Abstract
Mast cells play a central role in the initiation of inflammatory responses associated with asthma and other allergic disorders. Receptor-mediated mast cell growth, differentiation, homing to their target tissues, survival, and activation, are all controlled, to varying degrees, by phosphoinositide-3-kinase (PI3K)-driven pathways. It is not fully understood how such diverse responses can be differentially regulated by PI3K. However, recent studies have provided greater insight into the mechanisms which control, and those which are controlled by, different PI3K subunit isoforms in mast cells. In this review, we discuss how PI3K influences the mast cell processes described above. Furthermore, we describe how different mast cell receptors utilize alternative isoforms of PI3K for these functions, and discuss potential downstream targets of these isoforms.
Keywords: Mast cells, PI3K, FcεRI, Kit, p110δ, p110γ
Regulation of mast cell processes by Kit and FcεRI
Mast cells are important effector cells which contribute to the body’s ability to respond defensively to invading pathogens and parasites [1,2]. However, activation of mast cells can have disabling consequences by initiating allergic inflammatory reactions in response to antigen which are associated with atopic asthma, allergic rhinitis, eczema, and anaphylaxis [3]. In addition, mast cells have also been implicated in rheumatoid arthritis, multiple sclerosis, atherosclerosis, inflammatory bowel disease, and angiogenesis associated with tumor progression [4]. Such responses are the result of the generation and/or release of various inflammatory mediators including histamine, leukotriene C4, prostaglandin D2, cytokines, chemokines, and proteases, by mast cells following their activation [5,6]. Multiple processes are required for the mature tissue-localized mast cells to respond in this manner. These include growth, differentiation and maturation of the mast cells from their bone marrow-derived progenitor cells, homing to their target tissues, continued survival of the mature tissue-resident mast cells, and eventually, mast cell activation [1–5,7]. Of the various receptors expressed on mast cells, two key ones are the growth factor receptor, Kit (CD117), and the high affinity receptor for IgE, FcεRI (Box 1). Whereas Kit plays a major role in growth, development, and homeostasis of mast cells [7], the FcεRI is principally responsible for initiating the signaling events leading to inflammatory mediator release [8]. In this review we shall thus primarily focus on the role of PI3K in mediating responses initiated by these two receptors. However, since specific G protein-coupled receptors (GPCRs) can either induce or modify FcεRI-dependent mast cell activation, the role of PI3K in specific GPCR-mediated mast cell responses will also be discussed.
Box 1. Kit and FcεRI on mast cells.
Kit is a single chain receptor with intrinsic tyrosine kinase activity [9,81]. The extracellular domain possesses 5 immunoglobulin-like regions which contain the binding site for its ligand, stem cell factor (SCF; alternatively termed steel factor or Kit ligand). Within the cytosolic tail, there is a split tyrosine kinase catalytic domain and multiple tyrosine residues which serve as auto-phosphorylation sites following Kit activation. These phosphorylated sites subsequently recruit specific signaling molecules which are crucial for Kit-mediated responses [9,81]. In contrast to Kit, the FcεRI is a multi-chain receptor complex consisting of an α chain, a β chain and a γ chain homodimer [8]. The FcεRIα chain possesses two extracellular immunoglobulin-like regions which bind IgE with high affinity. Both the β and γ subunits, possess immunoglobulin receptor-based tyrosine activation motifs (ITAMs) which are phosphorylated by the Src kinase, Lyn, upon antigen-dependent aggregation of IgE-occupied FcεRI [8]. The phosphorylated γ chains serve as the major initiators of signaling mediated by the FcεRI following recruitment of the tyrosine kinase Syk, whereas, the β chain might serve to modify these responses [8]. It should be noted that, an addition to its role in initiation of FcεRI-mediated signaling events, Lyn also appears to down-regulate FcεRI-mediated mast cell activation as a consequence of the recruitment of the inositol phosphatase, SHIP to the phosphorylated FcεRI β chain.
The intricacies of the downstream signaling cascades initiated by Kit and FcεRI have been discussed in several recent review articles (for Kit signaling: [9]; and for FcεRI signaling: [6,8,10]) and the readers are referred to these articles for further details. What is clear is that the majority, if not all, of the processes attributable to Kit, FcεRI, and other activating receptors, in mast cells are largely dependent upon PI3K activation. Thus, PI3K can be considered a central regulator of critical downstream signaling processes for receptor-mediated mast cell responses. How these multiple responses can be differentially regulated following PI3K activation has not been adequately delineated. However, recent studies are beginning to shed light on this topic. In this review, we thus discuss the signaling processes which regulate the activation of PI3K by FcεRI, Kit and other receptors expressed on mast cells and how the activation of PI3K regulates receptor-mediated mast cell function.
Expression and regulation of PI3K in mast cells
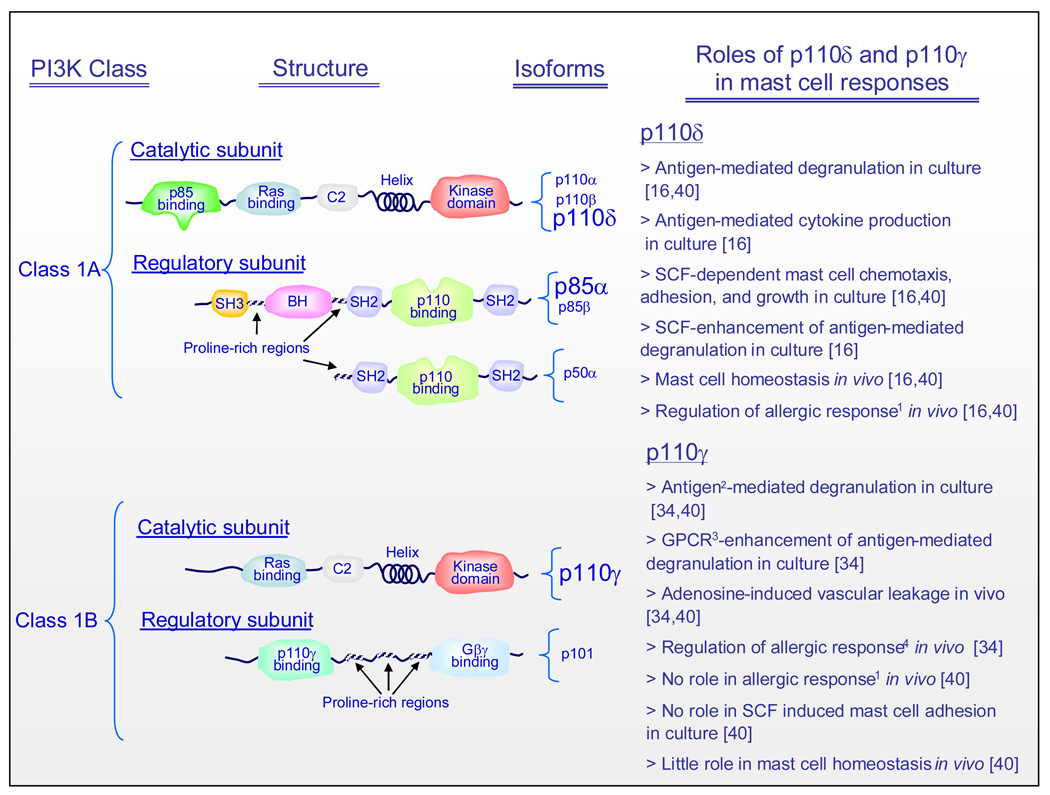
PI3Ks comprise a family of lipid kinases (Box 2) which play crucial roles in multiple biological responses [11–13]. Class 1 PI3Ks, the major haematopoietic forms of PI3K (Figure 1, Box 2), are heterodimeric comprising a regulatory and catalytic subunit. Mast cells express the class 1A p85α, p85β, and p50α regulatory subunit isoforms [14,15] in addition to all three class 1A PI3K catalytic subunit isoforms, p110α, p110β, and p110δ and the class 1B p110γ catalytic subunit [16,17] (Figure 1). As Kit and the FcεRI initiate their signaling processes via activation of tyrosine kinases, either intrinsically or by recruitment of cytosolic kinases [6,9], they utilize class 1A PI3Ks to mediate subsequent downstream signaling events, whereas GPCRs, such as those for adenosine, PGE2, S1P, and C3a, mediate their responses via class 1B PI3K (Figure 2) [18].
Box 2. The composition and activation of PI3Ks.
The PI3K family has been grouped into three major classes (class I, class II, and class III) depending on their molecular structure, modes of activation, and substrate specificity [82]. Class I PI3Ks are the best characterized and studied with regards to the regulation of immunological processes [83–85]. To date, there is little evidence for a role for Class II and Class III PI3Ks in mast cell biology and indeed other cell types of haematopoietic lineage.
Class I PI3Ks are heterodimers, subdivided into two groups, class IA and class IB. The class IA PI3Ks consist of a regulatory subunit (p85s: p85α, p55α, p50α, p85β, and p55γ) and a catalytic subunit (p110α, p110β, and p110δ) [86]. The class 1B PI3K p110γ catalytic subunit interacts with the p101 or the p87PIKAP regulatory subunits [87]. Whereas the catalytic p110α and p110β subunits are ubiquitously expressed in many cell types, tissues, and organs, the p110δ and p110γ subunits are preferentially found at high levels in lymphocytes [88]. All class IA regulatory subunits have a p110-binding region as well as two Src-homology (SH)2 domains that bind a specific recognition sequence (-YXXM-) found in various receptors and adaptor molecules, following phosphorylation of the tyrosine contained within this sequence. In contrast to class IA PI3Ks, the class IB PI3Kγ is activated by heterotrimeric G-protein-coupled receptors (GPCR) and regulated by the free βγ subunits of G proteins following GPCR activation. PI3Ks phosphorylate the inositol ring of membrane-associated phosphoinositides at the D3 hydroxyl position [89], thereby converting phosphatidylinositol-(4,5)-bisphosphate (PtdInsP2) to phosphatidylinositol-(3,4,5)-triphosphate (PtdInsP3) (Figure 2A). PtdInsP3 provides inducible docking sites for pleckstrin homology (PH) domains of associating signaling molecules allowing recruitment of these molecules to the receptor-signaling complex [89]. Responses initiated by PI3K are terminated following PTEN and SHIP-mediated de-phosphophorylation of PtdInsP3. PTEN removes the phosphate group from the D3 position of the inositol ring whereas SHIP removes the phosphate in the D5 position [90], thereby converting PtdInsP3 to PtdIns(4,5)P2 and PtdIns(3,4)P2, respectively (Figure 2B).
Figure 1.
Structure and function of PI3K subunit isoforms expressed in mast cells. The emphasized catalytic subunit isoforms are those that have been documented to play specific roles in mast cell responses as outlined in the text column. Whereas there is little selectivity in utilization of the p85 subunits for FcεRI-mediated responses, Kit responses show selective requirements for the p85α subunit (emphasized). Abbreviations: BH: Bcr/Rac GAP homology domain; C2: protein kinase C homology domain 2; GPCR; G protein-coupled receptor; SH: Src homology domain. 1As determined by passive cutaneous anaphylaxis reaction. 2Likely indirectly following release of adenosine or other GPCR ligand. 3GPCRs binding adenosine, MIP-1α (CCL3), Rantes (CCL5), or platelet activating factor. 4As determined by passive systemic anaphylaxis reaction.
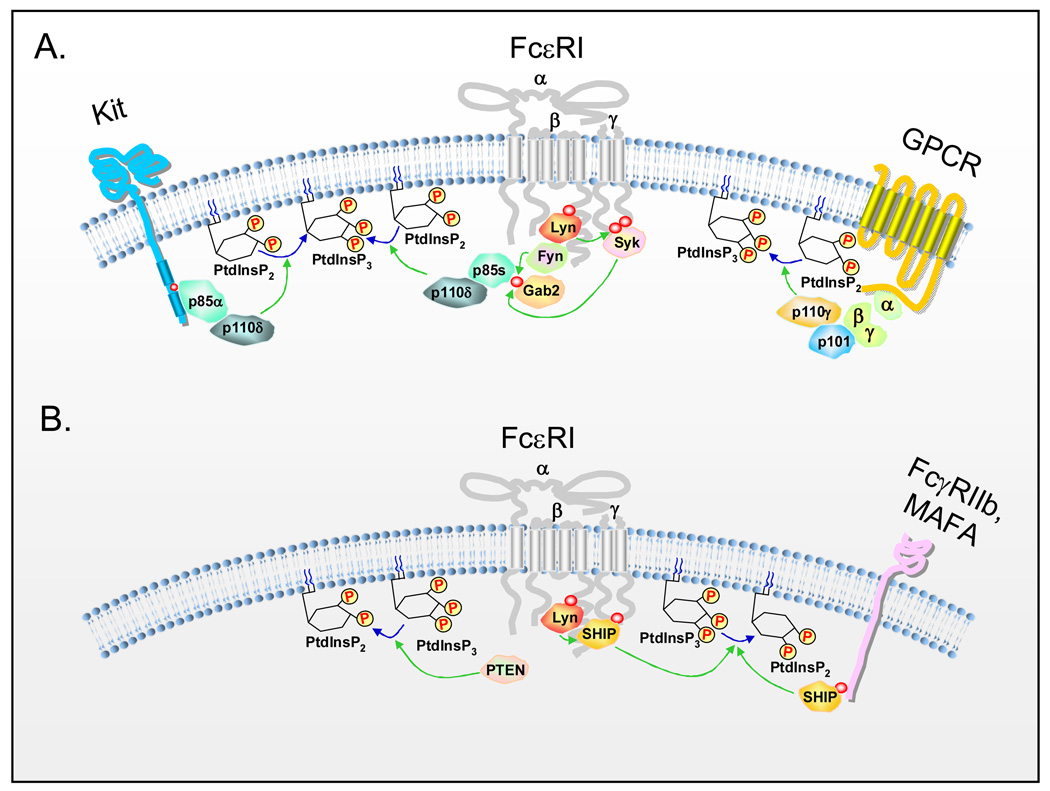
Figure 2.
Positive and negative regulation of PI3K-dependent signaling pathways in activated mast cells. (A) Activation of class 1A PI3Ks by Kit and FcεRI and class 1B PI3K by GPCRs at the inner cell membrane. The SH2 domains of the p85 regulatory subunits of class 1A PI3K can directly bind to phosphorylated Kit, whereas the association of p85s with the FcεRI is indirect via the cytosolic adaptor molecule, Gab2. The p101 regulatory subunit of class 1B PI3K directly binds to free G protein βγ homodimers that are dissociated from heterotrimeric G-proteins after GPCR activation. The catalytic subunits of PI3K, p110δ and p110γ, phosphorylate the inositol ring of phosphoinositides at the D3 hydroxyl position, thereby converting phosphatidylinositol-(4,5)-bisphosphate (PtdInsP2) to phosphatidylinositol-(3,4,5)-triphosphate (PtdInsP3) at the inner plasma membrane. This leads to the regulation of the downstream signaling events in activated mast cells depicted in Figure 3. (B) Activation of PTEN and SHIP by FcεRI and inhibitory receptors. PTEN removes the phosphate group from the D3 position of the inositol ring whereas SHIP removes the phosphate from the D5 position of the inositol ring, converting PtdInsP3 to PtdIns(4,5)P2 and PtdIns(3,4)P2, respectively. Thus, PtdInsP3 levels decrease, and the effect of PI3K activation is negatively regulated by PTEN and SHIP. SHIP interacts with FcεRI in a Lyn-dependent manner and with inhibitory receptors such as FcγRIIb and MAFA, through association with their ITIM motifs.
The regulatory p85 subunits of Class 1A PI3Ks can directly bind to activated and phosphorylated (p) Kit (via −pY721MDM-, in human Kit), however association with aggregated FcεRI requires indirect interaction via the cytosolic adaptor molecule, Gab2, following its phosphorylation by either Fyn [19] or Syk [20] (Figure 2A). It is also possible that the p85α subunit might directly bind to Syk following phosphorylation by Lyn, as suggested by studies conducted in the THP-1 monocytic cell line [21]. Such interactions are critical for propagation of signals initiated by Kit and the FcεRI. In this respect, disruption of the binding of PI3K to Kit abrogates Kit-dependent mast cell responses [22–25]. Similarly, the ability of dexamethasone to disrupt the FcεRI-induced interaction of PI3K with Gab2 in the rat RBL-2H3 mast cell line results in inhibition of PI3K and downstream signaling events [26]. The p110 catalytic subunit is maintained under constitutive inhibition by virtue of an inhibitory motif contained within the C-terminus of the p85 subunit [27,28]. Phosphorylation of a critical tyrosine residue (Y688) on the p85 subunit, by Src kinases alleviates this inhibition [27,28] which, together with the binding of the small GTP-binding protein Ras to the p110 subunit, results in enhancement of the catalytic activity associated with the p110 subunit [29]. In mast cells, Fyn is the major Src kinase involved in FcεRI-mediated PI3K activation [19]. The Ras guanyl nucleotide-releasing protein (RasGRP1) might also play an important role in FcεRI-mediated PI3K activation in mast cells and, it has been proposed that RasGRP1 might provide a link between the Lyn-Syk-LAT pathway and the Fyn-Gab2-PI3K pathway through binding to diacylglycerol generated by LAT-dependent phospholipase (PL)Cγ activation [30]. Thus, in both human [14,31] and rodent mast cells [16,32–35] triggered via Kit, FcεRI, or GPCRs, PI3K is rapidly activated resulting in the PI3K-dependent recruitment of PH domain-containing signaling molecules, such as PLCγ1 and γ2, AKT, PDK1, and BTK, to the receptor-signaling complex.
As will be discussed later, PI3K-dependent pathways in mast cells can however, also be negatively regulated by the inositol phosphatases PTEN (inositol phosphatase and tensin homologue deleted on chromosome 10), and SHIP (SH2-domain-containing inositol phosphatase) (Box 2, Figure 2B), the latter of which is recruited to the FcεRI in a Lyn- dependent manner (Box 1). The PI3K-signaling pathways in mast cells initiated upon FcεRI ligation therefore, represent a balance between Fyn-mediated positive regulation of PI3K and Lyn-dependent negative regulation, following activation of SHIP [19,36–38].
Functional responses of mast cells dependent on PI3K
Mast cell degranulation and cytokine production
A role for PI3K in mast cell activation has been revealed by a number of approaches as outlined in Box 3. The PI3K inhibitors, wortmannin and LY294002, have been widely reported to inhibit antigen-mediated degranulation and cytokine production in both rodent and human mast cells [14,32,39]. However, at least in human mast cells, these compounds fail to completely inhibit degranulation suggesting that, although PI3K is essential for optimal degranulation of mast cells, PI3K-independent pathways might also regulate this response. Studies utilizing mouse bone marrow-derived mast cells (BMMCs) expressing a kinase-inactive mutant isoform of the p110δ catalytic subunit have revealed that p110δ is the major isoform responsible for antigen-mediated degranulation and cytokine production in mast cells (Figure 1) [16,40]. This conclusion is further supported by the ability of the selective p110δ inhibitor, IC87114, to inhibit antigen-mediated mast cell activation and by its ability to inhibit the enhancement of antigen-mediated degranulation by stem cell factor (SCF) [16]. By contrast, mast cells derived from the bone marrow of p85α and p85β knock out mice show normal antigen-mediated calcium flux and degranulation [14,15], suggesting that the p110 catalytic subunit can utilize alternative regulatory subunits for its interaction with phosphorylated Gab2.
Box 3. Approaches for the study of the role of PI3K in mast cell functions.
A number of approaches have been utilized to explore the role of PI3K and its isoforms on mast cell functions. The most widely used approach has been through the use of the naturally occurring PI3K inhibitor wortmannin, and the synthetic inhibitor, LY294002. However, these inhibitors display non-selective inhibition of other kinases, for example, several PI3K-related kinases and PI4K [91,92] at similar or higher concentrations. Furthermore, they cannot discriminate between the different isoforms of PI3Ks. Recently, isoform-specific PI3K inhibitors have been employed to define the role of specific PI3K isoforms in mast cells; namely IC87114 which is a selective inhibitor of the p110δ isoform and AS-252424 which is a selective inhibitor of p110γ isoform [16,40,93]. Genetic approaches to examine the roles of specific isoforms in mast cell function have been somewhat hampered by the overlapping or redundant functions of specific subunit isoforms and the embryonic lethality associated with disruption of either p110α or p110β, or p85α/p85β double knock out mice [86]. However, mice lacking the p85α or p85β subunits individually, and mice lacking catalytic p110γ, p110δ subunits, or mice with a knock-in point mutation of p110δD910A have been generated, and utilized to examine the specific roles of these subunits in mast cell function. For further discussions on the PI3K gene-targeted murine models readers are referred to [86].
Specific GPCR ligands including adenosine, PGE2 and complement component C3A, can either enhance antigen-mediated mast cell activation (adenosine, PGE2) or by themselves, directly induce mast cell degranulation and chemokine and cytokine production (C3A) [41]. A role for PI3K in GPCR-mediated responses in mast cells has been suggested by the ability of wortmannin to block C3a-induced formation of the chemokine, CCL2, in human mast cells [42], and by the reduced ability of adenosine to potentiate antigen-mediated degranulation in PI3Kγ-deficent mast cells (Figure 1) [34,40]. Antigen-mediated degranulation is also attenuated in PI3Kγ-deficent mast cells [34,40] and in wild type mast cells pretreated with the PI3Kγ inhibitor, AS-252424 [40]. Similarly, the in vivo mast cell-dependent passive cutaneous anaphylaxis reaction has been reported to be virtually absent in mice deficient in the p110γ PI3K catalytic subunit [34]. These data led to the conclusion that a component of antigen-mediated mast cell degranulation might be regulated by a positive feedback loop following the release of adenosine or other GPCR ligand from the mast cells. More recent studies, however, using both isoform-specific inhibitors and genetic approaches, have indicated that, although both p110δ and p110γ isoforms contribute to antigen-mediated degranulation in vitro, only the p110δ isoform, is essential for the antigen-mediated mast cell-driven anaphylaxis response reactions in vivo [16,40]. The reasons for the differences between the in vivo data in the above studies are not clear. However, it is possible that differences in genetic backgrounds (129sv [34] vs C57BL/6 [40]) or differences in sensitization protocol (intravenous [34] vs intradermal [40]) could account for the discrepancies between the two studies [40]. The differences between the in vitro and in vivo responses might reflect a greater localized concentration of released adenosine or other GPCR agonist and/or reduced metabolism of the putative GPCR agonist in vitro [41]. It is also possible that other regulatory factors or elements operating in vivo, but absent in cell cultures, may contribute to these differences.
Mast cell homing and homeostasis
In addition to its role in mast cell mediator release, PI3K is also critical for mast cell chemotaxis, adhesion and homeostasis. This has been evidenced by the ability of wortmannin and LY294002 to effectively inhibit SCF-mediated cell migration, adhesion to fibronectin coated plates, proliferation, and survival in human and mouse mast cell cultures [32]. In addition, reduced numbers of mast cells are observed in the peritoneal cavity, but not dorsal skin, of mice expressing a mutation in the PI3K binding site on Kit [25]. As with degranulation, mast cell chemotaxis, adhesion, and homeostasis appear to be mediated by specific PI3K isoforms. For example, in p110δ inactive BMMCs, there is a dramatic defect in SCF-mediated mast cell adhesion and chemotaxis compared to the responses observed in wild type mast cells [16]. In addition, the ability of SCF to enhance mast cell growth is significantly reduced in cells expressing defective p110δ [14]. These attenuated responses are similarly observed in wild type mouse mast cells incubated with the p110δ-selective PI3K inhibitor, IC87114, but not in cells incubated with the p110γ inhibitor, AS-252424 [16,40]. In vivo, there is a loss of peritoneal and gastrointestinal mast cells in p85α-deficient mice [43] and reduced numbers of mast cells in the ear dermis, but not in the back skin, of p110δ inactive mutant mice compared with wild type mice [16]. Furthermore, intratracheal administration of IC87114 to ovalbumin-sensitized mice, blocks ovalbumin-induced total leukocyte, eosinophil, neutrophil and lymphocyte infiltration, and the release of the TH2 cytokines, IL-4, IL-5, IL-13, and the chemokine, RANTES (CCL5) in the lungs in response to antigen challenge [44]. Taken together, the above data again provide evidence for a predominant role for p110δ, compared to p110γ, in antigen- mediated mast cell responses in vivo and furthermore suggest that GPCRs only play a minor role in mast cell homing and homeostasis in vivo.
In contrast to the regulation of antigen-mediated degranulation, Kit-mediated responses show selective regulation by specific p85 regulatory subunits. In this respect, Kit-dependent AKT phosphorylation and cell proliferation are markedly lower in p85α−/− BMMCs compared to wild type cells [15]. Similarly, the aberrant growth of mast cell progenitor cells associated with a constitutively activated mutant form of Kit associated with the mast cell cancer, mastocytosis, also appears to be preferentially regulated by the p85α isoform [45]. SCF-dependent chemotaxis also shows selective dependency on p85α as demonstrated by the substantial reduction in the ability of SCF to cooperate with α4 integrin to induce Rac activation and subsequent cell migration in p85α−/− BMMCs [23].
Negative regulation of PI3K-dependent mast cell responses by PTEN and SHIP
PtdInsP3 levels, and hence PI3K-regulated signaling pathways in mast cells and other cell types, are finely regulated by the balance between phosphorylation induced by PI3Ks and de-phosphorylation regulated by the inositol phosphatases, PTEN and SHIP (Box 2 and Figure 2b). In quiescent human mast cells, shRNA-induced knock-down of PTEN results in increased basal PtdInsP3 levels and constitutive phosphorylation of AKT and the MAP kinases, p38 and JNK resulting in enhanced cell survival and cytokine production [46]. Further augmentation of calcium mobilization, degranulation, and cytokine production is observed in the PTEN-knock-down human mast cells challenged with antigen [46]. These results suggest that PI3K-mediated responses are under tonic inhibition by PTEN.
Mast cells express both the SHIP-1 and SHIP-2 isoforms. Disruption of Lyn-dependent interaction of SHIP-1 with the FcεRI results in defective phosphorylation of SHIP-1, increased PtdInsP3 levels, and subsequently, inhibition of degranulation in antigen-challenged mast cells [37,47]. Mice deficient in SHIP-1 also have increased numbers of mast cells which exhibit elevated spontaneous degranulation [48]. It has been proposed that SHIP-1 suppresses degranulation, in part, by preventing the FcεRI-mediated cytoskeletal re-organization required for granule movement leading to exocytosis [49]. SHIP-2, also appears to negatively regulate FcεRI-mediated mast cell degranulation and cytokine production. However, in contrast to SHIP-1, FcεRI triggered SHIP-2-deficient BMMCs are not defective in FcεRI-mediated calcium mobilization or actin depolarization [50]. Depletion of SHIP-2 in BMMCs was shown to increase the activation of the GTPase Rac-1 as well as enhanced microtubule formation upon FcεRI activation [50]. Thus, SHIP-1 and SHIP-2 likely mediate their inhibitory responses by different mechanisms. It is possible, however, that PtdIns(3,4)P2 generated by SHIP 1 and 2 may also contribute to the regulation of specific signaling events [51]. Whether this is true for mast cells is currently unclear.
SHIP-1 and -2 can associate with inhibitory ITIM (immunoreceptor tyrosine-based inhibitory motif)-containing receptors expressed on mast cells. FcγRIIb, MAFA (mast cell function-associated antigen), gp49B1, MAIR-I, PIR-A and -B, and CD200R have all been documented to be expressed on, and to down-regulate FcεRI-mediated responses in, mast cells [52,53]. Tyrosines within the ITIM sequences are phosphorylated by src kinases, thus allowing recruitment of SHIP-1 and -2, and the tyrosine phosphatases SHP-1 and -2, via their SH2 domains. SHIP-1 has been suggested to be the primary enzyme responsible for mediating MAFA’s inhibitory responses [54] whereas, the ITIM of activated FcγRIIb recruits both SHIP-1 and -2. Fusion proteins which co-aggregate FcγRIIb and the FcεRI [55] have been demonstrated to block human antigen-induced mast cell mediator release and protect mice from IgE-dependent anaphylaxis [56] by a mechanism that partly involves SHIP reversal of PI3K-dependent signaling.
Downstream effectors regulated by PI3K
The above discussions demonstrate that PI3K regulates diverse responses in activated mast cells. From studies conducted in multiple cell types, it is evident that PI3K can influence a wide range of signaling molecules and pathways [57]. Systematic studies, utilizing a variety of approaches (Box 3), are now beginning to address which of these PI3K-regulated signaling processes regulate specific mast cell responses. In the following section and in Figure 3, we describe what is currently known about these downstream targets of PI3K activation in mast cells.
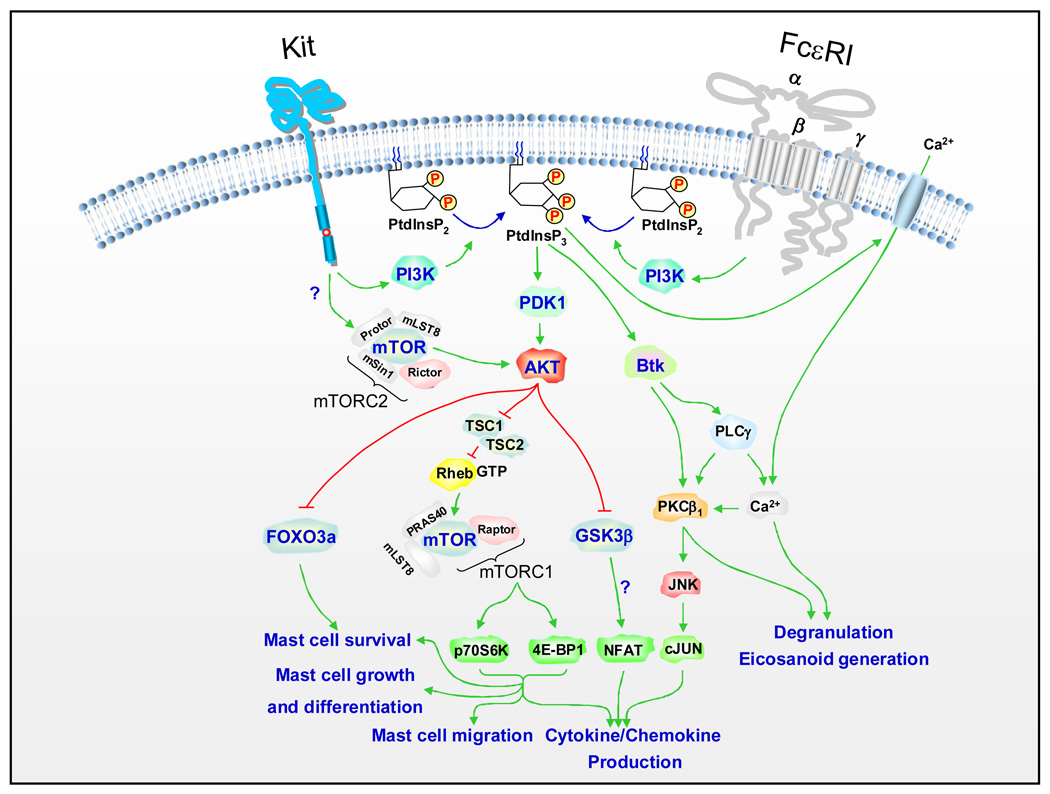
Figure 3.
Downstream targets of PI3K in activated mast cells. Activation of class 1 PI3Ks generates the membrane-associated PtdInsP3 at the inner membrane, which provides inducible docking sites for pleckstrin homology (PH) domains of associating signaling molecules. The Ser/Thr kinases, PDK1 and AKT are subsequently phosphorylated and activated after Kit and FcεRI activation. Major downstream targets of PI3K and AKT in mast cells, mTORC1, Btk, FOXO3a, and GSK3β pathways are shown (also see text) together with the specific responses in activated mast cells. Although depicted in the figure, and documented in other systems, it is not known whether AKT activation is regulated by the mTORC2 complex in mast cells. In addition, the function of GSK3β in activated mast cells still requires clarification. Whether these events are also initiated by GPCRs is currently unknown. For clarity other signaling events required for FcεRI-and Kit-mediated responses in mast cells are not depicted in this figure. Readers are referred to recent review articles for further details of these processes [6,10].
The role of PI3K in elevation of intracellular calcium concentrations
The ability of PI3K to control antigen-mediated mast cell degranulation, and in part, cytokine production, is likely linked to its capacity to regulate the calcium signal essential for these responses [46,58,59,60] (Figure 3). The initial calcium signal induced by FcεRI aggregation follows PLCγ-mediated PtdIns2 hydrolysis and subsequent binding of the generated IP3 to receptors on the endoplasmic reticulum resulting in liberation of calcium from these intracellular stores [61]. The calcium signal is maintained by subsequent influx of external calcium by store operated calcium entry (SOCE) as a consequence of intracellular storage depletion. In human mast cells, early FcεRI-mediated PLCγ1 activation and resulting IP3 production and calcium mobilization from intracellular stores appear to be PI3K independent [14]. In contrast, the subsequent SOCE-dependent maintenance phase of the calcium signal is, at least, partially PI3K dependent [14].
In mouse mast cells deficient in the Tec kinase, Btk, a similar partial reduction in antigen-mediated PLCγ1 activation, calcium mobilization, degranulation, and cytokine production, to that produced by PI3K inhibitors is observed [62,63]. From these studies, and by extension of studies conducted in B cells [64], it has been concluded that Btk is an essential intermediate in the ability of PI3K to regulate antigen-mediated calcium mobilization leading to mast cell degranulation [65]. Although the initiation of antigen-mediated signaling processes is PI3K-independent, it has been proposed that the PI3K-Btk axis might constitute a delayed maintenance and amplification pathway regulating calcium mobilization for degranulation, through continued PLCγ1 activation (Figure 3) [6]. The ability of Kit to enhance FcεRI-mediated degranulation appears to be linked to its capacity to interact with this maintenance/amplification pathway [6]. Indeed there is more of an absolute requirement for PI3K and Btk in this response than for antigen-mediated degranulation [16,62]. Btk might also contribute to antigen-mediated cytokine production via activation of PKCβ1 and the JNK pathway [66]. However, the enhancement of antigen-mediated cytokine production by SCF appears to be less dependent on Btk [62].
In addition to the regulation of degranulation via Btk, PI3K can also regulate degranulation at other stages of the secretory process. For example, it has been proposed that PtdInsP3, produced by PI3K, directly stimulates calcium transportation across the mast cell plasma membrane [59]. PI3K might also control the calcium signal in mast cells by the activation of phospholipase D (PLD) [67] which, by activating sphingosine kinase1 [68] may enhance sphingosine 1 phosphate (S1P) production, a putative regulator of calcium mobilization [69]. Studies in fMLP (f-methione-leucine-phenylalanine peptide) - challenged RBL-2H3 cells [70] have also suggested that the phosphorylation of the Snare complex proteins Snap-23, Syntaxins 2 and 4, which are involved in the fusion of the granules with the plasma membrane and subsequent release of their contents, is dependent on PI3K.
PDK, AKT and FOXO
PI3K activation results in recruitment of the serine/threonine kinase PDK1, (3-phosphoinositide-dependent kinase 1) to the plasma membrane where PDK1 subsequently phosphorylates and activates AKT [71] (Figure 3). Thus, in mast cells, FcεRI aggregation, activation of Kit, and GPCR ligation, induce activation of PDK and AKT [35,72]. Although AKT, in other cell types, has been demonstrated to control multiple downstream targets [73,74] the targets for AKT in mast cells are relatively unknown. Inhibitor and transfection studies have provided evidence that the PDK1-AKT interaction might contribute to the PI3K-dependent signaling events that regulate mast cell growth, homeostasis, and cytokine production. For example, Leflunomide, a drug which inhibits PDK1 activation and resulting AKT phosphorylation [31], was reported to induce apoptosis in SCF-maintained human mast cells [31]. Phosphorylation of glycogen synthase kinase 3β (GSK3β), a downstream target of AKT, was also reduced in the cells treated with leflunomide. Studies in mouse BMMCs have suggested that AKT activation leads to cytokine production by regulating transcription from the NFκB, NFAT and AP-1 binding regions in the IL-2 and TNF-α promoter sites [75]. These studies also indicated that GSK3β might be involved in the regulation of NFAT and AP-1 activity leading to mast cell cytokine production.
The forkhead box class O (FOXO) transcription factors family members, FOXO1a and FOXO3a, are phosphorylated in an AKT-dependent manner in SCF-stimulated BMMCs [76]. FOXO3a phosphorylation leads to its proteosomal degradation following ubiquitination thereby blocking its transcriptional control of the pro-apopototic factor, Bim. SCF also induces the AKT- and MAPK-dependent phosphorylation of Bim in mouse bone marrow-derived mast cells and it has been proposed that this also leads to its proteosomal degradation [76]. Thus these observations would account for the ablity of PI3K-AKT driven pathways to promote mast cell survival.
mTOR
mTOR is a conserved serine/threonine kinase which exists in two distinct multimolecular complexes, mTOR complex 1 (mTORC1) and mTORC2 [77] (Figure 3). PI3K regulates the mTORC1 pathway via the activation of AKT which directly phosphorylates the negative regulators of mTOR activation, TSC1 and TSC2 (tuberin), thereby inactivating its inhibitory activity. This allows mTOR activation resulting in the phosphorylation of p70 ribosomal S6 kinase (p70S6K) and eukaryotic initiation factor 4E-binding protein1 (4E-BP1) [78,79]. These events lead to mTOR-dependent gene transcription that regulates cell growth, protein synthesis, and metabolism in response to a variety of environmental stimuli [80].
A marked PI3K-dependent activation of the mTORC1 pathway is observed in human or mouse mast cells stimulated via FcεRI or Kit [32]. Rapamycin, a specific inhibitor of mTORC1, selectively and completely blocks the FcεRI- and Kit-induced mTORC1-dependent p70S6K phosphorylation and partially blocks the 4E-BP1 phosphorylation in both mouse and human mast cells [32]. This is associated with a significant inhibition of antigen- and SCF-mediated cytokine production, and SCF-mediated mast cell chemotaxis, growth, and survival, suggesting that these responses are at least in part regulated by the mTORC1 complex. However, in the case of cytokine production and chemotaxis, inhibition by rapamycin was not as marked as that produced by wortmannin, suggesting that PI3K might also regulate these responses independently of mTORC1. Interestingly, there is a marked enhancement in the activation of components of the mTORC1 complex in the LAD2 and HMC-1 mast cell tumor lines, even under resting conditions [32]. Rapamycin blocks this constitutive activation of the mTORC1 pathway and inhibits the survival of these cells. These data imply that the PI3K-mTORC1 axis contributes to the abnormal cell growth in human tumor mast cells.
Summary, conclusions, and future considerations
In summary, it is now clear that PI3K-regulated signaling events play a central role in mast cell biology. The downstream targets responsible for the receptor-mediated, PI3K-dependent responses in mast cells have still not been fully elucidated. However, PI3K-regulated degranulation, and to a certain extent cytokine production, appears to be linked to the regulation of a latent calcium signal, likely requiring activation of Btk. Multiple PI3K-regulated process appear to contribute to mast cell growth and survival including those requiring activation of PDK, AKT and the mTORC1 cascade, and inactivation of FOXO. It is less clear how PI3K can regulate mast cell chemotaxis and adhesion, but data suggest that mTORC1 might also play a partial role in the regulation of SCF-mediated mast cell chemotaxis.
Due to their central roles in the generation and activation of mast cells, PI3K-regulated pathways are attractive targets for the treatment of mast cell-related disorders, for example anaphylaxis, asthma and mastocytosis. The selective expression of the p110δ and p110γ catalytic subunit isoforms in cells of haematopoietic lineage, and the consensus of studies supporting a role for especially the p110δ isoform in both Kit- and FcεRI-mediated mast cell responses in vitro and in vivo, indicates the potential utility of isoform-selective PI3K inhibitors in mast cell-driven disease. Alternatively, downstream targets of PI3K might also lend themselves to pharmacological intervention. However, a major challenge would be the selective targeting of these molecules in mast cells. Although progress has been made in the identification of these downstream targets, much is still unknown regarding how these, and potentially other molecules, are differentially regulated following activation by individual surface receptors on mast cells. Further research is thus required to fully identify these targets and to precisely delineate their role in specific mast cell responses.
Acknowledgements
Research in the authors’ laboratory is supported by the NIAID Intramural Program within the National Institutes of Health, USA. The authors would like to thank Dr. Michael A. Beaven LMI/NHLBI/NIH for his critical review of the manuscript and the editor and the reviewers of this manuscript for their helpful comments. Due to space constraints, not all pertinent literature could be referenced in this article. This does not imply that other articles not referenced are of lesser merit.
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, et al. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD, et al. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Jensen BM, et al. Pharmacological targeting of the KIT growth factor receptor: a therapeutic consideration for mast cell disorders. Br J Pharmacol. 2008 doi: 10.1038/bjp.2008.204. doi 10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 6.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 7.Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34:97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 9.Roskoski R., Jr Signaling by Kit protein-tyrosine kinase–the stem cell factor receptor. Biochem Biophys Res Commun. 2005;337:1–13. doi: 10.1016/j.bbrc.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 10.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch E, et al. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol. 2007;194:243–256. doi: 10.1677/JOE-07-0097. [DOI] [PubMed] [Google Scholar]
- 12.Vanhaesebroeck B, et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 13.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 14.Tkaczyk C, et al. The phospholipase Cγ1-dependent pathway of FcεRI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem. 2003;278:48474–48484. doi: 10.1074/jbc.M301350200. [DOI] [PubMed] [Google Scholar]
- 15.Lu-Kuo JM, et al. Impaired kit- but not FcεRI-initiated mast cell activation in the absence of phosphoinositide 3-kinase p85α gene products. J Biol Chem. 2000;275:6022–6029. doi: 10.1074/jbc.275.8.6022. [DOI] [PubMed] [Google Scholar]
- 16.Ali K, et al. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 17.Okkenhaug K, et al. Antigen receptor signalling: a distinctive role for the p110δ isoform of PI3K. Trends Immunol. 2007;28:80–87. doi: 10.1016/j.it.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wymann MP, et al. Phosphoinositide 3-kinase γ: a key modulator in inflammation and allergy. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- 19.Parravicini V, et al. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- 20.Yu M, et al. Scaffolding adapter Grb2-associated binder 2 requires Syk to transmit signals from FcεRI. J Immunol. 2006;176:2421–2429. doi: 10.4049/jimmunol.176.4.2421. [DOI] [PubMed] [Google Scholar]
- 21.Moon KD, et al. Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J Biol Chem. 2005;280:1543–1551. doi: 10.1074/jbc.M407805200. [DOI] [PubMed] [Google Scholar]
- 22.Shivakrupa R, et al. Phosphatidylinositol 3'-kinase is required for growth of mast cells expressing the kit catalytic domain mutant. Cancer Res. 2003;63:4412–4419. [PubMed] [Google Scholar]
- 23.Tan BL, et al. Genetic evidence for convergence of c-Kit- and α4 integrin-mediated signals on class IA PI-3 kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. [DOI] [PubMed] [Google Scholar]
- 24.Ueda S, et al. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood. 2002;99:3342–3349. doi: 10.1182/blood.v99.9.3342. [DOI] [PubMed] [Google Scholar]
- 25.Kissel H, et al. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. Embo J. 2000;19:1312–1326. doi: 10.1093/emboj/19.6.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade MV, et al. Dexamethasone suppresses antigen-induced activation of phosphatidylinositol 3-kinase and downstream responses in mast cells. J Immunol. 2004;172:7254–7262. doi: 10.4049/jimmunol.172.12.7254. [DOI] [PubMed] [Google Scholar]
- 27.Cuevas BD, et al. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:27455–27461. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- 28.von Willebrand M, et al. Modification of phosphatidylinositol 3-kinase SH2 domain binding properties by Abl- or Lck-mediated tyrosine phosphorylation at Tyr-688. J Biol Chem. 1998;273:3994–4000. doi: 10.1074/jbc.273.7.3994. [DOI] [PubMed] [Google Scholar]
- 29.Chan TO, et al. Small GTPases and tyrosine kinases coregulate a molecular switch in the phosphoinositide 3-kinase regulatory subunit. Cancer Cell. 2002;1:181–191. doi: 10.1016/s1535-6108(02)00033-8. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, et al. An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response. J Exp Med. 2007;204:93–103. doi: 10.1084/jem.20061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawamukai N, et al. Leflunomide inhibits PDK1/Akt pathway and induces apoptosis of human mast cells. J Immunol. 2007;179:6479–6484. doi: 10.4049/jimmunol.179.10.6479. [DOI] [PubMed] [Google Scholar]
- 32.Kim MS, et al. Activation and function of the mTORC1 pathway in mast cells. J Immunol. 2008;180:4586–4595. doi: 10.4049/jimmunol.180.7.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitaura J, et al. Regulation of highly cytokinergic IgE-induced mast cell adhesion by Src, Syk, Tec, and protein kinase C family kinases. J Immunol. 2005;174:4495–4504. doi: 10.4049/jimmunol.174.8.4495. [DOI] [PubMed] [Google Scholar]
- 34.Laffargue M, et al. Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 35.Murphy TR, et al. Activation of protein kinase D1 in mast cells in response to innate, adaptive, and growth factor signals. J Immunol. 2007;179:7876–7882. doi: 10.4049/jimmunol.179.11.7876. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Hansen V, et al. Increased expression of genes linked to FcεRI Signaling and to cytokine and chemokine production in Lyn-deficient mast cells. J Immunol. 2005;175:7880–7888. doi: 10.4049/jimmunol.175.12.7880. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Hansen V, et al. Dysregulated FcεRI signaling and altered Fyn and SHIP activities in Lyn-deficient mast cells. J Immunol. 2004;173:100–112. doi: 10.4049/jimmunol.173.1.100. [DOI] [PubMed] [Google Scholar]
- 38.Xiao W, et al. Positive and negative regulation of mast cell activation by Lyn via the FcεRI. J Immunol. 2005;175:6885–6892. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okayama Y, et al. Comparison of FcεRI- and FcγRI-mediated degranulation and TNF-α synthesis in human mast cells: selective utilization of phosphatidylinositol-3-kinase for FcγRI-induced degranulation. Eur J Immunol. 2003;33:1450–1459. doi: 10.1002/eji.200323563. [DOI] [PubMed] [Google Scholar]
- 40.Ali K, et al. Isoform-specific functions of phosphoinositide 3-kinases: p110δ but not p110γ promotes optimal allergic responses in vivo. J Immunol. 2008;180:2538–2544. doi: 10.4049/jimmunol.180.4.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuehn HS, Gilfillan AM. G protein-coupled receptors and the modification of FcεRI-mediated mast cell activation. Immunol Lett. 2007;113:59–69. doi: 10.1016/j.imlet.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venkatesha RT, et al. Distinct regulation of C3a-induced MCP-1/CCL2 and RANTES/CCL5 production in human mast cells by extracellular signal regulated kinase and PI3 kinase. Mol Immunol. 2005;42:581–587. doi: 10.1016/j.molimm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Fukao T, et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 44.Lee KS, et al. Inhibition of phosphoinositide 3-kinase δ attenuates allergic airway inflammation and hyperresponsiveness in murine asthma model. Faseb J. 2006;20:455–465. doi: 10.1096/fj.05-5045com. [DOI] [PubMed] [Google Scholar]
- 45.Munugalavadla V, et al. Genetic and pharmacologic evidence implicating the p85α, but not p85β, regulatory subunit of PI3K and Rac2 GTPase in regulating oncogenic KIT-induced transformation in acute myeloid leukemia and systemic mastocytosis. Blood. 2007;110:1612–1620. doi: 10.1182/blood-2006-10-053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furumoto Y, et al. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 47.Furumoto Y, et al. The FcεRIβ immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IκB kinase phosphorylation and mast cell cytokine production. J Biol Chem. 2004;279:49177–49187. doi: 10.1074/jbc.M404730200. [DOI] [PubMed] [Google Scholar]
- 48.Oh SY, et al. Src homology 2 domain-containing inositol 5-phosphatase 1 deficiency leads to a spontaneous allergic inflammation in the murine lung. J Allergy Clin Immunol. 2007;119:123–131. doi: 10.1016/j.jaci.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gimborn K, et al. SHIP down-regulates FcεR1-induced degranulation at supraoptimal IgE or antigen levels. J Immunol. 2005;174:507–516. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]
- 50.Leung WH, Bolland S. The inositol 5'-phosphatase SHIP-2 negatively regulates IgE-induced mast cell degranulation and cytokine production. J Immunol. 2007;179:95–102. doi: 10.4049/jimmunol.179.1.95. [DOI] [PubMed] [Google Scholar]
- 51.Kimber WA, et al. Evidence that the tandem-pleckstrin-homology-domain-containing protein TAPP1 interacts with Ptd(3,4)P2 and the multi-PDZ-domain-containing protein MUPP1 in vivo. Biochem J. 2002;361(Pt 3):525–536. doi: 10.1042/0264-6021:3610525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katz HR. Inhibitory receptors and allergy. Curr Opin Immunol. 2002;14:698–704. doi: 10.1016/s0952-7915(02)00400-4. [DOI] [PubMed] [Google Scholar]
- 53.Li L, Yao Z. Mast cell and immune inhibitory receptors. Cell Mol Immunol. 2004;1:408–415. [PubMed] [Google Scholar]
- 54.Xu R, et al. SH2 domain-containing inositol polyphosphate 5'-phosphatase is the main mediator of the inhibitory action of the mast cell function-associated antigen. J Immunol. 2001;167:6394–6402. doi: 10.4049/jimmunol.167.11.6394. [DOI] [PubMed] [Google Scholar]
- 55.Kepley CL, et al. Co-aggregation of FcγRII with FcεRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J Biol Chem. 2004;279:35139–35149. doi: 10.1074/jbc.M404318200. [DOI] [PubMed] [Google Scholar]
- 56.Mertsching E, et al. A mouse Fcγ-Fcε protein that inhibits mast cells through activation of FcγRIIB, SH2 domain-containing inositol phosphatase 1, and SH2 domain-containing protein tyrosine phosphatases. J Allergy Clin Immunol. 2008;121:441–447. doi: 10.1016/j.jaci.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 57.Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr Opin Immunol. 2004;16:314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Djouder N, et al. Rac and phosphatidylinositol 3-kinase regulate the protein kinase B in FcεRI signaling in RBL 2H3 mast cells. J Immunol. 2001;166:1627–1634. doi: 10.4049/jimmunol.166.3.1627. [DOI] [PubMed] [Google Scholar]
- 59.Ching TT, et al. Phosphoinositide 3-kinase facilitates antigen-stimulated Ca(2+) influx in RBL-2H3 mast cells via a phosphatidylinositol 3,4,5-trisphosphate-sensitive Ca(2+) entry mechanism. J Biol Chem. 2001;276:14814–14820. doi: 10.1074/jbc.M009851200. [DOI] [PubMed] [Google Scholar]
- 60.Barker SA, et al. Multiple roles for PI 3-kinase in the regulation of PLCγ activity and Ca2+ mobilization in antigen-stimulated mast cells. J Leukoc Biol. 1999;65:321–329. doi: 10.1002/jlb.65.3.321. [DOI] [PubMed] [Google Scholar]
- 61.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 62.Iwaki S, et al. Btk plays a crucial role in the amplification of FcεRI-mediated mast cell activation by kit. J Biol Chem. 2005;280:40261–40270. doi: 10.1074/jbc.M506063200. [DOI] [PubMed] [Google Scholar]
- 63.Kawakami Y, et al. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–1219. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 64.Saito K, et al. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- 65.Nadler MJ, Kinet JP. Uncovering new complexities in mast cell signaling. Nat Immunol. 2002;3:707–708. doi: 10.1038/ni0802-707. [DOI] [PubMed] [Google Scholar]
- 66.Kawakami Y, et al. Regulation of protein kinase CβI by two protein-tyrosine kinases, Btk and Syk. Proc Natl Acad Sci U S A. 2000;97:7423–7428. doi: 10.1073/pnas.120175097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cissel DS, et al. Thapsigargin-induced secretion is dependent on activation of a cholera toxin-sensitive and phosphatidylinositol-3-kinase-regulated phospholipase D in a mast cell line. J Pharmacol Exp Ther. 1998;285:110–118. [PubMed] [Google Scholar]
- 68.Melendez AJ, Khaw AK. Dichotomy of Ca2+ signals triggered by different phospholipid pathways in antigen stimulation of human mast cells. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 69.Choi OH, et al. Calcium mobilization via sphingosine kinase in signalling by the FcεRI antigen receptor. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 70.Nanamori M, et al. Regulation of leukocyte degranulation by cGMP-dependent protein kinase and phosphoinositide 3-kinase: potential roles in phosphorylation of target membrane SNARE complex proteins in rat mast cells. J Immunol. 2007;178:416–427. doi: 10.4049/jimmunol.178.1.416. [DOI] [PubMed] [Google Scholar]
- 71.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- 72.Kuehn HS, et al. Synergistic activation of phospholipases Cγ and Cβ: A novel mechanism for PI3K-independent enhancement of FcεRI-induced mast cell mediator release. Cell Signal. 2008;20:625–636. doi: 10.1016/j.cellsig.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tokunaga E, et al. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 74.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Kitaura J, et al. Akt-dependent cytokine production in mast cells. J Exp Med. 2000;192:729–740. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moller C, et al. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood. 2005;106:1330–1336. doi: 10.1182/blood-2004-12-4792. [DOI] [PubMed] [Google Scholar]
- 77.Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 78.Dann SG, et al. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Shah OJ, et al. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- 80.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 81.Roskoski R., Jr Structure and regulation of Kit protein-tyrosine kinase–the stem cell factor receptor. Biochem Biophys Res Commun. 2005;338:1307–1315. doi: 10.1016/j.bbrc.2005.09.150. [DOI] [PubMed] [Google Scholar]
- 82.Marone R, et al. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Andrews S, et al. PI3K class IB pathway in neutrophils. Sci STKE. 2007 doi: 10.1126/stke.4072007cm3. 2007, cm3. [DOI] [PubMed] [Google Scholar]
- 84.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 85.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 86.Vanhaesebroeck B, et al. Signalling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem Sci. 2005;30:194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 87.Voigt P, et al. Characterization of p87PIKAP, a novel regulatory subunit of phosphoinositide 3-kinase γ that is highly expressed in heart and interacts with PDE3B. J Biol Chem. 2006;281:9977–9986. doi: 10.1074/jbc.M512502200. [DOI] [PubMed] [Google Scholar]
- 88.Rommel C, et al. PI3Kδ and PI3Kγ: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- 89.Hawkins PT, et al. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans. 2006;34:647–662. doi: 10.1042/BST0340647. [DOI] [PubMed] [Google Scholar]
- 90.Harris SJ, et al. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J Biol Chem. 2008;283:2465–2469. doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- 91.Meyers R, Cantley LC. Cloning and characterization of a wortmannin-sensitive human phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:4384–4390. doi: 10.1074/jbc.272.7.4384. [DOI] [PubMed] [Google Scholar]
- 92.Sarkaria JN, et al. Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res. 1998;58:4375–4382. [PubMed] [Google Scholar]
- 93.Pomel V, et al. Furan-2-ylmethylene thiazolidinediones as novel, potent, and selective inhibitors of phosphoinositide 3-kinase γ. J Med Chem. 2006;49:3857–3871. doi: 10.1021/jm0601598. [DOI] [PubMed] [Google Scholar]