Abstract
Amyloid plaques and neurofibrillary tangles (NFTs) are the pathological hallmarks of Alzheimer disease (AD). There is controversy regarding the use of current diagnostic criteria for AD and whether amyloid plaques and NFTs contribute to cognitive impairment. Because AD is specific to humans, rigorous and comprehensive clinicopathologic studies are necessary to test and refine hypotheses of AD diagnosis and pathogenesis. Neither the clinical nor the pathological aspects of AD evolve in a linear manner, but the predictable sequence of AD pathology allows for stage-based correlations with cognitive deterioration. We discuss subsets of patients with clinical dementia who lack amyloid plaques and NFTs and, conversely, whether individuals without antemortem cognitive impairment can harbor severe AD-type pathological findings at autopsy. There are many medical, technical, and anatomical challenges to clinicopathologic studies in AD. For example, at least two thirds of persons older than 80 years have non-AD brain diseases that can effect on cognitive function. We argue that existing data strongly support the hypothesis that both amyloid plaques and NFTs contribute to cognitive impairment.
Keywords: Acetylcholine, Aging, Cognition, Lewy, Mini-Mental State Examination, Stroke, Tau
INTRODUCTION
Clinicopathologic (CP) studies are essential for determining the pathobiological importance of amyloid plaques and neurofibrillary pathology in Alzheimer disease (AD). Research on CP correlation in AD has been active for several decades, and meaningful data have accumulated from many international centers. These investigations have been aided by a detailed understanding of both clinical and pathological progression of AD. Along with advances, however, there have been many controversies. This review of the literature on CP studies in AD is intended to describe the progress and complexities for a broad scientific audience.
We interpret CP studies to provide robust support for the importance of both amyloid plaques and neurofibrillary tangles (NFTs) in the clinical manifestations of AD (Fig. 1; Table 1). We review the large body of pertinent literature and describe the medical, technical, and anatomical aspects relevant to interpreting these data. Because the pathological features of AD and many other aspects of the aged brain are distinct in Homo sapiens, we focus primarily on studies on humans.
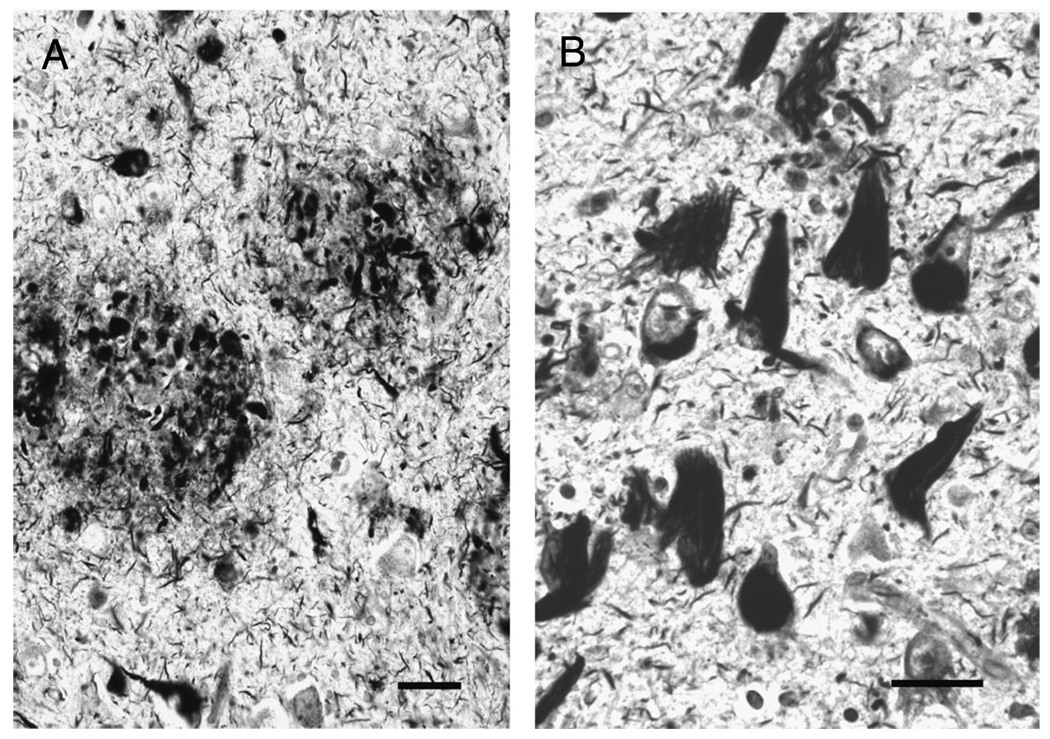
FIGURE 1.
Histopathologic hallmarks of Alzheimer disease demonstrated with Bielschowsky silver impregnation. (A) Neuritic plaques are extracellular fibrillary amyloid deposits, surrounded by swollen, degenerating, argyrophilic neurites. (B) Neurofibrillary tangles are composed of intracellular, insoluble, and protease-resistant fibrillary polymers of tau protein. In both panels, there are wispy argyrophilic neuropil threads. Scale bars = 25 µm.
TABLE 1.
Definition of Terms
| Pathological terms | ||
| Neurofibrillary pathology | ||
| Neurofibrillary pathology comprises aberrant, insoluble, and protease-resistant tau aggregates in various cellular compartments. Neuropil threads and the intracellular components of neuritic plaques are subsets of neurofibrillary pathology that are present within dendrites or neurites. | ||
| Neurofibrillary tangle | ||
| Neurofibrillary tangle is the term that describes neurofibrillary pathology found in cell bodies. | ||
| Amyloid plaques | ||
| Amyloid plaques are extracellular, often roughly spherical, proteinaceous deposits stainable with Congo red, silver stains, and thioflavine histologically. Fibrillary polymers of the Aβ peptide comprise the structural core of amyloid plaques in Alzheimer disease. Different subsets of amyloid plaques are defined later. | ||
| Neuritic plaques vs diffuse plaques | ||
| Neuritic plaques are extracellular amyloid deposits invested by swollen degenerating neurites. The swollen neurites contain filamentous tau protein aggregates identical structurally to the inclusions within neurofibrillary tangles. Diffuse plaques lack the presumed degenerating and/or aberrant tau-immunoreactive neuritis. | ||
| Braak Stages | ||
| Braak stages refer to the relatively predictable progression of neurofibrillary tangle-type pathology in the brain during the course of Alzheimer disease. In early stages (I–III) the pathology is mostly isolated to the mesial temporal lobe structures, but later stages (IV–VI) progressively affect the neocortex (see later). | ||
| Anatomical terms | ||
| Mesial temporal lobe structures (allocortex) | ||
| Mesial temporal lobe structures in the human brain comprise allocortical structures including the entorhinal cortex, amygdala, and the cornu ammonis fields (CA1–CA4) and subiculum of the hippocampus. Mesial temporal lobe areas functionally play an important role in consolidating short-term memory. | ||
| Isocortex or neocortex | ||
| Neocortex refers to areas of cerebral cortex, also known as isocortex, that have 6 cellular layers. Neocortical areas subserve higher-order functions including aspects of judgment, executive function, and so on. The contradistinction between mesial temporal lobe areas and neocortical areas is important in comprehending the predictable, but nonlinear, progression of pathology in Alzheimer disease. | ||
CLINICOPATHOLOGIC CORRELATION IN THE CONTEXT OF AD: THE GOALS AND THE POTENTIAL OBSTACLES
The goal of CP studies is to understand the clinical and biologic importance of identified pathological features. In principle, pathological severity should correlate meaningfully with the extent of clinical disease, that is, cognitive impairment in AD. A desirable correlation is depicted in Figure 2A, in which the ideal correlation requires a linear association between 2 discrete entities: impairment of health and severity of pathology. Such correlations are, however, seldom attained in practice because of functional reserve capacity, biologic variation between individuals in both protective and pathogenetic pathways, and incomplete understanding of disease mechanisms.
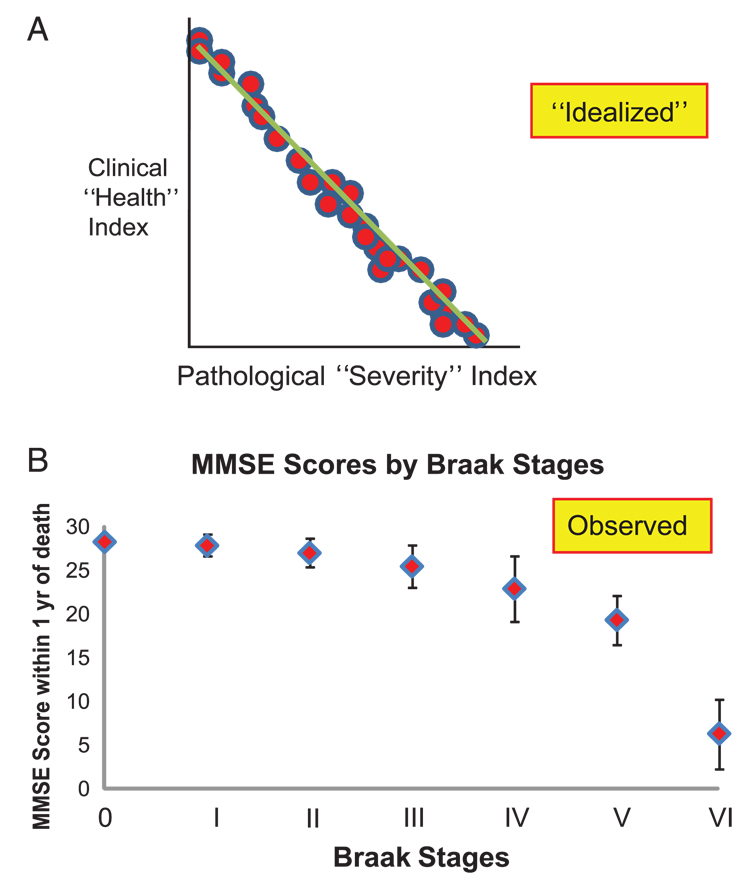
FIGURE 2.
(A) An idealized clinicopathological correlation may not be applicable to Alzheimer disease. (B) A conventional metric for cognition, the Mini-Mental State Examination (MMSE) and the Braak staging method provide a relatively strong correlation between pathological severity and antemortem cognitive decline. Data depicted are from the University of Kentucky Alzheimer's Disease Center (63). Each patient in this series lacked concomitant pathological processes, for example, advanced cerebrovascular disease, and died within a year of their last MMSE score. Numbers of patients from each group: Braak Stage 0, n = 8; I, n = 15; II, n = 18; III, n = 13; IV, n = 12; V, n = 6; and VI, n = 25. Error bars are SD.
Furthermore, CP studies require assumptions with respect to the pathological substrates. It has been suggested that amyloid plaques and NFTs may not be pathogenetic in AD, and that these abnormalities could instead be a neuroprotective response to other disease stimuli such as oxidative stress and/or inflammation (1). Neither oxidative nor inflammatory brain insults have, however, been shown to induce NFTs in any wild-type animal model. Are NFTs “neuroprotective” exclusively in humans? Interpreting AD-related changes as adaptive becomes even more difficult as one seeks explanations for the peculiar amyloid plaque pattern and the selective vulnerability of only a few types of nerve cells in AD. In principle, all nerve cells and nonneuronal cells can be expected to react similarly to oxidative stress and inflammation, although not necessarily always to the same extent. We interpret the genetic evidence (discussed later) and qualitative pathological assessments of AD brains, particularly the neurofibrillary pathology that appears to warp and impinge upon normal cell constituents (Fig. 1), to indicate more of a toxic than protective role. There may be both harmful and adaptive aspects of a given phenomenon, of course, and AD lesions presumably both result from, and in turn stimulate, other toxic factors.
Additional questions have been raised regarding the biologic relevance of amyloid plaques and NFTs (1). Evaluating these arguments requires consideration of the specificity of plaques and NFTs in AD, the importance of concomitant disease processes, and the challenges in performing cognitive assessments.
Specificity of AD Lesions: Amyloid Plaques and NFTs are Seen Outside of AD
It has been suggested that neurofibrillary pathology and amyloid plaques are not specific to AD. If this is true, does nonspecificity argue for or against the importance of NFTs and amyloid plaques in AD?
Neurofibrillary tangles are not specific for AD, particularly if a broader definition of NFTs includes different tau isoforms or if one expands the expectations of the morphological characteristics of NFTs (2, 3). In addition to AD, NFTs are also found in some frontotemporal dementias, myotonic dystrophy, viral panencephalitis, dementia pugilistica, some prion diseases, and other brain diseases (4, 5). For many of these disorders, the severity of NFT pathology is less than that observed in end-stage AD. No condition characterized by widespread neocortical NFTs lacks extensive neurodegeneration and clinical dementia. On the other hand, there are many subtypes of chronic brain diseases in which there are extensive neurodegeneration and clinical dementia without NFTs, such as many subtypes of frontotemporal dementias, synucleinopathies, subacute or chronic infarcts, metabolic, demyelinating, developmental, and trinucleotide repeats diseases. Nor is there any doubt that tau protein itself can directly trigger neurodegeneration: many germ line mutations in tau produce clinical dementia with NFTs (3, 6). These tauopathies are distinct from AD, but common pathways may be involved. The bottom line is that NFTs appear in multiple brain diseases, and some researchers, including the present authors, infer that NFTs contribute to neurodegeneration in more than 1 disease state.
Unlike NFTs, the appearance of amyloid plaques in AD brains is unique. Dystrophic neurites that contain amyloid precursor protein are seen in traumatic brain injury (7, 8), and “diffuse plaques” can be observed in association with dementia pugilistica (9). “Lewy plaques” have also been described, with a corona of dystrophic α-synuclein–positive neurites (10). By contrast, the particular appearance of neuritic plaques (i.e. Aβ peptide–containing extracellular lesions surrounded by tau neurofibrillary pathology) is considered to be specific for AD. Amyloid plaques are not a nonspecific reaction to neurofibrillary pathology because non-AD tauopathies lack amyloid plaques. Further attesting to the specificity of AD-type amyloid plaques is the fact that mutations or duplications in the amyloid precursor protein gene produce the specific features of AD, clinically and pathologically (11–13). Notably, without NFTs or amyloid angiopathy, amyloid plaques are not associated with neurodegeneration (see later). In sum, AD involves a specific combination of neuritic amyloid plaques and NFTs; the neuritic plaques are more specific to the disease, and the NFTs seem more likely to induce neurodegeneration.
Specificity of AD Lesions: General and Theoretical Considerations About the Clinical Context of AD, Neuroanatomy, and Synergy Between Plaques and NFTs
One challenge of CP studies in AD is to place nonlinear disease processes onto quasi-linear continua for purposes of correlation. The pathological changes in AD follow a complex stereotyped neuroanatomical pattern (14). Prior studies have produced a general scheme for how the pathology correlates with clinical outcomes (Fig. 2 and Fig 3), but it is important to consider the clinical context for this association.
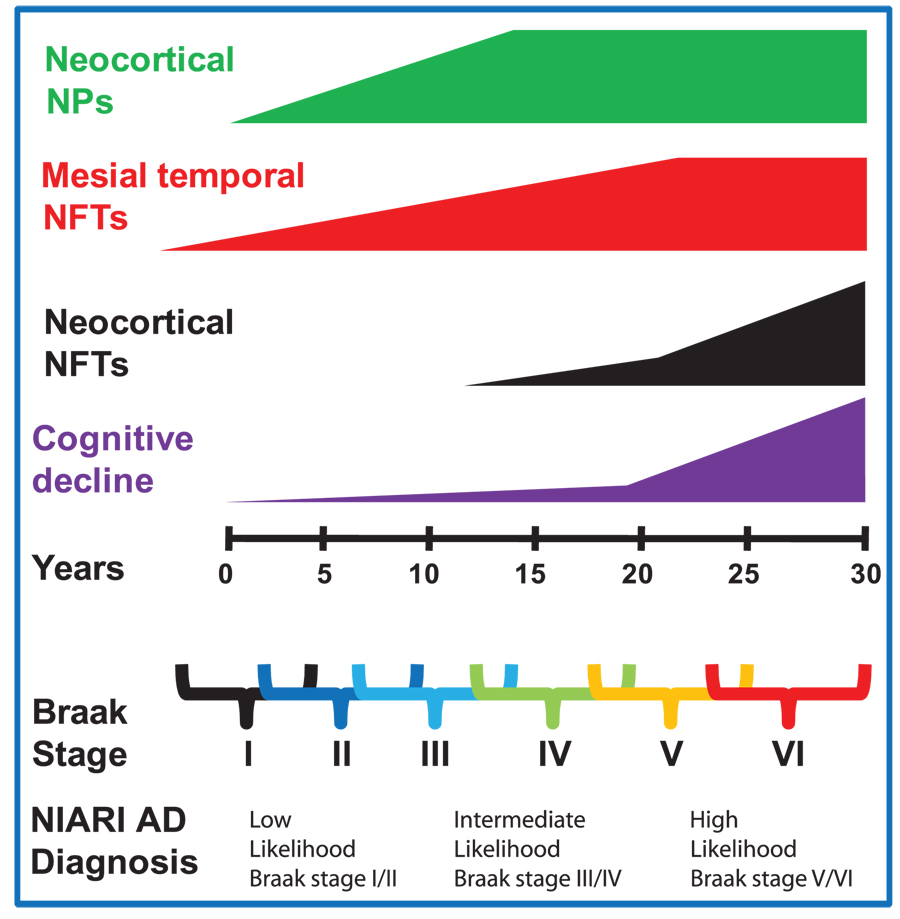
FIGURE 3.
Alzheimer disease (AD) progresses in a nonlinear manner that renders clinicopathological correlation a challenge. The disease duration is long, there is a preclinical phase, in which pathological findings are present, but there are no clinical manifestations. Further, each patient has a unique constellation of clinical and pathological features. Nonetheless, there is a relatively predictable progression of both clinical and pathological indices. This schematic illustrates that the association between mesial temporal neurofibrillary tangles (NFTs) and neocortical neuritic plaques with cognitive decline may be weak, whereas the association between neocortical NFTs and cognitive decline is strong. Early mesial temporal NFTs may play a role in memory dysfunction in early AD.
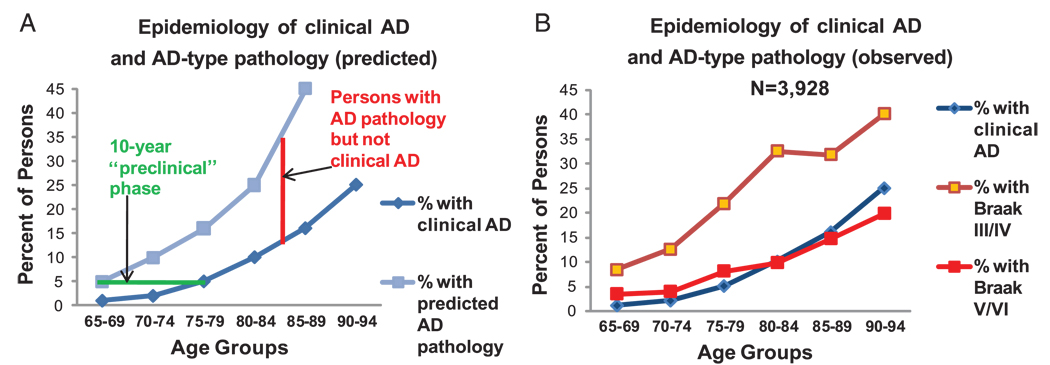
Epidemiological studies indicate that clinical AD prevalence doubles every half decade after age 65 years so that approximately 25% of 90-year-olds carry the diagnosis of AD (15, 16). The time from formal clinical diagnosis to death is only approximately 8 years (17). Structural and cognitive disease is present over a longer time span, however, certainly over a decade before death and perhaps considerably longer (18–24). Using these data, we deduce that an average person dying at age 78 years will have approximately 5% to 10% likelihood of having an antemortem diagnosis of AD, but at least approximately 20% to 40% likelihood of having significant AD-type pathology. Thus, many individuals with AD-related pathology and minimal or no detectable antemortem cognitive deficits die of other causes. Theoretical and empirically derived data agree on this important point, as shown in Figure 4. Figure 4B shows data of Del Tredici and Braak (25) based on 3,928 cases. The existence of nondemented persons with AD-type pathology is hence not a potential confound; it is obvious that many persons die in the preclinical phase of AD. Preclinical and subclinical diseases are well-accepted ideas in cancer, atherosclerosis, and in many other diseases, but seem to cause confusion in discussions of AD.
FIGURE 4.
(A) The epidemiology of Alzheimer disease (AD) and the duration of the disease process predict that many persons with appreciable AD pathology die before the onset of dementia. Assuming a 10-year preclinical stage, approximately as many individuals would have significant AD pathology without the clinical disease as would manifest the disease during life. The epidemiological data represent an average of 6 large studies, as summarized previously (16). (B) Empirical data confirm the prediction that many individuals who die of other causes show appreciable AD-type pathology. Empirical data are from 3,928 cases in the indicated age ranges (25). Note that Braak Stages III/IV correspond to National Institute on Aging–Reagan Institute “intermediate likelihood” for the diagnosis of AD (46).
In addition to the concept of preclinical AD, there are other potential pitfalls in CP studies. Distinct neuroanatomical regions are affected at different stages of AD, and this must be taken into account for CP correlations. For example, portions of the allocortex (Table 1), including the hippocampus and entorhinal cortex, may be involved severely early in AD (14, 26–28) when neocortical areas are still relatively well preserved. The involvement of neocortical areas presumably underlies the later-stage cognitive impairment. Therefore, study of hippocampus may be best for assessing earlier memory changes, but a study limited to hippocampus would be inappropriate for correlating overall pathology with overall cognitive decline. An important case study is that of Patient H.M., who had bilateral near-complete temporal lobectomies in 1953 (29, 30). Although mostly amnestic, Patient H.M. is still quite robust cognitively more than 5 decades later without AD (30) because most cognitive domains impaired in AD pertain to neocortical involvement (i.e. nondeclarative memory, judgment, gnosia, visuospatial skills, language, executive function, etc.).
As if factoring in both the clinical course and the anatomical aspects of the disease was not difficult enough, there is added complexity in the lesions of AD. By definition, AD is characterized by the presence of both amyloid plaques and neurofibrillary pathology, and there seems to be some synergy of the two, most definitely in neuritic plaques, but the tracking of 2 separate pathologies renders CP correlations challenging. In the multidecade course of AD progression, amyloid plaques and NFTs may be first observed in different parts of the brain: NFTs tend to appear first in allocortical structures, whereas amyloid plaques may first be found in the neocortex (26, 27, 31–35). Because 2 different types of pathology develop initially without apparent direct correlation to each other, a linear continuum is difficult to establish. A further layer of complexity is that amyloid plaques and NFTs are probably not static lesions (see later).
These considerations help explain why some prior studies have shown apparent general discrepancies with regard to the correlation of extent of pathology and the severity of premortem cognitive decline (1). However, apparent incongruities have been asserted by a number of researchers, and a discussion is provided later regarding specific studies.
Specificity of AD Lesions: Particular Cases and Pathological Combinations
AD is defined by the presence of neuritic plaques and neurofibrillary pathology, the abundance of which is hypothesized to correlate with the severity of cognitive deterioration. If this is so, then certain corollaries must hold true: 1) there should not be individuals with extremely abundant plaques and tangles but no cognitive deterioration; 2) there should not be clinical dementia cases with abundant plaques and no NFTs; 3) there should not be patients with clinical dementia in whose brains there is no detectable pathological substrate; and 4) although there exist a number of tauopathies (progressive supranuclear palsy, corticobasal degeneration, Guamanian parkinsonism, FTDP-17, etc.), there should not be a true “tau-only” AD. These ideas have been tested repeatedly and have fostered controversy. Different specific groups will be discussed in turn.
Amyloid Plaques and Neurofibrillary Pathology But No Dementia
Cases with considerable AD-type pathology but without documented cognitive decline have been termed pathological aging and presymptomatic aging (36–38). Because mild cognitive impairment is considered to constitute an early expression of AD (39), we here discuss only nondemented patients. Such patients with incipient AD-like changes have also been called preclinical AD based on magnetic resonance imaging data (19, 40), neuropsychological testing (21, 41), Pittsburgh Compound B positron emission tomography imaging (42), and cerebrospinal fluid biomarkers (43).
Pathologically, many nondemented cases meet the older Khachaturian diagnostic criteria (44) and a significant minority meet Consortium to Establish A Registry for Alzheimer’s Disease definite criteria for AD (41, 45). These criteria have been superseded for the stand-alone AD diagnosis since 1997 (46). Moreover, these criteria are not relevant directly to a discussion of false-positives because they were established for making the diagnosis of AD in clinically demented patients.
The pathological diagnosis of “intermediate likelihood” for AD by the current National Institute on Aging–Reagan Institute (NIARI) criteria corresponds to Braak Stages III and IV (46), neither of which is by itself a substrate for profound cognitive impairment (Fig. 2). The NIARI criteria for pathological diagnosis of “high likelihood” for AD correspond to Braak Stages V and VI (46). Note that the average final Mini-Mental State Examination (MMSE) score for Braak Stage V is approximately 20 out of a possible 30 (Fig. 2), so may include some relatively normal individuals. A recent case report described a 92-year-old nondemented patient whose brain, on autopsy, contained Braak Stage VI pathology (47), but neocortical NFTs from this patient did not appear particularly dense, and the patient did demonstrate cognitive deficits. In 3 different cognitive tests, she scored at or below the 20th percentile for her age despite previously normal test results (47). Because there is as yet no published report of true end-stage pathology in a cognitively intact individual, an assessment of larger studies is necessary.
In 2004, Fernando and Ince (48) described a population-based autopsy series in which “…severe neocortical NFTs were not seen in any nondemented individual.” This result has been obtained repeatedly from multiple centers (33, 47–57), as shown in Table 2 (N = 555 nondemented subjects, of whom 15 were Braak Stages V or VI). These data from many research centers indicate a 2.7% false-positive rate in the NIARI criteria for high-likelihood AD diagnosis, with mostly Braak Stage V cases. In addition, a number of other studies have noted that nondemented patients have NFTs confined predominantly to the mesial temporal lobe structures (58–61). These data support the accuracy of the high likelihood NIARI criteria, but leave unanswered the important question: are there nondemented persons with truly end-stage AD-type pathology?
TABLE 2.
Autopsy Series on Nondemented Persons With False-Positive Pathological Diagnosis of National Institute on Aging–Reagan Institute High Likelihood for Alzheimer Disease (Braak Stages V and VI)
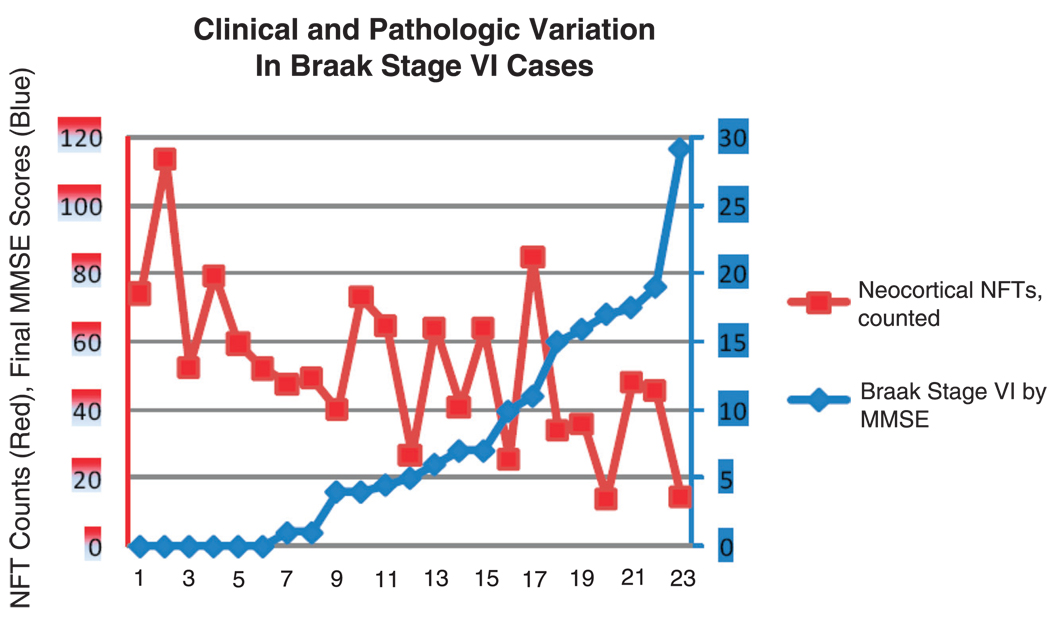
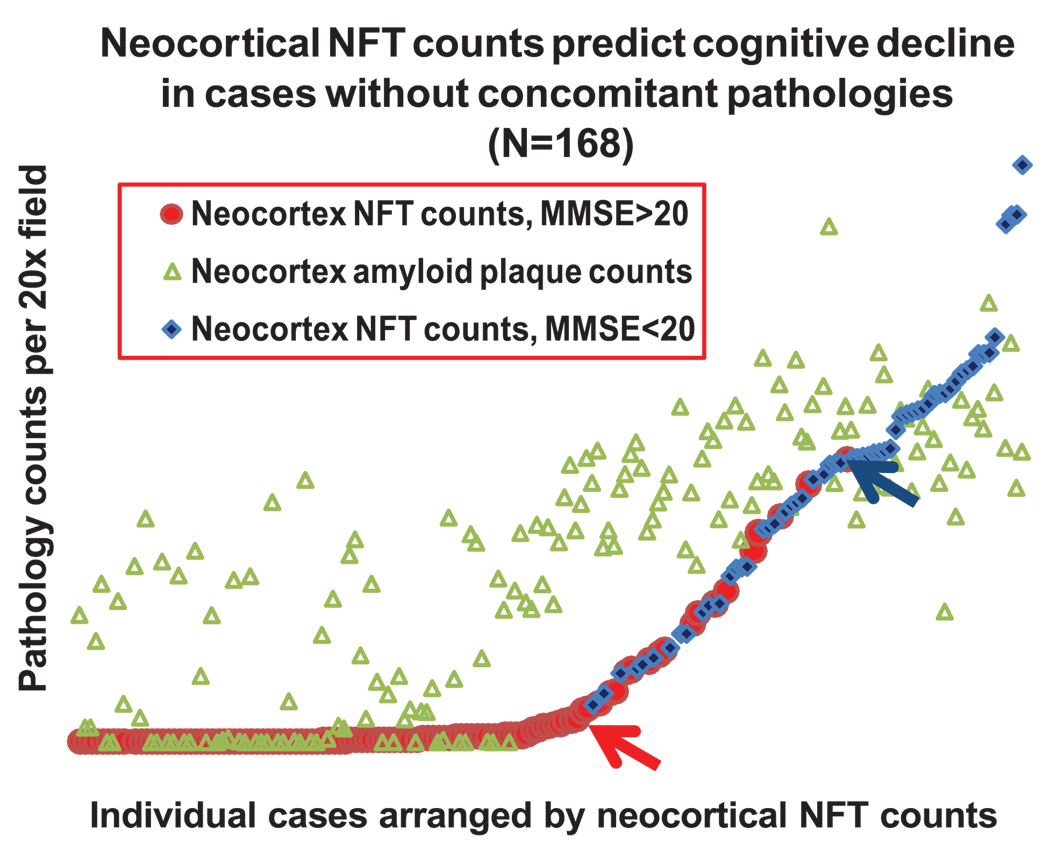
We addressed this issue using data from the University of Kentucky Alzheimer’s Disease Center autopsy series (62, 63). Of 59 longitudinally followed nondemented subjects with MMSE scores within a year of death, 9 showed Braak Stage IV, 3 Stage V, and 1 Stage VI (Table 2). The single Braak Stage VI patient from this series had a final MMSE score of 29 within a year of death. Note that in this brain, there was a relatively low density of neocortical NFTs (Fig. 5, rightmost data points). This person seems to demonstrate the lowest-severity Braak Stage VI. Thus, even among Braak Stage VI cases, individuals with lower neocortical NFT densities tend to have better preservation of cognitive faculties. Variability in Braak Stage VI severity is underscored by different studies in which the median final MMSE scores range from less than 2 (64) to 14 (53) in this group. Moreover, in a study of 168 autopsied persons (Fig. 6), every case with neocortical NFT density greater than a particular threshold (blue arrow) had clinical dementia (MMSE, <20), just as every case with low or absent neocortical NFTs (red arrow) had final MMSE scores greater than 20. In sum, whereas the correlation between AD-type pathology and clinical impairment is not absolutely predictable in each patient, there seems to be a threshold of pathology above which every patient, without exception, is profoundly impaired.
FIGURE 5.
Variations in clinical and pathological indices in Braak Stage VI cases from the University of Kentucky Alzheimer’s Disease Center database (63). Cases are arranged left-to-right by increasing Mini-Mental State Examination (MMSE) scores. All patients had undergone MMSE testing within a year before death. Patients with higher MMSE scores tended to have fewer neocortical neurofibrillary tangles (NFTs). One nondemented patient (MMSE score, 29) had a relatively low number of neocortical NFTs compared with the other more cognitively impaired patients.
FIGURE 6.
Data from the University of Kentucky Alzheimer’s Disease Center database demonstrate that neocortical neurofibrillary tangle (NFT) counts predict dementia in patients without severe concomitant brain pathologies (n = 168). Neocortical counts comprised total counts from occipital (Brodmann area 17 and 18), inferior parietal lobule, superior and midtemporal gyri, and middle frontal gyrus (63). Neocortical amyloid plaque counts are the combined amount of neuritic and diffuse amyloid plaques in the same sections. Above a certain neocortical count (blue arrow), all patients were demented (blue diamonds = patients with the Mini-Mental State Examination [MMSE] <20). By contrast, patients with mild or no dementia (MMSE, >20; red circles) tend to have lower neocortical NFTs, and all patients below a threshold of neocortical NFT counts (shown by red arrow) have MMSE greater than 20. There is only a weak correlation between the amyloid plaques (green triangles) and the NFTs, but cases with high neocortical NFTs generally have high amyloid plaques.
“Tangle-Only” Dementia
There are reports of autopsied patients with neurofibrillary pathology–predominant dementia (5, 54, 65, 66). These cases are characterized by abundant NFTs in medial temporal lobe structures with no or few amyloid plaques throughout the brain. The individuals are usually older and primarily had memory decline and reduced activities of daily living. Whether this is a subtype of AD or a different entity remains to be elucidated.
Other diseases are now known to be characterized by NFT formation and minimal or absent amyloid pathology. These cases are now presumed to reflect tauopathies, which are a diverse group of diseases that may be associated with genetic (tau mutations) or environmental (Guamian or postencephalitic parkinsonism) factors (3, 5). In most population-based series, and in older patients, tauopathies are uncommon (54, 57, 67).
“Plaque-Only” Dementia
We have no personal experience of cases with dementia, abundant neocortical neuritic plaques, or diffuse plaques but with no other clinical or pathological explanation for the cognitive decline. The phenomenon of plaque-only dementia has been described (68, 69) but may partly correspond to patients with dementia with cortical Lewy bodies (70). Even in the recent series of plaque-only AD (68), which described a group of 16 brains of persons with clinical dementia but with no neocortical NFTs and a low number of amyloid plaques, it is unclear whether or how other prevalent brain diseases that are capable of contributing to dementia were excluded. Thus, with the apparent exception of a rare familial variant of historical significance (71), plaque-only dementia has not been demonstrated beyond doubt despite many studies addressing this topic. Without fuller clarification, it is possible that the small numbers of plaque-only dementia cases described to date had cognitive decline caused by other neurodegenerative conditions.
Cases With Dementia Lacking Amyloid Plaques, NFTs, and Other Pathological Substrates
The literature does not indicate an appreciable subset of patients with clinical dementia and histopathologically pristine brains on autopsy. Studies that show a significant proportion of cases with dementia, low neurofibrillary pathology, and no other known pathological substrate, tend to be the older studies that lack probes for recently described diseases. In 2000, Crystal et al (72) described a cohort with “dementia of unknown etiology,” however, cognitive impairment in this cohort was not extreme, and these patients showed specific contributory neuropathological features including hippocampal sclerosis and vascular disease.
There are many additional causes of cognitive decline, and these can be clinically diagnosed in most cases. For this reason, in clinical series such as those at the University of Kentucky Alzheimer’s Disease Center, which include rigorous longitudinal clinical assessments followed by brain autopsy, extremely rare patients with profound dementia and the premortem diagnosis of AD have lacked histopathologic substrates at autopsy. We and others have found that persons with concomitant pathologies die with less neurofibrillary pathology (63, 64). Identifying and taking into account the presence and magnitude of concomitant pathologies is probably the biggest challenge to studies of the aging human brain.
Concomitant Pathology: The Large Impact of Cerebrovascular Diseases
The most prevalent concomitant pathology in the brains of aged persons, cerebrovascular disease (CVD), is directly relevant to any discussion of CP studies in dementia or AD. The challenges introduced by this widespread but unpredictable comorbidity in aged persons have been discussed previously (48, 64, 73–79). Yet, the profound impact of CVD on studies pertinent to cognition in the elderly seems to be underappreciated, or even ignored, in the broader field of AD research.
Cerebrovascular disease spans a wide spectrum including small- and large-vessel ischemic disease, embolic, venous, inflammatory, hypertensive, hemorrhagic, aneurysmal, hypoglycemia, hypoxia, amyloidogenic, and others. The importance of CVD is at least 2-fold. First, some cerebrovascular abnormalities are so common as to be the norm in advanced old age. Second, CVD induces an unpredictable change in cognition and hence presents challenges to correlating the severity of cognitive decline with other diseases.
Clinical and subclinical CVD is common in elderly subjects. Cerebrovascular disease is detectable in 75% to 90% of persons older than 90 years (63, 80), and whereas frank clinical strokes affect approximately 750,000 in America each year, more than 11 million discrete but clinically silent cerebrovascular events are thought to occur over the same interval (81). The high frequency of cerebrovascular changes among elderly patients means that both cognition and other pathology need to be weighed in the context of an altered milieu. Furthermore, unlike AD, CVD progresses in an anatomically, clinically, and temporally unpredictable fashion, and there is no systematic methodology for correlating pathological and clinical findings (82). These are some reasons why CVD-related pathology is frequent in cases termed dementia of unknown origin (72).
Importantly for CP studies, CVD shifts the detection threshold for cognitive changes during life. In elderly AD patients, CVD leads to more rapid cognitive deterioration (83, 84). Subcortical and/or lacunar infarcts alter the detection threshold for dementia (74, 85–87). The change in clinical course is mirrored by a different pathological appearance in the brain. Persons with CVD tend to have lower AD-related pathology with a given degree of cognitive impairment (63, 86). In other words, the frequent presence of concomitant CVD severely dampens the association for other pathologies, such as neurofibrillary pathology and amyloid plaques, to the severity of antemortem cognitive decline. For example, Braak Stage IV pathology may combine with CVD to induce significant cognitive impairment, whereas Braak Stage V pathology may itself have more biologic impact, yet still be clinically silent in the absence of concomitant pathological abnormalities.
Concomitant Pathology: An Expanding List of Non-AD Brain Diseases in Aged Persons
Although not as prevalent as CVD, non-AD neurodegenerative diseases are important for consideration. Synucleinopathies are the second most prevalent neurodegenerative disease category after AD; these include dementia with Lewy bodies and Parkinson disease dementia. Depending on the study population, dementia with Lewy bodies and Parkinson disease dementia constitute approximately 5% to 15% of all neurodegenerative diseases (57, 88). Cortical Lewy body pathology occurs frequently in association with AD (88, 89). Whatever the reason for this clustering, the phenomenon is important from the perspective of CP correlation. When cortical Lewy bodies are present, the correlation between AD pathology and cognitive impairment becomes far less strong (63), probably because the Lewy body pathology is accounting for additional cognitive impairment. Accordingly, we and others have found that patients with AD and dementia with Lewy bodies decline at a faster rate than pure AD (90–92). The most reliable diagnostic tool for detection of neocortical Lewy bodies (i.e. immunohistochemistry for α-synuclein) was not developed until the late 1990s, so earlier studies had less ability to detect these structures (93, 94). There are now known non-AD dementias linked to mutations in valosin, progranulin, TDP-43, CHMP2B, and various triplet-repeat alleles (6, 95). These relatively newly discovered diseases present an important caveat to interpreting older studies that tackled the problem of CP correlation in dementia.
In addition to neurodegenerative diseases per se, some other common diseases such as hippocampal sclerosis, substance abuse, mood disorders, cancer, hematomas, and hydrocephalus are directly contributory to cognitive impairment in the elderly (96–98). Together, neurodegenerative diseases, CVD, and other aging-associated brain diseases weaken the apparent association between AD pathology and antemortem cognitive impairment. An analogy can be made to studying heart disease: it would be difficult to study the CP correlation between coronary atheromas and heart function if three quarters of cases had bacterial endocarditis and/or severe arrhythmias as well. That would be true even if clinical parameters could be correctly and confidently assessed in each case.
Challenges Pertaining to Cognitive Assessment
Difficulties in CP correlation may have as much to do with the clinical as to the pathological component. In AD-relevant CP studies, the clinical aspect is commonly the severity of cognitive impairment as quantified by test scores. First, it is problematic to align cognition and its many substituent functions on a linear scale. Second, there is added difficulty in correlating brain disease with test scores. In other words, even if cognition could be placed on a valid linear continuum (a test score), it would still be unsafe to assume that regression along that continuum will occur in a linear fashion that correlates with brain disease severity, whether a histopathologic substrate exists.
Although outside the scope of this review, specific cognitive assessments are used for differing applications. More challenging, memory-oriented, and sensitive tests such as the AD Assessment Scale, Cognitive component have been used frequently in clinical trials and clinical imaging studies when intraindividual assessments can be correlated as an outcome measure. By contrast, most CP studies have relied on less sensitive tests such as MMSE, the Blessed Test, or the even more semiquantitative Clinical Dementia Rating Scale for CP correlation (26, 36, 52, 61, 63, 90, 99). These latter tests have been more constantly used over time, assess multiple cognitive domains, have relatively well-established normative values, and could be more specific for interpersonal and cross-study CP correlations. It may be best to use a comprehensive battery of cognitive and functional tests such as those used currently in the Uniform Data Set by Alzheimer’s Disease Centers (100).
A further complication to CP studies is that many nonstructural factors alter cognitive assessment in the elderly. These include cardiovascular, metabolic, infectious, medication related, and mood disorders and debilities such as fatigue, arthritis, hearing loss, and visual impairments that can also change test-taking behavior.
Collectively, many clinical factors render inevitable some degree of variation in the results of cognitive assessments and hence in correlating test scores with any other parameter. There are thus many layers of complexity to CP studies of AD. Yet, by taking into account some of the above challenges, we can begin to address the existing CP literature and evaluate the hypothesis that amyloid plaques and NFTs contribute in a biologic sense to the clinical manifestations of AD.
CLINICOPATHOLOGIC STUDIES IN AD: REVIEW OF THE LITERATURE
Correlation of Amyloid Plaques and Cognitive Decline
More than 30 CP studies have assessed correlations between the density of amyloid plaques in human brains and the severity of antemortem cognitive decline (26, 34, 36, 56, 61, 63, 99, 101–106). These studies varied in research cohorts, anatomical areas examined, pathological methods, amyloid plaque subcategories, plaque-counting techniques, metrics for cognition and the range of cognitive decline tested, and the rigor with which concomitant pathologies were evaluated and/or factored into the study. Controversy exists about the best way to calculate the extent of amyloid plaque pathology. Neuritic plaques can be visualized via a variety of techniques, including silver stains, thioflavine S, and immunohistochemistry. Excellent studies have put forth differing views of whether one should use neuritic plaques, diffuse plaques, total plaque load, amyloid burden, or other pathology metrics (107–109). Studies also use differing techniques for counting and/or scoring their density (107).
Despite the wide variations in study designs, several points emerge consistently among CP studies about amyloid plaques. First, compared with NFTs, there is a weaker direct correlation between the density of amyloid plaques and the severity of cognitive decline. Second, the amyloid plaque subtype that seems to correlate best with the severity of cognitive decline is the neuritic plaque. Third, the patterns seen in aged persons’ brain seem to segregate into these 3 groups (note that many refers to counted lesions in neocortex): 1) Few plaques, few NFTs, and no cognitive impairment; 2) Many plaques, few NFTs, and no cognitive impairment; and 3) many plaques, many NFTs and cognitive impairment.
Because in part of the scarcity of neocortical NFTs in the absence of plaques, it has been suggested that the pathogenetic effect of amyloid plaques may be mediated through stimulating neurofibrillary pathology (63, 110). The relative importance of amyloid plaques in terms of correlating with cognitive decline may be greatest in the earliest stages of the disease, before there are widespread NFTs and neocortical neurodegeneration (99). By contrast, there is relatively little evidence of a direct effect of amyloid plaques in contributing to the later-stage cognitive decline in AD. Germane to this topic but often neglected is the question of lesion turnover. The apparent success of Aβ vaccine trials attests to the ability of the immune system to clear amyloid plaques (111), but it is not known whether a toxic plaque substance is left unaffected in these studies. Microglia appear to actively scavenge the fibrillar material in amyloid plaques (112). There is more direct evidence of amyloid plaque turnover in animal models (113). This phenomenon would tend to dampen the correlation between pathology and clinical features.
To a degree far greater than for NFTs, there is a strong association between amyloid plaques and genetic factors of risk for AD. As a rule, environmental and genetic factors that predict AD risk also appear to directly potentiate amyloid plaques. These risk factors include head trauma, the ApoE4 allele, Down syndrome, amyloid precursor protein mutations and duplications, SORL1 variants, and mutations in PSEN1 and PSEN2 genes (13). This strong genetic association is relevant to the question of the correlation with cognitive symptoms because all of these alleles are also strong risk factors for dementia.
The Neuritic Plaque: A Pathological Entity With Strong Mechanistic Implications
Neuritic plaques deserve special consideration. Neuritic plaques comprise roughly spherical extracellular amyloid deposits that are invested by degenerating or dying back nerve cell processes (Table 1; Fig. 1). These abnormal dendrites and axons contain aberrant tau fibrils identical to those seen in NFTs. In a given neuritic plaque, axons from a variety of different sources expressing distinct neurotransmitter signatures may be present (114, 115). This is therefore an important clue to AD pathogenesis because neuritic plaques represent unambiguously a nidus in which extracellular amyloid plaque pathology induces intracellular neurofibrillary pathology and apparent structural and functional disruption (116, 117). Although the particular toxic substance(s) within neuritic plaques are still not definitively known (i.e. they may represent specific conformation(s) of the Aβ peptide [118]), this apparent “smoking gun” is difficult to interpret in any other way.
CORRELATION OF NFTs AND COGNITIVE DECLINE
Dozens of research groups have assessed the association between NFTs and the severity of cognitive decline (26, 34, 36, 56–58, 61, 63, 99, 101–106, 119–122). As with amyloid plaque research, studies of NFTs have been defined by different staining techniques, followed many different study designs, and led to differing conclusions. Regardless of the staining or counting method, however, the correlation between neocortical NFTs and antemortem cognitive decline is strong in studies that span the clinical spectrum of AD. Several important themes emerge, including the importance of neuroanatomical considerations and the different subtypes of tau pathology in AD brains.
In the course of AD, the development of NFTs follows a predictable pattern (14, 31, 123). This pattern seems to correlate on the 1 hand with where neurons die (124–127) and on the other with the cognitive domains affected in AD. For example, in the earliest stages of AD, symptoms tend to relate to memory loss. At this stage of the disease, the allocortical substrates of memory are strongly affected by NFTs, but other areas are not. The cognitive domains affected in midstage and late-stage AD expand to include areas of executive function, judgment, visuospatial capacities, and speech. This pattern is paralleled by the development of NFTs in the neocortical areas that subserve those functions.
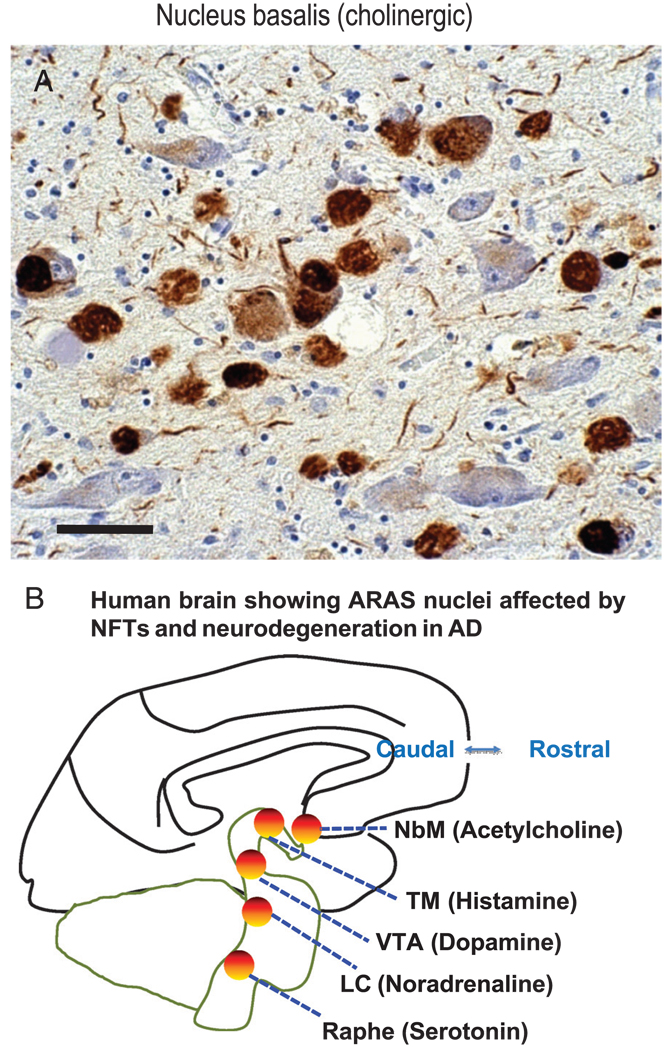
A special anatomical consideration referent to NFTs in AD is the impact of NFTs on the ascending reticular activating system (ARAS). Ascending reticular activating system neurons send strong inputs to the cerebral cortex that augment memory, arousal, attention, and motivation. These cells also are severely affected in AD. For example, although the “cholinergic hypothesis” of AD (128, 129) is now considered somewhat dated, the impact from NFTs on the ARAS cholinergic nucleus basalis of Meynert (NbM) may be profound. NFTs are numerous in the NbM starting early in the disease (Fig. 7), and NFT density parallels cognitive decline severity as does cerebral cortical cholinergic tone (130–132). The ARAS neurons, as a rule, supply neurotransmitters that are absent from intrinsic cortical neurons. For example, the NbM is a major source of cholinergic neurotransmission in the cerebral cortex, which itself harbors no cholinergic cells (133). The importance of cortical cholinergic tone is underscored by the fact that cholinergic antagonists such as scopolamine induce transient but complete amnesia in humans (134). NFTs are also thought to affect adversely other ARAS neurotransmitters including histamine, norepinephrine, dopamine, and serotonin (corresponding to neurodegeneration and NFT-containing cells of the tuberomammillary nucleus, locus caeruleus, ventral tegmental area, and pontine raphe, respectively) (135–141). There may be clinical manifestations associated with this subcortical pathology, although this would not be detected in most CP studies because consensus criteria for AD diagnosis currently focus on cortical pathology (46).
FIGURE 7.
Neurofibrillary tangles (NFTs) in ascending reticular activating system (ARAS) including the acetylcholinergic nucleus basalis of Meynert (NbM) do not map directly onto most clinicopathologic studies. (A) Many NFTs are stained using an antibody against phosphorylated tau protein in the NbM of a midstage Alzheimer disease (AD). Scale bar = 50 µM. (B) Other ARAS nuclei, including the tuberomammillary nucleus (TM), the locus caeruleus (LC), ventral tegmental area (VTA), and the brainstem raphe areas also show many NFTs and neuronal loss in AD patient. Neurotransmitters that are known to augment memory, arousal, attention, and mood are indicated alongside the ARAS cell groups that produce them. The ARAS neurons are the only source of those neurotransmitters in cerebral cortex, and thus pathological alterations in ARAS may contribute to clinical symptoms.
As with amyloid plaques, there are subcategories of neurofibrillary pathology; of these, NFTs are just one (Table 1). Less attention has been focused on the other subtypes possibly because neurofibrillary pathology is absent in most animal models and not generally amenable to positron emission tomographic scans. There is, however, abundant dendritic and axonal neurofibrillary pathology in AD brains (Fig. 1); these also are known as “neuropil threads” and “curly fibers” (142–145). All the neurofibrillary lesions, which contain tau polymer fibrils called paired helical filaments (142), may contribute to cognitive decline in AD (103). They provide another reason why a simple linear correlation between “plaques and tangles” would not be expected in relation to cognitive decline, although the pathways involved in the formation of those lesions may contribute to the disease.
Also in common with amyloid plaques, NFTs are quantified only at death, so it is important to consider whether some NFTs are removed from the brain in the course of AD. This has not been proven either way. Some, but not all, studies have indicated that more neurons disappear in AD brains than can be explained directly by the number of NFTs present at autopsy (126, 146, 147, but see Fukutani et al [127]); this might indicate NFT turnover and/or a non-NFT pathological mechanism. Extracellular NFTs are invested by microglial cells and astrocytes, but it is unclear whether these microglial scavengers successfully digest the protease-resistant insoluble neurofibrillary material. Morsch et al (148) modeled the correlation between NFT counts and neuron loss in human hippocampi and concluded that individual neurons may harbor intracellular NFTs over several decades (148, 149). This finding has been interpreted to indicate that NFTs are benign (1), although a pathological process that takes place over such a timeframe could well be toxic ultimately, as in many other diseases of development or aging. Before inducing cell death per se, these conspicuous changes in cell bodies, axons, and dendrites might be significantly impairing function.
LIMITATIONS TO CP STUDIES IN AD
Although many CP studies support the hypothesis that NFTs and amyloid plaques contribute to the cognitive decline in AD, there are important limitations to these studies and significant unanswered questions. The schema of amyloid plaques and NFTs “causing” AD is oversimplistic and incomplete. There are at least 2 relevant ideas that must be acknowledged: 1) Correlation does not establish causation. Although the density of NFTs on autopsy correlates with cognitive decline severity, and the experimental results align with a plausible hypothesis, this does not prove that NFTs are directly neurotoxic; and 2) Results raise the question of what induces the formation of amyloid plaques and NFTs. For many, if not most, AD patients, there are no known specific genetic or environmental triggers that would account for the development of amyloid plaques and NFTs. Yet, acknowledging that these lesions probably contribute to cognitive decline is important because it gives us a target for experimental studies.
SUMMARY AND CONCLUSIONS
Neuropathology of aging-related brain disease must take into account diverse medical, technical, biochemical, and anatomical considerations (Table 3). For example, concurrent pathologies are very common in aging brains. These diseases and many other factors are, collectively, formidable obstacles to aligning pathology and cognition along linear scales. An extensive literature on CP correlations in AD indicates a sequence that may begin with specific genetic and environmental factors that increase risk of widespread amyloid plaques. At some point, neuritic plaques appear that combine extracellular amyloid and intracellular neurofibrillary pathology. Whether independently or not, NFTs develop first in the allocortex and spread to neocortical areas. When neocortical NFTs are abundant, neurodegeneration occurs and, over a certain threshold, cognitive impairment is inevitable. The NIARI consensus “high likelihood for AD” pathological criteria are approximately 97% specific for measurable cognitive impairment, and the false-positives (nondemented persons with plaques and tangles) are brains in which the highest pathological burden has not been reached. These results are within realistic expectations for preclinical AD pathology. Among patients with measurable clinical disease, the extent of cognitive impairment parallels the severity of neurofibrillary pathology. In sum, the association of plaques and tangles with cognitive impairment in AD is complex but coherent.
TABLE 3.
Summary Points
| Nonlinear clinicopathological correlations are expected in Alzheimer disease (AD) | |
| • | There are prevalent (>two thirds of patients) comorbidities in aged brains, including cerebrovascular diseases, synucleinopathies, frontotemporal, and TDP-43–related diseases that inevitably skew correlations between pathology and cognition |
| • | The relationship between cognitive decline and brain disease is not linear, and both are difficult to quantify |
| • | There are 2 separate important subtypes of AD pathology—amyloid plaques and neurofibrillary tangles—that develop in different patterns in AD brains after a predictable course |
| Summary | |
| • | Evidence from AD clinicopathological studies strongly supports the existence of a specific prevalent disease as defined by the presence of clinical dementia, amyloid plaques, and neurofibrillary tangles |
| • | End-stage AD patients have extremely high burden of plaques and tangles, and there is not a subset of cases with very dense AD-type lesions lacking cognitive decline |
| • | There is not a significant subset of cases with severe cognitive decline lacking a pathological substrate |
| • | Results are compatible with the hypothesis that amyloid plaques and neurofibrillary tangle contribute to clinical decline in AD |
ACKNOWLEDGMENTS
The authors thank all of the participants in the longitudinal aging study and brain banks. The authors thank Richard Kryscio, PhD, and Marta Mendiondo, PhD, for data management and statistical support; Ela Patel, Ann Tudor, Paula Thomason, Dr Huaichen Liu, and Sonya Anderson for technical support; Frederick Schmitt, PhD, Gregory Jicha, MD, PhD, Charles Smith, MD, Gregory Cooper, MD, PhD, Nancy Stiles, MD, and Allison Caban-Holt, PhD, for clinical evaluations; and Daron Davis, MD, and Dianne Wilson, MD, for pathological assessments.
This study was supported by Grant No. 5-P30-AG028383 and Grant No. K08 NS050110 from the National Institutes of Health, Bethesda, Maryland, by a grant from the Healy Family Foundation, and funding from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Castellani RJ, Lee HG, Zhu X, et al. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goedert M. Tau protein and neurodegeneration. Semin Cell Dev Biol. 2004;15:45–49. doi: 10.1016/j.semcdb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 3.Buee L, Bussiere T, Buee-Scherrer V, et al. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PT, Keller JN. RNA in brain disease: No longer just “the messenger in the middle.”. J Neuropathol Exp Neurol. 2007;66:461–468. doi: 10.1097/01.jnen.0000240474.27791.f3. [DOI] [PubMed] [Google Scholar]
- 5.Feany MB, Dickson DW. Neurodegenerative disorders with extensive tau pathology: A comparative study and review. Ann Neurol. 1996;40:139–148. doi: 10.1002/ana.410400204. [DOI] [PubMed] [Google Scholar]
- 6.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: Consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham DI, Gentleman SM, Nicoll JA, et al. Altered beta-APP metabolism after head injury and its relationship to the aetiology of Alzheimer’s disease. Acta Neurochir Suppl. 1996;66:96–102. doi: 10.1007/978-3-7091-9465-2_17. [DOI] [PubMed] [Google Scholar]
- 8.Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts GW, Allsop D, Bruton C. The occult aftermath of boxing. J Neurol Neurosurg Psychiatry. 1990;53:373–378. doi: 10.1136/jnnp.53.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H, Del Tredici K. Invited article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka K, Miki T, Katsuya T, et al. The 717Val-Ile substitution in amyloid precursor protein is associated with familial Alzheimer’s disease regardless of ethnic groups. Biochem Biophys Res Commun. 1991;178:1141–1146. doi: 10.1016/0006-291x(91)91011-z. [DOI] [PubMed] [Google Scholar]
- 12.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 13.Mayeux R, Hyslop PS. Alzheimer’s disease: Advances in trafficking. Lancet Neurol. 2008;7:2–3. doi: 10.1016/S1474-4422(07)70298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 15.Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 16.Katzman R, Kawas C. The epidemiology of dementia and Alzheimer disease. In: Terry RD, editor. Alzheimer Disease. New York, NY: Raven Press, LTD; 1994. pp. 105–122. [Google Scholar]
- 17.Williams MM, Xiong C, Morris JC, et al. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67:1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
- 18.Scarmeas N, Habeck CG, Hilton J, et al. APOE related alterations in cerebral activation even at college age. J Neurol Neurosurg Psychiatry. 2005;76:1440–1444. doi: 10.1136/jnnp.2004.053645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CD, Chebrolu H, Wekstein DR, et al. Brain structural alterations before mild cognitive impairment. Neurology. 2007;68:1268–1273. doi: 10.1212/01.wnl.0000259542.54830.34. [DOI] [PubMed] [Google Scholar]
- 20.Thal DR, Del Tredici K, Braak H. Neurodegeneration in normal brain aging and disease. Sci Aging Knowledge Environ. 2004;2004:pe26. doi: 10.1126/sageke.2004.23.pe26. [DOI] [PubMed] [Google Scholar]
- 21.Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: A clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 22.Mosconi L, De Santi S, Rusinek H, et al. Magnetic resonance and PET studies in the early diagnosis of Alzheimer’s disease. Expert Rev Neurother. 2004;4:831–849. doi: 10.1586/14737175.4.5.831. [DOI] [PubMed] [Google Scholar]
- 23.Rusinek H, De Santi S, Frid D, et al. Regional brain atrophy rate predicts future cognitive decline: 6-Year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- 24.Backman L, Jones S, Berger AK, et al. Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 25.Del Tredici K, Braak H. Neurofibrillary changes of the Alzheimer type in very elderly individuals: Neither inevitable nor benign: Commentary on “No disease in the brain of a 115-year-old woman.”. Neurobiol Aging. 2008;29:1133–1136. doi: 10.1016/j.neurobiolaging.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 27.Giannakopoulos P, Hof PR, Giannakopoulos AS, et al. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of very old patients. Arch Neurol. 1995;52:1150–1159. doi: 10.1001/archneur.1995.00540360028012. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell TW, Mufson EJ, Schneider JA, et al. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer’s disease. Ann Neurol. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 29.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002;3:153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- 31.Braak H, Braak E. Staging of Alzheimer’s disease–related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [discussion 8–84] [DOI] [PubMed] [Google Scholar]
- 32.Price JL, Davis PB, Morris JC, et al. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 33.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 34.Markesbery WR, Schmitt FA, Kryscio RJ, et al. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 35.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 36.Green MS, Kaye JA, Ball MJ. The Oregon brain aging study: Neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- 37.Crystal HA, Dickson DW, Sliwinski MJ, et al. Pathological markers associated with normal aging and dementia in the elderly. Ann Neurol. 1993;34:566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- 38.Morris JC, Storandt M, McKeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC, Negash S. Mild cognitive impairment: An overview. CNS Spectr. 2008;13:45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- 40.Skoog I. Detection of preclinical Alzheimer’s disease. N Engl J Med. 2000;343:502–503. doi: 10.1056/NEJM200008173430709. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt FA, Davis DG, Wekstein DR, et al. “Preclinical” AD revisited: Neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 42.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 43.Chong MS, Sahadevan S. Preclinical Alzheimer’s disease: Diagnosis and prediction of progression. Lancet Neurol. 2005;4:576–579. doi: 10.1016/S1474-4422(05)70168-X. [DOI] [PubMed] [Google Scholar]
- 44.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 45.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: A commentary. Neurobiol Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 46.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 47.Berlau DJ, Kahle-Wrobleski K, Head E, et al. Dissociation of neuropathologic findings and cognition: Case report of an apolipoprotein E epsilon2/epsilon2 genotype. Arch Neurol. 2007;64:1193–1196. doi: 10.1001/archneur.64.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernando MS, Ince PG. Vascular pathologies and cognition in a population-based cohort of elderly people. J Neurol Sci. 2004;226:13–17. doi: 10.1016/j.jns.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 50.Xuereb JH, Brayne C, Dufouil C, et al. Neuropathological findings in the very old. Results from the first 101 brains of a population-based longitudinal study of dementing disorders. Ann N Y Acad Sci. 2000;903:490–496. doi: 10.1111/j.1749-6632.2000.tb06404.x. [DOI] [PubMed] [Google Scholar]
- 51.Hulette CM, Welsh-Bohmer KA, Murray MG, et al. Neuropathological and neuropsychological changes in “normal” aging: Evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 52.Wang DS, Bennett DA, Mufson E, et al. Decreases in soluble alpha-synuclein in frontal cortex correlate with cognitive decline in the elderly. Neurosci Lett. 2004;359:104–108. doi: 10.1016/j.neulet.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 53.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: A voxel-based morphometry study. Neurology. 2008;71:743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noda K, Sasaki K, Fujimi K, et al. Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): The Hisayama Study. Neuropathology. 2006;26:508–518. doi: 10.1111/j.1440-1789.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 55.Newell KL, Hyman BT, Growdon JH, et al. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Haroutunian V, Purohit DP, Perl DP, et al. Neurofibrillary tangles in nondemented elderly subjects and mild Alzheimer disease. Arch Neurol. 1999;56:713–718. doi: 10.1001/archneur.56.6.713. [DOI] [PubMed] [Google Scholar]
- 57.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–413. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 58.Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology. 1992;42:1681–1688. doi: 10.1212/wnl.42.9.1681. [DOI] [PubMed] [Google Scholar]
- 59.Iseki E, Tsunoda S, Suzuki K, et al. Regional quantitative analysis of NFT in brains of non-demented elderly persons: Comparisons with findings in brains of late-onset Alzheimer’s disease and limbic NFT dementia. Neuropathology. 2002;22:34–39. doi: 10.1046/j.0919-6544.2001.00425.x. [DOI] [PubMed] [Google Scholar]
- 60.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 61.Bancher C, Jellinger K, Lassmann H, et al. Correlations between mental state and quantitative neuropathology in the Vienna longitudinal study on dementia. Eur Arch Psychiatry Clin Neurosci. 1996;246:137–146. doi: 10.1007/BF02189115. [DOI] [PubMed] [Google Scholar]
- 62.Davis DG, Schmitt FA, Wekstein DR, et al. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Nelson PT, Jicha GA, Schmitt FA, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: Neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol. 2007;66:1136–1146. doi: 10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. J Neurol Sci. 2007;257:80–87. doi: 10.1016/j.jns.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 65.Ulrich J, Spillantini MG, Goedert M, et al. Abundant neurofibrillary tangles without senile plaques in a subset of patients with senile dementia. Neurodegeneration. 1992;1:257–264. [Google Scholar]
- 66.Yamada M, Itoh Y, Sodeyama N, et al. Senile dementia of the neurofibrillary tangle type: A comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12:117–126. doi: 10.1159/000051245. [DOI] [PubMed] [Google Scholar]
- 67.Tschanz JT, Treiber K, Norton MC, et al. A population study of Alzheimer’s disease: Findings from the Cache County Study on Memory, Health, and Aging. Care Manag J. 2005;6:107–114. doi: 10.1891/cmaj.6.2.107. [DOI] [PubMed] [Google Scholar]
- 68.Tiraboschi P, Sabbagh MN, Hansen LA, et al. Alzheimer disease without neocortical neurofibrillary tangles: “A second look.”. Neurology. 2004;62:1141–1147. doi: 10.1212/01.wnl.0000118212.41542.e7. [DOI] [PubMed] [Google Scholar]
- 69.Terry RD, Hansen LA, DeTeresa R, et al. Senile dementia of the Alzheimer type without neocortical neurofibrillary tangles. J Neuropathol Exp Neurol. 1987;46:262–268. doi: 10.1097/00005072-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Hansen LA, Masliah E, Galasko D, et al. Plaque-only Alzheimer disease is usually the Lewy body variant, and vice versa. J Neuropathol Exp Neurol. 1993;52:648–654. doi: 10.1097/00005072-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 71.Klunemann HH, Fronhofer W, Werner-Fuchtenbusch D, et al. Characterization of the kindred of Alois Alzheimer’s patient with plaque-only dementia. Alzheimer Dis Assoc Disord. 2006;20:291–294. doi: 10.1097/01.wad.0000213855.81989.c8. [DOI] [PubMed] [Google Scholar]
- 72.Crystal HA, Dickson D, Davies P, et al. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57:713–719. doi: 10.1001/archneur.57.5.713. [DOI] [PubMed] [Google Scholar]
- 73.Bowler JV, Munoz DG, Merskey H, et al. Fallacies in the pathological confirmation of the diagnosis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1998;64:18–24. doi: 10.1136/jnnp.64.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider JA, Boyle PA, Arvanitakis Z, et al. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol. 2007;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 75.Todorov AB, Go RC, Constantinidis J, et al. Specificity of the clinical diagnosis of dementia. J Neurol Sci. 1975;26:81–98. doi: 10.1016/0022-510x(75)90116-1. [DOI] [PubMed] [Google Scholar]
- 76.Chui HC, Zarow C, Mack WJ, et al. Cognitive impact of subcortical vascular and Alzheimer’s disease pathology. Ann Neurol. 2006;60:677–687. doi: 10.1002/ana.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rockwood K. Mixed dementia: Alzheimer’s and cerebrovascular disease. Int Psychogeriatr. 2003;15 suppl 1:39–46. doi: 10.1017/S1041610203008949. [DOI] [PubMed] [Google Scholar]
- 78.Kovari E, Gold G, Herrmann FR, et al. Cortical microinfarcts and demyelination affect cognition in cases at high risk for dementia. Neurology. 2007;68:927–931. doi: 10.1212/01.wnl.0000257094.10655.9a. [DOI] [PubMed] [Google Scholar]
- 79.Gold G, Giannakopoulos P, Herrmann FR, et al. Identification of Alzheimer and vascular lesion thresholds for mixed dementia. Brain. 2007;130:2830–2836. doi: 10.1093/brain/awm228. [DOI] [PubMed] [Google Scholar]
- 80.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: Update from the Honolulu-Asia aging study. J Geriatr Psychiatry Neurol. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. Prevalence of stroke—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56:469–474. [PubMed] [Google Scholar]
- 82.Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc. 2006;81:223–230. doi: 10.4065/81.2.223. [DOI] [PubMed] [Google Scholar]
- 83.Mungas D, Reed BR, Ellis WG, et al. The effects of age on rate of progression of Alzheimer disease and dementia with associated cerebrovascular disease. Arch Neurol. 2001;58:1243–1247. doi: 10.1001/archneur.58.8.1243. [DOI] [PubMed] [Google Scholar]
- 84.Regan C, Katona C, Walker Z, et al. Relationship of vascular risk to the progression of Alzheimer disease. Neurology. 2006;67:1357–1362. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- 85.Snowdon DA, Greiner LH, Mortimer JA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 86.Petrovitch H, Ross GW, He Q, et al. Characterization of Japanese-American men with a single neocortical AD lesion type. Neurobiol Aging. 2008;29:1448–1455. doi: 10.1016/j.neurobiolaging.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gold G, Kovari E, Herrmann FR, et al. Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke. 2005;36:1184–1188. doi: 10.1161/01.STR.0000166052.89772.b5. [DOI] [PubMed] [Google Scholar]
- 88.Jellinger KA. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2004;62:160. doi: 10.1212/wnl.62.1.160. [author reply] [DOI] [PubMed] [Google Scholar]
- 89.Lee VM, Giasson BI, Trojanowski JQ. More than just two peas in a pod: Common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends Neurosci. 2004;27:129–134. doi: 10.1016/j.tins.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Kraybill ML, Larson EB, Tsuang DW, et al. Cognitive differences in dementia patients with autopsy-verified AD, Lewy body pathology, or both. Neurology. 2005;64:2069–2073. doi: 10.1212/01.WNL.0000165987.89198.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olichney JM, Galasko D, Salmon DP, et al. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology. 1998;51:351–357. doi: 10.1212/wnl.51.2.351. [DOI] [PubMed] [Google Scholar]
- 92.Nelson PT, Kryscio RJ, Abner EA, et al. Acetylcholinesterase inhibitor treatment is associated with relatively slow cognitive decline in patients with Alzheimer’s disease and AD+DLB. J Alzheimers Dis. 2008 doi: 10.3233/JAD-2009-0926. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berg L, McKeel DW, Jr, Miller JP, et al. Neuropathological indexes of Alzheimer’s disease in demented and nondemented persons aged 80 years and older. Arch Neurol. 1993;50:349–358. doi: 10.1001/archneur.1993.00540040011008. [DOI] [PubMed] [Google Scholar]
- 94.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 95.Kosaka K, Iseki E. Recent advances in dementia research in Japan: Non-Alzheimer-type degenerative dementias. Psychiatry Clin Neurosci. 1998;52:367–373. doi: 10.1046/j.1440-1819.1998.00402.x. [DOI] [PubMed] [Google Scholar]
- 96.Amador-Ortiz C, Ahmed Z, Zehr C, et al. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol (Berl) 2007;113:245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knopman DS, Petersen RC, Cha RH, et al. Incidence and causes of nondegenerative nonvascular dementia: A population-based study. Arch Neurol. 2006;63:218–221. doi: 10.1001/archneur.63.2.218. [DOI] [PubMed] [Google Scholar]
- 98.Erkinjuntti T, Sulkava R, Kovanen J, et al. Suspected dementia: Evaluation of 323 consecutive referrals. Acta Neurol Scand. 1987;76:359–364. doi: 10.1111/j.1600-0404.1987.tb03594.x. [DOI] [PubMed] [Google Scholar]
- 99.Tiraboschi P, Hansen LA, Thal LJ, et al. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62:1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 100.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: The uniform data set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 101.Guillozet AL, Weintraub S, Mash DC, et al. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 102.Imhof A, Kovari E, von Gunten A, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: A new paradigm? J Neurol Sci. 2007;257:72–79. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 103.Cummings BJ, Pike CJ, Shankle R, et al. Beta-amyloid deposition and other measures of neuropathology predict cognitive status in Alzheimer’s disease. Neurobiol Aging. 1996;17:921–933. doi: 10.1016/s0197-4580(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 104.Davis PC, Gearing M, Gray L, et al. The CERAD experience, Part VIII: Neuroimaging-neuropathology correlates of temporal lobe changes in Alzheimer’s disease. Neurology. 1995;45:178–179. doi: 10.1212/wnl.45.1.178. [DOI] [PubMed] [Google Scholar]
- 105.Koepsell TD, Kurland BF, Harel O, et al. Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology. 2008;70:1732–1739. doi: 10.1212/01.wnl.0000284603.85621.aa. [DOI] [PubMed] [Google Scholar]
- 106.Silver MH, Newell K, Brady C, et al. Distinguishing between neurodegenerative disease and disease-free aging: Correlating neuropsychological evaluations and neuropathological studies in centenarians. Psychosom Med. 2002;64:493–501. doi: 10.1097/00006842-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 107.Braak H, Braak E, Ohm T, et al. Alzheimer’s disease: Mismatch between amyloid plaques and neuritic plaques. Neurosci Lett. 1989;103:24–28. doi: 10.1016/0304-3940(89)90479-5. [DOI] [PubMed] [Google Scholar]
- 108.Nagy Z, Esiri MM, Jobst KA, et al. Relative roles of plaques and tangles in the dementia of Alzheimer’s disease: Correlations using three sets of neuropathological criteria. Dementia. 1995;6:21–31. doi: 10.1159/000106918. [DOI] [PubMed] [Google Scholar]
- 109.Thal DR, Rub U, Schultz C, et al. Sequence of Abeta-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol. 2000;59:733–748. doi: 10.1093/jnen/59.8.733. [DOI] [PubMed] [Google Scholar]
- 110.Bennett DA, Schneider JA, Wilson RS, et al. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 111.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 112.Wisniewski HM, Barcikowska M, Kida E. Phagocytosis of beta/A4 amyloid fibrils of the neuritic neocortical plaques. Acta Neuropathol. 1991;81:588–590. doi: 10.1007/BF00310142. [DOI] [PubMed] [Google Scholar]
- 113.Meyer-Luehmann M, Spires-Jones TL, Prada C, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benzing WC, Mufson EJ, Armstrong DM. Immunocytochemical distribution of peptidergic and cholinergic fibers in the human amygdala: Their depletion in Alzheimer’s disease and morphologic alteration in non-demented elderly with numerous senile plaques. Brain Res. 1993;625:125–138. doi: 10.1016/0006-8993(93)90145-d. [DOI] [PubMed] [Google Scholar]
- 115.Armstrong DM, Benzing WC, Evans J, et al. Substance P and so-matostatin coexist within neuritic plaques: Implications for the pathogenesis of Alzheimer’s disease. Neuroscience. 1989;31:663–671. doi: 10.1016/0306-4522(89)90431-4. [DOI] [PubMed] [Google Scholar]
- 116.Wisniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol. 1973;32:566–584. doi: 10.1097/00005072-197310000-00007. [DOI] [PubMed] [Google Scholar]
- 117.Wisniewski HM, Vorbrodt AW, Moretz RC, et al. Pathogenesis of neuritic (senile) and amyloid plaque formation. Exp Brain Res. 1982 suppl 5:3–9. doi: 10.1007/978-3-642-68507-1_1. [DOI] [PubMed] [Google Scholar]
- 118.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimmers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gold G, Bouras C, Kovari E, et al. Clinical validity of Braak neuropathological staging in the oldest-old. Acta Neuropathol (Berl) 2000;99:579–582. doi: 10.1007/s004010051163. [discussion 83–84] [DOI] [PubMed] [Google Scholar]
- 120.Duyckaerts C, Bennecib M, Grignon Y, et al. Modeling the relation between neurofibrillary tangles and intellectual status. Neurobiol Aging. 1997;18:267–273. doi: 10.1016/s0197-4580(97)80306-5. [DOI] [PubMed] [Google Scholar]
- 121.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: Findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 122.Arriagada PV, Growdon JH, Hedley-Whyte ET, et al. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 123.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 124.Bobinski M, Wegiel J, Tarnawski M, et al. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:414–420. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 125.Rossler M, Zarski R, Bohl J, et al. Stage-dependent and sector-specific neuronal loss in hippocampus during Alzheimer’s disease. Acta Neuropathol. 2002;103:363–369. doi: 10.1007/s00401-001-0475-7. [DOI] [PubMed] [Google Scholar]
- 126.Gomez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 127.Fukutani Y, Kobayashi K, Nakamura I, et al. Neurons, intracellular and extracellular neurofibrillary tangles in subdivisions of the hippocampal cortex in normal ageing and Alzheimer’s disease. Neurosci Lett. 1995;200:57–60. doi: 10.1016/0304-3940(95)12083-g. [DOI] [PubMed] [Google Scholar]
- 128.Bartus RT, Dean RL, 3rd, Beer B, et al. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 129.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 130.Mesulam M, Shaw P, Mash D, et al. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004;55:815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- 131.Rasool CG, Svendsen CN, Selkoe DJ. Neurofibrillary degeneration of cholinergic and noncholinergic neurons of the basal forebrain in Alzheimer’s disease. Ann Neurol. 1986;20:482–488. doi: 10.1002/ana.410200407. [DOI] [PubMed] [Google Scholar]
- 132.Saper CB, German DC, White CL., 3rd Neuronal pathology in the nucleus basalis and associated cell groups in senile dementia of the Alzheimer’s type: Possible role in cell loss. Neurology. 1985;35:1089–1095. doi: 10.1212/wnl.35.8.1089. [DOI] [PubMed] [Google Scholar]
- 133.Saper CB, Chelimsky TC. A cytoarchitectonic and histochemical study of nucleus basalis and associated cell groups in the normal human brain. Neuroscience. 1984;13:1023–1037. doi: 10.1016/0306-4522(84)90286-0. [DOI] [PubMed] [Google Scholar]
- 134.Izquierdo I. Mechanism of action of scopolamine as an amnestic. Trends Pharmacol Sci. 1989;10:175–177. doi: 10.1016/0165-6147(89)90231-9. [DOI] [PubMed] [Google Scholar]
- 135.Airaksinen MS, Paetau A, Paljarvi L, et al. Histamine neurons in human hypothalamus: Anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991;44:465–481. doi: 10.1016/0306-4522(91)90070-5. [DOI] [PubMed] [Google Scholar]
- 136.Grudzien A, Shaw P, Weintraub S, et al. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 137.German DC, White CL, 3rd, Sparkman DR. Alzheimer’s disease: Neurofibrillary tangles in nuclei that project to the cerebral cortex. Neuroscience. 1987;21:305–312. doi: 10.1016/0306-4522(87)90123-0. [DOI] [PubMed] [Google Scholar]
- 138.Rub U, Del Tredici K, Schultz C, et al. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol. 2000;26:553–567. doi: 10.1046/j.0305-1846.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 139.Zweig RM, Ross CA, Hedreen JC, et al. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol. 1988;24:233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- 140.Ishino H, Otsuki S. Frequency of Alzheimer’s neurofibrillary tangles in the basal ganglia and brain-stem in Alzheimer’s disease, senile dementia and the aged. Folia Psychiatr Neurol Jpn. 1975;29:279–287. doi: 10.1111/j.1440-1819.1975.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 141.Torack RM, Morris JC. The association of ventral tegmental area histopathology with adult dementia. Arch Neurol. 1988;45:497–501. doi: 10.1001/archneur.1988.00520290025008. [DOI] [PubMed] [Google Scholar]
- 142.Ohtsubo K, Izumiyama N, Kuzuhara S, et al. Curly fibers are tau-positive strands in the pre- and post-synaptic neurites, consisting of paired helical filaments: Observations by the freeze-etch and replica method. Acta Neuropathol. 1990;81:111–115. doi: 10.1007/BF00334498. [DOI] [PubMed] [Google Scholar]
- 143.Braak H, Braak E, Grundke-Iqbal I, et al. Occurrence of neuropil threads in the senile human brain and in Alzheimer’s disease: A third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986;65:351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- 144.Mitchell TW, Nissanov J, Han LY, et al. Novel method to quantify neuropil threads in brains from elders with or without cognitive impairment. J Histochem Cytochem. 2000;48:1627–1638. doi: 10.1177/002215540004801206. [DOI] [PubMed] [Google Scholar]
- 145.Markesbery WR, Wang HZ, Kowall NW, et al. Morphometric image analysis of neuropil threads in Alzheimer’s disease. Neurobiol Aging. 1993;14:303–307. doi: 10.1016/0197-4580(93)90115-r. [DOI] [PubMed] [Google Scholar]
- 146.Kril JJ, Patel S, Harding AJ, et al. Neuron loss from the hippocampus of Alzheimer’s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- 147.Vogt BA, Vogt LJ, Vrana KE, et al. Multivariate analysis of laminar patterns of neurodegeneration in posterior cingulate cortex in Alzheimer’s disease. Exp Neurol. 1998;153:8–22. doi: 10.1006/exnr.1998.6852. [DOI] [PubMed] [Google Scholar]
- 148.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 149.Lovell MA, Robertson JD, Buchholz BA, et al. Use of bomb pulse carbon-14 to age senile plaques and neurofibrillary tangles in Alzheimer’s disease. Neurobiol Aging. 2002;23:179–186. doi: 10.1016/s0197-4580(01)00281-0. [DOI] [PubMed] [Google Scholar]