Abstract
Melanoma differentiation associated gene-7/interleukin 24 (mda-7/IL-24) is a novel cytokine displaying selective apoptosis-inducing activity in transformed cells without harming normal cells. The studies by further defines the mechanism(s) by which a GST-MDA-7 fusion protein inhibits cell survival of primary human glioma cells in vitro. GST-MDA-7 killed glioma cells with diverse genetic characteristics that were dependent on activation of JNK1-3 with subsequent activation of BAX and the induction of mitochondrial dysfunction. Activation of JNK1-3 was dependent upon protein kinase R-like endoplasmic reticulum kinase (PERK) and GST-MDA-7 lethality was suppressed in PERK-/- cells. GST-MDA-7 caused PERK-dependent vacuolization of LC3-expressing endosomes whose formation was suppressed by incubation with 3-methyladenine, expression of HSP70 or of BiP/GRP78, or by knockdown of ATG5 or Beclin 1 expression, but not by inhibition of the JNK1-3 pathway. Knockdown of ATG5 or Beclin 1 expression or overexpression of HSP70 reduced GST-MDA-7 lethality. Our data demonstrate that GST-MDA-7 induces an ER stress response that, via the induction of autophagy, is causal in the activation of pro-apoptotic pathways that converge on the mitochondrion and ultimately culminate in decreased glioma cell survival.
Keywords: autophagy, caspase, ER stress, cell death
Glioblastoma multiforme is one of the most lethal malignancies, with very low 5 year survival rates (<1%).1 The mda-7 gene (recently renamed Interleukin 24, IL-24) was isolated from human melanoma cells induced to undergo terminal differentiation by treatment with fibroblast interferon and mezerein.2 The protein expression of MDA-7/IL-24 is decreased in advanced melanomas, with nearly undetectable levels in metastatic disease.2-4 This novel cytokine is a member of the interleukin-10 (IL-10) gene family.5-12 Enforced expression of MDA-7/IL-24, by use of a recombinant adenovirus Ad.mda-7, inhibits the growth and kills a broad spectrum of cancer cells, without exerting deleterious effects in normal human epithelial or fibroblast cells.9-14 Considering its potent cancer-specific apoptosis-inducing ability and tumor growth-suppressing properties in human tumor xenograft animal models, mda-7/IL-24 was evaluated in a Phase I clinical trial in patients with advanced cancers.10,11,15 This study indicates that Ad.mda-7 injected intra-tumorally was safe, and with repeated injection, significant clinical activity was evident.
The apoptotic pathways by which Ad.mda-7 causes cell death in tumor cells are not fully understood, and current evidence suggests an inherent complexity and an involvement of proteins important for the onset of growth inhibition and apoptosis, including Bcl-XL Bcl-2, Bax and APO2/TRAIL.9-14 In melanoma cell lines, but not in normal melanocytes, Ad.mda-7 infection induces a significant decrease in both Bcl-2 and Bcl-XL levels, with only a modest upregulation of Bax and Bak expression.16 These data support the hypothesis that Ad.mda-7 enhances the ratio of pro-apoptotic to anti-apoptotic proteins in cancer cells, thereby facilitating induction of apoptosis.9-14,16,17 The ability of Ad.mda-7 to induce apoptosis in DU145 prostate cancer cells, which does not produce Bax, indicates that MDA-7/IL-24 can also mediate apoptosis in tumor cells by a Bax-independent pathway.9-12 In prostate cancer cells, overexpression of either Bcl-2 or Bcl-XL protects cells from Ad.mda-7-induced toxicity in a cell type-dependent fashion.18 Thus MDA-7/IL-24 lethality seems to occur by multiple distinct pathways in different cell types. More recently, MDA-7/IL-24 toxicity has been linked to alterations in endoplasmic reticulum stress signaling.19 In these studies, MDA-7/IL-24 physically associates with BiP/GRP78 and inactivates the protective actions of this ER-chaperone protein. In addition to virus-administered mda-7/IL-24, delivery of this cytokine as a bacterially-expressed GST fusion protein, GST-MDA-7, retains cancer-specific killing, selective ER localization and induces similar signal transduction changes in cancer cells. We have noted that high concentrations of GST-MDA-7 or infection with Ad.mda-7 kill rodent and human glioma cells.20-23 However, the precise mechanisms by which Ad.mda-7 and GST-MDA-7 modulates cell survival in non-established human glioma cells are presently unknown.
A GST-MDA-7 concentration that causes profound toxicity ∼72 h after exposure in glioma cells correlates with strong activation of JNK1-3. This treatment nearly abolishes ERK1/2 signaling. Multiple studies using a variety of cytokine and toxic stimuli document that JNK1-3 activation in astrocytes, neurons and transformed versions of these cells can trigger cell death.24 The balance between the readouts of ERK1/2 and JNK1-3 signaling may represent a key homeostatic mechanism that regulates cell survival versus cell death processes.25 GST-MDA-7—induced JNK1-3 signaling is PERK-dependent and causal in Bax activation, with loss of Bax expression reducing GST-MDA-7—induced cell killing. GST-MDA-7—induced suppression of ERK1/2 signaling is also found to be PERK-dependent. These findings argue that a form of ER stress signaling may be a primary mediator of GST-MDA-7-induced toxicity in primary malignant glioma cells (Fig. 1). Our studies go on to demonstrate that MDA-7/IL-24 must simultaneously induce multiple pathways of mitochondrial dysfunction to provoke tumor cell killing, including activation of Bax and Bak, cleavage of BID, dephosphorylation of BAD, and dephosphorylation and increased expression of BIM. In parallel, MDA-7/IL-24 also reduces expression of mitochondrial protective proteins such as Bcl-XL and Mcl-1. Activation of death receptor-caspase 8 signaling was not involved in any GST-MDA-7—stimulated death processes in GBM cells.
Figure 1.
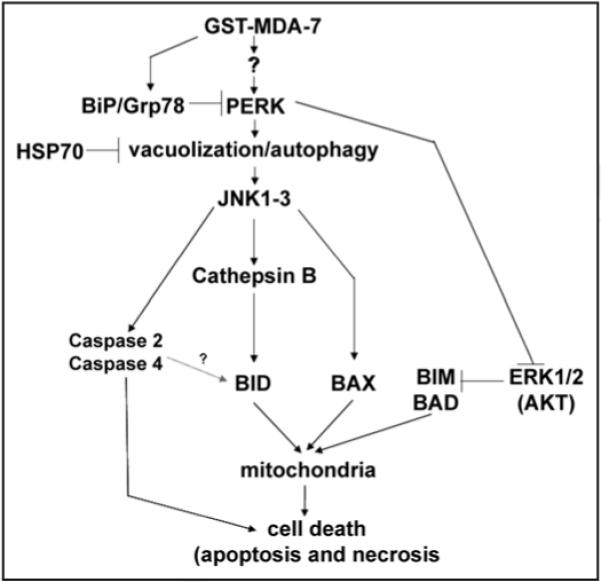
GST-MDA-7 causes PERK-dependent vacuolization and JNK pathway activation in transformed cells that leads to cell death. Exposure to GST-MDA-7 causes a PERK-dependent activation of the JNK pathway and a PERK-dependent inactivation of the ERK1/2 pathway. Enhanced JNK pathway signaling plays a key role in the activation of pro-caspase 2, pro-caspase 4 and cathepsin B. Activation of cathepsin B promotes the cleavage/activation of the BH3 domain protein BID. Elevated JNK pathway signaling also activates BAX. Inactivation of the ERK1/2 pathway facilitates activation of BAD and BIM. Thus GST-MDA-7, via PERK signaling, promotes increased activity of at least 4 BH3 domain proteins which all act to cause mitochondrial dysfunction and ultimately promote cell killing.
GST-MDA-7 activated a PERK-dependent pathway to initiate mitochondrial dysfunction that requires JNK activation downstream of PERK. Although cell killing is reduced in PERK-/- cells, GST-MDA-7 toxicity is still evident and other ER stress regulatory proteins as well as other sensors of the unfolded protein response, e.g., activating transcription factor 6 (ATF6), inositol-requiring enzyme1 (IRE1), PKR, HRI and GCN2, may mediate the toxic response of GST-MDA-7.26 Prior studies implicate MDA-7/IL-24 as a protein that associates with and activates PKR.27 As PERK and PKR are proteins with structural similarities, it is possible that PKR and PERK represent MDA-7/IL-24 targets in the regulation of eIF2α phosphorylation and transformed cell survival. Matsuzawa et al. implicate a TRAF2-ASK1-JNK cascade downstream of IRE1 in ER-stress responses in multiple cell types, and based on our data PERK-dependent signaling can also feed into this survival regulatory process.28
GST-MDA-7 causes cell killing in part via a PERK-dependent mechanism, PERK is a sensor of ER stress, and MDA-7/IL-24 has been previously shown to bind to a regulatory chaperone of PERK, namely BiP/GRP78. Therefore, we explored whether GST-MDA-7 altered intracellular vacuolization of cells and specifically whether GST-MDA-7 could cause the formation of autophagic vesicles. Using a plasmid expressing a GFP-tagged form of LC3 GST-MDA-7 caused vacuolization of GFP-LC3 in multiple GBM cell types within 12-24 h, at a time prior to measurable cell killing. Expression of a dominant negative PERK protein, knockdown of ATG5 or Beclin 1 protein expression, or overexpression of the MDA-7/IL-24 binding partner BiP/GRP78, suppresses vesicle formation and protects GBM cells from GST-MDA-7 toxicity.29-32 3-methyladenine can suppress autophagic vesicle formation and incubation of GBM cells with this agent also suppresses GFP-LC3 -containing vesicle formation and protects cells from GST-MDA-7 toxicity. Our data strongly argue that GST-MDA-7 promotes GBM and transformed cell death and one of the earliest manifestations of GST-MDA-7-induced cellular dysfunction is the formation of autophagic vesicles.
Increased expression of HSP70 has been shown by several groups to stabilize endosomes and to promote cell survival in response to noxious stresses, including ER stress.33,34 In our analyses, we demonstrate that GST-MDA-7 variably causes early, and definitively causes later, suppression of HSP70 protein levels that correlate with increasing amounts of autophagic vacuolization in glioma cells; overexpression of HSP70 blocks the formation of GFP-LC3 vesicles and significantly suppresses GST-MDA-7 toxicity. Many laboratories are attempting to generate small molecule HSP70 inhibitors and it will be of interest to determine whether MDA-7 lethality will be enhanced by any such putative HSP70 inhibitory drug.
In summary, in transformed cells GST-MDA-7 induces multiple pro-apoptotic pathways to promote cell death. In primary human GBM cells, activation of the JNK1-3 pathway represents a key nodal signal, downstream of PERK and the induction of a toxic form of autophagy in promoting the activation of multiple pro-apoptotic proteases and causing mitochondrial dysfunction. From our studies, it is clear that the downstream effectors are complex, but the defining events in MDA-7/IL-24-promoted lethality of GBM cells involve a shift in the balance between anti-apoptotic and pro-apoptotic signals eliciting mitochondrial dysfunction uniquely in the context of cancer cells.
Acknowledgements
Support for the present study was provided; to P.D. from PHS grants (P01-CA104177, R01-CA108325, R01-DK52825) and The Jim Valvano “V” foundation; to S.G. from PHS grants (R01-CA63753; R01-CA77141) and a Leukemia Society of America grant 6405-97; to PBF from PHS grants (P01-CA104177, R01-CA097318; R01-CA098172; P01-NS031492), and the Samuel Waxman Cancer Research Foundation; and to DTC from PHS grant (P01-CA104177). P.D. is The Universal Inc. Professor in Signal Transduction Research and P.B.F. holds the Thelma Neumeyer Corman Chair in Cancer Research and is a SWCRF Investigator. This manuscript is dedicated to Gillian in her continued fight against GBM.
Abbreviations
- ERK
extracellular regulated kinase
- MEK
mitogen activated extracellular regulated kinase
- JNK
c-Jun NH2-terminal kinase
- PI3K
phosphatidyl inositol 3 kinase
- MDA-7
melanoma differentiation associated gene 7
- PERK
protein kinase R-like endoplasmic reticulum kinase
- MAPK
mitogen activated protein kinase
- ca
constitutively active
- dn
dominant negative
- EGFR
epidermal growth factor receptor
- IL
interleukin
- PTEN
phosphatase and tensin homologue on chromosome ten
Footnotes
Addendum to: Yacoub A, Gupta P, Park MA, Rhamani M, Hamed H, Hanna D, Zhang G, Sarkar D, Lebedeva IV, Emdad L, Koumenis C, Curiel DT, Grant S, Fisher PB, Dent P. Regulation of GST-MDA-7 toxicity in human glioblastoma cells by ERBB1, ERK1/2, PI3K, and JNK1-3 pathway signaling. Mol Cancer Ther 2008; 7:314-29.
References
- 1.Robins HI, Chang S, Butowski N, Mehta M. Therapeutic advances for glioblastoma multiforme: current status and future prospects. Curr Oncol Rep. 2007;9:66–70. doi: 10.1007/BF02951428. [DOI] [PubMed] [Google Scholar]
- 2.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–86. [PubMed] [Google Scholar]
- 3.Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, et al. Downregulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int J Cancer. 2001;94:54–9. doi: 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- 4.Ellerhorst JA, Prieto VG, Ekmekcioglu S. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20:1069–74. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- 5.Huang EY, Madireddi MT, Gopalkrishnan RV, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–63. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 6.Parrish Novak J, Xu W, Brender T, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–23. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 7.Caudell EG, Mumm JB, Poindexter N, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168:6041–6. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 8.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–79. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 9.Gupta P, Su ZZ, Lebedeva IV, et al. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lebedeva IV, Sauane M, Gopalkrishnan RV, et al. mda-7/IL-24: exploiting cancer’s Achilles’ heel. Mol Ther. 2005;11:4–18. doi: 10.1016/j.ymthe.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Fisher PB, Gopalkrishnan RV, Chada S, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:23–37. [PubMed] [Google Scholar]
- 12.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–38. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 13.Su ZZ, Lebedeva IV, Gopalkrishnan RV, et al. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci USA. 2001;98:10332–7. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su ZZ, Madireddi MT, Lin JJ, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci USA. 1998;95:14400–5. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham CC, Chada S, Merritt JA, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–59. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 16.Lebedeva IV, Su ZZ, Chang Y, Kitada S, Reed JC, Fisher PB. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21:708–18. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 17.Saeki T, Mhashilkar A, Swanson X, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21:4558–66. doi: 10.1038/sj.onc.1205553. [DOI] [PubMed] [Google Scholar]
- 18.Su ZZ, Lebedeva IV, Sarkar D, et al. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–48. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- 19.Gupta P, Walter MR, Su ZZ, et al. BiP/GRP78 Is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006;66:8182–91. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- 20.Su ZZ, Lebedeva IV, Sarkar D, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003;22:1164–80. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 21.Yacoub A, Mitchell C, Lister A, et al. Melanoma differentiation-associated 7 (interleukin 24) inhibits growth and enhances radiosensitivity of glioma cells in vitro and in vivo. Clin Cancer Res. 2003;9:3272–81. [PubMed] [Google Scholar]
- 22.Yacoub A, Mitchell C, Lebedeva IV, et al. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol Ther. 2003;2:347–53. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
- 23.Yacoub A, Mitchell C, Hong Y, et al. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739–51. doi: 10.4161/cbt.3.8.968. [DOI] [PubMed] [Google Scholar]
- 24.Yoon S, Choi J, Yoon J, Huh JW, Kim D. Okadaic acid induces JNK activation, bim overexpression and mitochondrial dysfunction in cultured rat cortical neurons. Neurosci Lett. 2006;394:190–5. doi: 10.1016/j.neulet.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 25.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 26.Fels DR, Koumenis C. The PERK/eIF2α/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–28. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- 27.Pataer A, Vorburger SA, Chada S, et al. Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR. Mol Ther. 2005;11:717–23. doi: 10.1016/j.ymthe.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 28.Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal. 2002;4:415–25. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- 29.Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–34. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 30.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–88. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–32. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 32.Shibata M, Lu T, Furuya T, et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem. 2006;281:14474–85. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- 33.Nylandsted J, Gyrd-Hansen M, Danielewicz A, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425–35. doi: 10.1084/jem.20040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–57. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]


