SUMMARY
Th17 cells have been recently discovered in both mouse and human. Although IL-1 has been shown to be important in human Th17 cell differentiation with little knowledge of the underlying mechanism, its function in mouse is less clear. Here we show that IL-1R1 expression in T cells, which was induced by IL-6, was necessary for Th17-mediated autoimmunity and for early Th17 differentiation in vivo. Moreover, IL-1 signaling in T cells was required in dendritic cell-mediated Th17 differentiation from naïve or regulatory precursors and IL-1 synergized with IL-6 and IL-23 to regulate Th17 differentiation and maintain cytokine expression in effector Th17 cells. Importantly, IL-1 regulated the expression of IRF4 and RORγt during Th17 differentiation; over-expression of these two factors resulted in IL-1-independent Th17 polarization. Our data thus indicate a critical role of IL-1 in Th17 differentiation and this pathway may serve as a novel target for Th17-mediated immunopathology.
INTRODUCTION
Th17 is a novel lineage of T cells that have been shown to be important in autoimmunity and clearance of mucosal infection by producing pro-inflammatory cytokines IL-17, IL-17F and IL-22 (Dong, 2008; Ouyang et al., 2008). Compared with other Th lineages, Th17 cells have their unique genetic programs. STAT3 is essential for Th17 differentiation in mouse and human possibly via induction of two orphan nuclear receptors RORγt and RORα (Laurence et al., 2007; Ma et al., 2008; Milner et al., 2008; Yang et al., 2008b; Yang et al., 2007). RORγt was first identified as a Th17-specific transcription factor that was sufficient and necessary for Th17 differentiation (Ivanov et al., 2006). RORα plays a synergistic and somewhat redundant function with RORγt during Th17 polarization (Yang et al., 2008c). Interferon regulatory factor 4 (IRF4) was originally reported to be important in Th2 differentiation (Lohoff et al., 2002; Rengarajan et al., 2002). However, a recent study revealed that IRF4-deficient T cells were completely impaired in Th17 polarization; IRF4 knockout mice were resistant to induction of Th17-mediated experimental autoimmune encephalomyelitis (EAE) (Brustle et al., 2007). An environmental toxin sensor, aryl hydrocarbon receptor (Ahr), was found to be highly expressed in Th17 and regulatory T (Treg) cells and to regulate Th17 cytokine, especially IL-22, production (Quintana et al., 2008; Veldhoen et al., 2008). In contrast, the Treg cell transcription factor Foxp3 inhibits Th17 polarization by binding to RORγt and RORα to inhibit their transcriptional activity (Yang et al., 2008b; Zhou et al., 2008).
IL-6 and TGF-β have been reported as the minimal requirements for murine Th17 differentiation from naïve CD4 T cells (Bettelli et al., 2006; Veldhoen et al., 2006). During Th17 differentiation initiated by TGF-β and IL-6, IL-1 appears to play an accessory role (Veldhoen et al., 2006). IL-21 induced by IL-6 acts in an autocrine fashion and drives Th17 generation together with TGF-β(Korn et al., 2007; Nurieva et al., 2007; Zhou et al., 2007). However, human T cells were originally shown to require IL-1 plus IL-23 or IL-6 to differentiate into Th17 cells and addition of TGF-β inhibits this polarization (Acosta-Rodriguez et al., 2007; Wilson et al., 2007). More recent studies described that TGF-β was required for Th17 generation from naïve CD4 T cells (Manel et al., 2008; Yang et al., 2008a). However, the differential roles of IL-1 for Th17 commitment in mouse and human still remain elusive. Mice defective in IL-1R1 signaling were reported to be resistant to EAE and exhibited a severe defect in the IL-17-producing T cell population (Sutton et al., 2006), suggesting IL-1 is important for mouse Th17 cell regulation in vivo. However, it was not clear from this study whether IL-1 signaling in T cells was necessary for their differentiation into Th17 cells.
In the current study, we examined the role of IL-1 in murine Th17 cell regulation. Our results demonstrate that IL-1 signaling in T cells is required for the early Th17 differentiation in vitro and in vivo. IL-1 regulates the expression of IRF4 and RORγt and overexpression of these two factors results in IL-1-independent Th17 differentiation. Therefore, similar to the human system, IL-1 plays a unique, non-redundant role during murine Th17 polarization.
RESULTS
IL-1R1 expression is upregulated in Th17 cells
In a search for genes upregulated in Th17 over Th1 cells, we found that IL-1R1 mRNA was expressed at higher levels in Th17 cells when compared with Th1 cells (data not shown). We then examined the expression of IL-1 receptor components in in vitro polarized Th1, Th2 and Th17 cells. We observed that IL-1R1 mRNA was highly expressed in Th17 compared with Th1 and Th2 (Fig 1a). Expression of IL-1R-accessory protein (IL-1RAcP) was also moderately increased. To understand the regulation of IL-1R1 expression, we activated naïve CD4+ T cells in the presence of anti-CD3/CD28 Abs with or without IL-6, IL-23 or TGF-β. TcR and costimulation signaling elevated IL-1R1 mRNA expression (Figure 1b). Among the cytokines tested, IL-6 appeared to be a critical factor for IL-1R1 upregulation whereas IL-23 or TGF-β alone minimally affected its expression (Fig 1b).
Figure 1. IL-1R1 expression on Th17 is critical for induction of EAE.
a CD25−CD44loCD62hiCD4+ T cells (Naïve CD4+ T) were FACS-sorted and polarized under Th1, Th2 or Th17 condition for 4 days. b, Naïve CD4+ T cells were stimulated with anti-CD3/CD28 Ab in the presence of indicated cytokine for 24 hours before mRNA expression of IL-1R1 was analyzed. c, FACS-sorted naïve CD4 T cells from WT mice or STAT3 KO, RORα KO, or RORα/γ double KO mice were cultured under Th17 condition for 4 days. d, Naive OT-II CD4+ T cells were activated with Ova peptide-pulsed splenic APCs under the neutral (anti-IL-4 and anti-IFN-γ) and coinfected with two bicistronic retroviruses expressing RORα-GFP or GFP vector and RORγt-hCD2 or hCD2. GFP+hCD2+ cells were FACS sorted. In a, c, and d, cells were restimulated with plate-bound anti-CD3 Ab for four hours before mRNA expression was analyzed by real-time PCR. Data were normalized with expression amounts of Actb. e and f, CD4 T cells were sorted from WT or IL-1R1 KO mice and i.v. transferred into Rag1−/− mice. The recipient mice were induced EAE and disease incidence (e) and score (f) were measured daily and means ± SEM of all mice in each group were shown (f). *, p<0.05; **, p<0.01 in comparison with WT recipients.
Th17 cell differentiation requires several transcription factors such as STAT3, RORα and RORγ (Ivanov et al., 2006; Yang et al., 2007; Yang et al., 2008c). To determine if these transcription factors are required for IL-1R1 expression in CD4+ T cells, we isolated naïve CD4+ T cells from mice defective in these transcription factors and cultured them in a Th17 polarizing condition (Yang et al., 2007; Yang et al., 2008c). As shown in Fig 1c, IL-1R1 expression was severely impaired in STAT3 KO T cells. RORα KO T cells showed a moderate decrease in IL-1R1 expression and RORα/RORγ doubly deficient T cells exhibited a more severe defect in IL-1R1 expression. Consistently, when we overexpressed RORα, RORγor both in T cells cultured under neutral conditions (Yang et al., 2008c), both RORαand RORγinduced the upregulation of IL-1R1 mRNA in T cells (Fig 1d).
IL-1 signaling in T cells is necessary for Th17 responses in vivo
IL-1R1 is necessary for the induction of EAE disease in mice (Brustle et al., 2007), which could be a result of defective IL-1 signaling in innate immune system and/or T cells. Since Th17 cells highly express IL-1R1/IL-1RAcP, we next sought to determine the role of IL-1R1 in CD4+ T cells by adoptive transfer of CD4+ T cells from wild-type (WT) or IL-1R1-deficient (KO) mice into Rag1−/− mice. Upon immunization with MOG, all recipient mice containing WT T cells developed EAE whereas only 3 out of 8 (37.5 %) mice with KO cells developed EAE, with significantly delayed onset (Fig 1e). Moreover, the severity of EAE was significantly lower in mice with KO T cells than those with WT cells (Fig 1f).
To illustrate more precisely the functional defects of IL-1R1 KO T cells in EAE, we generated mixed bone-marrow (BM) chimeras by transferring a mixture of CD45.1+ WT and CD45.2+ KO BM cells into sublethally irradiated Rag1−/− mice (Supplementary Fig 1). Eight weeks later, we induced EAE in the recipient mice. To analyze the T cell phenotypes during disease progression, we divided the EAE mice into two groups- early and peak phases- based on the disease scores (score, 0.5–2 for early phase; 2.5–4.0 for peak phase). In CNS, the numbers of CD4+ T cells were higher in WT population than in KO (9.1×104 ± 3.4 x104 vs 3.3×104 ± 1.0 x104 cells, p=0.016) while CD11b+ macrophage were comparable between them (9.4×104 ± 1.3×104 vs 10.2×104 ± 1.6 x104 cells, p=0.105). Moreover, we observed that WT CD4+ T cells contained a significantly higher percentage of IL-17 producers compared to KO (Figure 2b/c). The percentage of IFNγ+IL-17+ cells was also remarkably higher in WT compared with the KO CD4 T cell population while the percentages of IFNγ+IL-17- cells were not significantly different. Moreover, the absolute numbers of IL-17-producing WT T cells were profoundly higher than that of KO (Figure 2c). Since Treg can also migrate into the inflamed CNS in a CCR6-dependent manner (Yamazaki et al., 2008), we also analyzed the Treg population by staining Foxp3. Of note, we observed a significantly higher percentage of Foxp3+ cells in KO relative to WT population and this pattern was more obvious in the inflamed CNS (Figure 2d/e). However, the absolute number of Foxp3+ T cells in WT and KO population was comparable due to lower percentage of total KO CD4+ T cell in the CNS (Figure 2a/e). Therefore, IL-1R1-deficient CD4+ T cells migrated into the inflamed tissue much less efficiently than WT T cells and were defective in IL-17-production. Further examination of MOG-reactive T cells in spleen revealed that IL-17- but not IFNγ-expressing T cells were also reduced in the KO T cell population (Figure 2b), suggesting that IL-1 signaling in CD4+ T cells is necessary for proper Th17 cell differentiation in vivo.
Figure 2. IL-1 signaling in T cells is required for Th17 development in EAE model.
Mixed bone marrow chimeric mice were generated and induced EAE. Mononuclear cells in central nervous system (CNS) and spleen were stained with anti-CD45.2 to distinguish WT and IL-1R1 KO compartments and analyzed for CD4+ and CD11b+ cell presence (a), cytokine production profiles in CD4 T cells after PMA/Ionomycin (b and c) or MOG stimulation (b, only for spleen), or Foxp3+ cell in CD4 T cells (d and e). The mice were divided into two groups based on the disease severity (score, 0.5-2 for early phase; 2.5-4.0 for peak phase). *, p<0.05; **, p<0.01 in comparison with WT compartments. Data shown represent two independent experiments with consistent results.
IL-1 signaling in T cells functions to promote early Th17 differentiation
IL-1R1 KO CD4+ T cells were defective in generating Th17 cells in the EAE model. IL-1 signaling may mediate early Th17 differentiation or the maintenance/expansion of polarized Th17 cells. To test these two possibilities, we immunized WT and IL-1R1 KO mice with KLH in CFA and analyzed IL-17 and IFNγ-producing cells upon ex vivo KLH restimulation. As depicted in Figure 3a, as early as day 3 the KO CD4+ T cell population contained a significantly lower frequency of IL-17+ cells compared to WT. On day 7, increased percentages of IL-17+ cells were found in WT but not in KO mice while the percentage of IFNγ+ cells was comparable between WT and KO mice. The Th17-specific defect of KO T cells was confirmed using supernatants from KLH-restimulated splenocytes (Supplementary Fig 2). To directly ask if the observed defect in the early Th17 polarization is T cell intrinsic, we transferred naïve T cells from either WT or KO mice carrying the chicken ovalbumin (OVA)-specific TcR transgene (OT-II) into congenic mice and immunized the recipients with OVA323–339 peptide in CFA. As shown in Figure 3b, the frequency of IL-17-producing WT OT-II T cells was significantly higher compared with KO OT-II cells within 3 days after immunization (15.07 ± 4.06 vs 4.19 ± 2.35, p=0.045). These data overall indicates that the requirement of IL-1R1 signaling for early differentiation of Th17 cells in vivo is CD4+ T cell intrinsic.
Figure 3. IL-1 signal is required for early differentiation of Th17 cells .
a, WT and IL-1R1 KO mice were subcutaneously immunized with KLH in CFA. Three or seven days later, lymphoid cells from draining lymph nodes were restimulated with KLH overnight and IL-17- or IFN-γ-expressing cells were measured by intracellular staining. b, FACS-sorted naïve OT-II T cells from WT or IL-1R1-deficient OT-II mice were intravenously transferred into congenic (CD45.1) mice. The recipient mice were immunized with OVA323-339 peptide in CFA. Three days later, lymphoid cells from draining lymph nodes were restimulated with PMA and Ionomycin for 5 hours and IL-17- or IFN-γ-expressing cells were measured by intracellular staining. Data shown are on gated CD45.1+ CD4+ T cells. c and d, FACS-sorted naïve CD4 T cells from WT or IL-1R1−/− mice were cocultured with BM-derived DC (WT) in the presence of soluble anti-CD3 Ab (0.1μg/ml) and LPS (100 ng/ml) plus TGF-β (1 ng/ml). IL-17- or IFN-γ-expressing cells were measured by intracellular staining (c). At day 4, mRNA expression was assessed by real-time RT-PCR after restimulation of T cells by anti-CD3 for 4 hr (d). Data shown represent two independent experiments and normalized with expression amounts of Actb.
The above results indicate that IL-1 signaling is required in the early phase of Th17 differentiation in vivo. This is surprising considering that in vitro, IL-1 only moderately enhances Th17 differentiation in purified CD4+ T cells (Veldhoen et al., 2006). We thus turned to a different Th17 differentiation system in which T cells were co-cultured with dendritic cells (DC) in the presence of soluble anti-CD3Ab, LPS and TGF-β(Veldhoen et al., 2006). Naive WT or KO CD4+ T cells were activated with either WT or KO bone-marrow derived DC and the deficiency of IL-1R1 on T cells but not on DC resulted in a great reduction in all Th17 cytokines including IL-17, IL-17F, IL-22 and IL-21 (Supplementary Fig 3). Therefore, the IL-1 signal in CD4+ T cells rather than DC is required for Th17 generation.
To further address the role of IL-1 during early Th17 commitment, we utilized the same DC/T cell co-culture system and examined the kinetics of Th17 generation. IL-17-producing T cells started to appear 2 days after culture with evident difference in WT and KO T cells and thereafter (Figure 3c). Moreover, the staining intensity of anti-IL-17 antibody in WT cells was higher than that of KO (mean fluorescence intensity at day 4, WT; 402 vs KO; 211), indicating that WT Th17 cells are more potent IL-17-producers. Real-time PCR analysis after anti-CD3 restimulation showed an efficient induction of all Th17 cytokines (IL-17, IL-17F, IL-22 and IL-21) in WT but not in KO T cells (Figure 3d). These in vivo and in vitro experiments together demonstrate that IL-1 signaling is critical for the early differentiation stages of the Th17 lineage.
Conversion of Treg into IL-17-producing cells requires IL-1 signaling
In our EAE study with mixed bone-marrow chimeras, we observed significantly higher percentages of Foxp3+ T cells in the KO population, compared with WT, particularly in the inflamed CNS (Figure 2d/e). This observation led us to hypothesize that IL-1 signaling might be involved in a reciprocal regulation between Treg and Th17 cells. IL-1 does not seem to be involved in the generation of naturally occurring Foxp3+ Treg cells as naïve IL-1R1 KO mice have normal numbers of Foxp3+ T cells in secondary lymphoid organs (data not shown). Recent studies showed that Foxp3+ T cells can be reprogrammed into IL-17-producing cells (Yang et al., 2008b). To examine if IL-1 signaling is necessary for the conversion of Foxp3+ cells into IL-17-producing T cells in vivo, we analyzed IL-17+ cells that co-expresses Foxp3 in the CD4+ T cells from our mixed BM-chimeric mice after EAE induction. We reasoned that IL-17+Foxp3+ T cells were likely to be Treg cells on their way to become Th17 cells since very few Foxp3+ T cells in naïve mice produce IL-17. As shown in Figure 4a, ~10 % of splenic and CNS Foxp3+ WT T cells produced IL-17 after EAE induction; however, this population was almost completely absent within KO Foxp3+ T cells in the same animals.
Figure 4. Conversion of Foxp3+ T cells into Th17 cells requires IL-1 signal.
a, Mononuclear cells in EAE-induced mixed bone marrow chimeric mice as described in Figure 2 were analyzed for IL-17 and Foxp3 expression. Data shown are on gated CD4+ Foxp3+ cells. Mean values are shown as horizontal bars. **, p<0.01 in comparison with WT compartments. Data shown represent two independent experiments with consistent results. b and c, FACS-sorted CD4+GFP+ cells from naïve Foxp3-GFP reporter mice were cultured with DC and in the presence of soluble anti-CD3 Ab, LPS and TGF-β plus anti-IL-1R1 Ab or rat IgG as a control. IL-17- or GFP-expressing cells were measured by intracellular staining (b). At day 4, mRNA expression was assessed by real-time RT-PCR after restimulation of T cells by anti-CD3 for 4 hr (c). mRNA expression in fresh FACS-sorted CD4+GFP+ cells, stimulated with anti-CD3 Ab for 4 hr, was used as a control. Data shown represent two independent experiments and normalized with expression amounts of Actb.
To directly determine the role of IL-1 in the induction of IL-17 in Foxp3+ T cells, we utilized Foxp3-GFP reporter mice (Fontenot et al., 2005). CD4+GFP+ cells from naïve Foxp3-GFP reporter mice were cultured with DC in the presence of soluble anti-CD3Ab, LPS and TGFβ. In some samples, we added anti-IL-1R1 Ab in the culture to block IL-1 signaling. IL-17+ cells started to appear on day 2 following activation of Treg cells (Figure 4b). Blocking IL-1R1 greatly reduced the IL-17+ population, especially within the GFP+ (Foxp3+) population. This pattern became more apparent on day 6 with more than 50% reduction of IL-17+ cells in GFP- population and nearly 90% reduction in GFP+ population (Figure 4b). Of note, little difference in GFP downregulation was observed between cells treated with control Ab and IL-1R1 blocking Ab, indicating no role of IL-1 signaling in Foxp3 downregulation. In support of these data, real-time RT-PCR analysis revealed that Foxp3+ Treg cells after activation remarkably downregulated Foxp3 and TGF-β 1 expression with no difference between control Ab and anti-IL-1R1Ab treatment (Figure 4c). In contrast, those cultured in the presence of control Ab expressed significantly increased levels of RORγ and IRF4 while cells treated with IL-1R1 blocking did not. Moreover, the expression of IL-17, IL-17F, IL-22 and IL-21 was greatly increased in T cells in the absence of IL-1R1 blockade (Figure 4c). The induction of Th17 transcription factors and cytokines appeared to be specific since T-bet and IFNγ expression remained largely unchanged.
Collectively, these data demonstrate that similar to naïve T cells, IL-1 signaling is critical for inducing the phenotypic conversion of natural Treg into Th17, which is independent of Foxp3 downregulation.
IL-1 signaling maintains Th17 cells in the absence of TCR stimuli
The regulation of Th17 maintenance after initial differentiation has not been well understood. IL-23 has been suggested as a key factor required for Th17 maintenance rather than differentiation (Veldhoen et al., 2006). On the other hand, IL-1R1 expression, similar to that of IL-23R, is also upregulated by IL-6 (Figure 1b), raising the possibility that IL-1 signaling may have an additional function in differentiated Th17 cells. To address this hypothesis, naïve CD4+ T cells from IL-17F-RFP reporter mice (Yang et al., 2008b) were stimulated under Th17 conditions and RFP+ cells were sorted to enrich Th17 cells. More than 50% of the sorted cells expressed Th17 cytokines (IL-17, IL-17F) with less than 1% of Foxp3+ cells and virtually no IFNγ+ cells (Figure 5a). These cells were labeled with CFSE and cultured with IL-23 or IL-1 alone, IL-23 plus IL-1, or IL-6 plus TGF-β in the absence of TCR stimulation. Of interest, Th17 cells cultured with IL-1 but not IL-23 proliferated in the absence of TCR stimulation, which was enhanced by IL-23 (Figure 5b, top panels). Compared with the starting cells, Th17 cultured with medium alone produced reduced levels of IL-17 and IL-17F (Figure 5a–b). In Th17 cells cultured with IL-23 alone or IL-6 plus TGF-β there was no improvement over the medium-only condition. However, addition of IL-1 to the culture effectively maintained the cytokine production in Th17 cells (Figure 5b). Moreover, Th17 cells cultured with IL-1 plus IL-23 exhibited enhanced maintenance of Th17 cytokine profiles. None of the culture condition showed any increased expression of IL-4, IFNγ (Figure 5b, lower panels), or Foxp3 (data not shown).
Figure 5. IL-1 signal expands and maintains Th17 program in the absence of TCR stimulation.
FACS-sorted naïve CD4+ T cells from IL-17F-RFP reporter mice were stimulated under Th17 condition (anti-IL-4, anti-IFNγ, IL-6, TGF-β, IL-23). At day 4, RFP+ cells were sorted and the expression of IL-17, IL-17F, IFNγ, Foxp3 or T-bet was analyzed by intracellular staining (a). b-d, The sorted RFP+ cells were labeled with CFSE and cultured with medium alone, IL-23, IL-1 alone, IL-23 plus IL-1, or IL-6 plus TGF-β for additional 3 days. b, IL-17-, IL-17F-, IL-4-, IFNγ-expressing cells were analyzed by intracellular staining after PMA and Ionomycin stimulation for 4 hr. c, Cytokines in the supernatant of 3 day additional culture were measured by ELISA. d, Cells were further restimulated with plate bound anti-CD3 Ab overnight and cytokines in the supernatant were measured by ELISA. ‘Th17’ is the supernatant of the sorted RFP+ cells after anti-CD3 Ab overnight stimuation. Data shown represent four independent experiments.
Unexpectedly, in the above experiment, we detected significantly increased levels of IL-17, IL-17F and IL-22 in the supernatants from cells treated with IL-1 (Figure 5c). Therefore, polarized Th17 cells produced Th17 cytokines upon IL-1 signal in the absence of TcR stimulation. Consistent with intracellular staining profiles, IL-23 alone did not induce these Th17 cytokines but greatly synergized with IL-1, especially in inducing IL-17 and IL-22 secretion (Figure 5c). After anti-CD3 restimulation, cells cultured with IL-1 alone or together with IL-23 produced Th17 cytokines at levels at least comparable to original Th17 cells while the other conditions failed to do so (Figure 5d). These observations indicate that IL-1 signaling not only maintains Th17 phenotypes after polarization but also triggers Th17 cytokine production even in the absence of TcR stimulation.
IL-1 regulates Th17 differentiation in the absence of exogenous TGF-β
Our in vivo and in vitro data thus far have revealed an essential role of IL-1 signaling for early Th17 differentiation. However, it was also shown that purified T cells differentiated into Th17 cells in the presence of TGF-β and IL-6. To better understand the IL-1 effect on T cells, we treated naïve T cells with anti-CD3/CD28 Abs in the presence of defined cytokine combination. In this experimental setting, IL-6/TGF-β induced comparable levels of IL-17 between WT and IL-1R1 KO T cells, indicating no autocrine effect of IL-1 in this condition. However, addition of IL-1 in WT but not KO culture greatly increased the IL-17+ population and induced higher amounts of IL-17 as measured by mean fluorescence intensity (Figure 6a). Since TGF-β is essential for Th17 commitment, down-regulation of Foxp3 by IL-21 or IL-6 is one of the crucial steps during Th17 differentiation. Foxp3 induction by TGF-β was not hampered by addition of IL-1 (Figure 6b), suggesting IL-1 contributes to Th17 polarization via a mechanism distinct from IL-6 and IL-21.
Figure 6. IL-1 signal regulates Th17 cytokine production in the absence of exogenous TGF-β.
FACS-sorted naïve CD4+ T cells from WT or IL-1R1 KO mice were stimulated with anti-CD3/CD28 Abs and anti-IL-4/anti-IFNγ Abs in the presence of indicated cytokines for 4 days. a, IL-17-producing cells were analyzed by intracellular staining. Values are the percentage of IL-17+ cells and mean fluorescence intensity (MFI) of the IL-17 staining of the gated cells. b, Foxp3- and T-bet-expressing cells were analyzed by intracellular staining. c, IL-17- and IFNγ-expressing cells were analyzed by intracellular staining. d, At day 4, cells stimulated under the condition described in c were harvested and restimulated with plated bound anti-CD3 Ab overnight and cytokines in the supernatant were measured by ELSIA. Data shown represent at least two independent experiments.
In the absence of exogenous TGF-β, IL-6 and IL-23 only induced low levels of IL-17 production (Figure 6c), whereas IL-1 and IL-23 barely induced IL-17 (data not shown). However, the combination of IL-6, IL-23 and IL-1 greatly induced IL-17+ cells (Figure 6c). This was dependent on TGF-β as neutralizing anti-TGF-β completely abolished IL-17 induction. Th17 cells generated by IL-6/IL-23/IL-1 produced all Th17 cytokines (IL-17, IL-17F, IL-22) whereas Th17 cells generated by IL-6/TGF-β produced little amount of IL-22 (Figure 6d). Therefore, IL-1 synergizes with IL-6 and IL-23 to induce Th17 differentiation at a low concentration of TGF-β.
IL-1 signaling induces IRF4 and RORγt expression during early Th17 polarization
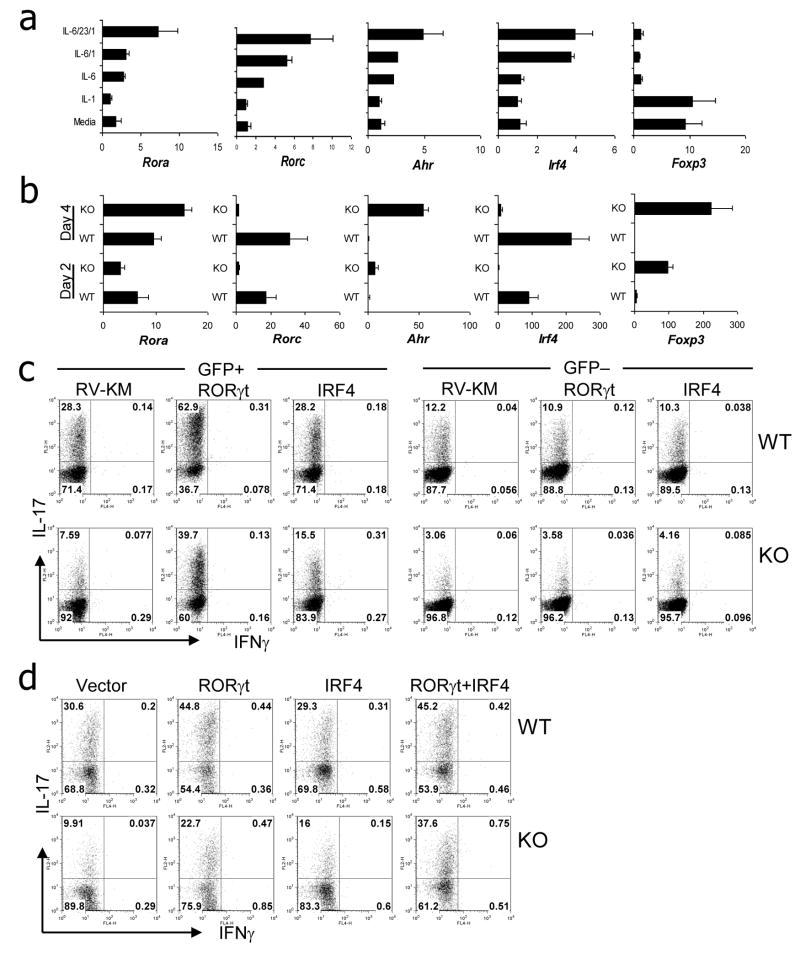
To understand the molecular mechanism by which IL-1 signaling induces Th17 polarization, we stimulated naïve CD4+ T cells in the presence of anti-CD3/CD28 with or without IL-1, IL-6, and/or IL-23. Cells stimulated with IL-6 moderately upregulated RORγ but not IRF4 expression; however, the combination of IL-6 and IL-1 significantly increased the expression of these transcription factors, particularly IRF4 (Figure 7a). The combination of IL-6, IL-1 and IL-23 further enhanced the expression of RORαand Ahr.
Figure 7. IL-1R1 signal regulates upregulation of RORγt and IRF4 expression.
a, FACS-sorted naïve CD4+ T cells from WT mice were stimulated with anti-CD3/CD28 Abs and anti-IL-4/anti-IFNγAbs in the presence of indicated cytokines for 4 days. mRNA expression was assessed by real-time RT-PCR. b, FACS-sorted naïve CD4 T cells from WT or IL-1R1−/− mice were co-cultured with BM-derived DC (WT) in the presence of soluble anti-CD3 Ab and LPS plus TGF-β. At days 2 and 4, mRNA expression was assessed by real-time RT-PCR. Data shown represent two independent experiments and normalized with expression amounts of Actb. c and d, FACS-sorted naïve CD4+ T cells from WT or IL-1R1−/− mice were co-cultured with BM-derived DC (WT) in the presence of soluble anti-CD3 Ab and LPS plus TGF-β and infected with bicistronic retrovirus expressing GFP vector (RV-KM), RORγt-GFP or IRF4-GFP. Four days after infection, IL-17- or IFNγ-expressing cells were assessed by intracellular staining. Data shown are gated on GFP+ or GFP− cells and represent two independent experiments. In d, cells were coinfected with two bicistronic retroviruses expressing IRF4-GFP or GFP vector and RORγt-hCD2 or hCD2. GFP+ and hCD2+ cells were sorted and assessed by intracellular staining.
The above data suggest that IL-1 may synergize with IL-6 to induce IRF4 and RORγt expression during Th17 polarization. To further test this idea, we compared gene expression of WT and IL-1R1 KO CD4+ T cells during Th17 differentiation activated by DC in the presence of LPS and TGF-β. Real-time RT-PCR analysis demonstrated comparable induction of RORα in WT and KO T cells (Figure 7b). In contrast, KO T cells failed to express RORγt. Similarly, an almost complete lack of IRF4 expression was observed in KO T cells compared with WT T cell as early as day 2 (Figure 7b). Moderate increase in IL-23R expression was observed in WT but not in KO T cells (data not shown). In contrast, Foxp3 and Ahr expression was higher in KO T cells than those of WT T cells.
We next asked if over-expression of IRF4 and/or RORγt in T cells would overcome IL-17 deficiency in KO cells. We utilized retroviral over-expression during the DC/T cell coculture. Analysis of cytokine expression in T cells infected with empty vector showed significant reduction of IL-17+ population in IL-1R1 KO T cells compared with WT T cells (Figure 7c). RORγt over-expression greatly improved the percentages of the IL-17-producing population in both WT and KO T cells. Interestingly, overexpression of IRF4 moderately increased the IL-17+ population in KO T cells, with no effect in WT T cells (Figure 7c). Since we observed severe defects in both RORγt and IRF4 expression in IL-1R1 KO T cells and overexpession of either one only partially restored IL-17 production, we assessed if combined expression of IRF4 and RORγt induces IL-17 production in IL-1R1 KO T cells. After infection, dually infected cells were sorted and analyzed. Again, overexpression of IRF4 alone increased the IL-17+ population only in KO but not in WT T cells (Figure 7d). In WT T cells, RORγt overexpression increased the IL-17+ population with no further increase by co-expression of IRF4. In contrast, the IL-17+ population in KO T cells was remarkably higher after RORγt/IRF4 co-expression condition than RORγt or IRF4 alone condition. Therefore, RORγt and IRF4 can synergistically restore the Th17 polarization in the absence of IL-1R1 signal.
DISCUSSION
In the present study, we addressed the role of IL-1 in the Th17 lineage differentiation pathway. We found that Th17 cells expressed higher levels of IL-1R1 mRNA, which was dependent on STAT3, RORα and RORγt. IL-1R1-deficient CD4+ T cells failed to induce EAE, which was associated with a selective defect in IL-17-producing T cells and an accumulation of Treg cells in the inflamed tissue. IL-1R1 signal was required for the early differentiation of Th17 cells and conversion of Foxp3+ T cells into IL-17-producing cells. After polarization, IL-1 also allowed Th17 cells to maintain their cytokine secretion profile. The IL-1R1 signaling in T cells functioned by upregulating IRF4 and RORγt.
IL-1 is a pleiotropic cytokine with many target cells. Although IL-1 was shown previously to be important in Th17 generation in vivo, its exact action has not been understood. Higher IL-1R1 expression on Th17 cells has been observed in the SKG mouse strain that spontaneously develops arthritis (Hirota et al., 2007). On the other hand, IL-1R1 expression on DC has been reported to be critical for onset of autoimmune myocarditis (Eriksson et al., 2003), a Th17-mediated autoimmune disorder (Rangachari et al., 2006). In our study, we clearly demonstrated that IL-1R1 expression in CD4 T cells but not on DC is critically required for Th17 generation in vitro. Moreover, Rag1−/− mice receiving IL-1R1 KO CD4 T cells displayed significantly attenuated EAE compared with recipients of WT T cells. Taken together, these observations indicate that IL-1 responsiveness in T cells is required for Th17 cell development and Th17-mediated autoimmunity.
The regulation of IL-1R1 expression has not been well understood. In the current study, we show that although TcR/costimulation activation resulted in elevated IL-1R1 mRNA expression, IL-6, but not IL-23 or TGFβ, serves a unique role to further enhance its expression. Consistent with this observation, IL-1 only functioned in the presence of IL-6 in upregulating Th17-specific genes. IL-6 is an essential Th17 priming cytokine- it can not only initiate Th17 differentiation and downregulate Foxp3 expression, but also has been shown to allow T cells to produce IL-21 (Nurieva et al., 2007; Zhou et al., 2007) and respond to IL-1 and IL-23 (Yang et al., 2007; Zhou et al., 2007). Interestingly, naïve T cells stimulated with IL-6, IL-23 and IL-1 can be differentiated into Th17 cells in the absence of exogenous TGF-β. Although this type of Th17 differentiation is still dependent on TGF-β, most likely from T cell itself or contained in serum, it led to greater IL-22 expression than those polarized by only TGF-β and IL-6. This finding suggests flexibility of Th17 differentiation. As previously shown in the literature, Th17 cells can be generated in the presence of IL-6 and relatively high concentrations of TGF-β which could be found at sites such as the intestine or tumor microenvironment On the other hand, with low TGF-β concentrations but with strong innate responses, IL-1, along with the other proinflammatory cytokines, can also drive Th17 polarization. This idea is supported by the early defect of Th17 differentiation in vivo in the absence of IL-1 signaling. However, the function and regulation of Th17 generated by two different conditions remain to be understood. Interestingly, IL-1 induces IL-22 expression in Th17 cells differentiated in the presence of exogenous TGFβ suggesting the plasticity of IL-22 expression. Most importantly, our results also indicate that similar to human Th17 cells, mouse Th17 cells can be developed in the presence of pro-inflammatory cytokines IL-6, IL-1 and IL-23 in the absence of exogenous TGF-β.
IL-23 has been suggested to support Th17 expansion and maintenance (Veldhoen et al., 2006). In the present study, we observed that IL-1 regulates early Th17 cell differentiation and maintenance of polarized effector Th17 cells. For the latter, IL-1 may have an overlapping function with IL-23. However, IL-23 alone had little effect on Th17 cells in the absence of TCR stimulation whereas the combination of IL-1 and IL-23 greatly synergized to expand Th17 cells and maintain their cytokine profiles. As IL-1R1 KO T cells expressed lower levels of IL-23R, IL-1 may function to enhance the IL-23R expression on Th17. Nevertheless, the expansion and cytokine production of Th17 cells by IL-1 in the absence of TcR stimulation suggests an important role of IL-1 in the homeostatic maintenance of Th17 cells. Since tissue inflammation induces abundant IL-1 and IL-23 expression, it would be interesting to surmise that Th17 cells that are not antigen-specific in inflamed tissue might expand and produce Th17 cytokines in a TCR-independent manner, and thus aggravates tissue damage. Further in vivo study will be needed to clarify the role of inflammatory cytokines on Th17 cells.
A recent study demonstrates that IRF4 is necessary for Th17 differentiation; IRF4-deficient T cells fail to produce IL-17 in Th17 conditions but produces IFNγ in Th1 conditions (Brustle et al., 2007). Moreover, overexpression of RORγt in IRF4 KO T cells only partially restores IL-17 production (Brustle et al., 2007). The physiological factor(s) regulating IRF4 induction during Th17 polarization has been unclear. In the present study, we utilized DC/T cell coculture system and observed a severe defect in IRF4 expression in IL-1R1 KO T cells compared to WT T cells. Overexpression of IRF4 or RORγt alone partially overcame Th17 polarization in IL-1R1 KO. Interestingly, coexpression of these two transcription factors enhanced Th17 polarization in IL-1R1 KO but not in WT T cells. Therefore IL-1 from activated antigen-presenting cells delivers signals to T cells to upregulate IRF4 and RORγt during their early lineage programming and to sustain their differentiation. The present study unveiled IL-1 as a critical factor for inducing IRF4 in CD4+ T cells during Th17 polarization. Lack of an effect after IRF4 overexpression in WT cells is probably because IRF4 levels in WT T cells after stimulation by TcR and DC was sufficient for Th17 polarization.
The balance between Treg and Th17 is important for the generation of immunity against extracellular bacteria and regulating autoimmunity. Recent studies by our own group and others showed physical interaction between Foxp3 and RORγt or RORα(Yamazaki et al., 2008; Yang et al., 2008b; Zhou et al., 2008). Of note, Foxp3+ cells can produce IL-17 in response to inflammatory stimuli. In the mixed bone-marrow chimera study, we observed higher percentage of Foxp3+ T cells in the IL-1R1 KO population compared with the WT population in the inflamed CNS. More importantly, about 10 % of Foxp3+ WT T cells in CNS and spleen expressed IL-17 whereas nearly no Foxp3+ KO T cells expressed IL-17. It has been reported that Foxp3+ IL-17F+ cells are much less efficient in suppressing the activation of naïve T cells (Yang et al., 2008b). Therefore the IL-1R1 signal is critically required for Foxp3+ T cells to produce IL-17. However, IL-1 did not affect the downregulation of Foxp3 or the TGF-β-induction of Foxp3, indicating that the mechanism of IL-1-mediated IL-17 expression is distinct from that of IL-6. IL-1R1 delivers signal through the recruitment of MyD88 and TRAF6 leading to the activation of NF-κ B and MAPK pathways, which is distinct from IL-6 and IL-23 signaling. This may explain the synergism of IL-1 with the other Th17-inducing factors and their different effects on Foxp3, RORα and RORγt. Ahr is recently discovered as a critical transcription factor for both Treg and Th17 cells. Of note, we observed higher Ahr expression in the IL-1R1 KO T cells, suggesting inhibitory role of IL-1 on Ahr. The precise regulation by IL-1 signaling on Ahr expression remains to be determined.
The present study unveiled the critical roles of IL-1 during early Th17 differentiation and expansion/maintenance. Our results may explain the molecular mechanism of the spontaneous arthritis onset in IL-1Ra-deficient mice, which is a Th17-mediated autoimmune disorder (Nakae et al., 2003). Of interest, a recent study showed that administration of ATP, a well known inflammasome activator, drives Th17 differentiation in the gut (Atarashi et al., 2008), suggesting a role of IL-1 for the generation of Th17 in the gut. Our data thus corroborate these findings. The identification of IL-1 as a key regulator of Th17 biology may provide a support for therapeutic targeting of IL-1 in autoimmune disorders.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6, OT-II, B6.SJL (CD45.1), IL-1R1-deficient and Rag1−/− mice were purchased from Jackson Laboratory. Foxp3-gfp reporter mice were generously provided by Dr. Rudensky (University of Washington) (Fontenot et al., 2005). IL-1R1-deficient and OT-II mice were bred to yield IL-1R1−/−OT-II mice. Heterozygous Staggerer (Rorasg/+) mice and Rorasg/sgRorc−/− mice were obtained as described (Yang et al., 2008c). Stat3 fl and Tie2-Cre mice were bred to yield f/Δ Cre+ as described (Panopoulos et al., 2006; Yang et al., 2007). The animal experiments were performed at the age of 6–10 weeks with protocols approved by Institutional Animal Care and Use Committee.
Adoptive transfer and mixed bone-marrow chimera studies
CD4 T cells from C57BL/6 or IL-1R1-deficient mice were sorted by AutoMacs and intravenousely transferred into Rag1−/− mice (7×106 cells/transfer). One day later, the recipient mice were induced EAE. To generate mixed bone-marrow chimera, T cell-depleted bone-marrow cells were obtained from C57BL/6 or IL-1R1-deficient mice and mixed 1:1 ratio before transferred into irradiated Rag1−/− mice (10×106 cells/transfer, 750 rad). Eight weeks later, the recipient mice were induced EAE.
In some experiments, we isolated naïve CD4+ T cells from either wild-type OT-II or IL-1R1-deficient OT-II mice (CD45.2) and transferred into congenic mice (CD45.1). The recipients were immunized with OVA323-339 peptide in CFA. Three days later, lymphoid cells from the draining lymph nodes of the recipients were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of Golgi-stop for 4 hr, after which CD45.2+ CD4+ cells were analyzed for intracellular IL-17- and IFN-γ.
EAE induction
Mixed bone-marrow chimeras and CD4+ T cell reconstituted Rag1−/− mice were immunized subcutaneously at the dorsal flanks with 150 μg of MOG peptide emulsified in CFA at day 0 and day 7. Pertussis toxin was given intraperitoneally at day 1 and day 8 (500 ng/injection). Signs of EAE were assigned scores on a scale of 1–5 as follows: 0, none; 1, limp tail or waddling gait with tail tonicity; 2, wobbly gait; 3, hind-limb paralysis; 4, hind-limb and forelimb paralysis; 5, death. Disease incidence and scores were measured daily. For analysis of central nervous system infiltrates, both brain and spinal cord were collected from perfused mice, and mononuclear cells were prepared using 37% percoll gradient.
T cell differentiation
Dendritic cell and T cell coculture system was adopted from previous study (Veldhoen et al., 2006). Briefly, naive CD4+CD25− CD62LhiCD44lo T cells from C57BL/6 and IL-1R1-deficient mice were FACS sorted and activated with 0.2 μg/ml anti-CD3 Ab and bone-marrow derived DCs in the presence or absence of 1 ng/ml TGF-β (Peprotech), 100 ng/ml LPS. For anti-CD3/CD28 stimulated differentiation, naive CD4+ T cells were activated with plate-bound 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28 and in the presence of 5 ng/ml TGF-β (Peprotech), 40 ng/ml IL-6 (Peprotech), 50 ng/ml IL-23 (R&D systems), 5 μg/ml anti-IL-4 (11B11), 5 μg/ml anti-IFN-γ (XMG 1.2), 10 ng/ml IL-1β (Peprotech), 10 ng/ml IL-1α(Peprotech) or combination of these stimuli (in some experiments, 10 μg/ml of anti-IL-2 Ab or anti-TGF-β Ab was used). Four days after activation, cells were washed and restimulated with plate-bound anti-CD3 (2μg/ml) overnight and cytokines in the supernatant were measured by ELISA. For intracellular staining, cells were stimulated with PMA and ionomycin in the presence of Golgi-stop for 4 hr, after which IL-17- and IFN-γ-producing cells were analyzed with intracellular staining. Intracellular staining for Foxp3 or T-bet was performed with a Foxp3 staining kit (eBioscience) and PE-conjugated anti-T-bet Ab (Santa Cruz).
Quantitative real-time RT-PCR
Total RNA was prepared from T cells with TriZol reagent (Invitrogen). Complementary DNA (cDNA) was synthesized with Superscript reverse transcriptase and oligo(dT) primers (Invitrogen), and gene expression was examined with a Bio-Rad iCycler Optical System with iQTM SYBR green real-time PCR kit (Bio-Rad Laboratories). The data were normalized to Actb reference. The following primer pairs were used: IL-1R1; forward, 5′-TGGAACAGAGCCAGTGTCAG-3′, reverse, 5′-CAGGAGAAGTCGCAGGAAGT-3′, IL-1RAcP; forward, 5′-TTGCCACCCCAGATCTATTC-3′, reverse, 5′-CCAGACCTCATTGTGGGAGT-3′, IRF4: forward, 5′-TCCTCTGGATGGCTCCAGATGG-3′, reverse, 5′-CACCAAAGCACAGAGTCACCTG-3′, TGF-β 1; forward, 5′-GCAACATGTGGAACTCTACCAGA-3′, reverse, 5′-GACGTCAAAAGACAGCCACTCA-3′. The primers for Ifng, Il17, Il17f, Il23r, Il22, Il21, Rora, Foxp3, Rorc, Tbx21, and Actb were previously described (Nurieva et al., 2007; Yang et al., 2008c).
Retroviral transduction
Genes encoding IRF4 (GenBank accession number NM 013674) and RORγt (GenBank accession number AJ132394) were cloned into bicistronic retroviral vector pGFP-RV (Ouyang et al., 1998) or pMIG-hCD2 (Deftos et al., 1998) containing IRES-regulated GFP and human CD2, respectively. Naive CD4+CD25−CD62LhiCD44lo T cells from C57BL/6 and IL-1R1-deficient mice were FACS sorted and activated with 0.2 mg/ml anti-CD3 and bone-marrow derived DCs in the presence or absence of 1 ng/ml TGF-β (Peprotech) and 100 ng/ml LPS (Veldhoen et al., 2006). Twenty-four hours after activation, the cells were infected by retroviruses expressing IRF4-GFP, RORγt-GFP or control empty vector (containing only IRES-GFP). Four days after infection, the cells were restimulated with PMA and ionomycin in the presence of Golgi-stop for 4 hr, after which IL-17- and IFN-γ-producing cells were analyzed with intracellular staining on a GFP+ gate. Coinfection was performed with two bicistronic retroviruses expressing IRF4 or empty vector (both have IRES-GFP) and RORγt or control vector (both with IRES-hCD2). The analysis was performed on a GFP+hCD2+ gate. RORα and RORγt single or double infection was performed as described (Yang et al., 2008c).
Statistics
The Kaplan-Meier method was used to determine the EAE incidence in CD4+ T cell transfer models, and the log-rank test was used for statistical analysis. The Student t test was used to assess all other statistical values. P values were determined, and error bars represent standard error of the mean (SEM).
Supplementary Material
Acknowledgments
We thank Dr. Alexandar Rudensky for Foxp3-GFP reporter mice, Zhiwei He and Karen Ramirez for helping cell sorting, and the Dong lab for their help. The work is supported by research grants from NIH (to CD), an Intramural Research Program of the NIEHS, NIH (to AMJ), Leukemia and Lymphoma Society (to CD) and MD Anderson Cancer Center (to CD and SSW) and the Gillson Longenbaugh Foundation (to SSW). RN is a recipient of a Scientist Development Grant from the American Heart Association. CD is a Trust Fellow of the MD Anderson Cancer Center, a Cancer Research Institute Investigator, a Leukemia and Lymphoma Society Scholar and an American Lung Association Career Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- Deftos ML, He YW, Ojala EW, Bevan MJ. Correlating notch signaling with thymocyte maturation. Immunity. 1998;9:777–786. doi: 10.1016/s1074-7613(00)80643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Eriksson U, Kurrer MO, Sonderegger I, Iezzi G, Tafuri A, Hunziker L, Suzuki S, Bachmaier K, Bingisser RM, Penninger JM, Kopf M. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J Exp Med. 2003;197:323–331. doi: 10.1084/jem.20021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lohoff M, Mittrucker HW, Prechtl S, Bischof S, Sommer F, Kock S, Ferrick DA, Duncan GS, Gessner A, Mak TW. Dysregulated T helper cell differentiation in the absence of interferon regulatory factor 4. Proc Natl Acad Sci U S A. 2002;99:11808–11812. doi: 10.1073/pnas.182425099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, Liu F, Goldsmith MA, Link DC, Murray PJ, Watowich SS. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- Rangachari M, Mauermann N, Marty RR, Dirnhofer S, Kurrer MO, Komnenovic V, Penninger JM, Eriksson U. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J Exp Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Mowen KA, McBride KD, Smith ED, Singh H, Glimcher LH. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J Exp Med. 2002;195:1003–1012. doi: 10.1084/jem.20011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008a;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008b;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008c;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.