In March 2004, AJPH published an article by Des Jarlais et al. presenting and supporting the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) statement and checklist for reporting standards of behavioral and public health intervention evaluations involving nonrandomized designs.1 With the publication of this editorial and the inclusion of links and instructions on our “Instructions for Authors” page, we are making two important changes to our submission policy for randomized controlled trials (RCTs): First, the Journal joins a growing list of health and medical journals to endorse the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized controlled trials. Second, we will support the International Committee of Medical Journal Editors (ICMJE) policy requiring RCT registration in an approved registry as a condition for publication consideration in the Journal.
These policies apply to any RCT, defined by the ICMJE and World Health Organization as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes.”2 We describe the CONSORT statement and RCT registration in detail below.
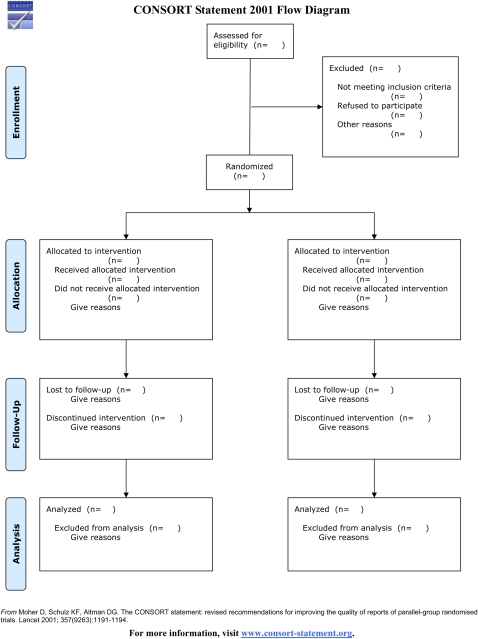
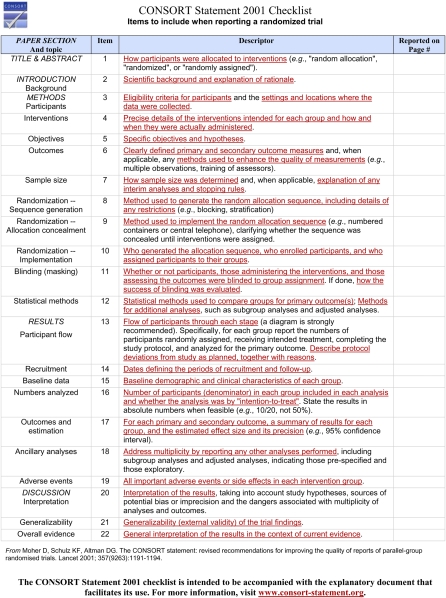
The CONSORT statement, or the Consolidated Standards of Reporting Trials statement, was originally developed by an international group of biostatisticians, epidemiologists, biomedical editors, and experts in clinical trials, in an effort to increase the quality and transparency of the reporting of information from 2-group RCTs.3 The standards were revised in 2001, and continue to be modified and improved,4–7 and current guidelines and instructions are provided on the CONSORT statement Web site (http://www.consort-statement.org). Often referred to as CONSORT, the statement includes a checklist to help authors ensure that their report is as clear as possible (Figure 1), as well as a flow diagram to help authors explicitly present the path of those targeted for inclusion in the study as they move from eligibility to enrollment to analysis (Figure 2). The checklist is to be submitted along with the manuscript for editorial review, but only the figure is to be included in the body of the manuscript.
FIGURE 1.
The Consolidated Standards of Reporting Trials (CONSORT) Submission Checklist.
Source. CONSORT Web site (http://www.consort-statement.org).
FIGURE 2.
The Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram.
Source. CONSORT Web site (http://www.consort-statement.org).
“Meters, Tijuana,” by photographer, Naa Oyo Kwate. From the photographer: “The meters caught my eye both because they were interesting visually but also because it was such a public display of life in Tijuana, particularly because all of the names are listed. That was the other thing that struck me—that the meters were on this free-standing rack close to the sidewalk, away from the buildings they are apparently reading. Unlike here in the US where the meters are in the basement or otherwise hidden from view, and each residence has its own meter, the meters in Tijuana were in one large group for several homes and were clearly in the view of the public. So the relative lack of privacy was also striking.” Printed with permission.
The CONSORT statement should be used by our authors as a tool to help present, and have his or her audience appreciate, the importance and applicability of the study results.8 For each of the 22 items in the checklist, the CONSORT Web site provides many examples of good reporting, and all are easily adaptable to most research settings. The flow diagram is self-explanatory, but easy definitions are also available on the CONSORT Web site to help guide the investigator. In addition, there is an online tool that can help generate the downloadable chart for inclusion in the manuscript.
Not surprisingly, the CONSORT statement checklist focuses on the parts of papers that occupy the fewest manuscript pages: the Methods and Results. In fact, over 75% of the CONSORT checklist points deal with these sections. All the better, we believe, as it provides authors with another opportunity to ensure that those parts of the manuscript are as concise and as accurate as possible. Following the CONSORT checklist and guidelines enhances RCT transparency by making clear to the reader the operationalizations of constructs, the a priori declarations of meaningful and important effect sizes, and the number of participants at each phase of the study. But transparency is about allowing the audience and our peers to judge for themselves what the results of the study mean, and presenting clear operationalizations of constructs and measures, making explicit a priori declarations about meaningful effect sizes, and documenting the number and type of participant at each phase of the study—all of which goes a long way toward allowing a more objective assessment to happen.
The CONSORT guidelines have been expanded and are now available for other designs and other forms of presentations, namely cluster randomized designs,9 noninferiority or equivalence trials,10 herbal medicine,11 nonpharmacological treatments,12 harms,13 and abstracts.14 Along with our prior publication of the TREND statement, models of transparent and informative data presentation are now readily available and we have encouraged, and now require, their use.
In 2004, an editorial published simultaneously in all ICMJE member journals announced that as a condition for publication consideration, the RCT on which a submitted manuscript was based must be registered (prior to the enrollment of a single participant in that trial) in a public registry.15 The five most common acceptable registries are clinicaltrials.gov, the Australian-New Zealand Clinical Trials Registry (http://www.actr.org.au), the International Standard Randomised Controlled Trial Number Register (http://www.ISRCTN.org), the University Hospital Medical Information Network (http://www.umin.ac.jp/ctr/index/htm), and the Netherlands Trial Register (http://www.trialregister.nl), although other approved primary registries exist (for instance, the International Clinical Trials Registry Platform; http://www.who.int/ictrp/network/primary/en/index.html). The purpose of this registration policy is clear; selective reporting or submission for publication of only those trials where desired outcomes were achieved is misleading and unethical. Shedding light on the number of trials being conducted for a given intervention or outcome can inform our judgment as to whether the results are “selected” in a way that impacts their representativeness or generalizability.
Beginning December 1, 2009, the Journal will neither accept nor send for review the results of RCTs that have not been previously registered with one of the above-mentioned public registries. Trials that have already started enrolling patients by that date should register as soon as possible but not later than March 1, 2010. RCT registration in a public registry is fast becoming the norm rather than the exception, and the Journal now strongly supports this effort.16
We all benefit from the implementation of these two important additions to our submission policy. Through this and similar efforts, we can begin to more easily compare the results of RCTs across journals, meta-analytic studies can include our published studies (as the presentation of information becomes uniform), and the universe of RCTs on a particular topic can be enumerated so the correct number of attempts and type of outcome can be observed. This transparency motivates us to hold the quality of our research to the highest standards, in support of the public's health and to the benefit of all.
References
- 1.Des Jarlais DC, Lyles C, Crepaz N, et al. ; TREND Group Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: The TREND Statement. AJPH 2004;94:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Clinical Trials Registry Platform World Health Organization. Available at: http://www.who.int/ictrp/en. Accessed June 2, 2009 [Google Scholar]
- 3.Begg CB, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA 1996;276(8):637–639 [DOI] [PubMed] [Google Scholar]
- 4.Altman DG, Schulz KF, Moher D, et al. ; CONSORT Group The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134(8):663–694 [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Schulz KF, Altman DG; CONSORT Group The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Ann Intern Med 2001;134(8):657–662 [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Schulz KF, Altman D; CONSORT Group The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285(15):1987–1991 [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357(9263):1191–1194 [PubMed] [Google Scholar]
- 8.Hopewell S, Altman DG, Moher D, Schulz KF. Endorsement of the CONSORT Statement by high impact factor medical journals: a survey of journal editors and journal ‘Instructions for Authors.’ Trials 2008;9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell MK, Elbourne DR, Altman DG; CONSORT Group CONSORT statement: extension to cluster randomised trials. BMJ 2004;328:702–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piaggio G, Elbourne DR, Altman DG, Pocock SJ, Evans SJ; CONSORT Group Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006;295:1152–1160 [DOI] [PubMed] [Google Scholar]
- 11.Gagnier JJ, Boon H, Rochon P, et al. ; CONSORT Group Reporting randomized controlled trials of herbal interventions: an elaborated CONSORT Statement. Ann Intern Med 2006;144:364–367 [DOI] [PubMed] [Google Scholar]
- 12.Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295–309 [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis JP, Evans SJ, Gotzche PC, et al. CONSORT Group Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004;141:781–788 [DOI] [PubMed] [Google Scholar]
- 14.Hopewell S, Eisinga A, Clarke M. Better reporting of randomized trials in biomedical journals and conference abstracts. J of Inf Sci 2008;34(2):162–173 [Google Scholar]
- 15.De Angelis C, Drazen JM, Frizelle FA, et al. ; International Committee of Medical Journal Editors Clinical trial registration: A statement from the International Committee of Medical Journal Editors. N Engl J Med 2004;351:1250–1251 [DOI] [PubMed] [Google Scholar]
- 16.Laine C, Horton R, DeAngelis CD, et al. Clinical Trial Registration: Looking back and moving ahead. Available at: http://www.icmje.org/clin_trial07.pdf. Accessed June 2, 2009 [DOI] [PubMed] [Google Scholar]