervets at the Behavioral Science Foundation, St. Kitts, Eastern Caribbean, and numerous unnamed staff at our institutions for their effort in patient recruitment, clinical assessment, and sample preparation. This work was supported by the John Douglas French Alzheimer's Foundation (T.W.-C.), the Department of Veterans Affairs (T.W.-C., E.R.P., G.L., and J.A.Y.), and National Institute on Aging Grants AG20603 (to T.W.-C.), AG10491, AG030539 (to J.A.G.), P50 AG005136 (to E.R.P.), and AG08017 (to J.F.Q. and J.A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904866106/DCSupplemental.
References
- 1.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 2.Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid β protein in Alzheimer's disease. J Biol Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- 3.Näslund J, et al. Relative abundance of Alzheimer A β amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci USA. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saido TC, et al. Dominant and differential deposition of distinct β-amyloid peptide species, A β N3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- 5.Iwatsubo T, et al. Full-length amyloid-β (1-42(43)) and amino-terminally modified and truncated amyloid-β 42(43) deposit in diffuse plaques. Am J Pathol. 1996;149:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- 6.Piccini A, et al. β-Amyloid is different in normal aging and in Alzheimer disease. J Biol Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- 7.Varadarajan S, et al. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer's A β(1-42) and A β(25-35) J Am Chem Soc. 2001;123:5625–5631. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- 8.Barnham KJ, et al. Neurotoxic, redox-competent Alzheimer's β-amyloid is released from lipid membrane by methionine oxidation. J Biol Chem. 2003;278:42959–42965. doi: 10.1074/jbc.M305494200. [DOI] [PubMed] [Google Scholar]
- 9.Atwood CS, et al. Copper mediates dityrosine cross-linking of Alzheimer's amyloid-β. Biochemistry. 2004;43:560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- 10.Russo C, et al. Pyroglutamate-modified amyloid β-peptides–AβN3(pE)–strongly affect cultured neuron and astrocyte survival. J Neurochem. 2002;82:1480–1489. doi: 10.1046/j.1471-4159.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 11.Schenk D, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 12.DeMattos RB, et al. Peripheral anti-Aβ antibody alters CNS and plasma Aβ clearance and decreases brain Aβ burden in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levites Y, et al. Anti-Aβ42- and anti-Aβ40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buttini M, et al. β-Amyloid immunotherapy prevents synaptic degeneration in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:9096–9101. doi: 10.1523/JNEUROSCI.1697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes C, et al. Long-term effects of Aβ42 immunisation in Alzheimer's disease: Follow-up of a randomized, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 16.Hock C, et al. Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- 17.Bard F, et al. Epitope and isotype specificities of antibodies to β-amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc Natl Acad Sci USA. 2003;100:2023–2028. doi: 10.1073/pnas.0436286100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, et al. Novel Aβ peptide immunogens modulate plaque pathology and inflammation in a murine model of Alzheimer's disease. J Neuroinflammation. 2005 doi: 10.1186/1742-2094-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamikonyan G, et al. Anti-Aβ1 11 antibody binds to different β-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils but not the most toxic oligomers. J Biol Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaskin F, et al. Human antibodies reactive with β-amyloid protein in Alzheimer's disease. J Exp Med. 1993;177:1181–1186. doi: 10.1084/jem.177.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyman BT, et al. Autoantibodies to amyloid-β in Alzheimer's disease. Ann Neurol. 2001;49:808–810. doi: 10.1002/ana.1061. [DOI] [PubMed] [Google Scholar]
- 22.Nath A, et al. Autoantibodies to amyloid β-peptide (Aβ) are increased in Alzheimer's disease patients and Aβ antibodies can enhance Aβ neurotoxicity: Implications for disease pathogenesis and vaccine development. Neuromol Med. 2003;3:29–39. doi: 10.1385/nmm:3:1:29. [DOI] [PubMed] [Google Scholar]
- 23.Moir RD, et al. Autoantibodies to redox-modified oligomeric Aβ are attenuated in the plasma of Alzheimer's disease patients. J Biol Chem. 2005;280:17458–17463. doi: 10.1074/jbc.M414176200. [DOI] [PubMed] [Google Scholar]
- 24.Henkel AW, et al. Immune complexes of auto-antibodies against Aβ 1-42 peptides patrol cerebrospinal fluid of non-Alzheimer's patients. Mol Psychiatry. 2007;12:601–610. doi: 10.1038/sj.mp.4001947. [DOI] [PubMed] [Google Scholar]
- 25.Szabo P, Relkin N, Weksler ME. Natural human antibodies to amyloid β peptide. Autoimmun Rev. 2008;7:415–420. doi: 10.1016/j.autrev.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Gustaw KA, et al. Antigen-antibody dissociation in Alzheimer disease: A novel approach to diagnosis. J Neurochem. 2008;106:1350–1356. doi: 10.1111/j.1471-4159.2008.05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weksler ME, et al. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol. 2002;37:943–948. doi: 10.1016/s0531-5565(02)00029-3. [DOI] [PubMed] [Google Scholar]
- 28.Du Y, et al. Reduced levels of amyloid β-peptide antibody in Alzheimer's disease. Neurology. 2001;57:801–805. doi: 10.1212/wnl.57.5.801. [DOI] [PubMed] [Google Scholar]
- 29.Relkin NR, et al. 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 30.Lesne S, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 31.Quintana FJ, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci USA. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lutz HU, Binder CJ, Kaveri S. Naturally occurring auto-antibodies in homeostasis and disease. Trends Immunol. 2009;30:43–51. doi: 10.1016/j.it.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Vidal R, et al. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 35.Vidal R, et al. A decamer duplication in the 3′ region of the BRI gene originates an amyloid peptide that is associated with dementia in a Danish kindred. Proc Natl Acad Sci USA. 2000;97:4920–4925. doi: 10.1073/pnas.080076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lashuel HA, et al. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 37.Quist A, et al. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc Natl Acad Sci USA. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, et al. Improvement of a low pH antigen-antibody dissociation procedure for ELISA measurement of circulating anti-Aβ antibodies. BMC Neurosci. 2007 doi: 10.1186/1471-2202-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemere CA, et al. Alzheimer's disease aβ vaccine reduces central nervous system aβ levels in a nonhuman primate, the Caribbean vervet. Am J Pathol. 2004;165:283–297. doi: 10.1016/s0002-9440(10)63296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podlisny MB, Tolan DR, Selkoe DJ. Homology of the amyloid β protein precursor in monkey and human supports a primate model for β amyloidosis in Alzheimer's disease. Am J Pathol. 1991;138:1423–1435. [PMC free article] [PubMed] [Google Scholar]
- 41.Sohn JH, et al. Reduced serum level of antibodies against amyloid β peptide is associated with aging in Tg2576 mice. Biochem Biophys Res Commun. 2007;361:800–804. doi: 10.1016/j.bbrc.2007.07.107. [DOI] [PubMed] [Google Scholar]
- 42.Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 43.Gruden MA, et al. Differential neuroimmune markers to the onset of Alzheimer's disease neurodegeneration and dementia: Autoantibodies to Aβ(25-35) oligomers, S100b, and neurotransmitters. J Neuroimmunol. 2007;186:181–192. doi: 10.1016/j.jneuroim.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 44.Levites Y, et al. Insights into the mechanisms of action of anti-Aβ antibodies in Alzheimer's disease mouse models. FASEB J. 2006;20:2576–2578. doi: 10.1096/fj.06-6463fje. [DOI] [PubMed] [Google Scholar]
- 45.Sparks DL, Schreurs BG. Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer's disease. Proc Natl Acad Sci USA. 2003;100:11065–11069. doi: 10.1073/pnas.1832769100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghiso J, et al. Systemic catabolism of Alzheimer's Aβ40 and Aβ42. J Biol Chem. 2004;279:45897–45908. doi: 10.1074/jbc.M407668200. [DOI] [PubMed] [Google Scholar]
- 47.Rogers J, et al. Peripheral clearance of amyloid β peptide by complement C3-dependent adherence to erythrocytes. Neurobiol Aging. 2006;27:1733–1739. doi: 10.1016/j.neurobiolaging.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 48.Ray S, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 49.Pfeifer M, et al. Cerebral hemorrhage after passive anti-Aβ immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- 50.Wilcock DM, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McLaurin J, et al. Therapeutically effective antibodies against amyloid-β peptide target amyloid-β residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- 52.Kuo YM, et al. Isolation, chemical characterization, and quantitation of A β3-pyroglutamyl peptide from neuritic plaques and vascular amyloid deposits. Biochem Biophys Res Commun. 1997;237:188–191. doi: 10.1006/bbrc.1997.7083. [DOI] [PubMed] [Google Scholar]
- 53.Schilling S, et al. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- 54.Rowley MJ, Buchanan H, Mackay IR. Reciprocal change with age in antibody to extrinsic and intrinsic antigens. Lancet. 1968;2:24–26. doi: 10.1016/s0140-6736(68)92893-6. [DOI] [PubMed] [Google Scholar]
- 55.Friedland RP, et al. Antibodies to potato virus Y bind the amyloid-β peptide: Immunohistochemical and NMR studies. J Biol Chem. 2008;283:22550–22556. doi: 10.1074/jbc.M802088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boonham N, Barker I. Strain specific recombinant antibodies to potato virus Y potyvirus. J Virol Methods. 1998;74:193–199. doi: 10.1016/s0166-0934(98)00097-4. [DOI] [PubMed] [Google Scholar]
- 57.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 58.Robinson WH, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 59.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genomewide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saldanha AJ. Java Treeview: Extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 62.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B. 2005;67:301–320. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.