SUMMARY
Transcriptional regulatory networks that control the morphologic and functional diversity of mammalian neurons are still largely undefined. Here we dissect the roles of the highly homologous POU-domain transcription factors Brn3a and Brn3b in retinal ganglion cell (RGC) development and function using conditional Brn3a and Brn3b alleles that permit the visualization of individual wild type or mutant cells. We show that Brn3a- and Brn3b-expressing RGCs exhibit overlapping but distinct dendritic stratifications and central projections. Deletion of Brn3a alters dendritic stratification and the ratio of monostratified: bistratified RGCs, with little or no change in central projections. In contrast, deletion of Brn3b leads to RGC transdifferentiation and loss, axon defects in the eye and brain, and defects in central projections that differentially compromise a variety of visually-driven behaviors. These findings reveal distinct roles for Brn3a and Brn3b in programming RGC diversity, and they illustrate the broad utility of germ-line methods for genetically manipulating and visualizing individual identified mammalian neurons.
INTRODUCTION
The retina is currently the best understood subdivision within the vertebrate CNS. Experiments over the past several decades have provided a remarkably detailed picture of the morphology, synaptic connectivity, and physiology of each of the major classes of retinal neurons, and in many cases these properties can be related to the performance of the visual system at the level of the intact animal (Rodieck, 1998). By contrast, the molecular mechanisms responsible for the development of structural and functional diversity among retinal neurons are still poorly understood.
Retinal ganglion cells (RGCs), the output neurons of the vertebrate retina, relay visual information to brain centers that mediate image-forming vision, or that control eye movements, pupil diameter, or circadian photoentrainment. All RGCs integrate and process information from bipolar and amacrine cells. A small percentage of RGCs are also intrinsically photoreceptive (ipRGCs) and subserve non-image-forming vision (Hattar et al., 2002). Since the realization over a century ago that RGCs fall into distinct morphologic classes (Ramon y Cajal, 1893), there has been an intensive effort to define and correlate RGC physiology and anatomy, with the latter category encompassing both dendritic morphology and the trajectories of RGC axons in the brain.
One of the central physiologic-anatomic correlations relates the stratification of RGC dendrites within the inner plexiform layer (IPL) and the type of visual information relayed by that cell. RGC dendrites that reside within the inner layers of the IPL are activated by light (the ON pathway) and those that reside in the outer layers of the IPL are inhibited by light (the OFF pathway) (Nelson et al., 1978). Additional physiologic-anatomic correlations have further divided RGCs into a still-growing collection of subtypes. In one of the most recent additions to this collection, a population of OFF RGCs with wedge-shaped and dorsally oriented dendritic arbors was found to project to the superior colliculus (SC) and respond specifically to visual stimuli with upward motion (Kim et al., 2008). Current data suggest that the number of distinct RGC subtypes in the mammalian retina is likely to be at least twenty (Dacey et al., 2003; Masland, 2001).
Several transcription factors that control RGC development have been identified, including the POU-domain protein Brn3b. Shortly after completing their terminal mitosis, ~80% of RGC precursors express Brn3b, and ~1 day later the closely related Brn3a and Brn3c genes are expressed in ~80% and ~20% of developing RGCs, respectively, subsets that substantially overlap the subset expressing Brn3b (Pan et al., 2005; Quina et al., 2005; Xiang, 1998; Xiang et al., 1995). In the absence of Brn3b three distinct defects occur: (1) many cells that would have become RGCs instead differentiate as amacrine or horizontal cells and then die prenatally, (2) among RGCs, multiple genes are down-regulated and some RGC axons follow aberrant intra-ocular and extra-ocular trajectories, and (3) by adulthood the number of RGCs is reduced to <30% of wild type, including large reductions in the number of RGCs expressing Brn3a or Brn3c (Erkman et al., 1996; Erkman et al., 2000; Gan et al., 1999; Gan et al., 1996; Mu et al., 2004; Qiu et al., 2008; Wang et al., 2000). The role of Brn3a and Brn3c in RGC development has been less clear. Brn3c−/− mice show a severe defect in the maturation and survival of inner ear sensory hair cells (Erkman et al., 1996; Xiang, 1998; Xiang et al., 1997), but they show no obvious defects in retinal structure. However, Brn3c appears to function in the retina in some capacity because Brn3b−/−;Brn3c−/− retinas have a more severe loss of RGC axons than Brn3b−/− retinas (Wang et al., 2002). Brn3a−/− mice die at birth due to defects in dorsal root and trigeminal neurons, but show no obvious defects in retinal structure at this point in development (McEvilly et al., 1996; Xiang et al., 1996). However, a definitive analysis of Brn3a function in RGC development has not yet been reported; such an analysis requires tissue-specific gene deletion since much of retinal development occurs postnatally in the mouse.
Despite the vastly different phenotypes of Brn3a−/−, Brn3b−/−, and Brn3c−/− mice, the high degree of sequence similarity among the three Brn3 proteins (95% identity in the DNA binding domain and 41% average identity outside of the DNA binding domains, excluding large insertions/deletions) and their identical DNA binding specificity (Liu et al., 2000; Xiang et al., 1995) has led to the suggestion that these proteins could function interchangeably, with any in vivo differences in their activities principally arising from differences in the timing and cell-type specificity of their expression. In support of this general idea, ectopic mis-expression of any of the Brn3 proteins in chick retinal progenitors promotes an RGC fate (Liu et al., 2000), and mice carrying a targeted replacement of Brn3b coding sequences by Brn3a coding sequences show normal numbers of RGCs in the adult retina and grossly normal RGC axon guidance at the optic chiasm (Pan et al., 2005).
The experiments described above pose a variety of questions regarding the roles of Brn3 transcription factors in the specification of RGC subtypes. What is the relationship between the partially overlapping expression patterns of Brn3 family members and RGC morphologies and central projections? In what ways are RGC morphologies affected by Brn3 mutations? What are the consequences of Brn3 mutations for vision? Using conditional Brn3 alleles that permit the visualization of individual genetically altered RGCs together with tests of light-evoked behaviors, the experiments described here address these questions as they relate to Brn3a and Brn3b, the two Brn3 genes expressed in the majority of RGCs.
Results
Brn3a and Brn3b alleles that conditionally activate alkaline phosphatase expression
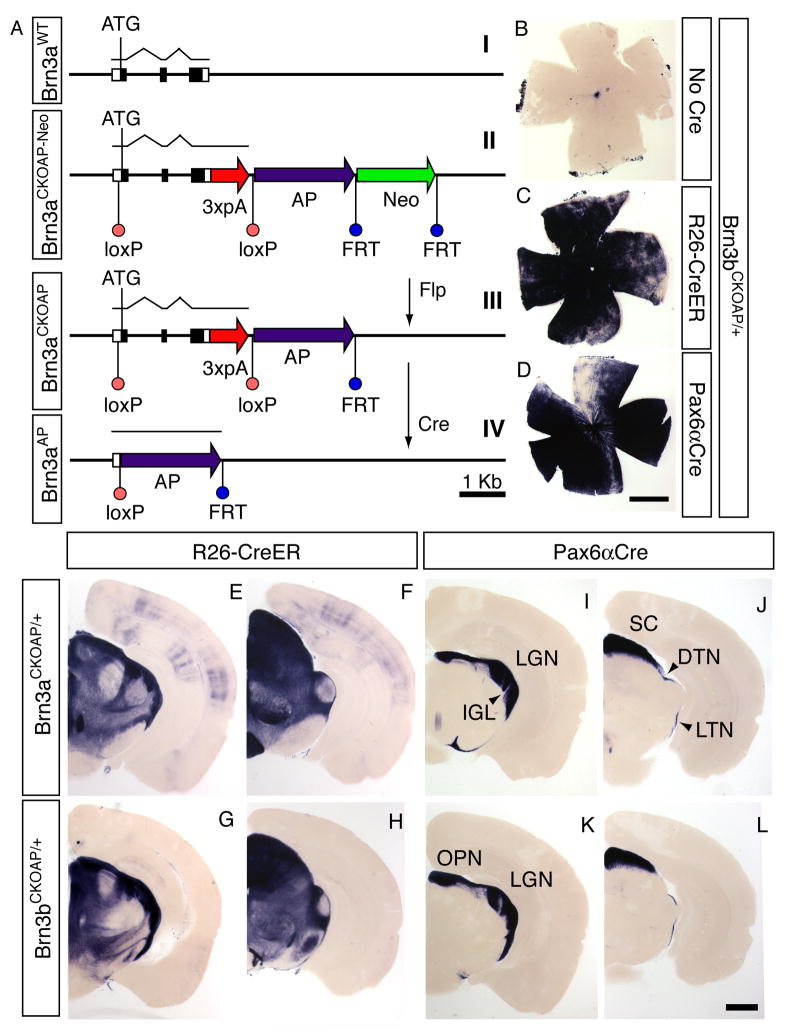
To both genetically modify and selectively visualize individual Brn3a- or Brn3b-expressing RGCs, we constructed conditional alleles of the Brn3a and Brn3b genes by inserting loxP sites 5′ and 3′ of each Brn3 coding region together with a human placental alkaline phosphatase (AP) coding region immediately downstream of the 3′ loxP site (Figure 1A). A series of transcription termination signals inserted immediately upstream of the 3′ loxP site minimized any read-through into the AP coding region. Following Cre-mediated recombination, the AP coding region was faithfully expressed under the control of the adjacent Brn3 promoter (Figure 1 and Supplementary Figure 1).
Figure 1. Conditional targeting of the Brn3a and Brn3b loci.
(A) Schematic of the conditional targeting strategy illustrated for Brn3a; an identical strategy was used for Brn3b. (I) the endogenous locus, (II) the targeted locus, (III) the targeted locus after Flp-mediated excision of the PGK-neo cassette, and (IV) the targeted locus after Cre-mediated excision of the Brn3a coding region, which places the AP coding region under the control of the Brn3a promoter. Filled black boxes, Brn3a coding region; open boxes, 5′ and 3′ UTRs.
(B–D) flat mounted Brn3bCKOAP/+ retinas histochemically stained for AP activity: (B) in the absence of Cre, (C) with ROSA26-creER(T), and (D) with Pax6αCre.
(E–L) Coronal brain sections (200 μm thickness) from (E,F,I,J) Brn3aCKOAP/+ or (G,H,K,L) Brn3bCKOAP/+ in the background of (E–H) ROSA26-CreER(T) or (I–L) Pax6αCre. The left and right members of each pair of sections (e.g. E and F) are at the level of the LGN and SC, respectively. The ROSA26-CreER(T) samples are from mice that had not been exposed to 4-hydroxytamoxifen, showing that the Brn3aCKOAP and Brn3bCKOAP loci exhibit a relatively high efficiency of Cre-mediated recombination. No AP-expressing cells were seen in the absence of a Cre or CreER gene. DTN, dorsal terminal nucleus; IGL, intergeniculate leaflet; LGN, lateral geniculate nucleus; LTN, lateral terminal nucleus; OPN, olivary pretectal nucleus; SC, superior colliculus. Scale bars in D and L, 1 mm.
In the experiments described below, we used either of two heterozygous configurations at the Brn3a or Brn3b loci: the conditional allele over the wild type allele (e.g. Brn3bCKOAP/+) or the conditional allele over a conventional null allele (e.g. Brn3bCKOAP/−). In the former case, Cre-mediated recombination results in AP expression in cells that are heterozygous for the Brn3 gene of interest (Brn3aAP/+ or Brn3bAP/+) and these cells appear to be phenotypically wild type (WT), while in the latter case Cre-mediated recombination results in AP expression in cells that carry two mutant alleles (Brn3aAP/− or Brn3bAP/−). Brn3aCKOAP/+ and Brn3bCKOAP/+ mice carrying the ubiquitously expressed Rosa26CreER(T) locus (Badea et al., 2003) exhibit AP activity in the retina and in retino-recipient areas in the brain, as well as in a variety of other brain regions (Figure 1C,E–H). Brn3aCKOAP/+ or Brn3bCKOAP/+ mice carrying the retina-specific Pax6αCre transgene (Marquardt et al., 2001) exhibit AP activity only in the retina and in retinorecipient areas of the brain (Figure 1D,I–L). As described by Marquardt et al. (Marquardt et al., 2001) and as seen in Figure 1D, Pax6αCre is expressed at a very low level in a wedge-shaped stripe in the dorsal and central retina; within this zone, the morphologies of well-isolated AP+ RGCs can be analyzed. The Pax6αCre transgene is expressed in the retina beginning at embryonic day (E)9.5 (Marquardt et al., 2001), three days before the onset of Brn3b expression and four days before the onset of Brn3a expression in postmitotic RGC precursors (Xiang, 1998).
RGCs expressing Brn3a and Brn3b show distinct patterns of dendritic stratification
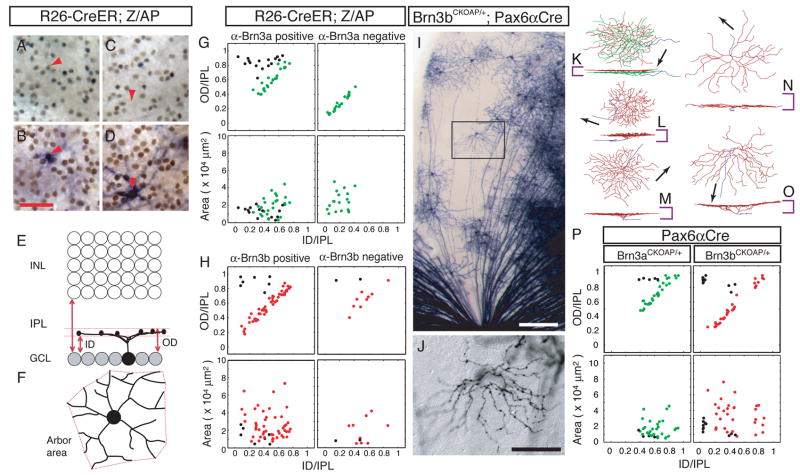
Two approaches based on visualizing the morphologies of individual RGCs in flat-mounted retinas were used to define the wild type (WT) patterns of dendritic arborization in those RGCs that express Brn3a or Brn3b. In the first approach, we analyzed RGC morphologies following sparse and random AP labeling of neurons in mice carrying Rosa26CreER(T) and Z/AP (a transgene that can express AP in essentially any cell type following Cre-mediated recombination (Badea and Nathans, 2004; Lobe et al., 1999)) and determined which AP+ RGCs expressed Brn3a (Figure 2A–D) or Brn3b by immunostaining. In the second approach, we visualized the morphologies of well-isolated AP+ RGCs in Brn3aCKOAP/+;Pax6αCre and Brn3bCKOAP/+;Pax6αCre retinas (Figure 2I–O). For each RGC analyzed, the locations of the dendrites were digitized, and the area of the dendritic arbor in the plane of the retina and its vertical position and thickness in the IPL were determined (Figure 2E,F). As seen in the scatter plots for monostratified RGCs (Figure 2G,H,P), both approaches reveal a striking morphological difference between Brn3a and Brn3b expressing RGCs: while arbors of Brn3b-expressing RGCs are located throughout the full extent of the IPL, the arbors of Brn3a-expressing RGCs are confined to the outer ~60–70% of the IPL. Moreover, the data in Figure 2G show that 20/20 monostratified RGCs that did not express Brn3a stratify within the inner 30–40% of the IPL. Among Brn3aCKOAP/+;Pax6αCre and Brn3bCKOAP/+;Pax6αCre RGCs, 19% and 9%, respectively, are bistratified (Supplementary Figure 2).
Figure 2. The populations of Brn3a- and Brn3b-expressing RGCs have distinct distributions of dendritic stratification.
(A–D) Anti-Brn3a immunoreactive (red arrowhead in A,B) and anti-Brn3a non-reactive (red arrow in C,D) RGC nuclei in flat mounts of R26-CreER(T);ZAP retinas. After HRP immunohistochemistry (A,C) the tissue was processed for AP histochemistry (B,D) and the same region rephotographed. The focal plane is in the GCL, and RGC dendrites in the IPL are out of focus.
(E,F) Morphological parameters for the analysis of RGC dendritic arbors (Badea and Nathans, 2004; Badea et al., 2003). The inner distance (ID) and outer distance (OD), expressed as a fraction of IPL thickness, together define the level of stratification and arbor thickness. The arbor area is calculated as the area of the polygon (in red) with the shortest perimeter that connects the tips of the RGC dendritic arbor when projected in the plane of the retina.
(G,H) Scatter plots of morphological parameters for monostratified Brn3a-positive, Brn3a-negative, Brn3b-positive and Brn3b-negative RGCs. RGCs with axon arbors thicker than 0.25 of the IPL thickness are coded by black symbols in this and all other scatter plots; red and green symbols represent RGCs with narrowly stratified arbors (<0.25 of the IPL thickness).
(I,J) Brn3bAP/+ RGCs in the sparsely recombined zone of a flat mounted Brn3bCKOAP/+;Pax6αCre retina. The optic disc is at the bottom center. The enclosed region in I is shown at higher magnification in J; the digitized morphology of this cell is shown in O. Scale bars in I and J are 200 and 80 μm, respectively.
(K–O) Digitized morphologies of AP-expressing RGCs from Brn3aCKOAP/+;Pax6αCre (K,L,M) and Brn3bCKOAP/+;Pax6αCre (N,O). For each cell, en face (top) and vertical views (bottom) are shown. Vertical and horizontal bars show, respectively, the thickness of the IPL and a 30 μm scale. Axons are shown in blue and arrows indicate the direction of the optic disc. For the bistratified cell in K, dendrites are shown in green (ON lamina) and red (OFF lamina).
(P) Scatter plots of morphological parameters for monostratified Brn3aAP/+ RGCs from Brn3aCKOAP/+;Pax6αCre retinas and Brn3bAP/+ RGCs from Brn3bCKOAP/+;Pax6αCre retinas. A scatter plot of bistratified RGCs is shown in Supplementary Figure 2.
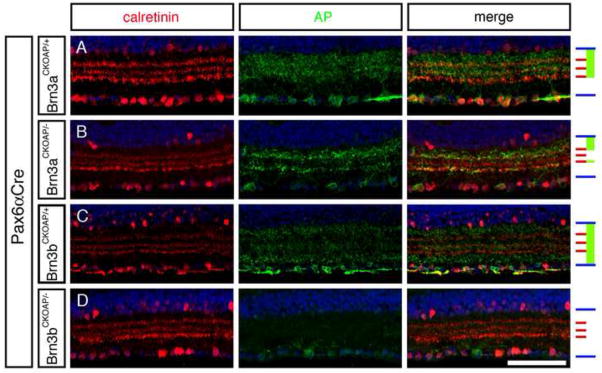
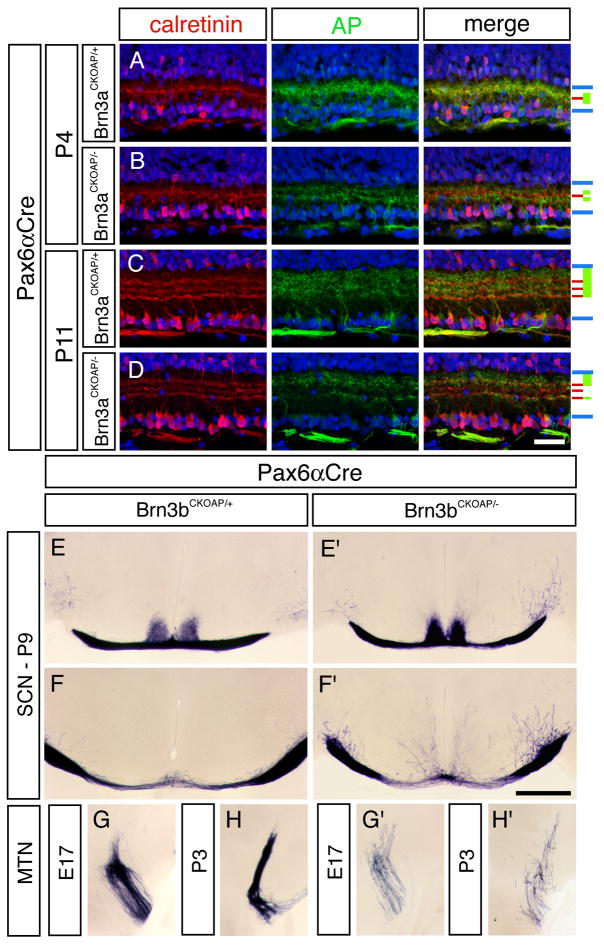
As an independent measure of these patterns of dendritic stratification, vertical sections of Brn3aCKOAP/+;Pax6αCre and Brn3bCKOAP/+;Pax6αCre retinas were immunostained for AP and for calretinin. Calretinin localizes to three narrow bands within the IPL, with the central band marking the junction between ON and OFF sublaminae (Morgan et al., 2006). For this analysis, we focused on regions of the retina with a high level of Cre-mediated recombination where the AP signal within any section is derived from the dendrites of a large number of RGCs. As seen in Figure 3 and Supplementary Figure 3 and in agreement with the analyses of individual RGC morphologies described above, Brn3aAP/+ RGC dendrites are confined to the outer ~60–70% of the IPL whereas the Brn3bAP/+ RGC dendrites populate the full width of the IPL, although with a relatively reduced representation in the innermost ~50% of the OFF substrata demarcated by the outer two bands of calretinin staining. Finally, we observed by double labeling of adult Brn3aCKOAP/+;Pax6αCre and Brn3bCKOAP/+;Pax6αCre with anti-AP and with anti-Brn3a or anti-Brn3b antibodies that 87% of Brn3aAP RGCs express Brn3b and 78% of Brn3bAP RGCs express Brn3a (Supplementary Figure 1A–I).
Figure 3. Vertical sections showing the inner retina immunostained for calretinin and AP reveal distinct stratification levels for dendrites of Brn3aAP/+ and Brn3bAP/+ RGCs, a loss of the centrally stratifying IPL dendrites in Brn3aAP/− RGCs, and a loss of most Brn3bAP/− RGCs.
The GCL is at the bottom. Right, schematic showing, for each set of panels, the boundaries of the IPL (blue), the three calretinin-immunoreactive laminae (red), and the regions occupied by AP-immunoreactive RGC dendrites (green). Scale bar: 80 μm.
Brn3a−/− RGCs exhibit defects in dendritic stratification
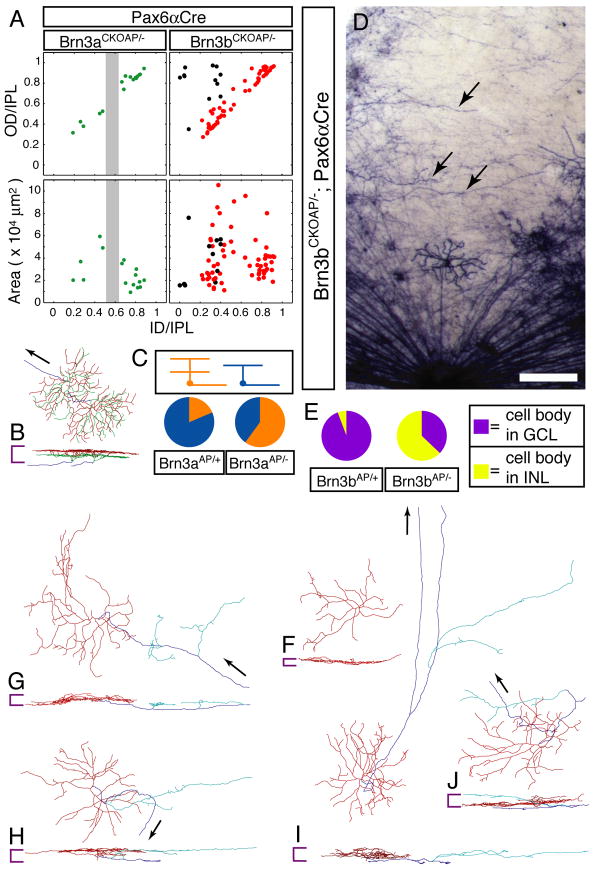
A comparison of vertical sections of Brn3aCKOAP/+;Pax6αCre and Brn3aCKOAP/−;Pax6αCre retinas shows that the absence of Brn3a leads to a loss of AP-expressing RGC dendrites in a narrow stripe centered over the boundary between ON and OFF laminae in the IPL (compare Figure 3A and B). A similar gap is seen when the dendritic stratification patterns of individual monostratified Brn3aCKOAP/− RGCs are plotted (Figure 4A). This observation indicates that loss of Brn3a leads either to a loss of Brn3a-expressing RGCs that would normally stratify in this central layer, to a re-specification of dendritic stratification within this RGC population, or both. A comparison of the morphologies of individual Brn3aAP/+ and Brn3aAP/− RGCs indicates a substantial degree of re-specification among the latter group, as the ratio of bistratified: monostratified RGCs increases from 0.22 (8:35) in Brn3aCKOAP/+;Pax6αCre retinas to 1.5 (24:16) in Brn3aCKOAP/−;Pax6αCre retinas (P<0.0001; Figure 4B,C and Supplementary Figure 2). The absence of Brn3a is also associated with a ~30% decrease in the number of RGCs fated to express Brn3a (as determined by counting Brn3aAP/+ and Brn3aAP/−RGCs), with a corresponding decrement in the number expressing Brn3b (Supplementary Figure 1I–L). Thus, in the absence of Brn3a, there is both RGC respecification and loss.
Figure 4. Brn3bAP/− RGCs have altered axonal and dendrite morphologies.
(A) Scatter plots of Brn3aAP/− and Brn3bAP/− dendritic arbor parameters derived from single cell morphologies. In the Brn3aAP/− scatter plots, the gap centered at the ON/OFF boundary is indicated by a vertical grey bar.
(B) Digitized morphologies of two adjacent and partly overlapping bistratified RGCs from a Brn3aCKOAP/−;Pax6αCre retina.
(C) Pie charts showing the ratios of bistratified (orange):monostratified (blue) Brn3aAP RGCs in Brn3aCKOAP/+;Pax6αCre and Brn3aCKOAP/−;Pax6αCre retinas.
(D) Sparsely recombined zone of a flat mounted Brn3bCKOAP/−;Pax6αCre retina showing circumferentially oriented fibers (arrowheads). The optic disc is at the bottom center.
(E) Pie charts showing the ratios of Brn3bAP cell bodies in the INL (yellow):GCL (purple) in Brn3bCKOAP/+;Pax6αCre and Brn3bCKOAP/−;Pax6αCre retinas.
(F–J) Digitized morphologies of Brn3bCKOAP/−;Pax6αCre RGCs. Color coding and symbols are as described for Figure 2, with the addition that neurites originating from axons and penetrating into the IPL are shown in light blue. The cell in (F) has no axon. Scale bar in D: 200 μm.
Brn3b−/− RGCs exhibit defects in intra-retinal axon morphology
As previously reported (Erkman et al., 1996; Erkman et al., 2000; Gan et al., 1996; Xiang, 1998), loss of Brn3b leads to a loss of >70% of RGCs (Figure 3C,D and Supplementary Figures 1, 3C,D). AP staining of Brn3bCKOAP/−;Pax6αCre retinas shows that many surviving Brn3bAP/− RGCs continue to transcribe the recombined Brn3b locus (Figure 4D). In earlier work, Gan et al. (Gan et al., 1999) and Wang et al.(Wang et al., 2000) observed disorganized RGC axon bundles in Brn3b−/− retinas and aberrant axonal and dendritic processes in cultured Brn3b−/− RGCs. We have extended this analysis by observing the aberrant morphologies of individual Brn3bAP/− RGCs in retina flat mounts (Figure 4G–J). Although the distribution of stratification levels among Brn3bAP/− RGC dendrites resembles that of the Brn3bAP/+ control (compare Figures 2P and 4A), the population of Brn3bAP/− RGCs includes an excess of cells with large areas and thick arbors (Supplementary Figure 2C), and an overall increase in mean area size for dendritic arbors of all types (compare Figures 2P and 4A). While the axons of most Brn3bAP/− RGCs correctly target the optic disc, some axons or axon-like processes from Brn3bAP/− cells follow aberrant trajectories (Figure 4D,G), bifurcate within the retina (Figure 4I), or give rise to dendrite-like branches that ramify within the IPL (Figure 4G–J). Among a sampling of 87 Brn3bAP/− neurons with cell bodies in the ganglion cell layer (GCL), three lacked an axon entirely (Figure 4F), suggesting that they may have adopted, at least partially, an amacrine cell fate. The idea that amacrine cell developmental pathways are derepressed in the absence of Brn3b is strengthened by the observation that Brn3bAP/− neurons are significantly more likely to reside in the inner nuclear layer (INL); the ratio of INL:GCL Brn3bAP/− cell bodies is 178:105 as compared to 9:147 for Brn3bAP/+ controls (Figure 4E). Moreover, many displaced Brn3bAP/− cells exhibit morphologies characteristic of small-field glycinergic amacrine cells (Menger et al., 1998), and immunostain for the vesicular inhibitory amino acid transporter (Chaudhry et al., 1998) (Supplementary Figure 4). These observations are consistent with those of Qiu et al., (Qiu et al., 2008) showing that amacrine markers are expressed transiently in prenatal Brn3b−/− RGCs. Among surviving Brn3bAP/− RGCs, the fraction that expresses Brn3a is unaltered compared to Brn3bAP/+ RGCs (Supplementary Figure 1I–L), implying that, for at least some RGCs that normally co-express Brn3a and Brn3b, Brn3b is not required for the maintenance of Brn3a expression.
Defects in central projections from Brn3b−/− but not Brn3a−/− RGCs
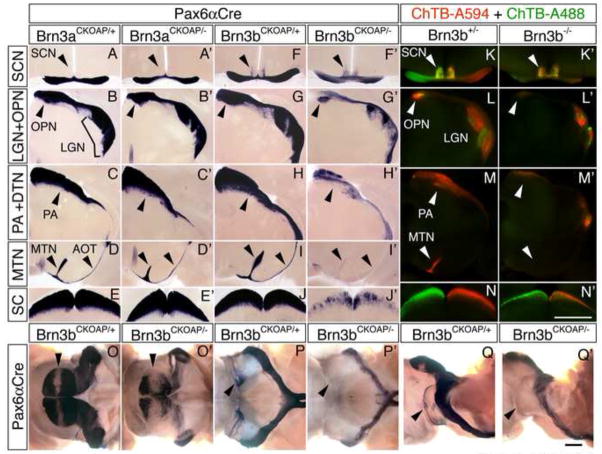
As revealed by AP histochemistry, Brn3aAP/+ and Brn3bAP/+ RGCs project to a diverse set of central targets that mediate both image-forming and non-image-forming vision: the lateral geniculate nucleus (LGN), the superior colliculus (SC), the dorsal, lateral, and medial terminal nuclei (DTN, LTN, and MTN), the olivary pretectal nucleus (OPN), and the pretectal area (PA) and adjacent nucleus of the optic tract (NOT) (Figure 1I–L; Figure 5A–J). The suprachiasmatic region and the intergeniculate leaflet (IGL) receive projections from Brn3b-expressing RGCs but not from Brn3a-expressing RGCs (Figure 1I,K; Figure 5A,F). These observations regarding the central targets of Brn3a-expressing RGCs extend an earlier analysis that utilized a Brn3atau-lacZ allele in which projections to the accessory optic system were not detected (Quina et al., 2005), a difference that we ascribe to the different sensitivities of the AP and tau-lacZ reporters.
Figure 5. Decreased brain projections from Brn3bAP/− but not Brn3aAP/−RGCs.
(A–J′) AP-stained RGC projections in the principal retinorecipient areas in coronal sections of adult brains from Brn3aCKOAP/+;Pax6αCre, Brn3aCKOAP/−; Pax6αCre, Brn3bCKOAP/+;Pax6αCre, and Brn3bCKOAP/−;Pax6αCre mice. From top to bottom: the optic chiasm and SCN, the LGN and anterior pretectal area (PA) containing the OPN, the posterior PA containing the NOT and DTN, the MTN and the inferior and lateral fascicles of the AOT, and the SC. The narrow unstained vertical zone within the LGN in panels B, B′, and G, and the wider zone in the LGN in G′, correspond to the area targeted by RGCs with cell bodies in the dorso-ventral stripe of retina that is weakly recombined in the Pax6αCre background.
(K–N) RGC projections in the principal retinorecipient areas in coronal sections of adult brains from Brn3b+/− and Brn3b−/− mice injected with Alexa Fluor 594-conjugated Cholera toxin B in the left eye (red) and Alexa Flour 488-conjugated Cholera toxin B in the right eye (right).
(O–Q′) AP-stained Brn3bCKOAP/+;Pax6αCre and Brn3bCKOAP/−;Pax6αCre intact midbrains in dorsal (O,O′), ventral (P,P′), and lateral (Q,Q′) views. Anterior is to the right. Arrowheads in O and O′: the coronal stripe of weak AP staining in the center of the SC corresponding to the target area of RGCs with cell bodies in the dorsoventral stripe of retina that is weakly recombined in the Pax6αCre background. Arrowheads in P and P′: the ventral aspect of the AOT. Arrowheads in Q and Q′: the LTN and lateral aspect of the AOT. Scale bars: 1 mm.
In contrast to the normal or nearly normal projections to central targets from Brn3aAP/− RGCs (Figure 5A′-E′), projections from Brn3bAP/−RGCs are greatly reduced, mirroring the reduction in the number of Brn3b-expressing RGCs (Figure 5F′-J′ and O–Q′). Large decrements in Brn3bAP/− projections are apparent in the OPN, PA, LGN, and SC. In the accessory optic system projections to the LTN and MTN are eliminated and the accessory optic tract (AOT) is missing (Figure 5I,I′, P–Q′). Within the suprachiasmatic region, Brn3bAP/− axon arbors shift from a narrow lateral distribution to a central and more diffuse distribution (Figure 5F, F′). In the SC the topographic arrangement of Brn3bAP/− fibers is minimally perturbed, as shown by the central unstained stripe that bisects the SC in the coronal plane and that corresponds to the dorso-ventral stripe of low Cre activity in the Pax6αCre retina (Figure 5O,O′). Axon tracing by intraocular injection of fluorescent cholera toxin B in Brn3b−/− mice (Figure 5K–N′) shows a nearly normal density of projections to the SCN, but for other central targets there is a decrement in retinal projections roughly proportional to the decrement observed for the projections from Brn3bAP/− RGCs. In Brn3b−/− mice, the MTN receives little or no retinal input of any kind (Figure 5M and M′), implying that this input is normally derived from (or is dependent on) Brn3b-expressing RGCs.
Development of Brn3aAP/− RGC dendrites and Brn3bAP/− RGC axons
Defects in dendritic morphology and axon targeting can arise from defects in specification or pathfinding early in development, aberrant pruning later in development, or some combination of these processes. To define the development of dendritic morphologies and axon trajectories for wild type and mutant RGCs in a cell type-specific manner, we imaged developing Brn3aAP/+, Brn3aAP/−, Brn3bAP/+, and Brn3bAP/−RGCs in late fetal and early postnatal life. In the postnatal day (P)4 retina - a time when IPL lamination is still incomplete as judged by the presence of a single band of calretinin immunoreactivity – the dendrites of Brn3aAP/+ RGCs are confined to a discrete zone at the center of the IPL (Figure 6A–D and Supplementary Figure 5). By P11, this pattern has evolved to match that of the adult IPL. Interestingly, the distinctive gap in the distribution of Brn3aAP/− dendrites within the central IPL is already evident at P4 (Figure 6B and Supplementary Figure 5B). These data imply that the cell-autonomous differences in dendritic stratification between Brn3aAP/+ and Brn3aAP/− RGCs are programmed early in IPL development, prior to the time when bipolar cells are fully developed and activity-dependent dendritic refinement is thought to act (Morgan et al., 2006; Tian and Copenhagen, 2003).
Figure 6. Development of IPL stratification by Brn3aAP/+ and Brn3aAP/− RGC dendrites and central projections by Brn3bAP/+ and Brn3bAP/− RGC axons.
(A–D) Immunostaining for calretinin and AP in Brn3aCKOAP/+;Pax6αCre and Brn3aCKOAP/−;Pax6αCre retinas at P4 and P11. The inner retina is shown with the GCL at the bottom. Schematic at right is as for Figure 3. Scale bar in D: 40 μm.
(E–H′) Coronal sections of Brn3bCKOAP/+;Pax6αCre and Brn3bCKOAP/−;Pax6αCre brains at the level of: (E,E′) the optic chiasm and (F,F′) 200 μm posterior to the chiasm at P9, and (G–H′) the MTN at E17 and P3. Scale bar in F:1 mm.
In earlier descriptions of the developing Brn3b−/− CNS, diI labeling of RGC axons revealed erroneous trajectories at the optic chiasm and SC (Erkman et al., 2000). By specifically visualizing the central projections of Brn3bAP/− RGCs, we observed that a small number of axons from Brn3bAP/− RGCs project to the developing MTN at E17 and are then progressively lost over the first postnatal week (Figures 6G–H′ and 5I,I′). We also observed a distinctive defect beginning at P4 in which numerous neurites emerge from the optic tract and penetrate nearby nontarget areas (Figure 6E–F′). These neurites increase in number through P9 but are lost by adulthood. We speculate that these relatively late developmental phenotypes may reflect earlier errors in targeting or synaptogenesis by Brn3bAP/− RGC axons.
Visual sensory defects in Brn3b−/− mice
The widespread reduction in projections to retinorecipient areas in the Brn3b−/− brain, and, in particular, the complete absence of projections to the LTN and MTN, suggested the possibility that Brn3bAP/− mice might exhibit distinctive deficits in visual behavior. To test this hypothesis we quantified eye tracking in response to a pattern of moving stripes (the optokinetic reflex, OKR), the pupillary light response, and circadian photoentrainment in Brn3b−/− and control (Brn3b+/− and Brn3b+/+) littermates.
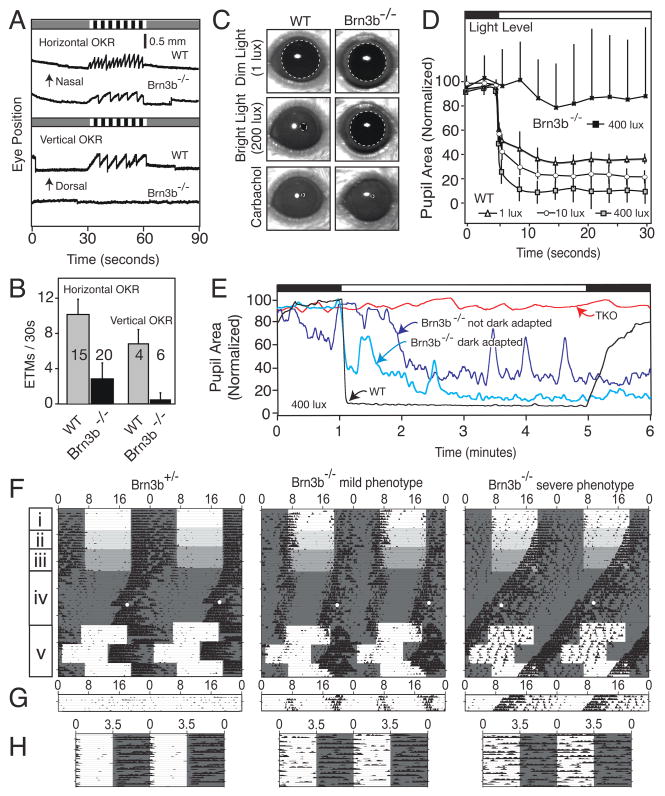
In Brn3b−/− mice the OKR to horizontally moving stimuli is reduced to roughly one-third of the WT value and the response to vertically moving stimuli is abolished (Figure 7A and B). This difference between horizontal and vertical OKRs suggests that in the Brn3b−/− brain, the loss of projections to the MTN and LTN, which control vertical eye movements, is more severe than the loss of projections to the DTN and NOT, which control horizontal eye movements (Simpson, 1984). Brn3b−/− mice also show a profound defect in the central control of pupil constriction (Figure 7C–E). While the innervation and competence of the pupillary muscles appear to be normal, as demonstrated by full pupil constriction in response to topical carbachol (Figure 7C), the pupillary response to changes in ambient light level is greatly reduced in sensitivity, typically delayed by tens of seconds, and unstable (Figure 7D and E). This defect is likely referable to a loss of projections to the PA and OPN (Gamlin, 2005).
Figure 7. Deficits in visual behavioral in Brn3b−/− mice.
(A,B) OKR measured by infrared imaging of eye position in response to temporal-to-nasal (horizontal OKR) or dorsal-to-ventral (vertical OKR) motion of black and white stripes during the 30 second period indicated by the striped pattern at the top of each panel in (A). In (B) the number of eye tracking movements (ETMs; each ETM is defined as a slow tracking movement followed by a rapid saccade) is plotted for WT and Brn3b−/− mice. The number of mice tested for each condition is indicated in panel B. [One Brn3b−/− mouse produced a small number of ETMs to vertical stimuli; subsequent testing showed that this was due to imperfect vertical alignment of the head. These data account for the non-zero value of the averaged Brn3b−/− vertical OKRs in panel B.]
(C–E) Pupillary light responses. C, Brn3b−/− mice show little pupillary constriction to 200 lux, but a normal ciliary muscle response to 1% topical carbachol; dashed lines indicate the boundaries of the pupil. D, WT mice show rapid pupil constriction in response to illumination, with greater constriction in response to higher intensity illumination; Brn3b−/− mice show variable responses with, on average, little constriction even at the highest level of illumination (400 lux); each curve shows the average and standard deviation of three trials with each of five mice (for WT: three Brn3b+/+ and two Brn3b+/−). E, Extended time course of pupil responses of WT, TKO (triple knockout; Opn4−/−Gnat1−/−;Cnga3−/− mice in which rods, cones, and intrinsically photosensitive RGCs are inactivated37,38,39,44) and Brn3b−/− mice to switching from darkness to 400 lux and then back to darkness (top bar).
(F–H) Actograms for a Brn3b+/− and two Brn3b−/− mice subjected to various light-dark regimens as described in the text. Numbers mark hours; each horizontal line in F and G corresponds to 48 hours. F, 12:12 LD cycles of intensities 1000 (i), 100 (ii), and 10 (iii) lux; after 12 days of constant darkness, mice were exposed to a 15 minute, 1500 lux light pulse (white dot) at CT16 (iv); and a jet-lag paradigm (v) in which the LD cycle was advanced and then delayed by six hours. G, constant light (1000 lux). H, ultradian 3.5:3.5 LD cycle.
To test photoentrainment, wheel running activity was recorded from seven Brn3b−/− and seven Brn3b+/− control mice subjected to 12 hour:12 hour light: dark (12:12 LD) cycles with progressively dimmer light regimens (Figure 7F, panels i, ii, iii). Two Brn3b−/− mice free-ran with a period less than 24 hours in all light regimens (Figure 7F, right panels; “severe phenotype”). The remaining five Brn3b−/− mice were able to photoentrain, albeit weakly and with an unstable phase relation to the imposed LD cycle, and often had what appeared to be a split rhythm (Figure 7F, center panels; “mild phenotype”). Under constant darkness (Figure 7F, panel iv), Brn3b−/− and Brn3b+/− mice exhibited similarly shortened periodicities (controls: 23.69 ± 0.08 hours; Brn3b−/−: 23.71 ± 0.25 hours), implying the existence of a normally functioning circadian clock in Brn3b−/− mice. When a 15 minute light pulse was given four hours after the onset of the subjective night (Figure 7F, panel iv), all Brn3b+/− mice responded with a robust phase shift (−2.37 ± 0.79 hours), while Brn3b−/− mice were unresponsive (−0.11 ± 0.27 hours; P<0.01). Mice were then subjected to a jet-lag paradigm in which their imposed LD cycle was advanced and then delayed by six hours (Figure 7F, panel v). In response to the new LD cycle, Brn3b+/− mice rapidly resynchronized, mild phenotype Brn3b−/− mice gradually resynchronized, and severe phenotype Brn3b−/− mice did not resynchronize. Constant light strongly suppressed the activity of Brn3b+/− mice, but had little affect on the activity or periodicity of Brn3b−/− mice (Figure 7G). Finally, to directly observe masking responses independent of the circadian clock, we used an ultradian paradigm consisting of 3.5:3.5 LD cycles (Figure 7H). Brn3b−/− mice were substantially more active in the light than their control counterparts: the percentage of daily activity that occurred within the light phase was 4.72 ± 2.65% in controls versus 39.12 ± 14.22% in Brn3b−/− mice (P<0.05). These observations indicate that the retinal inputs to the SCN that remain in Brn3b−/− mice (Figure 5K,K′), although substantial, are insufficient to mediate normal photoentrainment. Defects in other retinorecipient nuclei, such as the OPN or IGL, which receive input from ipRGCs (Hattar et al., 2006), project to the SCN (Moga and Moore, 1997), and are affected in Brn3b−/− mice, may account, at least in part, for the photoentrainment defects.
DISCUSSION
The principal results of the present study are that: (1) the dendrites of Brn3a- and Brn3b-expressing RGCs exhibit different distributions of IPL stratification, with dendrites of Brn3a-expressing RGCs stratifying exclusively in the outer 60–70% of the IPL, (2) except for the suprachiasmatic region and the IGL, Brn3a- and Brn3b-expressing RGCs project to largely overlapping sets of central targets, including all of the major retino-recipient areas, (3) loss of Brn3a leads to an increase in the ratio of bistratified to monostratified RGCs, with only modest RGC loss and little effect on central projections, and (4) loss of Brn3b leads to loss of ~70% of RGCs, disorganization of axonal structure in the eye and brain, and a differential loss and/or dysfunction of central projections that severely impairs the vertical OKR, and compromises light-dependent pupil constriction and photo-entrainment. The behavioral deficits in Brn3b−/− mice affect all of the non-image forming divisions of the visual system. In the case of the pupillary light response and the OKR, the observed defects in axonal projections accurately predict the behavioral deficits. However, photo-entrainment is substantially impaired despite a relatively modest decrement in RGC projections to the SCN, suggesting that this phenotype reflects defects in the innervation of other retinorecipient nuclei.
Brn3 transcription factors and the specification of neuronal identity
Understanding the transcriptional regulatory networks that control neuronal diversity is one of the central aims of developmental neurobiology. In the developing spinal cord, investigations over the past two decades have shown that there are complex combinatorial, partially redundant, and stage-specific interactions among transcription factor genes that determine the distinctive properties of different groups of motor neurons. For example, the territories defined by Hox gene expression and retinoid receptor signaling control rostro-caudal diversity among motor neurons, and the combinatorial action of LIM-homeodomain genes controls axon pathfinding and target selection (Maden, 2006; Shirasaki and Pfaff, 2002). In the retina, a large number of transcription factors have been identified that control cell fate decisions within the major classes of neurons, including rods and cones (Furukawa et al., 1999; Ng et al., 2001; Oh et al., 2007), bipolar cells (Cheng et al., 2005; Chow et al., 2004), horizontal and amacrine cells (Elshatory et al., 2007; Fujitani et al., 2006; Li et al., 2004), and RGCs (Mu et al., 2008; Qiu et al., 2008). In general, the mechanisms by which these transcription factor genes produce their cellular effects –i.e. the identities and functions of their target genes – remain unknown.
Among transcription factor genes implicated thus far in controlling retinal development, the Brn3 genes are distinctive in the RGC-specificity of their expression, their high mutual sequence similarity, and their role in the development of primary sensory cells in other sensory systems as revealed by their knockout phenotypes. How the last of these attributes relates to their roles in controlling RGC development is unclear. In the present work, the identification of distinctive patterns of dendritic stratification by Brn3a- and Brn3b-expressing RGCs, together with the differential innervation of the SCN and IGL by these RGCs, provides the first clear connection between morphologically defined RGC subtypes and patterns of transcription factor expression. Moreover, the observation that loss of Brn3a leads to a dramatically altered pattern of dendritic stratification, whereas loss of Brn3b largely affects axonal development, pathfinding, and target selection, argues that, despite their high degree of amino acid sequence similarity, Brn3a and Brn3b perform qualitatively different functions during RGC development.
Thus far, experiments aimed at defining genes regulated by Brn3a in the somatosensory system (Eng et al., 2004; Lanier et al., 2007) and by Brn3b in the retina (Erkman et al., 2000; Mu et al., 2004), have been performed on bulk samples, with the result that distinctions between neuronal subtypes have not been revealed. Distinguishing between subtypes is likely to be important because the actions of the different Brn3 proteins on their transcriptional regulatory targets may depend on the cellular context, as suggested, for example, by the contrast between the presence of axon pathfinding defects among Brn3a−/− dorsal root ganglion neurons (Eng et al., 2001) and the absence of axonal pathfinding defects among Brn3a−/− RGCs. In this example, a substantial part of the context effect may reflect redundancy with co-expressed Brn3b in RGCs. Alternately, the afferent of each DRG neuron, as the input branch of a pseudo-unipolar neurite, may, in some of its molecular properties, be developmentally homologous to the dendritic arbors of RGCs, which, as shown here, are dependent on Brn3a function.
A potentially useful model for Brn3 function within a larger transcriptional network can be found in Drosophila, where the POU-domain gene Acj6 appears to participate in a combinatorial code with multiple other transcription factors to control the dendritic specificity of olfactory projection neurons and the stereotyped branching of the axons of olfactory receptor neurons in their target glomeruli (Clyne et al., 1999; Komiyama et al., 2004; Komiyama et al., 2003; Komiyama and Luo, 2007). As Acj6 is the sole Drosophila orthologue of the vertebrate Brn3 family, it may be of interest to examine mammalian homologues of those Drosophila genes identified as part of the Acj6 regulatory network as candidates that might interact with Brn3 family members.
Brn3 control of dendritic stratification, axonal structure, and target selection
Dendritic stratification is an organizational strategy used in a variety of contexts in the CNS, including the retina, the dorsal horn of the spinal cord, the cerebral cortex, and the LGN. It has been most intensively studied in the retina, where the IPL is comprised of at least ten distinct strata and most RGCs have dendritic arbors that are either monostratified or bistratified. Correlations between the level of dendritic stratification and the physiologic response properties of individual RGCs show that different IPL strata extract (and therefore different RGCs transmit) different features of the visual stimulus (Roska and Werblin, 2001). Although the molecular mechanisms controlling IPL stratification are still largely unknown, recent work has implicated the Sidekick and Dscam members of the Ig protein superfamily in stratification specificity (Yamagata and Sanes, 2008; Yamagata et al., 2002). These cell surface proteins are expressed in largely nonoverlapping subsets of RGCs, bipolar cells, and amacrine cells, and each protein mediates homophilic but not heterophilic binding, suggesting a simple homophilic adhesion model for the mutual recognition of synaptic partners in the IPL. However, it is not currently known how synaptic partners determine which stratum to occupy within the IPL and how the two partners are programmed to express the same adhesion molecule. RGC dendritic stratification is also influenced by synaptic activity, as pharmacologic blockade of glutamate release or light deprivation in the developing retina impairs its refinement (Bisti et al., 1998; Bodnarenko and Chalupa, 1993; Tian and Copenhagen, 2003; Xu and Tian, 2007).
The conclusion that Brn3a is part of a genetic regulatory network that controls IPL stratification is based on (1) the stratification differences between Brn3a-expressing and Brn3b-expressing RGCs and (2) the effect of Brn3a deletion on the distribution of RGC dendrites within IPL strata and on the ratio of bistratified to monostratified RGCs. With respect to the increased ratio of bistratified to monostratified RGCs, we note that it is possible to account for this increase either by a conversion of monostratified to bistratified RGCs or by a selective loss of monostratified RGCs. We favor the former explanation because (1) the altered stratification pattern is observed at a time (P4) when RGC dendrites are just beginning to stratify in the IPL (Figure 6 and supplementary Figure 5), thus inconsistent with late elimination of monostratified RGCs, and (2) an RGC elimination mechanism alone cannot account for the appearance of Brn3aAP/− RGC dendrites at the level of the innermost of the three calretinin strata, a stratum that is minimally populated by Brn3aAP/+ RGC dendrites (Figure 3).
In contrast to Brn3aAP/− RGCs, Brn3bAP/− RGCs show an essentially normal distribution of dendritic morphologies. However, by selectively visualizing Brn3bAP/− RGCs we observe a wide variety of axonal defects that are consistent with in vitro culture experiments showing that Brn3b−/− axons have polarization defects and have acquired some of the properties of dendrites (Wang et al., 2000). Earlier diI tracing analyses had indicated defects among Brn3bAP/− axons in pathfinding at the optic chiasm and in retinotopic mapping in the colliculus (Erkman et al., 2000). By selectively visualizing Brn3bAP/− axons, we additionally observe defasciculated processes invading inappropriate territories along the optic tract (Figure 6), but we do not see evidence for large-scale defects in retinopy in the SC (Figure 5O and O′). In light of the qualitative differences that we observe in Brn3a and Brn3b loss-of-function RGC phenotypes, it would be of interest to re-examine the retinas of mice in which Brn3a coding sequences were substituted for Brn3b coding sequences (Pan et al., 2005) to determine whether RGC morphologies are or are not affected.
Genetic analysis of mammalian neural development at single cell resolution
The conditional knockout approach presented here and in a recent study of Frizzled5 function in the thalamus (Liu et al., 2008) – in which Cre-mediated excision of a target gene’s coding region and 3′UTR also leads to the expression of a histochemical reporter - represents a new strategy for analyzing the morphology and function of individual genetically altered mammalian neurons. In its current form, this strategy is most easily applied to relatively compact genes, in which case a single gene targeting construct can be used to engineer loxP sites both 5′ and 3′ of the coding region. This strategy also requires a conventional loss-of-function allele that lacks the histochemical reporter, since sparse Cre-mediated recombination requires that the conditional allele be present in only one copy. The present work demonstrates the general utility of this approach.
Related approaches for visualizing and/or altering mammalian neurons include (1) visualizing the progeny of a progenitor cell population by activating an unlinked lacZ reporter with a target gene-CreER knock-in (Zirlinger et al., 2002), and (2) activating loxP recombination by sparse co-expression of GFP with Cre or CreER from a thy-1 transgene or following transduction by AAV or lenitivirus vectors (Kasper et al., 2007; McCarty et al., 2004). In Drosophila, individual neurons can be manipulated by using mitotic recombination to simultaneously mark a cell and render it homozygous for a target gene mutation, (‘mosaic analysis with a repressible cell marker’/’MARCM’; (Komiyama et al., 2004; Lee and Luo, 1999), an approach that has recently been extended to the mouse (‘mosaic analysis with a double reporter’/’MADM’;(Zong et al., 2005)).
Potential extensions of the strategy used here include: (1) indelibly marking the cell that has undergone Cre-mediated recombination by placing an unrelated site-specific recombinase 3′ of the target gene and crossing in a reporter controlled by the second recombinase; (2) using reporters other than AP - in particular, fluorescent or epitope-tagged fusion proteins that localize to subcellular structure such as synapses; and (3) using cell-type specific Cre lines to selectively target neuronal subpopulations defined by the intersection of the expression patterns of the recombinase and its target. Given the importance of visualizing and genetically manipulating individual identified mammalian neurons, we predict that these strategies will become widely used.
EXPERIMENTAL PROCEDURES
Mouse lines
The Pax6αCre (Marquardt et al., 2001), ROSA26CreER(T) (Badea et al., 2003), and the Brn3a (Xiang et al., 1996) and Brn3b (Gan et al., 1996) conventional knock-out lines were described previously. The Brn3aCKOAP and Brn3bCKOAP conditional alleles were generated by homologous recombination in mouse embryonic stem cells using standard techniques. For the targeted alleles, the following changes were made: a loxP site was inserted in the 5′UTR 42 bp 5′ (for Brn3a) or 98 bp 5′ (for Brn3b) before the initiator ATG; three repeats of the SV40 early region transcription terminator were added to the 3′UTR 48 bp (for Brn3a) or 340 bp (for Brn3b) 3′ of the Brn3 translation termination codon, followed by a second loxP site and the coding region of human placental alkaline phosphatase (AP). A positive selection cassette (PGK-Neo), flanked by FRT sites, followed the AP coding region, and was subsequently removed by crossing to mice expressing FLP recombinase in the germline.
Histology
Retina whole mounts and brain sections were stained, processed, and imaged, and RGC dendrites and axons were traced and quantified as previously described (Badea and Nathans, 2004; Badea et al., 2003). High resolution images were captured on a Zeiss Imager. Z1 fitted with an Apotome for fluorescent imaging and Axiovision software. Rabbit polyclonal anti-Brn3a and anti-Brn3b antisera were described in Xiang (Xiang et al., 1995). The sources of commercial antibodies are: sheep polyclonal anti-AP (American Research Products, Belmont, MA), rabbit polyclonal anti-calretinin and anti-calbindin (Swant, Bellinzona, Switzerland), and rat monoclonal anti-Thy-1 (BD Biosciences). Cholera Toxin B conjugates were from Molecular Probes (Eugene, OR).
Optokinetic Reflex (OKR)
The OKR apparatus and recording methodology are described in detail in Cahill and Nathans (Cahill and Nathans, 2008). In brief, a head-posted mouse was immobilized in an acrylic holder in the center of a 29.5 cm diameter vertical white cylinder. A computer-generated image of alternating black and white vertical stripes (each stripe subtending 4 degrees of visual angle) was projected into the inner walls of the drum by a rotating projector mounted on the ceiling. The striped pattern had an average brightness of 100–200 lux and was rotated at 5°/sec. 30-second stimulus presentations were alternated with 30 seconds of a uniform grey. Eye movements were captured with an infrared video imaging system (ISCAN, Cambridge, MA), and stored, processed and displayed using Microsoft Excel. The number of eye tracking movements (ETMs; defined as a saccade followed by a slow tracking movement in the opposite direction) was counted per 30-second interval. Eye movements along the vertical axis (with respect to the mouse) were recorded by rotating the acrylic mouse holder 90 degrees so that the nose of the mouse pointed vertically. With the stripes now moving dorsal-to-ventral or ventral-to-dorsal with respect to the mouse, the same rotating visual stimulus was presented only to the eye from which the infrared video recording was obtained. To produce a degree of pupil constriction in Brn3b−/− mice that matches the constriction of WT mice, Brn3b−/− mice were treated with several microliters of 10% pilocarpine eyedrops just before OKR testing.
Pupil Constriction
The mouse’s head was stabilized as described for the OKR. Two different protocols were used to measure the pupillary light response. In the first, the environment was darkened or illuminated with intensities of 1, 10 and 400 lux in alternating 30-second intervals, a regimen that was repeated three times, progressing from dim to bright light. In the second protocol, mice were either dark adapted or exposed to room light (~100 lux), and then maintained for one minute in complete darkness, exposed to 400 lux for five minutes, and returned to complete darkness for a final one minute. Light intensities were controlled with neutral density filters and both eyes were illuminated equivalently.
Photoentrainment and light-induced activity suppression
Mice were placed in cages equipped with a 4.5 inch running wheel and exposed to different light regimens as described in the text. Light intensity was 1000 lux unless otherwise noted. Wheel running activity was monitored using VitalView software (Mini Mitter Co., Bend, OR). Data was analyzed and actograms were generated with ClockLab software (Actimetrics, Wilmette, IL).
Supplementary Material
Supplementary Figure 1. Overlap of Brn3a and Brn3b expression in WT and mutant RGCs.
(A–H) Immunofluorescent staining of transverse sections through regions of high Pax6αCre expression in adult retinas of Brn3aCKOAP/+;Pax6αCre (A,B), Brn3aCKOAP/−;Pax6αCre (C,D), Brn3bCKOAP/+;Pax6αCre (E,F), and Brn3bCKOAP/−;Pax6αCre (G,H) mice. Sections are immunostained with anti-AP, together with either anti-Brn3a (A,C,E,G), or anti-Brn3b (B,D,F,H) antibodies. Vertical bars to the right of panels G,H, and J show the extent of the IPL; the GCL is at the bottom. I, quantification of double immunostaining, expressed as a percentage of the AP-positive cell population. Between 36 and 86 AP-expressing cells were counted for each panel.
(J) example of a Brn3bAP/− cell with its soma in the INL and a small dendritic arbor.
(K,L) the number of AP-, Brn3a-, or Brn3b-expressing cells plotted as a fraction of all cells in the GCL for the indicated genotypes. In the mouse retina, ~50% of cells in the GCL are RGCs. Data are expressed as a percent DAPI positive cells and are averaged from >3 20x fields per bar; the number of DAPI stained nuclei counted is indicated above each bar. Adjacent sections were processed for anti-Brn3a and anti-Brn3b immunostaining to minimize variation in cell density or in Cre-mediated recombination across the retina. Scale bar in H: 40 μm.
Supplementary Figure 2. Morphometric parameters for dendrites from RGCs.
The following RGC genotypes are shown: Brn3aAP/+ (first column), Brn3aAP/−(second column), Brn3bAP/+ (third column), and Brn3bAP/− (fourth column). See figure 2E and F for a definition of the parameters.
(A) Inner distance (ID) normalized to the full thickness of the IPL (ID/IPL), and outer distance (OD) normalized to the full thickness of the IPL (OD/IPL) are plotted separately for the two arbors of bistratified RGCs. The points representing the paired inner and outer arbors of each RGC are connected by horizontal and vertical lines.
(B) Area of the inner arbor (x axis) vs. area of the outer arbor (y axis) for bistratified neurons.
(C) dendritic arbor thickness (OD-ID/IPL) vs. area for monostratified RGCs.
Supplementary Figure 3. Differential stratification levels for dendrites of Brn3aAP/+ and Brn3bAP/+ RGCs. Vertical sections of retinas immunostained for Thy-1, which stains all RGC dendrites, and AP reveal distinct stratification levels for dendrites of Brn3aAP/+ and Brn3bAP/+ RGCs, a loss of the centrally stratifying IPL dendrites in Brn3aAP/−RGCs, and a loss of most Brn3bAP/− RGCs. The GCL is at the bottom. Right, schematic showing, for each set of panels, the boundaries of the IPL (blue), the domain of Thy-1 immunoreactivity (i.e. the full thickness of the IPL; green), and the regions occupied by AP-immunoreactive RGC dendrites (red). The regions imaged correspond to zones of high Cre activity from the Pax6αCre transgene. The three nuclear layers are revealed by DAPI staining (blue). Scale bar: 80 μm.
Supplementary Figure 4. Amacrine features of Brn3bAP/− cells.
(A–H) Immunostaining for amacrine cell markers and AP in Brn3bCKOAP/+;Pax6αCre and Brn3bCKOAP/−;Pax6αCre vertical retina sections. (A–B) calretinin, (C–D) glutamic acid decarboxylase (GAD) 65/67, (E–F) calbindin, and (G–H) vesicular inhibitory amino acid transporter (VIAAT). White arrowheads indicate the individual cells that are enlarged and shown without the DAPI channel in the smaller red, green, and merged panels to the right. In panel A (Brn3bCKOAP/+;Pax6αCre), a rare displaced (i.e. cell body in the INL) RGC shows co-localization of AP and calretinin, a marker for subsets of amacrine cells and RGCs. In panels C, F, and G (Brn3bCKOAP/+;Pax6αCre), rare displaced RGCs show no co-localization of AP and the amacrine cell markers calbindin, GAD65/67, or VIAAT. In panels B, D, F, and H (Brn3bCKOAP/−;Pax6αCre), examples are shown of the many AP+ cell bodies in the INL. Co-localization of AP with VIAAT is seen in panel H (and in 11/15 cells scored); and co-localization of AP and calretinin, calbindin, or GAD65/67 is not seen in panels B,D, and F (or in 0/7, 0/12, or 0/8 cells scored for these markers, respectively).
(I) Front and lateral views of a reconstructed Brn3bCKOAP/−;Pax6αCre AP-expressing cell with amacrine-like morphology and a cell body in the INL. Horizontal scale bar: 25 μm. Vertical bar corresponds to the location of the IPL.
Supplementary Figure 5. Development of stratification in the IPL by Brn3aAP/+ and Brn3aAP/− dendrites.
(A–D) Immunostaining for calbindin and AP in Brn3aCKOAP/+;Pax6αCre and Brn3aCKOAP/−;Pax6αCre retinas at P4 and P11. The inner retina is shown with the GCL at the bottom. Schematic at right is as for Figure 3. Scale bar: 40 μm.
Acknowledgments
The authors thank Chunqiao Liu and Amir Rattner for helpful comments on the manuscript. Supported by the National Institutes of Health (S.H.) and the Howard Hughes Medical Institute (J.N.).
Footnotes
Author contributions. T.B. and J.N. designed and T.B. conducted the genetic, anatomic, and histologic experiments. H.C. conducted the OKR and pupil constriction experiments. J.E. and S.H. conducted the photoentrainment experiments. T.B. and J.N. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisti S, Gargini C, Chalupa LM. Blockade of glutamate-mediated activity in the developing retina perturbs the functional segregation of ON and OFF pathways. J Neurosci. 1998;18:5019–5025. doi: 10.1523/JNEUROSCI.18-13-05019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993;364:144–146. doi: 10.1038/364144a0. [DOI] [PubMed] [Google Scholar]
- Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: application to genetic and drug-induced variation. PLoS ONE. 2008;3:e2055. doi: 10.1371/journal.pone.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Chow RL, Lebel M, Sakuma R, Cheung HO, Thanabalasingham V, Zhang X, Bruneau BG, Birch DG, Hui CC, et al. The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev Biol. 2005;287:48–60. doi: 10.1016/j.ydbio.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Chow RL, Volgyi B, Szilard RK, Ng D, McKerlie C, Bloomfield SA, Birch DG, McInnes RR. Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc Natl Acad Sci U S A. 2004;101:1754–1759. doi: 10.1073/pnas.0306520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 1999;22:339–347. doi: 10.1016/s0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Deng M, Xie X, Gan L. Expression of the LIM-homeodomain protein Isl1 in the developing and mature mouse retina. J Comp Neurol. 2007;503:182–197. doi: 10.1002/cne.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng SR, Gratwick K, Rhee JM, Fedtsova N, Gan L, Turner EE. Defects in sensory axon growth precede neuronal death in Brn3a-deficient mice. J Neurosci. 2001;21:541–549. doi: 10.1523/JNEUROSCI.21-02-00541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng SR, Lanier J, Fedtsova N, Turner EE. Coordinated regulation of gene expression by Brn3a in developing sensory ganglia. Development. 2004;131:3859–3870. doi: 10.1242/dev.01260. [DOI] [PubMed] [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O’Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Erkman L, Yates PA, McLaughlin T, McEvilly RJ, Whisenhunt T, O’Connell SM, Krones AI, Kirby MA, Rapaport DH, Bermingham JR, et al. A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron. 2000;28:779–792. doi: 10.1016/s0896-6273(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Fujitani S, Luo H, Qiu F, Burlison J, Long Q, Kawaguchi Y, Edlund H, MacDonald RJ, Furukawa T, et al. Ptf1a determines horizontal and amacrine cell fates during mouse retinal development. Development. 2006;133:4439–4450. doi: 10.1242/dev.02598. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC, Cepko CL. Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat Genet. 1999;23:466–470. doi: 10.1038/70591. [DOI] [PubMed] [Google Scholar]
- Gamlin PD. The pretectum: connections and oculomotor-related roles. Prog Brain Res. 2005;151:379–405. doi: 10.1016/S0079-6123(05)51012-4. [DOI] [PubMed] [Google Scholar]
- Gan L, Wang SW, Huang Z, Klein WH. POU domain factor Brn-3b is essential for retinal ganglion cell differentiation and survival but not for initial cell fate specification. Dev Biol. 1999;210:469–480. doi: 10.1006/dbio.1999.9280. [DOI] [PubMed] [Google Scholar]
- Gan L, Xiang M, Zhou L, Wagner DS, Klein WH, Nathans J. POU domain factor Brn-3b is required for the development of a large set of retinal ganglion cells. Proc Natl Acad Sci U S A. 1996;93:3920–3925. doi: 10.1073/pnas.93.9.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, Regl G, Eichberger T, Frischauf AM, Aberger F. Efficient Manipulation of Hedgehog/GLI Signaling Using Retroviral Expression Systems. Methods Mol Biol. 2007;397:67–78. doi: 10.1007/978-1-59745-516-9_6. [DOI] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Carlson JR, Luo L. Olfactory receptor neuron axon targeting: intrinsic transcriptional control and hierarchical interactions. Nat Neurosci. 2004;7:819–825. doi: 10.1038/nn1284. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Johnson WA, Luo L, Jefferis GS. From lineage to wiring specificity. POU domain transcription factors control precise connections of Drosophila olfactory projection neurons. Cell. 2003;112:157–167. doi: 10.1016/s0092-8674(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Luo L. Intrinsic control of precise dendritic targeting by an ensemble of transcription factors. Curr Biol. 2007;17:278–285. doi: 10.1016/j.cub.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Lanier J, Quina LA, Eng SR, Cox E, Turner EE. Brn3a target gene recognition in embryonic sensory neurons. Dev Biol. 2007;302:703–716. doi: 10.1016/j.ydbio.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Li S, Mo Z, Yang X, Price SM, Shen MM, Xiang M. Foxn4 controls the genesis of amacrine and horizontal cells by retinal progenitors. Neuron. 2004;43:795–807. doi: 10.1016/j.neuron.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Khare SL, Liang X, Peters MA, Liu X, Cepko CL, Xiang M. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development. 2000;127:3237–3247. doi: 10.1242/dev.127.15.3237. [DOI] [PubMed] [Google Scholar]
- Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoids and spinal cord development. J Neurobiol. 2006;66:726–738. doi: 10.1002/neu.20248. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105:43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- McCarty DM, Young SM, Jr, Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- McEvilly RJ, Erkman L, Luo L, Sawchenko PE, Ryan AF, Rosenfeld MG. Requirement for Brn-3.0 in differentiation and survival of sensory and motor neurons. Nature. 1996;384:574–577. doi: 10.1038/384574a0. [DOI] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wassle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46. doi: 10.1002/(sici)1096-9861(19981109)401:1<34::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Morgan JL, Dhingra A, Vardi N, Wong RO. Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar cells. Nat Neurosci. 2006;9:85–92. doi: 10.1038/nn1615. [DOI] [PubMed] [Google Scholar]
- Mu X, Beremand PD, Zhao S, Pershad R, Sun H, Scarpa A, Liang S, Thomas TL, Klein WH. Discrete gene sets depend on POU domain transcription factor Brn3b/Brn-3.2/POU4f2 for their expression in the mouse embryonic retina. Development. 2004;131:1197–1210. doi: 10.1242/dev.01010. [DOI] [PubMed] [Google Scholar]
- Mu X, Fu X, Beremand PD, Thomas TL, Klein WH. Gene regulation logic in retinal ganglion cell development: Isl1 defines a critical branch distinct from but overlapping with Pou4f2. Proc Natl Acad Sci U S A. 2008;105:6942–6947. doi: 10.1073/pnas.0802627105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27:94–98. doi: 10.1038/83829. [DOI] [PubMed] [Google Scholar]
- Oh EC, Khan N, Novelli E, Khanna H, Strettoi E, Swaroop A. Transformation of cone precursors to functional rod photoreceptors by bZIP transcription factor NRL. Proc Natl Acad Sci U S A. 2007;104:1679–1684. doi: 10.1073/pnas.0605934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Yang Z, Feng L, Gan L. Functional equivalence of Brn3 POU-domain transcription factors in mouse retinal neurogenesis. Development. 2005;132:703–712. doi: 10.1242/dev.01646. [DOI] [PubMed] [Google Scholar]
- Qiu F, Jiang H, Xiang M. A comprehensive negative regulatory program controlled by Brn3b to ensure ganglion cell specification from multipotential retinal precursors. J Neurosci. 2008;28:3392–3403. doi: 10.1523/JNEUROSCI.0043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O’Leary DD, Goulding M, Turner EE. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci. 2005;25:11595–11604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramon y Cajal, S. (1893). La retine de vertebres. English translation by D. Maguire and R.W. Rodieck, Appendix I in Rodieck, R. W. (1973). The vertebrate retina; principles of structure and function (San Francisco,, W. H. Freeman).
- Rodieck RW. The first steps in seeing. Sunderland, Mass.: Sinauer Associates; 1998. [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annu Rev Neurosci. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- Simpson JI. The accessory optic system. Annu Rev Neurosci. 1984;7:13–41. doi: 10.1146/annurev.ne.07.030184.000305. [DOI] [PubMed] [Google Scholar]
- Tian N, Copenhagen DR. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- Wang SW, Gan L, Martin SE, Klein WH. Abnormal polarization and axon outgrowth in retinal ganglion cells lacking the POU-domain transcription factor Brn-3b. Mol Cell Neurosci. 2000;16:141–156. doi: 10.1006/mcne.2000.0860. [DOI] [PubMed] [Google Scholar]
- Wang SW, Mu X, Bowers WJ, Kim DS, Plas DJ, Crair MC, Federoff HJ, Gan L, Klein WH. Brn3b/Brn3c double knockout mice reveal an unsuspected role for Brn3c in retinal ganglion cell axon outgrowth. Development. 2002;129:467–477. doi: 10.1242/dev.129.2.467. [DOI] [PubMed] [Google Scholar]
- Xiang M. Requirement for Brn-3b in early differentiation of postmitotic retinal ganglion cell precursors. Dev Biol. 1998;197:155–169. doi: 10.1006/dbio.1998.8868. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O’Malley BW, Jr, Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci U S A. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gan L, Zhou L, Klein WH, Nathans J. Targeted deletion of the mouse POU domain gene Brn-3a causes selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Natl Acad Sci U S A. 1996;93:11950–11955. doi: 10.1073/pnas.93.21.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–4785. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HP, Tian N. Retinal ganglion cell dendrites undergo a visual activity-dependent redistribution after eye opening. J Comp Neurol. 2007;503:244–259. doi: 10.1002/cne.21379. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Zirlinger M, Lo L, McMahon J, McMahon AP, Anderson DJ. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci U S A. 2002;99:8084–8089. doi: 10.1073/pnas.122231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. doi: 10.1016/j.cell.2005.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Overlap of Brn3a and Brn3b expression in WT and mutant RGCs.
(A–H) Immunofluorescent staining of transverse sections through regions of high Pax6αCre expression in adult retinas of Brn3aCKOAP/+;Pax6αCre (A,B), Brn3aCKOAP/−;Pax6αCre (C,D), Brn3bCKOAP/+;Pax6αCre (E,F), and Brn3bCKOAP/−;Pax6αCre (G,H) mice. Sections are immunostained with anti-AP, together with either anti-Brn3a (A,C,E,G), or anti-Brn3b (B,D,F,H) antibodies. Vertical bars to the right of panels G,H, and J show the extent of the IPL; the GCL is at the bottom. I, quantification of double immunostaining, expressed as a percentage of the AP-positive cell population. Between 36 and 86 AP-expressing cells were counted for each panel.
(J) example of a Brn3bAP/− cell with its soma in the INL and a small dendritic arbor.
(K,L) the number of AP-, Brn3a-, or Brn3b-expressing cells plotted as a fraction of all cells in the GCL for the indicated genotypes. In the mouse retina, ~50% of cells in the GCL are RGCs. Data are expressed as a percent DAPI positive cells and are averaged from >3 20x fields per bar; the number of DAPI stained nuclei counted is indicated above each bar. Adjacent sections were processed for anti-Brn3a and anti-Brn3b immunostaining to minimize variation in cell density or in Cre-mediated recombination across the retina. Scale bar in H: 40 μm.
Supplementary Figure 2. Morphometric parameters for dendrites from RGCs.
The following RGC genotypes are shown: Brn3aAP/+ (first column), Brn3aAP/−(second column), Brn3bAP/+ (third column), and Brn3bAP/− (fourth column). See figure 2E and F for a definition of the parameters.
(A) Inner distance (ID) normalized to the full thickness of the IPL (ID/IPL), and outer distance (OD) normalized to the full thickness of the IPL (OD/IPL) are plotted separately for the two arbors of bistratified RGCs. The points representing the paired inner and outer arbors of each RGC are connected by horizontal and vertical lines.
(B) Area of the inner arbor (x axis) vs. area of the outer arbor (y axis) for bistratified neurons.
(C) dendritic arbor thickness (OD-ID/IPL) vs. area for monostratified RGCs.
Supplementary Figure 3. Differential stratification levels for dendrites of Brn3aAP/+ and Brn3bAP/+ RGCs. Vertical sections of retinas immunostained for Thy-1, which stains all RGC dendrites, and AP reveal distinct stratification levels for dendrites of Brn3aAP/+ and Brn3bAP/+ RGCs, a loss of the centrally stratifying IPL dendrites in Brn3aAP/−RGCs, and a loss of most Brn3bAP/− RGCs. The GCL is at the bottom. Right, schematic showing, for each set of panels, the boundaries of the IPL (blue), the domain of Thy-1 immunoreactivity (i.e. the full thickness of the IPL; green), and the regions occupied by AP-immunoreactive RGC dendrites (red). The regions imaged correspond to zones of high Cre activity from the Pax6αCre transgene. The three nuclear layers are revealed by DAPI staining (blue). Scale bar: 80 μm.
Supplementary Figure 4. Amacrine features of Brn3bAP/− cells.
(A–H) Immunostaining for amacrine cell markers and AP in Brn3bCKOAP/+;Pax6αCre and Brn3bCKOAP/−;Pax6αCre vertical retina sections. (A–B) calretinin, (C–D) glutamic acid decarboxylase (GAD) 65/67, (E–F) calbindin, and (G–H) vesicular inhibitory amino acid transporter (VIAAT). White arrowheads indicate the individual cells that are enlarged and shown without the DAPI channel in the smaller red, green, and merged panels to the right. In panel A (Brn3bCKOAP/+;Pax6αCre), a rare displaced (i.e. cell body in the INL) RGC shows co-localization of AP and calretinin, a marker for subsets of amacrine cells and RGCs. In panels C, F, and G (Brn3bCKOAP/+;Pax6αCre), rare displaced RGCs show no co-localization of AP and the amacrine cell markers calbindin, GAD65/67, or VIAAT. In panels B, D, F, and H (Brn3bCKOAP/−;Pax6αCre), examples are shown of the many AP+ cell bodies in the INL. Co-localization of AP with VIAAT is seen in panel H (and in 11/15 cells scored); and co-localization of AP and calretinin, calbindin, or GAD65/67 is not seen in panels B,D, and F (or in 0/7, 0/12, or 0/8 cells scored for these markers, respectively).
(I) Front and lateral views of a reconstructed Brn3bCKOAP/−;Pax6αCre AP-expressing cell with amacrine-like morphology and a cell body in the INL. Horizontal scale bar: 25 μm. Vertical bar corresponds to the location of the IPL.
Supplementary Figure 5. Development of stratification in the IPL by Brn3aAP/+ and Brn3aAP/− dendrites.
(A–D) Immunostaining for calbindin and AP in Brn3aCKOAP/+;Pax6αCre and Brn3aCKOAP/−;Pax6αCre retinas at P4 and P11. The inner retina is shown with the GCL at the bottom. Schematic at right is as for Figure 3. Scale bar: 40 μm.