Abstract
Rationale: The role played by resident pleural macrophages in the initiation of pleural inflammation is currently unclear.
Objective: To evaluate the role of resident pleural macrophages in the initiation of inflammation.
Methods: We have used a conditional macrophage ablation strategy to determine the role of resident pleural macrophages in the regulation of neutrophil recruitment in a murine model of experimental pleurisy induced by the administration of carrageenan and formalin- fixed Staphylococcus aureus.
Measurements and Main Results: Conditional macrophage ablation mice express the human diphtheria toxin receptor under the control of the CD11b promoter such that the administration of diphtheria toxin induces ablation of nearly 97% of resident macrophages. Ablation of resident pleural macrophages before the administration of carrageenan or S. aureus dramatically reduced neutrophil influx into the pleural cavity. In the carrageenan model, the reduction in neutrophil infiltration was associated with marked early reduction in the level of macrophage inflammatory protein 2 as well as reduced levels of various cytokines, including tumor necrosis factor α, interleukin 6, and interleukin 10. Adoptive transfer of nontransgenic macrophages partially restored neutrophil infiltration. We also stimulated macrophage-depleted and nondepleted pleural cell populations with carrageenan in vitro and determined the production of chemokines and cytokines. Chemokine and cytokine production was markedly reduced by macrophage depletion, reinforcing the role of resident pleural macrophages in the generation of mediators that initiate acute inflammation.
Conclusion: These studies indicate a critical role for resident pleural macrophages in sensing perturbation to the local microenvironment and orchestrating subsequent neutrophil infiltration.
Keywords: inflammation, macrophage, pleural diseases
The pleural membranes and associated cells are important because they are metabolically active and act as a barrier to invading pathogens by generating an innate and adaptive immunologic response. The pleural cavity is lined with mesothelium and contains resident macrophages (Mφ), mast cells, and lymphocytes (1, 2). During pleural inflammation, it has been reported that mesothelial cells are predominantly responsible for the secretion of C-X-C chemokines, such as interleukin 8 (IL-8), and C-C chemokines, such as macrophage inflammatory protein 1α (MIP-1α) and macrophage chemoattractant protein 1 (MCP-1), which act to recruit neutrophils (polymorphonuclear leukocytes [PMNs]) and mononuclear cells (3–6). In addition, a recent study demonstrated that activated pleural fibroblasts may also be a source of C-X-C and C-C chemokine production (7).
Previous work suggested that the initiation of inflammation is dependent on endogenous IL-6 secretion that subsequently stimulates the additional production of tumor necrosis factor α (TNF-α) and IL-1β from resident pleural cells (8). In contrast, increased IL-1β levels have been reported to precede elevated IL-6 levels (9), thereby suggesting that IL-1β might induce IL-6 production. There is no doubt that TNF-α and IL-1β are key cytokines in the development of pleural inflammation because they act to enhance IL-8 and MCP-1 production from mesothelial cells (3, 5, 10–12). In addition, studies using function-blocking antibodies suggest that activated resident Mφ could be responsible for this TNF-α and IL-1β secretion (10, 12).
Carrageenan-induced pleurisy is a well-established model of acute inflammation (13) and is characterized by a rapid influx of PMNs followed by mononuclear cell infiltration (14, 15). This model is often used to assess the antiinflammatory effects of pharmaceutical agents (16–20) and to assess the in vivo importance of established inflammatory mediators (21–23). Although the neutrophil influx evident in this model is generally used as an experimental readout of acute inflammation, there are data indicating that neutrophils are involved in the release of injurious enzymes and modulation of vascular permeability in carrageenan-mediated pleural inflammation (24, 25).
To date, there has been little study of the role of the resident pleural Mφ in the initiation of inflammation and orchestration of PMN recruitment. Previous work demonstrated a reduced eosinophil influx after administration of LPS to mice that had been previously treated with diphosphonate-containing liposomes to deplete resident pleural Mφ (26). Although this suggests that resident pleural Mφ may play a key role in the initiation of pleural inflammatory responses, there are no definitive data available for PMN infiltration and proinflammatory cytokine production.
This study used transgenic mice expressing the human diphtheria toxin receptor (DTR) under the CD11b promoter (designated CD11b-DTR mice) (27) to examine the role of resident pleural Mφ in carrageenan-induced pleurisy. Administration of diphtheria toxin (DT) to CD11b-DTR mice results in rapid depletion of resident pleural Mφ. Our data indicate that ablation of resident pleural Mφ markedly blunted both PMN recruitment and the levels of key chemokines and cytokines. In addition, resident Mφ ablation markedly reduced the acute PMN infiltration that followed the instillation of fixed, killed Staphylococcus aureus. This study demonstrates that resident pleural Mφ play an essential role in the orchestration of pleural PMN recruitment in pleural inflammation induced by carrageenan and fixed, killed S. aureus.
METHODS
Macrophage Ablation and Pleurisy Induction
Mice were housed in the University of Edinburgh animal facilities and experiments were performed in accordance with institutional and U.K. Home Office guidelines. CD11b-DTR transgenic mice were generated as previously described and were on an FVB/N background (27). Resident pleural Mφ were ablated in homozygous CD11b-DTR mice by intraperitoneal injection of DT (25 ng/g body weight) 24 h before the administration of carrageenan. DT-treated FVB/N wild-type (WT) mice served as control animals. Carrageenan-induced pleurisy was induced as described previously (28). λ-Carrageenan (0.1 ml of a 1% solution) was injected into the pleural cavity. Animals were culled at various time points after pleurisy developed. In addition, 3 × 106 formalin-fixed, fluorescently labeled S. aureus (Sigma, Dorset, UK) were injected into the pleural cavity of CD11b-DTR mice and FVB/N WT mice 24 h after administration of DT or phosphate-buffered saline (PBS). Animals were culled 4 h later.
Cell Processing and Analysis
Pleural cavities were washed with 1 ml of 3.15% (weight/volume) sodium citrate (Sigma, Dorset, UK) in saline. We performed flow cytometric analysis of pleural lavage and circulating blood as described previously (27). The antibodies used were anti-CD11b fluorescein isothiocyanate, anti-GR1 phycoerythrin (PE) and anti–c-kit PE (all from eBiosciences, London, UK), anti-B220 (mouse CD45R) PE and mouse anti-CD3 PE (both from Pharmingen, San Diego, CA), and F4/80 allophycocyanin (APC) and F4/80 PE (both from Caltag, Botolph Claydon, UK). Cell number was determined as described previously (27).
Adoptive Transfer of Pleural Cell Populations
Pleural lavages from groups of naive FVB/N WT mice were incubated with PE-conjugated anti-F4/80 antibody to stain Mφ and then incubated with anti-PE conjugated magnetic cell sorting (MACS) magnetic beads (Miltenyi Biotech Ltd., UK). Mφ were removed by passing the cells over a magnetic MACS column (27). As a control, pleural cells were incubated with an isotype control antibody and then processed as previously stated. This method removed 98.2 ± 0.7% of the Mφ. In addition, resident pleural Mφ were purified by negative selection after incubation of pleural cells with PE-conjugated anti-B220, anti–c-kit, and anti-CD3 antibodies followed by incubation with anti–PE-conjugated MACS magnetic beads and passage through the magnetic MACS column; isolated Mφ were 90% pure. Purified Mφ and the Mφ-depleted and Mφ nondepleted pleural cell populations were resuspended in 1% carrageenan and administered into the pleural cavity of each mouse. Groups therefore consisted of (1) DT-treated CD11b-DTR transgenic mice depleted of resident pleural Mφ, (2) DT-treated FVB/N WT mice, (3) Mφ-depleted mice reconstituted with a nondepleted Mφ-rich pleural cell population, (4) Mφ-depleted mice reconstituted with a Mφ-depleted pleural cell population, and (5) Mφ-depleted mice reconstituted with a population of pleural Mφ purified by negative selection. As a control, the effect of cell transfer alone was assessed by reconstituting Mφ-depleted mice with either nondepleted Mφ-rich pleural cells or purified Mφ alone in the absence of any additional stimulus. Animals were killed 6 h after induction of pleurisy.
Chemokine Studies
Mice underwent pleural lavage at 1, 3, 6, 24, and 72 h after administration of carrageenan. Lavage fluid was centrifuged and stored at −80°C until analyzed by specific ELISA for MIP-2, keratinocyte-derived chemokine (KC), and TNF-α (R&D Systems, Abingdon, UK). Cytometric bead array (BD Biosciences, Oxford, UK) was also used to determine the concentration of IL-6, IL-10, IL-12p70, IFN-γ, and MCP-1, with samples being processed as described previously (29). Chemokine and cytokine production by intact pleural cell populations or Mφ-depleted pleural cell populations stimulated with carrageenan in vitro was also determined: Mφ depletion was achieved using the MACS magnetic column and resulted in more than 98% Mφ depletion. Control pleural cells and Mφ-depleted pleural cells were plated in 48-well plates and exposed to 0.25% carrageenan for 6 h. In control experiments, cell preparations were exposed to medium alone. Pleural cell–conditioned supernatants were analyzed as above. No bioassays were undertaken.
Statistical Analysis
One-way analysis of variance with a Bonferroni multiple comparison post hoc test, with a 95% confidence interval, or a Student's t test was used as appropriate. Statistical analysis including correlation analysis was performed using GraphPad Prism software (San Diego, CA). The significance level was set at p < 0.05. Data are presented as mean ± SEM.
RESULTS
Transgenic Pleural Resident Mφ Are Ablated by DT In Vivo
There was no difference in the number of pleural Mφ, B cells, T cells, or mast cells between CD11b-DTR and FVB/N control mice (data not shown). Flow cytometric analysis of pleural cells was performed 24 h after the injection of DT (25 ng/g mouse body weight). CD11b-DTR transgenic mice exhibited almost complete ablation (96.1% ± 0.8) of F4/80-positive pleural Mφ after a single dose of DT (Figure 1). In addition, flow cytometric analysis of whole blood performed 24 h after DT administration indicated a significant 88% reduction in circulating monocyte numbers (1.17 × 105 ± 5.9 × 104 monocytes/ml whole blood vs. 5.23 × 105 ± 7.3 × 104, DT injection vs. control; p < 0.05). Circulating monocyte and pleural macrophage numbers remained markedly reduced for 48 h after the administration of DT with recovery of monocyte/macrophage numbers evident at 72 h (data not shown). However, no reduction in the number of circulating PMNs was evident 24 h after DT administration (10.1 × 105 ± 1.9 × 105 PMNs/ml whole blood vs. 4.7 × 105 ± 0.8 × 105, DT injection vs. control; p < 0.05). In addition, no difference in circulating PMN number was evident 6, 48, or 72 h after the administration of DT, indicating an absence of any initial neutropenia or delayed effects (6 h: 7.9 × 105 ± 1.2 × 105 PMNs/ml whole blood vs. 5.0 × 105 ± 1.4 × 105, DT injection vs. control; p > 0.05; 48 h: 4.0 × 105 ± 0.6 × 105 PMNs/ml whole blood vs. 4.9 × 105 ± 0.1 × 105, DT injection vs. control; p > 0.05; 72 h: 2.9 × 105 ± 1.4 × 105 PMNs/ml whole blood vs. 4.4 × 105 ± 0.3 × 105, DT injection vs. control; p > 0.05). We did, however, note a significant reduction in the number of B cells and mast cells within the pleural cavity 24 h after the administration of DT although T-cell numbers were unaffected (B cells: 8.1 × 104 ± 5.7 × 104 vs. 32.9 × 104 ± 8.8 × 104, DT vs. control; p < 0.05; mast cells: 6.1 × 102 ± 0.1 × 102 vs. 67.8 × 102 ± 18.2 × 102, DT vs. control; p < 0.05). Interestingly, the depletion of pleural Mφ is almost complete at 6 h at which time no significant difference in the number of B lymphocytes or mast cells was evident. The loss of B cells and mast cells may be a consequence of the secondary necrosis of apoptotic macrophages that may occur in the absence of a population of viable macrophages to phagocytose the dying cells. Also, a subset of B lymphocytes and mast cells may express CD11b and this may account for the reduced numbers seen after the administration of DT (30–32).
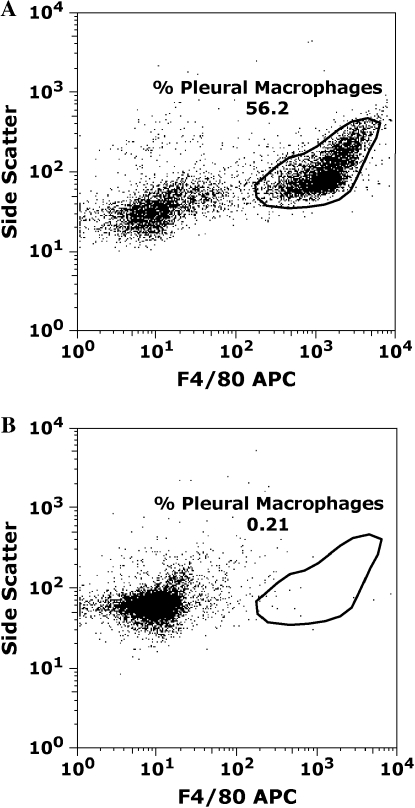
Figure 1.
Administration of DT 24 h before performing pleural lavage results in ablation of pleural F4/80-positive macrophages (Mφ). CD11b-DTR and FVB/N wild-type (WT) mice were treated with diphtheria toxin (DT) intraperitoneally at a dose of 25 ng/g body weight. Pleural lavage was performed 24 h later. Cells were stained for the Mφ surface marker F4/80 and analyzed by flow cytometry. (A) Representative flow cytometry dot plot indicating that over 50% of pleural cells retrievable by pleural lavage 24 h after DT administration in FVB/N mice are F4/80 positive. (B) Administration of DT results in marked ablation of resident F4/80 positive pleural Mφ in CD11b-DTR mice. DT administration ablated 96.1 ± 0.8% of the resident Mφ population compared with baseline Mφ numbers (n = 9 mice, p < 0.0001). APC = allophycocyanin.
Pleural Resident Mφ Ablation Reduces PMN Influx in Carrageenan-induced Pleurisy
We used the conditional Mφ ablation strategy to investigate the role of resident pleural Mφ in initiating PMN recruitment after the administration of carrageenan. PMN infiltration after the administration of 1% carrageenan was markedly attenuated at all experimental time points after resident Mφ ablation (Figure 2). It is particularly noteworthy that the early time points of 6 and 24 h demonstrated a dramatic difference between groups. Although PMN infiltration in CD11b-DTR mice did reach approximately 50% of control levels at the later time points of 72 h, this was still significantly less than DT-treated nontransgenic FVB/N WT mice.
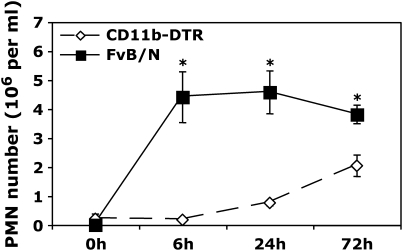
Figure 2.
Resident Mφ ablation 24 h before administration of carrageenan blunts neutrophil (PMN) recruitment. 0.1 ml of 1% carrageenan was administered to CD11b-DTR and FVB/N WT mice 24 h after DT treatment. Pleural lavage was performed at 0, 6, 24, and 72 h after carrageenan administration. Lavaged cells were stained for GR1 and counted by flow cytometry (*p < 0.05 vs. CD11b-DTR group; n = 4–5 mice/group).
Adoptive Transfer of Nontransgenic Purified Mφ or Mφ-rich Pleural Cell Populations Partially Restores PMN Influx in Mφ-ablated CD11b-DTR Mice after Carrageenan Administration
To further analyze the role of resident pleural Mφ in the initiation of acute pleural inflammation, we also performed Mφ repletion studies using the adoptive transfer of either Mφ-rich or Mφ-depleted pleural cell populations derived from DT-insensitive nontransgenic FVB/N WT mice. In these experiments, the adoptive transfer of Mφ-rich pleural cell populations restored Mφ number to approximately 50% of the Mφ number normally present in pleural lavage fluid. However, despite the fact that Mφ reconstitution of DT-treated CD11b-DTR mice was incomplete, the administration of Mφ-rich pleural cells concurrently with carrageenan significantly increased PMN infiltration at 6 h (Figure 3). The partial restoration of peak PMN infiltration was approximately 35% of levels present in control DT-treated FVB/N WT mice at the same time point. In contrast, administration of Mφ-depleted pleural cells concurrently with carrageenan made no significant impact on PMN infiltration compared with Mφ-depleted CD11b-DTR mice (Figure 3). Interestingly, a significant correlation (R2 = 0.9979) was found between the Mφ number present in the pleural space at the initiation of inflammation and the number of infiltrating PMNs present at 6 h. We also reconstituted DT-treated CD11b-DTR mice with purified Mφ (90% pure) concurrently with the administration of carrageenan and this resulted in a comparable PMN influx to that evident after reconstitution with Mφ-rich pleural cells. It should be noted that, although DT-induced Mφ ablation is associated with a reduction of B-cell and mast cell number, the administration of Mφ-depleted pleural cells comprising B cells, mast cells, and T cells had no significant impact on PMN infiltration. Last, the adoptive transfer of a control population of Mφ-rich pleural cells or purified Mφ was noninflammatory (Figure 3).
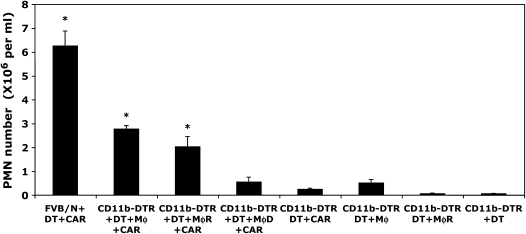
Figure 3.
Adoptive transfer of Mφ-rich pleural cells and purified pleural macrophages partially restores PMN infiltration in carrageenan-induced pleurisy. FVB/N WT and six groups of CD11b-DTR mice were injected with DT (25 ng/g body weight) 24 h before carrageenan injection. Three groups of Mφ-depleted CD11b-DTR mice were reconstituted with (1) purified Mφ isolated by negative selection (90% pure, designated Mφ), (2) Mφ-rich pleural cells (designated MφR), or (3) Mφ-depleted pleural cells (designated MφD) at the same time as the administration of carrageenan. Mice underwent pleural lavage 6 h after the induction of inflammation. Controls comprised the adoptive transfer of either (1) purified Mφ or (2) Mφ-rich pleural cell populations to DT-treated CD11b-DTR mice in the absence of carrageenan. DT-treated CD11b-DTR mice exhibited a marked reduction in PMN infiltration in response to carrageenan, whereas reconstitution of Mφ-depleted mice with either purified Mφ or a Mφ-rich pleural cell population partially restored PMN infiltration. The adoptive transfer of an Mφ-depleted pleural cell population did not increase PMN infiltration. The adoptive transfer of either purified Mφ or an Mφ-rich pleural cell population alone did not induce significant PMN infiltration compared with DT-treated CD11b-DTR mice (n = 8–10 mice/group; *p < 0.05 vs. DT-treated CD11b-DTR mice that received carrageenan).
Mφ-dependent Chemokine and Cytokine Responses during Carrageenan-induced Pleurisy
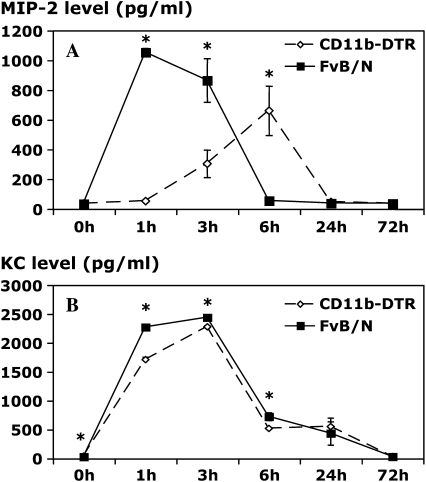
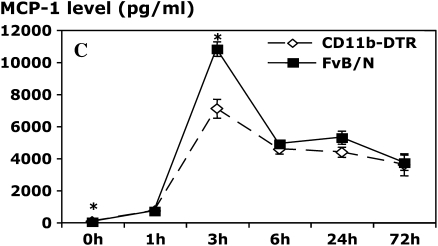
In this model, we found peak levels of the PMN C-X-C chemokines MIP-2 and KC at the 1- and 3-h time points, respectively. Ablation of resident pleural Mφ before administration of carrageenan markedly reduced MIP-2 levels at both 1 and 3 h (Figure 4A), thereby suggesting that the early production of MIP-2 in vivo is predominantly Mφ dependent. Interestingly, however, Mφ-ablated mice exhibited a delayed and significantly blunted MIP-2 response. It is of interest that very few Mφ (< 30,000) are present within the pleural cavity of DT-treated CD11b-DTR mice at the 6-h time points, suggesting that the delayed MIP-2 response may be derived from production by local cells, such as mesothelial cells and others. MIP-2 levels are very low at the 24-h time point and beyond in both experimental groups. In contrast to the MIP-2 data, a very modest, albeit statistically significant, reduction in KC levels was evident in Mφ-depleted mice at the 1-, 3-, and 6-h time points (Figure 4B), but no differences were evident thereafter, suggesting that cells other than Mφ may be responsible for production of this chemokine. The fact that ablation of resident pleural Mφ dramatically blunted PMN infiltration suggests that early PMN influx is very dependent on resident Mφ production of MIP-2. The ablation of resident pleural Mφ did not exert marked effects on the production of MCP-1 as levels were only reduced by approximately 36% at the 3-h time point (Figure 4C), suggesting a source other than Mφ.
Figure 4.
Resident Mφ ablation attenuates chemokine production in carrageenan-induced pleurisy. CD11b-DTR and FVB/N WT mice were injected with DT (25 ng/g body weight) 24 h before administration of carrageenan. Pleural lavage was performed 1, 3, 6, 24, and 72 h after the induction of pleurisy. The levels of macrophage inflammatory protein 2 (MIP-2; A) and keratinocyte-derived chemokine (KC; B) were determined in the pleural lavage supernatant by specific ELISA. The level of macrophage chemoattractant protein 1 (MCP-1; C) in the pleural lavage supernatant was determined by cytometric bead array (CBA) analysis (*p < 0.05 vs. CD11b-DTR group; n = 5 mice/group).
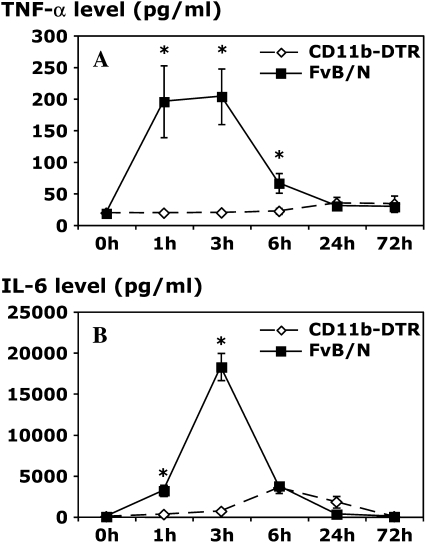
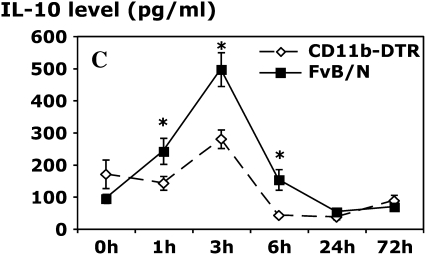
Analysis of the levels of cytokines in pleural lavage samples indicated a key role for resident Mφ in the early production of the cytokines TNF-α, IL-6, and IL-10. Mφ ablation resulted in greater than 90% reduction in TNF-α and IL-6 levels, with a less dramatic but significant inhibitory effect on IL-10 levels (Figure 5). IL-12 levels were also reduced with Mφ ablation at 24 h (data not shown). Despite these important differences in these cytokines, IFN-γ levels were comparable between DT-treated CD11b-DTR and FVB/N control mice at each time point (data not shown), suggesting a source other than resident Mφ.
Figure 5.
Resident Mφ ablation attenuates cytokine production in carrageenan-induced pleurisy. CD11b-DTR and FVB/N WT mice were injected with DT (25 ng/g body weight) 24 h before carrageenan injection. Pleural lavage was performed 1, 3, 6, 24, and 72 h after the induction of pleurisy. The level of tumor necrosis factor α (TNF-α; A) in the pleural lavage supernatant was determined by specific ELISA, whereas the levels of interleukin 6 (IL-6; B) and IL-10 (C) were determined by CBA analysis (*p < 0.05 vs. CD11b-DTR group; n = 5 mice/group).
Chemokine and Cytokine Responses of Pleural Cell Populations In Vitro Are Mφ Dependent
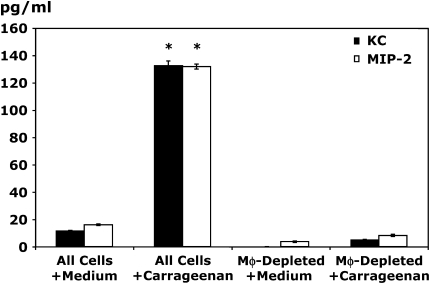
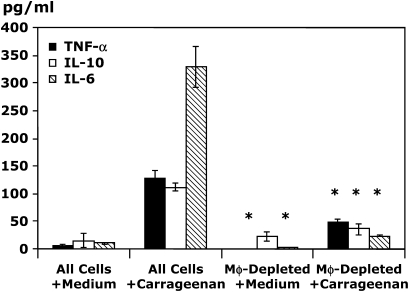
Because pleural mesothelial cells may be an important source of chemokines, we performed additional in vitro studies to determine the production of chemokines and cytokines by carrageenan-stimulated pleural cell populations that had been depleted of Mφ. Immunomagnetic Mφ depletion using antibodies for the Mφ specific marker F4/80 resulted in 98% depletion of Mφ from pleural cell populations, whereas B-cell and mast cell numbers were comparable between groups (data not shown). Stimulation of control Mφ-rich pleural cell populations for 6 h with carrageenan resulted in significant production of MIP-2 and KC (Figure 6). In contrast, no significant chemokine production was evident after stimulation of pleural cell populations depleted of resident Mφ but containing B cells, T cells, and mast cells, thereby indicating that production of these PMN C-X-C chemokines in vitro was completely Mφ dependent. Limited production of MCP-1 was evident in vitro but this was also significantly reduced by depletion of resident Mφ (25.3 ± 5.3 vs. 7.1 ± 4.7 pg/ml, Mφ-rich pleural cells vs. Mφ-depleted pleural cells; p < 0.05). Analysis of in vitro cytokine production demonstrated that resident Mφ were key cytokine producers, because Mφ depletion before carrageenan stimulation resulted in a reduction of 63, 67, and 92% in the production of TNF-α, IL-10, and IL-6, respectively (Figure 7).
Figure 6.
In vitro production of MIP-2 and KC after carrageenan stimulation is Mφ dependent. Resident pleural cells were harvested and immunodepleted of resident pleural Mφ by passage over a magnetic column. Equivalent numbers of cells were plated and stimulated with 0.25% carrageenan or normal medium for 6 h. Supernatants were harvested and analyzed by specific ELISA for MIP-2 and KC (*p < 0.05 vs. all cells with medium; n = 4 wells/condition).
Figure 7.
In vitro production of the cytokines TNF-α, IL-10, and IL-6 after carrageenan stimulation is Mφ dependent. Resident pleural cells were harvested and immunodepleted of resident pleural Mφ by passage over a magnetic column. Equivalent numbers of cells were stimulated with 0.25% carrageenan or normal medium for 6 h. Supernatants were harvested and analyzed by specific ELISA for TNF-α and by CBA for IL-10 and IL-6 (*p < 0.05 all cells vs. Mφ-depleted for their respective condition, i.e., Mφ with medium or Mφ with carrageenan). n = 4 wells/condition.
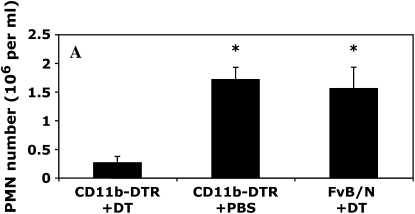
Pleural Resident Mφ Ablation Reduces PMN Influx in Response to S. aureus
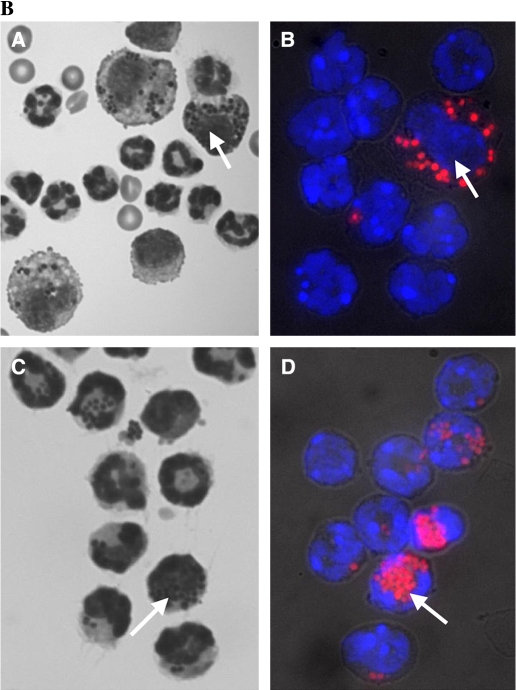
Although the carrageenan model of pleurisy is a useful model of inflammation and has been used by many investigators to dissect inflammatory pathways, we sought evidence that resident Mφ were involved in models of inflammation that were more closely related to clinical disease. We initially used the model of intrapleural LPS instillation, but this resulted in a very low level of PMN infiltration compared with carrageenan. We therefore instilled formalin-fixed, fluorescently labeled S. aureus into the pleural cavity and this induced a marked PMN infiltrate at the 4-h time point (> 1.5 × 106 PMNs). The ablation of resident Mφ significantly reduced PMN infiltration after the administration of S. aureus (Figure 8). We also found comparable PMN infiltration in DT-treated FVB/N WT mice and PBS-treated CD11b-DTR mice, indicating that insertion of the transgene had no significant effect on the generation of acute inflammatory responses (Figure 8A), with comparable findings evident after the administration of carrageenan (data not shown). Cytospin preparations of pleural lavage cells indicated prominent ingestion of S. aureus particles by Mφ in DT-treated FVB/N WT mice (Figure 8B) with very limited uptake by PMNs. In contrast, in the absence of Mφ, DT-treated CD11b-DTR mice exhibited marked ingestion of S. aureus particles by PMNs (Figure 8B).
Figure 8.
Resident Mφ ablation 24 h before the administration of formalin-fixed Staphylococcus aureus significantly blunts PMN recruitment. (A) A total of 3 × 106 formalin-fixed, fluorescently labeled S. aureus were instilled into the pleural cavity of CD11b-DTR and FVB/N WT mice 24 h after DT treatment with phosphate buffered saline (PBS)–treated CD11b-DTR serving as an additional control. Pleural lavage was performed at 4 h after the administration of S. aureus. Lavaged cells were stained for GR1 and counted by flow cytometry (*p < 0.05 vs. DT-treated CD11b-DTR group; n = 4 mice/group). (B) Photomicrographs of Diffquick-stained (A and C) or Hoechst-stained (B and D) cytospin preparations of pleural lavage cells from either DT-treated FVB/N WT mice (A and B) or DT-treated CD11b-DTR mice (C and D) 4 h after the administration of 3 × 106 formalin-fixed, fluorescently labeled S. aureus. PMNs may be readily distinguished from Mφ by their smaller size and the characteristic lobulated or circular nuclear morphology. Note that in B, the cell indicated with an arrow is the only Mφ present in the field and exhibits a large, rounded nucleus, whereas the remaining smaller PMNs exhibit a polylobular nuclear morphology. There are no Mφ present in C and D. Prominent ingestion of S. aureus particles by Mφ is evident in control DT-treated FVB/N WT mice (examples shown with arrows in A and B), with very limited uptake by PMNs. In contrast, in the absence of Mφ, DT-treated CD11b-DTR mice exhibit marked ingestion of S. aureus particles by PMNs (examples shown with arrows in C and D).
DISCUSSION
We used a conditional macrophage ablation strategy to dissect the role of the resident pleural Mφ in the initiation of pleural inflammation and PMN recruitment in carrageenan-induced pleurisy. Carrageenan induces inflammatory responses that are likely to be involved in human disease such as tuberculosis, which is a cause of significant morbidity and mortality. We also examined the effect of Mφ ablation before the administration of fixed S. aureus, a model with direct clinical relevance. Although the resident pleural Mφ can secrete chemokines and cytokines, their role in pleurisy is currently unclear. Pleural mesothelial cells also have the capacity to secrete various chemokines (3, 6, 7, 12, 33, 34). In addition, some studies have identified resident pleural Mφ-derived proinflammatory cytokines such as TNF-α that are essential for the secretion of C-X-C and C-C chemokines from pleural mesothelial cells (3, 5, 8, 10–12, 33), suggesting important cross-talk between different pleural cells.
The first major finding of this study is that the administration of DT to CD11b-DTR transgenic mice results in the rapid and effective ablation of resident pleural Mφ, with greater than 96% of resident pleural Mφ being depleted 24 h after DT treatment. This is comparable with our previous work studying peritoneal inflammation (27). Interestingly, despite PMN expression of CD11b, the administration of DT did not induce the death of circulating PMNs, indicating that PMNs are insensitive to DT, potentially as a result of their lower level of protein synthesis.
The second major finding of this study is that resident pleural Mφ ablation dramatically blunted early PMN infiltration into the pleural cavity, indicating an important role for resident pleural Mφ in initiating acute pleural inflammation. The administration of DT did not affect the numbers of circulating PMNs, thereby excluding this potential cause for diminished PMN infiltration of the pleural cavity. We performed Mφ repletion studies involving the adoptive transfer of nontransgenic pleural cell populations to Mφ-depleted mice concurrent with the induction of pleurisy. The adoptive transfer of pleural cell populations depleted of Mφ by magnetic immunodepletion had no significant effect on PMN recruitment; PMN numbers were comparable to those evident in control Mφ-depleted mice. In contrast, adoptive transfer of either pleural cell populations containing Mφ or a population of purified Mφ significantly increased pleural PMN infiltration, reinforcing the key role of resident pleural Mφ. Adoptive transfer of pleural cells was unable to restore Mφ numbers to normal values and this may explain the partial restoration of PMN infiltration compared with DT-treated FVB/N control mice. However, the striking correlation between the number of pleural Mφ at the initiation of disease and the number of infiltrating PMNs at 6 h after carrageenan administration strongly supports a key proinflammatory role for resident pleural Mφ. It is possible that carrageenan pleurisy may be partially dependent on the proinflammatory actions of recruited monocytes, unlike experimental peritonitis where acute PMN infiltration is monocyte independent (27). Our data indicate a profound effect of Mφ depletion on PMN recruitment at the early time point of 6 h and because monocyte recruitment occurs significantly later in the carrageenan model it is likely that monocyte recruitment will be very limited at this early time point. Thus, a reduction in monocyte recruitment in DT-treated CD11b-DTR mice is unlikely to be involved in the early reduction in PMN infiltration in these studies, although recruited monocytes may play a role in PMN infiltration at later time points.
Although defective PMN migration consequent on exposure to DT is an alternative explanation for these findings, our previous work in experimental peritonitis indicated that reconstitution of Mφ-depleted mice with nontransgenic Mφ was able to fully restore PMN infiltration in response to thioglycollate (27). In addition, intrapleural administration of the chemokine MIP-2 to Mφ-depleted CD11b-DTR mice resulted in significant PMN infiltration (3.2 × 105 ± 0.9 × 105 PMNs/ml at 4 h after the intrapleural administration of 30 ng MIP-2), suggesting that PMN migration is not defective under these experimental conditions.
In these experiments, DT administration and the subsequent induction of widespread Mφ death did affect the numbers of pleural B cells and mast cells. However, despite this potentially confounding issue, several factors support the prominent role of the pleural Mφ in the carrageenan model. First, data from in vitro experiments indicate a dramatic reduction in chemokine and cytokine production after Mφ depletion from resident pleural cell populations. In these studies, pleural cells were labeled with a PE-conjugated antibody to the specific Mφ marker F4/80 before immunomagnetic depletion, and F4/80 is not expressed by B cells or mast cells. Second, adoptive transfer of Mφ-depleted pleural cells comprising B cells, T cells, and mast cells did not induce significant PMN recruitment after carrageenan administration. In contrast, adoptive transfer of Mφ-rich pleural cells or purified Mφ isolated by negative selection significantly increased PMN infiltration in response to carrageenan administration. Last, previous work suggests that mast cells do not play a significant role in the carrageenan pleurisy model (35, 36).
We then examined the effect of resident pleural Mφ ablation on the level of C-X-C chemokines in this model. Resident pleural Mφ ablation markedly reduced MIP-2 levels in the pleural exudate but had a lesser, albeit significant, inhibitory effect on KC levels. In vitro study of Mφ-replete or Mφ-depleted pleural cell populations indicated that Mφ are a key source of chemokines because Mφ-depleted pleural cell populations produced minimal amounts of the chemokines MIP-2 and KC. Interestingly, these in vitro studies demonstrated comparable production of MIP-2 and KC, whereas analysis of pleural lavage fluid indicated that KC levels were approximately two- to threefold higher than MIP-2 levels in vivo. These data are comparable to our previous studies of thioglycollate peritonitis (27) and suggest that other cells within the pleural cavity, such as mesothelial cells, may be an important source of KC in vivo. The suggestion that pleural cells, other than those retrievable by pleural lavage, represent a significant source of KC is consistent with recent work in a wound model of inflammation (37) that demonstrated MIP-2 expression by inflammatory cells while KC was predominantly expressed by resident tissue cells, such as endothelial cells and fibroblasts. Pleural mesothelial cells undoubtedly participate in pleural inflammation and our data suggest that mesothelial cells actively contribute to KC production. It should be stressed, however, that marked inhibition of PMN recruitment was evident at early time points in the presence of relatively preserved KC levels suggesting that MIP-2 is more important in vivo in this model. Also, pleural cell populations stimulated with carrageenan in vitro produced relatively low levels of the C-C chemokine MCP-1 compared with the levels found in vivo, suggesting a prominent role for mesothelial cells in MCP-1 production in vivo and subsequent mononuclear cell recruitment. Our data are therefore also in accordance with previous reports highlighting the importance of pleural mesothelial cells (3, 6, 38).
Our data also indicate that resident pleural Mφ are critically involved in the generation of cytokines because TNF-α, IL-10, and IL-6 levels in pleural exudates were significantly reduced in CD11b-DTR mice treated with DT. Also, carrageenan-stimulated pleural cell populations exhibited a significant reduction in cytokine levels in vitro after magnetic immunodepletion of pleural Mφ. Our studies raise the question as to why there was no significant PMN infiltration in response to significant KC production. Pertinent previous work studying the effect of function-blocking antibodies to either MIP-2 or KC in thioglycollate peritonitis indicates that inhibition of either chemokine individually results in marked (> 70%) inhibition of PMN infiltration with inhibition of both chemokines giving little additional effect (39, 40). We did not perform in vitro PMN chemotaxis assays to assess the chemotactic activity of pleural lavage fluid from Mφ-depleted and control mice with pleurisy because the preparation of pure populations of nonactivated murine neutrophils is problematic. In addition, our previous studies indicate that experiments involving the adoptive transfer of lavage fluid are confounded by the resultant dilution of chemokines and cytokines. However, the dramatic reduction in the levels of intrapleural cytokines in Mφ-depleted mice may contribute to the defective PMN infiltration via modulation of local endothelial cell expression of adhesion molecules involved in PMN diapedesis. Although many mediators, including cytokines, nitric oxide, complement proteins, and prostaglandins, are involved in acute inflammatory processes and leukocyte recruitment, our findings indicate that resident Mφ play a key role in orchestrating PMN influx in carrageenan pleurisy. In addition, our limited experiments performed in mice administered killed S. aureus indicated that Mφ depletion markedly reduces staphylococcal-induced PMN infiltration. Also, prominent Mφ ingestion of S. aureus was evident in control DT-treated FVB/N mice and this reinforces the key role for resident Mφ as sentinel cells that act to recognize and clear proinflammatory pathogens and particulate material.
In conclusion, this study used a transgenic model of conditional Mφ ablation to demonstrate a key role for the resident pleural Mφ in sensing pleural irritation and orchestrating PMN infiltration in carrageenan-induced pleurisy. This proinflammatory function is predominantly mediated by production of the potent PMN C-X-C chemokine MIP-2 and proinflammatory cytokines such as TNF-α and IL-6 that can promote the production of the PMN C-X-C chemokine KC by mesothelial cells. Our study suggests that resident Mφ are critically important producers of PMN chemokines and proinflammatory cytokines and act to orchestrate PMN recruitment in murine carrageenan-induced pleurisy.
Acknowledgments
The authors thank Marine Colloids, Inc. (Philadelphia, PA), which kindly provided the λ-carrageenan.
Supported by the Canadian Institutes of Health Research (J.-F.C.). S.W. has an MRC Clinical Research Training Fellowship. K.H. is funded by the Royal Navy. D.W. has a National Kidney Research Fund Training Fellowship (TF6/2003). J.S. is supported by the Wellcome Trust (program grant 064487). R.A.L. is supported by grants from the National Institutes of Health (RO1s EY10559, EY11234, EY12370, and EY14102) and by funds from the Abrahamson Pediatric Eye Institute Endowment at Children's Hospital Medical Center of Cincinnati. J.H. is in receipt of a Wellcome Trust Senior Research Fellowship in Clinical Science (grant 061139).
Originally Published in Press as DOI: 10.1164/rccm.200504-538OC on December 15, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J 1997;10:2411–2418. [DOI] [PubMed] [Google Scholar]
- 2.Antony VB. Immunological mechanisms in pleural disease. Eur Respir J 2003;21:539–544. [DOI] [PubMed] [Google Scholar]
- 3.Antony VB, Hott JW, Kunkel SL, Godbey SW, Burdick MD, Strieter RM. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am J Respir Cell Mol Biol 1995;12:581–588. [DOI] [PubMed] [Google Scholar]
- 4.Mohammed KA, Nasreen N, Ward MJ, Mubarak KK, Rodriguez-Panadero F, Antony VB. Mycobacterium-mediated chemokine expression in pleural mesothelial cells: role of C-C chemokines in tuberculous pleurisy. J Infect Dis 1998;178:1450–1456. [DOI] [PubMed] [Google Scholar]
- 5.Mohammed KA, Nasreen N, Ward MJ, Antony VB. Macrophage inflammatory protein-1alpha C-C chemokine in parapneumonic pleural effusions. J Lab Clin Med 1998;132:202–209. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed KA, Nasreen N, Ward MJ, Antony VB. Helper T cell type 1 and 2 cytokines regulate C-C chemokine expression in mouse pleural mesothelial cells. Am J Respir Crit Care Med 1999;159:1653–1659. [DOI] [PubMed] [Google Scholar]
- 7.Loghmani F, Mohammed KA, Nasreen N, Van Horn RD, Hardwick JA, Sanders KL, Antony VB. Inflammatory cytokines mediate C-C (monocyte chemotactic protein 1) and C-X-C (interleukin 8) chemokine expression in human pleural fibroblasts. Inflammation 2002;26:73–82. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzocrea S, Sautebin L, De Sarro G, Costantino G, Rombola L, Mazzon E, Ialenti A, De Sarro A, Ciliberto G, Di Rosa M, et al. Role of IL-6 in the pleurisy and lung injury caused by carrageenan. J Immunol 1999;163:5094–5104. [PubMed] [Google Scholar]
- 9.Utsunomiya I, Nagai S, Oh-ishi S. Sequential appearance of IL-1 and IL-6 activities in rat carrageenin-induced pleurisy. J Immunol 1991;147:1803–1809. [PubMed] [Google Scholar]
- 10.Frode TS, Souza GE, Calixto JB. The modulatory role played by TNF-alpha and IL-1 beta in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine 2001;13:162–168. [DOI] [PubMed] [Google Scholar]
- 11.Goodman RB, Wood RG, Martin TR, Hanson-Painton O, Kinasewitz GT. Cytokine-stimulated human mesothelial cells produce chemotactic activity for neutrophils including NAP-1/IL-8. J Immunol 1992;148:457–465. [PubMed] [Google Scholar]
- 12.Park JS, Kim YS, Jee YK, Myong NH, Lee KY. Interleukin-8 production in tuberculous pleurisy: role of mesothelial cells stimulated by cytokine network involving tumour necrosis factor-alpha and interleukin-1 beta. Scand J Immunol 2003;57:463–469. [DOI] [PubMed] [Google Scholar]
- 13.Murai N, Nagai K, Fujisawa H, Hatanaka K, Kawamura M, Harada Y. Concurrent evolution and resolution in an acute inflammatory model of rat carrageenin-induced pleurisy. J Leukoc Biol 2003;73:456–463. [DOI] [PubMed] [Google Scholar]
- 14.Ackerman N, Tomolonis A, Miram L, Kheifets J, Martinez S, Carter A. Three day pleural inflammation: a new model to detect drug effects on macrophage accumulation. J Pharmacol Exp Ther 1980;215:588–595. [PubMed] [Google Scholar]
- 15.Harada Y, Hatanaka K, Kawamura M, Saito M, Ogino M, Majima M, Ohno T, Ogino K, Yamamoto K, Taketani Y, et al. Role of prostaglandin H synthase-2 in prostaglandin E2 formation in rat carrageenin-induced pleurisy. Prostaglandins 1996;51:19–33. [DOI] [PubMed] [Google Scholar]
- 16.Cuzzocrea S, Costantino G, Mazzon E, Caputi AP. Beneficial effects of raxofelast (IRFI 016), a new hydrophilic vitamin E-like antioxidant, in carrageenan-induced pleurisy. Br J Pharmacol 1999;126:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuzzocrea S, McDonald MC, Filipe HM, Costantino G, Mazzon E, Santagati S, Caputi AP, Thiemermann C. Effects of tempol, a membrane-permeable radical scavenger, in a rodent model of carrageenan-induced pleurisy. Eur J Pharmacol 2000;390:209–222. [DOI] [PubMed] [Google Scholar]
- 18.Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Maffia P, Patel NS, Di Paola R, Ialenti A, Genovese T, Chatterjee PK, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-gamma, reduces acute inflammation. Eur J Pharmacol 2004;483:79–93. [DOI] [PubMed] [Google Scholar]
- 19.Frode-Saleh TS, Calixto JB. Synergistic antiinflammatory effect of NF-kappaB inhibitors and steroidal or non steroidal antiinflammatory drugs in the pleural inflammation induced by carrageenan in mice. Inflamm Res 2000;49:330–337. [DOI] [PubMed] [Google Scholar]
- 20.Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol 2001;132:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuzzocrea S, Mazzon E, Calabro G, Dugo L, De Sarro A, van De Loo FA, Caputi AP. Inducible nitric oxide synthase-knockout mice exhibit resistance to pleurisy and lung injury caused by carrageenan. Am J Respir Crit Care Med 2000;162:1859–1866. [DOI] [PubMed] [Google Scholar]
- 22.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med 1999;5:698–701. [DOI] [PubMed] [Google Scholar]
- 23.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J 2004;18:489–498. [DOI] [PubMed] [Google Scholar]
- 24.Dalmarco EM, Frode TS, Medeiros YS. Effects of methotrexate upon inflammatory parameters induced by carrageenan in the mouse model of pleurisy. Mediators Inflamm 2002;11:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito M, Shima C, Takagi M, Ogino M, Katori M, Majima M. Enhanced exudation of fibrinogen into the perivascular space in acute inflammation triggered by neutrophil migration. Inflamm Res 2002;51:324–331. [DOI] [PubMed] [Google Scholar]
- 26.Bozza PT, Castro-Faria-Neto HC, Penido C, Larangeira AP, das Gracas M, Henriques MO, Silva PM, Martins MA, dos Santos RR, Cordeiro RS. Requirement for lymphocytes and resident macrophages in LPS-induced pleural eosinophil accumulation. J Leukoc Biol 1994;56:151–158. [DOI] [PubMed] [Google Scholar]
- 27.Cailhier JF, Partolina M, Vuthoori S, Wu S, Ko K, Watson S, Savill JS, Lang RA, Hughes J. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol 2005;174:2336–2342. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson A, Appleton I, Moore AR, Gilroy DW, Willis D, Mitchell JA, Willoughby DA. Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy. Br J Pharmacol 1994;113:693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoe K, Hiraki A, Murakami T, Murakami K, Makihata K, Takao K, Eda R, Maeda T, Sugi K, Darzynkiewicz Z, et al. Relative abundance and patterns of correlation among six cytokines in pleural fluid measured by cytometric bead array. Int J Mol Med 2003;12:193–198. [PubMed] [Google Scholar]
- 30.Rosenkranz AR, Coxon A, Maurer M, Gurish MF, Austen KF, Friend DS, Galli SJ, Mayadas TN. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)-deficient mice. J Immunol 1998;161:6463–6467. [PubMed] [Google Scholar]
- 31.Howell K, Campo M, Chiasson R, Duffy K, Riggs J. B-1 B cell subset composition of DBA/2J mice. Immunobiology 2002;205(3):303–313. [DOI] [PubMed] [Google Scholar]
- 32.Chevallier N, Berthelemy M, Le Rhun D, Laine V, Levy D, Schwartz-Cornil I. Bovine leukemia virus-induced lymphocytosis and increased cell survival mainly involve the CD11b+ B-lymphocyte subset in sheep. J Virol 1998;72:4413–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pace E, Gjomarkaj M, Melis M, Profita M, Spatafora M, Vignola AM, Bonsignore G, Mody CH. Interleukin-8 induces lymphocyte chemotaxis into the pleural space: role of pleural macrophages. Am J Respir Crit Care Med 1999;159:1592–1599. [DOI] [PubMed] [Google Scholar]
- 34.Jonjic N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Mantovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med 1992;176:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeshita K, Sakai K, Bacon KB, Gantner F. Critical role of histamine H4 receptor in leukotriene B4 production and mast cell-dependent neutrophil recruitment induced by zymosan in vivo. J Pharmacol Exp Ther 2003;307:1072–1078. [DOI] [PubMed] [Google Scholar]
- 36.Horakova Z, Bayer BM, Almeida AP, Beaven MA. Evidence that histamine does not participate in carrageenan-induced pleurisy in rat. Eur J Pharmacol 1980;62:17–25. [DOI] [PubMed] [Google Scholar]
- 37.Armstrong DA, Major JA, Chudyk A, Hamilton TA. Neutrophil chemoattractant genes KC and MIP-2 are expressed in different cell populations at sites of surgical injury. J Leukoc Biol 2004;75:641–648. [DOI] [PubMed] [Google Scholar]
- 38.Hill GD, Mangum JB, Moss OR, Everitt JI. Soluble ICAM-1, MCP-1, and MIP-2 protein secretion by rat pleural mesothelial cells following exposure to amosite asbestos. Exp Lung Res 2003;29:277–290. [DOI] [PubMed] [Google Scholar]
- 39.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. Am J Pathol 2001;158:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Call DR, Nemzek JA, Ebong SJ, Bolgos GR, Newcomb DE, Wollenberg GK, Remick DG. Differential local and systemic regulation of the murine chemokines KC and MIP2. Shock 2001;155:278–284. [DOI] [PubMed] [Google Scholar]