Abstract
Background
The mean urine BK viral load in kidney transplant recipients increases with the intensity of infection as the infection progresses from transient viruria to sustained viremia.
Objectives
This study investigated whether the intensity of infection is associated with the humoral immune response.
Study design
We measured BKV-specific IgG antibody titers in stored samples obtained serially over a 1 -year period from 70 kidney transplant recipients with BKV infection and 17 control recipients without active BKV infection.
Results
The mean pre-transplant BKV antibody level was lower in recipients who developed viremia than the mean level in those who never developed viremia (p = 0.004). Mean antibody titers in recipients who never showed evidence of active BKV infection rose slightly after transplant despite immunosuppression. The magnitude of the rise in the mean antibody titers in recipients who developed active BKV infection correlated with the intensity of infection (p < 0.001).
Conclusions
The mean antibody level increased in accordance with the intensity of the infection post-transplant. Pre-transplant seropositivity did not protect against sustained viremia and the antibody response was not associated with clearance of the virus.
Keywords: Polyomavirus, BK virus, BK-antibody, BK-viremia, Humoral immunity, Transplantation, Kidney
1. Introduction
BK virus (BKV), a human polyomavirus, causes nephropathy (BKN) and allograft loss in renal transplant recipients. Although it was discovered in 1971,1 understanding of the humoral immune response to BKV is limited.
By age 10, 90% of children have antibodies to BKV.2,3 After primary infection, BKV remains latent in the uroepithelium, but can reactivate after transplant and cause allograft dysfunction.4
The significance of the donor or the recipient BKV antibody serostatus is unclear. In pediatric kidney transplant recipients, a negative recipient serostatus was a risk factor for BK viruria5, and nephropathy.6 This is less clear in adults.7–9 In 1984, Gardner10 reported that the antibody titer was initially low, rose greater than 100-fold, and persisted in 6 kidney transplant recipients in whom BK virus was isolated by urine culture, suggesting that the antibody was non-neutralizing. In 2005, Hariharan11 showed that BKV-specific IgG, but not IgM, levels increased in 6 recipients with BKN after reduction in immunosuppression and clearance of viremia. They did not investigate titers from those without active BK infection or those without nephropathy.
We showed that the mean urine BK viral load in 70 kidney transplant recipients increases as the infection progresses from no infection, to transient viruria, sustained viruria, transient viremia, and finally sustained viremia.12 To investigate whether the intensity of infection is associated with the humoral immune response, we evaluated BKV-specific IgG levels, pre-transplant and serially post-transplant in renal transplant recipients with and without BKV infections (controls). Serial plasma samples had been tested by quantitative PCR for BK virus DNA, allowing us to compare the anti-BKV-antibody level with the BKV-viral load and correlate it with the overall severity of infection.
2. Methods
2.1. Subjects and samples
Two hundred renal transplant recipients were enrolled in a trial in which urine and blood samples were obtained weekly for 16 weeks and at months 5, 6, 9, and 12 after transplantation.12 Samples were analyzed by real-time PCR for BKV DNA. Urine, plasma, and whole blood samples and extracted DNA from the samples were stored at −70°C. Recipients were defined as having active BKV infection if any specimen were positive for BKV by PCR. Clinical characteristics and outcomes, BKV DNA levels, and baseline donor and recipient serology have been reported previously.8,12 The Washington University Human Research Protection Office approved the trial, and allowed for additional testing.
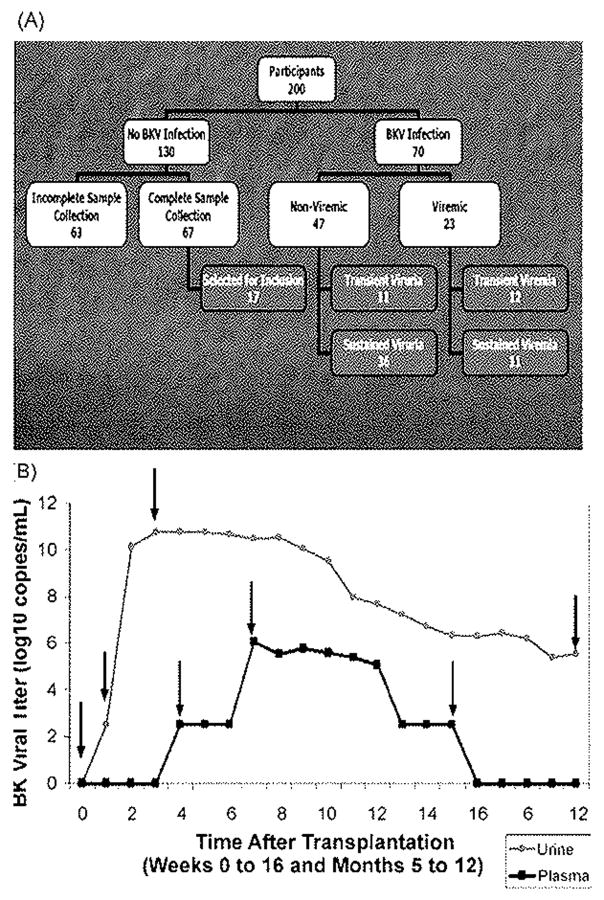
BKV antibody testing was performed on serial plasma samples from all 70 recipients with BKV infections determined by PCR and from a subset of 17 recipients without evidence of active BKV infection (Fig. 1A). These were selected from 130 subjects without evidence of active post-transplant BKV infection, after excluding those from whom all necessary plasma samples were not available (n = 63). To avoid selection bias, we selected every fourth subject from the remaining 67 recipients, based on the date of transplantation. For the purposes of analysis, BKV infections were divided into “viremic” and “non-viremic”, and further divided into transient and sustained infections. The term “sustained” was defined as BKV infection with ≥2 consecutive BKV positive samples spanning ≥3 weeks. The number of patients in each group was: transient viruria (n = 11), sustained viruria (n = 36), transient viremia (n = 12), and sustained viremia (n = 11). These categories represent increasing intensity of infection.12 Those with transient viruria had the least intense infection, with the latest onset, the shortest duration, and the lowest urine BKV DNA levels. In contrast, those with sustained viremia had the earliest onset, the longest duration, and the highest BKV DNA levels.
Fig. 1.
Selection of subjects and specimens for testing. Panel A shows selection of subjects from the 200 participants in the original clinical study. Panel B shows the time course in a representative patient with sustained viremia to display the viral events which were used to select plasma samples for BKV antibody testing. BKV antibody levels were tested pre-transplantation, at the onset of viruria, peak urine viral DNA level, onset of viremia, peak plasma viral DNA level, last episode of viremia, and 1 year. In this recipient, the last episode of viruria and 1 year sample were identical.
Antibody titers were measured in plasma samples taken pre-transplant and at the time of specific viral events (Fig. 1B): onset of EK viruria, peak urine viral level, last detection of viruria, and last available sample (usually at 1 -year post-transplant). In viremic recipients, titers were also measured at the onset of viremia, peak plasma viral level, and last episode of viremia. Because recipients with transient infections typically had only one sample positive for BKV DNA, BKV antibodies were measured only at the onset of infection. In the 17 recipients without active BKV infection, titers were measured pre-transplantation and at 1, 3, 6, and 12 months post-transplant. Of 458 possible samples from 87 recipients, 447 (97.6%) were available and BKV-specific IgG antibody titers were determined.
2.2. BKV IgG enzyme Immunoassay
BKV-specific IgG antibodies were measured using virus-like particles (VLP) made by expressing BKV VP1 proteins in baculovirus vectors in insect cells and then preparing the VLPs for use in the ELISA as previously described.13,14 Starting at a 1:40 dilution, samples were diluted in plate wells in serial 4-fold increments. The dilution was considered positive if the spectrophotometer absorbance was 0.05 greater than that of appropriate serum controls. Recipients with antibody titers ≥ 1:2560 were considered to be seropositive to eliminate concerns for cross-reactivity with SV40 or JC. Accordingly controls included VLP’s for SV40 and JC virus.
2.3. Statistical analysis
For analysis, BKV IgG antibody titers were expressed as the dilutional index (DI) where DI = −log4 (Ab titer × 10). For example, antibody14 titers of 1/40, 1/160, 1/640, and 1/2560 correspond to 1,2,3, and 4 DI, respectively, as previously described.8 Paired sample t-tests were used for comparison of antibody titers within a group. Independent sample t-tests or ANOVA were used for comparisons between groups. Pearson’s correlation coefficient was calculated to determine associations between interval data, while Spearman’s product-moment correlation coefficient was used for ordinal data. Associations between categorical data were assessed using Pearson χ2 test. To test for linear trends, the Mantel-Haenszel test for linear association was used for ordinal data and ANOVA test of linearity for interval data. For all tests, two-tailed α ≤ 0.05 was considered significant. For a significant ANOVA test of linearity, deviation from linearity was always non-significant, p > 0.05. Variables significantly correlated with the change in BKV antibody titer (1-year DI titer minus pre-transplant DI titer) were entered into a multivariate linear regression analysis using both a forward and backward entry method with p < 0.05 required for entry and p > 0.1 for removal. All statistical calculations were performed using SPSS 13.0 (Chicago, IL). Standard deviations are presented with mean values. Patients were analyzed only according to their initial triple immunosuppression regimen because dose adjustments or medication changes due to complications such as neutropenia (3%), CMV (4.5%), or rejection (5%) were rare.
3. Results
3.1. Subjects and samples
The demographic characteristics were similar among those without evidence of active BKV infection post-transplant and those with any of the four intensities of BKV infection post-transplant (Table 1).
Table 1.
Baseline demographics
| No BKV infection (n = 17) | Transient viruria (n = 11) | Sustained viruria (n = 36) | Transient viremia (n = 12) | Sustained viremia (n = 11) | pa | |
|---|---|---|---|---|---|---|
| Age (years ± S.D.) | 52.7 ±13.9 | 38.2 ± 11.2 | 46.9 ± 13.7 | 46.3 ± 12.2 | 52.1 ± 15.2 | 0.06 |
| Sex(%) | ||||||
| Male | 35.3 | 72.7 | 63.9 | 50 | 72.7 | NS |
| Race(%) | ||||||
| Caucasian | 88.2 | 72.7 | 75.0 | 83.3 | 81.8 | NS |
| Black | 11.8 | 27.3 | 25.0 | 8.3 | 18.2 | |
| Other | 0 | 0 | 0 | 8.3 | 0 | |
| ESRD (%)b | ||||||
| DM | 41.2 | 36.4 | 25.0 | 25 | 27.3 | |
| HTN | 5.9 | 27.3 | 19.4 | 8.3 | 27.3 | |
| PKD | 5.9 | 9.1 | 8.3 | 33.3 | 0 | NS |
| FSGS | 11.8 | 0 | 5.6 | 0 | 9.1 | |
| GN | 0 | 9.1 | 13.9 | 8.3 | 27.3 | |
| Other | 35.5 | 18.2 | 19.4 | 25.0 | 9.1 | |
| Type of transplant(%) | ||||||
| Deceased | 52.9 | 54.5 | 58.3 | 50 | 81.8 | NS |
| Living | 47.1 | 45.5 | 41.7 | 50 | 18.2 | |
| Calcineurin inhibitor (%) | ||||||
| Tacrolimus | 88.2 | 54.5 | 75 | 50 | 90.9 | 0.06 |
| Cyclosporine | 11.8 | 45.5 | 25 | 50 | 9.1 | |
| Total HLA mismatch (±S.D.) | 2.65 ± 1.73 | 2.18 ± 1.54 | 3.22 ± 1.66 | 2.42 ± 1.98 | 3.18 ± 1.83 | NS |
No significant differences existed for any category comparing all 130 recipients without a BKV infection to either the 17 recipients without a BKV infection selected for inclusion in the study or the three groups of recipients with a BKV infection. p-Values are for comparisons among the 87 recipients included in this study.
ESRD, end stage renal disease; DM, diabetes mellitus; HTN, hypertension; PKD, polycystic kidney disease; FSGS, focal and segmental glomerulosclerosis; and GN, glomerulonephritis.
3.2. BKV-specific antibody responses by type of BKV infection
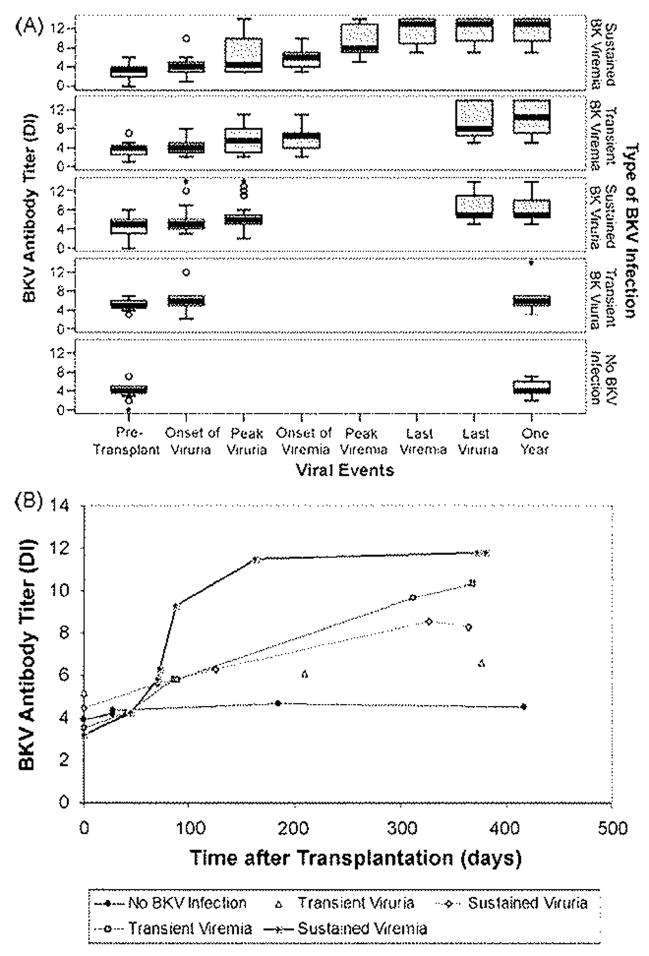
Pre-transplant, the mean antibody titers were lower in those who subsequently developed viremia compared to those who developed viruria without ever developing viremia (DI: 3.36 ± 1.70 vs. 4.64 ± 1.57, p = 0.004). Post-transplant, the mean BK antibody titers increased throughout the first post-transplant year in all groups (Fig. 2). The increase was significant even in the no BKV infection group, despite the use of immunosuppression (mean antibody change, 0.59 ± 1.00 DI, p = 0.028).
Fig. 2.
Post-transplant BKV antibody response related to virologic events (panel A) and time after transplant (panel B). In panel A, transplant recipients are divided according to type of post-transplant BKV infection. For the group of recipients with each type of infection, the boxes summarize the BKV antibody levels at the time of specified virologic events during the course of post-transplant BKV infection. Recipients with no BKV infection are also included. Each box summarizes all antibody measurements in the group at the time of the specified viral event. Median values are indicated by thick black lines. Boxes represent the interquartile range. Horizontal lines connected from above and below the hinges by whiskers represent the most extreme values within 1.5 times the interquartile range. Circles represent outliners, and asterisks represent extreme outliers. In panel B, each line represents one of the types of BKV infection. Each point represents the mean time of occurrence after transplantation of one of the virologic events shown in panel A and the mean BKV antibody titer at the time of that event. The points are connected by straight lines.
Post-transplant, the mean antibody titer increased corresponding to the intensity of infection. Recipients with viruria alone had a modest increase in the mean BKV antibody titer that persisted after the onset of viruria (mean antibody change 2.34 ± 2.62 DI, p < 0.001). Recipients who developed viremia had modest increases in the mean BKV antibody titers during the initial period when viruria alone was present (mean antibody change from onset of viruria to onset of viremia, 1.57 ± 1.78 DI, p < 0.001). However, the mean titer increased markedly after detection of viremia and persisted at a high level for 1 year in those with either transient or sustained viremia (mean antibody change from onset of viremia to 1 year, 5.22 ± 2.78 DI, p < 0.001). The mean antibody titer for those who developed a viremia was also higher at the last episode of viruria (DI: 10.70 ± 3.5 vs. 8.02 ± 2.79, p< 0.001) and at 1 year (DI: 11.04 ± 3.13 vs. 7.91 ± 2.72, p < 0.001) compared to those with viruria alone.
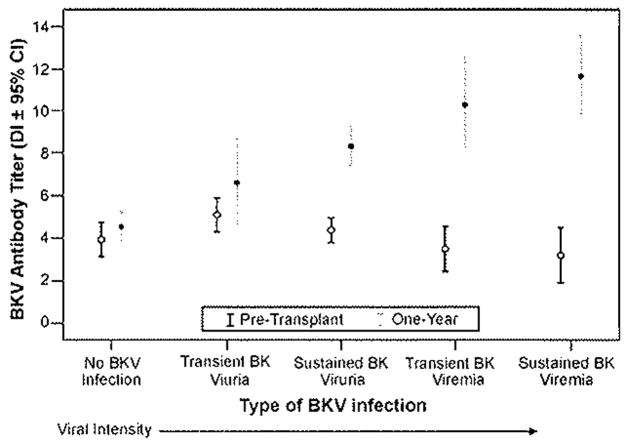
3.3. Pre-transplant and 1 year BKV-specific antibodies
There was a strong relationship between intensity of BKV infection and antibody titers (ANOVA for linear trend p < 0.001) (Fig. 3). The increase in antibody titer over the first year post-transplant was approximately 7-fold, 200-fold, 13,000-fold, and 150,000-fold for recipients with transient viruria, sustained viruria, transient viremia, and sustained viremia, respectively (Table 2). Recipients who developed BKV infection after transplantation also had higher 1-year antibody titers compared to recipients who did not develop BKV infection (DI: 8.97 ± 3.21 vs. 4.53 ± 1.38, p <0.001).
Fig. 3.
Relationship of BKV antibody levels measured by pre-transplant and at 1-year post-transplant with the intensity of BKV infection. Points represent mean antibody titers and the horizontal lines above and below represent the 95% Cl. Pre-transplant mean antibody titers differed between groups: transient viruria vs. transient viremia, p = 0.015, transient viruria vs. sustained viremia, p = 0.007, and sustained viruria vs, sustained viremia, p = 0.036. 1 year mean antibody titers differed significantly (p <0.05) between groups with two exceptions: transient viruria vs. sustained viruria, p = 0.073, and transient viremia vs. sustained viremia, p = 0.173.
Table 2.
Relationship between mean pre- and post-transplant BKV serologic parameters among patients with different types of BKV infections
| Type of BKV infection | Pre-transplant seroprevalence (%) | Pre-transplant antibody titer (DI ± S.D.)a | One year antibody titer (DI ± S.D.) | Change in antibody titer (DI, fold increase) | pb |
|---|---|---|---|---|---|
| No BKV infection | 76.5 | 3.94 ± 1.56 | 4.53 ± 1.38 | 0.59, 2.3 | 0.028 |
| Transient viruria | 90.9 | 5.18 ± 1.08 | 6.60 ± 2.88 | 1.42, 7.2 | 0.057 |
| Sustained viruria | 72.7 | 4.45 ± 1.68 | 8.29 ± 2.60 | 3.84, 205 | <0.001 |
| Transient viremia | 58.3 | 3.50 ± 1.68 | 10.33 ± 3.53 | 6.83, 12.944 | <0.001 |
| Sustained viremia | 50.0 | 3.20 ± 1.81 | 11.82 ± 2.56 | 8.62, 154.795 | <0.001 |
DI, dilutional increment, S.D., standard deviation.
Comparison of pre-transplant and 1 year antibody titer using paired sample t-test.
Demographic, immunologic, and viral factors were assessed for association with the change in BKV antibody titer over the first year in all recipients and separately in those recipients who developed active BKV infection post-transplant (Table 3). In the univariate analysis of all recipients, type of BKV infection, pre-transplant recipient BKV antibody serostatus and titer, donor BKV antibody serostatus and titer, donor and recipient BK-serology, duration of viruria and viremia, peak BKV urine and plasma PCR levels, and donor gender were associated with an increase in BKV antibody titer. In multivariate analyses, only the type of BKV infection remained significant. When the analysis was restricted to recipients having active BKV infection after transplant (viruria or viremia), type of BKV infection, peak BKV urine and plasma PCR levels, duration of viruria and viremia, pre-transplant BKV antibody serostatus and titer, and recipient age were associated with the post-transplant rise in mean BKV antibody titer in the univariate analysis. The multivariate analysis of this group showed that only the intensity of BKV infection and pre-transplant BKV antibody titer remained significant.
Table 3.
Factors associated with a rise in BKV antibody titers during the first post-transplant year
| Factorsa | Univariate correlation |
Multivariate regression |
||
|---|---|---|---|---|
| Rb | p | Bb | p | |
| All recipients | ||||
| Type of BKV infection | 0.747 | <0.001 | 1.92 | <0.001 |
| Peak plasma level | 0.629 | <0.001 | ||
| Peak urine level | 0.593 | <0.001 | ||
| Duration of viremia | 0.485 | <0.001 | ||
| Duration of viruria | 0.440 | <0.001 | ||
| Pre-transplant BKV Ab titer (DI) | −0.386 | <0.001 | ||
| Pre-transplant BKV serostatus | −0.373 | <0.001 | ||
| Donor BKV Ab status | 0.477 | 0.004 | ||
| Donor BKV Ab titer(DI) | 0.382 | 0.023 | ||
| Donor-recipient serostatus | 0.374 | 0.027 | ||
| Donor sex | −0.220 | 0.048 | ||
| Recipients with active BKV infections | ||||
| Type of BKV infection | 0.639 | <0.001 | 1.98 | <0.001 |
| Peak plasma level | 0.588 | <0.001 | ||
| Pre-transplant BKV Ab titer (DI) | −0.498 | <0.001 | −0.65 | 0.004 |
| Duration of viremia | 0.452 | <0.001 | ||
| Peak urine level | 0.431 | <0.001 | ||
| Pre-transplant BKV serostatus | −0.426 | <0.001 | ||
| Recipient age | 0.279 | 0.025 | ||
| Duration of viruria | 0.261 | 0.037 | ||
Variables considered in univariate model included: recipient and donor age, sex, CMV serostatus, and HLAC7 status; ESRD diagnosis; type of transplant; HLA A, B, DR, and total mismatch; pre-transplant donor and recipient BKV serostatus and antibody titer; type of calcineurin inhibitor; adjuvant immunosuppressive agent; immunosuppression combination; and placement of a ureteral stent. Additional variables considered for recipients who developed a BKV infection included time to onset of viruria, peak viruria, viremia, and peak viremia; duration of viruria and viremia; and peak urine and plasma BKV DNA levels.
R is the Spearman’s p or Pearson correlation coefficient determined for univariate analysis, and B is the regression coefficient from the linear regression model.
3.4. Antibody titer and clearance of BKV infections
Despite the increases in mean antibody titer at 1 year after transplantation, BKV DNA was present in the urine of 4 (36.4%) recipients with transient viruria, 28 (77.8%) recipients with sustained viruria, 8 (66.7%) recipients with transient viremia, and 10 (90.2%) recipients with sustained viremia. In contrast, viremia cleared by 1 year in 22 (96%) of the 23 recipients with viremia.
4. Discussion
The present study is the most extensive comparison to date of serial BKV-specific antibody measurements in renal transplant recipients. A strong point of the study is the comparison of the changes in antibody titers with serial quantitative measurements of BKV DNA in urine and plasma from the same patients. A clear finding is that the BKV antibody response correlated with the intensity of infection as assessed by urine viral load. BKV antibody titers reached higher levels at 1 year after transplantation in recipients with viremic compared to non-viremic infections, and the highest antibody levels were seen in recipients with sustained viremia. Our analysis of prospectively obtained samples supports a cross-sectional study by Randhawa et al.15 that showed that renal transplant recipients with BKV viruria or viremia had higher IgG BKV-specific antibody levels than those without an infection. A third finding of our study is that the mean pre-transplant BKV antibody levels were lower in recipients who eventually developed viremic BKV infection than in those in whom only viruria developed. A fourth finding is that even seropositive-recipients developed sustained viremia. Finally, viruria persisted at 1 year despite high plasma antibody levels.
We previously showed that the level of antibody in the donor in an ordered fashion predicts the development of BKV infection better than level in the recipient.8 We also showed that seropositivity in the recipient conferred a small incremental risk of infection in transplants from either seropositive or seronegative donors.8 The present study adds to these previous findings and shows that recipient seropositivity may not prevent infection but may mitigate the severity of the infection.
The design of the clinical study complicates the interpretation of the BKV-antibody response. The onset of viremia, but not viruria, led to discontinuation of adjuvant immunosuppression, which could have allowed for the increase in the antibody response among viremic patients. However, significantly increased antibody titers were detected at the time of onset of viremia prior to discontinuation of the antimetabolite. And, even when the antimetabolite was continued among those who were only viruric, the antibody response in the recipients with sustained viruria was greater than in those with transient viruria. These observations support that the increase in antibody titer is mainly in response to the intensity of the infection rather than from discontinuation of the antimetabolite.
Because BK, JC, and SV40 share 70% DNA sequence homology, there is a theoretical possibility of cross-reactivity with our assay. The assay used has minimal cross-reactivity for JC or SV40,13,14 and JC and SV40 viremia are extremely uncommon in transplant patients. Furthermore, the anti-BK antibody response rose with the BK viral load and correlated with the intensity of BK infection.
The presence of BKV-specific antibodies as measured by ELISA was not associated with prompt clearance of viremia or viruria. The ELISA assay used is a binding assay that does not measure virus neutralization. Nevertheless, these results may limit enthusiasm for the use of intravenous immunoglobulin (IVIG), which has been suggested as a possible therapeutic agent for the treatment of post-transplant BKV infection.16
Interestingly, BKV-specific antibody titers increased over the first post-transplant year in all groups and were unrelated to the initial immunosuppressive medication or combination. Our control group of patients with no infection was small by design but of sufficient size to show the differences between those who did and not develop infection. Inclusion of controls such as ours has not been done in other analyses of the humoral response to BK. The reason for the small rise in BKV-antibody titer in the recipients without a BKV infection is unclear. One might expect antibody titers to be reduced since mycophenolate mofetil has been shown to suppress the humoral immune response while azathioprine tended to a lower effect.17–19
While our earlier study demonstrated a strong effect of donor serostatus on occurrence and intensity of infection,8 the present study suggests that recipient BKV antibody titer also may affect the intensity of infection. Taken together, it appears that post-transplant BKV infection is most likely to occur when both donor and recipient are seropositive, especially if the donor has a high titer. Severe post-transplant BKV infection appears most likely in those recipients whose pre-transplant BKV antibody titer is low. We surmise that humoral immunity is associated with incomplete protection. Some studies have shown that seronegativity is associated with increased infection but others have not. A naïve immune system is less able to mount a vigorous anti-viral response to primary infection. However, we reported a 31% rate of viruria in BKV seropositive organs transplanted into seronegative transplant recipients and a 25% rate among seronegative donor organs transplanted into seropositive recipients.8 Ginevri et al.5 initially reported that recipient seronegative status conferred a significant risk of viral reactivation (58%) in relation to seropositive recipients (21%). This would support that the donor is a significant source of the infection, and donor virus is being reactivated and recipient seropositivity provides incomplete protection. However, they could not confirm this in a larger follow-up study.20
Our findings imply that measurement of BKV antibodies can be used as a means of monitoring post-transplant BKV infection. We have used this to consider patients for re-transplantation who have lost their transplant from BK when there is a choice of donors. Thus we prefer to select a donor with a low-titer anti-BK antibody in preference to one with a higher titer and to determine whether the recipient has developed anti-BK antibodies, although recipient seropositivity may be incompletely protective. We do not propose this for routine clinical use because monitoring can be accomplished more readily with real-time PCR measurements of viral load in the plasma. We suggest, however, that determination of BKV titers of donors and recipients may help to risk-stratify transplant recipients. Our study, demonstrates that the humoral response offers incomplete protection and does not result in viral clearance. Adequate control of BK may result from the cellular immune response as suggested recently.5,21,22
Acknowledgments
This work was supported in part by NIH 1 K24-02886 (D.C.B.) and NKF 22 3062 38053 (D.L.B.). D.L.B. is a recipient of the 2004 Amgen Renal Fellowship Award.
Abbreviations
- BKV
BK virus
- BKN
BK nephropathy
- PCR
polymerase chain reaction
- IgG
immunoglobulin G
- VLP
virus-like particles
- ELISA
enzyme-linked immunosorbent assay test
- DI
dilutional index
- SPSS
statistical package of the social sciences
- IVIG
intravenous immunoglobulin
Footnotes
Conflict of interest
1. D.L. Bohl, G.A. Storch, C. Ryschkewitsch, M. Gaudreault-Keener, and E.O. Major have no conflicts of interest.
2. D.C. Brennan grant support–Astellas, Novartis, Pfizer, Wyeth, and Genzyme. D.C. Brennan is a consultant for Pfizer, Wyeth, and Genzyme and an honorarium recipient from Genzyme.
References
- 1.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (BK) isolated from urine after renal transplantation. Lancet. 1971;1:1253–7. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 2.Knowles WA, Khalili K, Stoner G. Human polyomaviruses; molecular and clinical prospective. New York: Wiley-Liss Inc; 2001. The epidemiology of BK virus and the occurrence of antigenic and genomic subtypes ; pp. 527–560. [Google Scholar]
- 3.Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown DW, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71:115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3:611–23. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 5.Ginevri F, De Santis R, Comoli P, Pastorino N, Rossi C, Botti G, et al. Polyomavirus BK infection in pediatric kidney-allograft recipients: a single-center analysis of incidence, risk factors, and novel therapeutic approaches. Transplantation. 2003;75:1266–70. doi: 10.1097/01.TP.0000061767.32870.72. [DOI] [PubMed] [Google Scholar]
- 6.Smith JM, McDonald RA, Finn LS, Healey PJ, Davis CL, Limaye AP. Polyomavirus nephropathy in pediatric kidney transplant recipients. Am J Transpl. 2004;4:2109–17. doi: 10.1111/j.1600-6143.2004.00629.x. [DOI] [PubMed] [Google Scholar]
- 7.Andrews C, Shah KV, Rubin R, Hirsch M. BK papovavirus infections in renal transplant recipients: contribution of donor kidneys. J Infect Dis. 1982;145:276. doi: 10.1093/infdis/145.2.276. [DOI] [PubMed] [Google Scholar]
- 8.Bohl DL, Storch GA, Ryschkewitsch C, Gaudreault-Keener M, Schnitzler MA, Major EO, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transpl. 2005;5:2213–21. doi: 10.1111/j.1600-6143.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–96. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 10.Gardner SD, MacKenzie EF, Smith C, Porter AA. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J CIin Pathol. 1984;37:578–86. doi: 10.1136/jcp.37.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hariharan S, Cohen EP, Vasudev B, Orentas R, Viscidi RP, Kakela J, et al. BK virus-specific antibodies and BKV DNA in renal transplant recipients with BKV nephritis. Am J Transpl. 2005;5:2719–24. doi: 10.1111/j.1600-6143.2005.01080.x. [DOI] [PubMed] [Google Scholar]
- 12.Brennan DC, Agha I, Bohl DL, Schnitzler MA, Hardinger KL, Lockwood M, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transpl. 2005;5:582–94. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton RS, Gravell M, Major EO. Comparison of antibody titers determined by hemagglutination inhibition and enzyme immunoassay for JC virus and BK virus. J Clin Microbiol. 2000;38:105–9. doi: 10.1128/jcm.38.1.105-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscidi RP, Rollison DE, Viscidi E, Clayman B, Rubalcaba E, Daniel R, et al. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin Diagn Lab Immunol. 2003;10:278–85. doi: 10.1128/CDLI.10.2.278-285.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randhawa PS, Gupta G, Vats A, Shapiro R, Viscidi RP. Immunoglobulin G, A, and M responses to BK virus in renal transplantation. Clin Vaccine Immunol. 2006;13:1057–63. doi: 10.1128/CVI.00114-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sener A, House AA, Jevnikar AM, Boudville N, McAlister VC, Muirhead N, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81:117–20. doi: 10.1097/01.tp.0000181096.14257.c2. [DOI] [PubMed] [Google Scholar]
- 17.Keven K, Sahin M, Kutlay S, Sengul S, Erturk S, Ersoz S, et al. Immunoglobulin deficiency in kidney allograft recipients: comparative effects of mycophenolate mofetil and azathioprine. Transpl Infect Dis. 2003;5:181–6. doi: 10.1111/j.1399-3062.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 18.Rentenaar RJ, van Diepen FN, Meijer RT, Surachno S, Wilmink JM, Schellekens PT, et al. Immune responsiveness in renal transplant recipients; mycophenolic acid severely depresses humoral immunity in vivo. Kidney Int. 2002;62:319–28. doi: 10.1046/j.1523-1755.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith KG, Isbel NM, Catton MG, Leydon JA, Becker GJ, Walker RG. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transpl. 1998;13:160–4. doi: 10.1093/ndt/13.1.160. [DOI] [PubMed] [Google Scholar]
- 20.Ginevri P, Azzi A, Hirsch HH, Basso S, Fontana I, Cioni M, et al. Prospective monitoring of polyomavirus BK replication and impact of pre-emptive intervention in pediatric kidney recipients. Am J Transplant. 2007;7(12):2727–35. doi: 10.1111/j.1600-6143.2007.01984.x. [DOI] [PubMed] [Google Scholar]
- 21.Binggeli S, Egli A, Schaub S, Binet I, Mayr M, Steiger J, et al. Polyomavirus BK-specific cellular immune response to VP1 and large T-antigen in kidney transplant recipients. Am J Transpl. 2007;7:1131–3. doi: 10.1111/j.1600-6143.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 22.Comoli P, Azzi A, Maccario R, Basso S, Botti G, Basile G, et al. Polyomavirus BK-specific immunity after kidney transplantation. Transplantation. 2004;78:1229–32. doi: 10.1097/01.tp.0000137932.44791.d3. [DOI] [PubMed] [Google Scholar]