Abstract
Infusion of TGFα into the adult dopamine (DA)-depleted striatum generates a local population of nestin+/PCNA+ newborn cells [1]. The precise origin and fate of these new striatal cells are unknown, making it difficult to direct them for neural repair in Parkinson’s disease (PD). Experiments in rats using BrdU to label neural progenitor cells (NPCs) showed that during TGFα infusion in the DA-depleted striatum, newborn striatal cells formed a homogenous population of precursors, with the majority coexpressing nestin, Mash1, Olig2 and EGFR, consistent with the phenotype of multipotent C cells. Upon TGFα pump withdrawal, the subventricular zone (SVZ) was repopulated by neuroblasts. Strikingly, during this period, numerous clusters of DCX+/PSANCAM+ neuroblasts were also produced in the ipsilateral medial striatum. In parallel, striatal BrdU+/GFAP+ astrocytes were generated, but no BrdU+/O4+/CNPase+ oligodendrocytes. Infusion of the neuralizing BMP antagonist noggin after TGFα pump withdrawal increased the neuroblast to astrocyte ratio among new striatal cells by blocking glial differentiation, but did not alter striatal neurogenesis. At no time or no treatment condition were differentiated neurons generated, including DA neurons. Using 6-OHDA lesioned nestin-CreERT2/R26R-YFP mice that allow genetic fate-mapping of SVZ nestin+ cells, we show that TGFα-generated striatal cells originate from SVZ nestin+ precursors that confirmed data from the rats on the phenotype and fate of striatal nestin+/PCNA+ cells upon TGFα withdrawal. This work demonstrates that a large population of multipotent striatal C-like cells can be generated in the DA-depleted striatum that do not spontaneously differentiate into DA neurons.
Keywords: Parkinson’s disease, adult neurogenesis, subventricular zone, striatum, TGFα, neuroblasts
INTRODUCTION
In the context of Parkinson’s disease (PD), subventricular zone (SVZ) neural stem and precursor cells [herein collectively called neural progenitor cells (NPCs)] do not contribute to dopamine (DA) cell replacement [2]. The SVZ is the largest neurogenic zone in the brain and is adjacent to the striatum, the region where most DA neurotransmitter depletion occurs in PD. Given that some SVZ cells continuously differentiate into olfactory bulb (OB) DA neurons throughout life, their artificial recruitment into the striatum and differentiation into DA neurons is an appealing alternative to transplantation therapies [1, 3, 4].
The cytoarchitecture, cellular composition and lineage relationships between different NPC subtypes in the mammalian SVZ have been extensively studied [5–8]. There are 3 well-characterized NPC subtypes in the adult SVZ. The primary precursors which are thought to be neural stem cells (B cells) have immunocytochemical and ultrastructural characteristics of astrocytes [7]. These SVZ astrocytes give rise to transit-amplifying precursor cells (C cells), which have a globular morphology and proliferate rapidly, thus increasing the size of the SVZ precursor pool. The C cells generate neuroblasts (A cells) that express the polysialylated neuronal cell adhesion molecule (PSANCAM) and doublecortin (DCX) [7]. Those neuroblasts are committed to the neuronal lineage and migrate towards the OB, where they ultimately differentiate into interneurons, which use GABA or both GABA and DA as neurotransmitters. The latter interneurons constitute the A16 population of DA neurons that share functional characteristics with A9 DA neurons [9, 10]. Finally, in addition to OB neurons, some SVZ B cells generate oligodendrocytes, although the precise lineage leading to this fate is unclear [11].
DA secreted by midbrain afferent projections promotes NPC proliferation in the SVZ by binding directly to D2-like receptors on C cells [2]. Probably as a direct consequence of this, animal models of PD [2, 12] and PD patients [2] display reduced SVZ cell proliferation and fewer newborn OB neurons [2]. Surprisingly, SVZ Pax6+ DA neuron precursors and olfactory bulb DA neurons are increased in PD animal models [13–15]. This provides the intriguing possibility of using chemo-attractants or growth factors to artificially recruit endogenous DA neuron precursors to the DA-depleted striatum.
In the 6-hydroxydopamine (6-OHDA) rodent model of PD, striatal TGFα infusion stimulates forebrain cell division and induces septal and striatal waves of nestin+/PCNA+ cells oriented towards the infusion cannula [1, 16]. Here we infused the EGFR agonist TGFα into the DA-depleted striatum [1, 16], and addressed the following, new questions: (a) what are the origin and phenotype(s) of TGFα-activated cells in the striatal wave? (b) What is their fate 2 weeks after TGFα withdrawal? (c) What is the effect of TGFα and noggin consecutive infusions, given the known neurogenic influence of noggin on SVZ cells [17]?
MATERIALS AND METHODS
Rats
Adult 250–300 g female Sprague Dawley rats (Taconic Farms, Germantown, NY) administered 6-OHDA in the medial forebrain bundle [18] (n=88, 8 per group for all groups except n=4 for TGFα̣ weeks / PBS 4 weeks). The animals were maintained in accordance with current National Institutes of Health guidelines and McLean Hospital/Harvard University Institutional Animal Care and Use Committee protocols.
TGFα and Noggin infusions in rats
The infusion procedures for TGFα and noggin were performed as described [1]. Osmotic pumps (Alzet, models 1002 and 2004; Cupertino, CA) delivered either: (a) 0.5 mg/ml TGFα (R&D Systems, Minneapolis, MN), (b) 6 µg/ml noggin (R&D Systems), (c) 0.5 mg/ml TGFα̣ and 6 µg/ml noggin, or (d) PBS into the right striatum (+1.2 A/P; +2.7 M/L) via a cannula (Brain Infusion Kit II, Alzet) at 0.25µl h−1. Proteins were dissolved in 0.1 M PBS.
For sequential infusions, rats were anesthetized and the osmotic pump was replaced, while the cannula, polyethylene tubing and flow moderator remained in place.
Nestin-CreERT2/R26R-YFP mice experiments
5–8 week-old nestin-CreERT2/R26R-YFP mice [19] were anesthetized with ketamine/xylazine solution and 6-OHDA (5.0 mg/ml in 0.9% NaCl/0.02% ascorbate) was injected by microliter syringe (Hamilton) at 0.5 µl/min by pump for a total dose of 15.0 mg/3 µl. The right striatum was targeted at stereotaxic coordinates AP: +0.9 mm; ML: +2.2 mm; DV: −2.5 mm relative to bregma [20]. Two weeks following 6-OHDA injection, mice were administered tamoxifen at 180 mg/kg/day for 5 days (intraperitoneally (i.p.); dissolved in 10% EtOH/90% sunflower oil). The first group of mice was sacrificed 2 days after the tamoxifen regimen (Fig. 1C1). For the 2 other groups, 1 week following the tamoxifen regimen, osmotic pumps delivered either 0.5 mg/ml TGFα or 0.9% saline into the right striatum (AP: +1 mm; ML: +1.3 mm; DV: −3 mm relative to bregma). Mice were sacrificed after 2 weeks of infusion (Fig. 1C2) or 2 weeks after pump withdrawal (Fig.1C3).
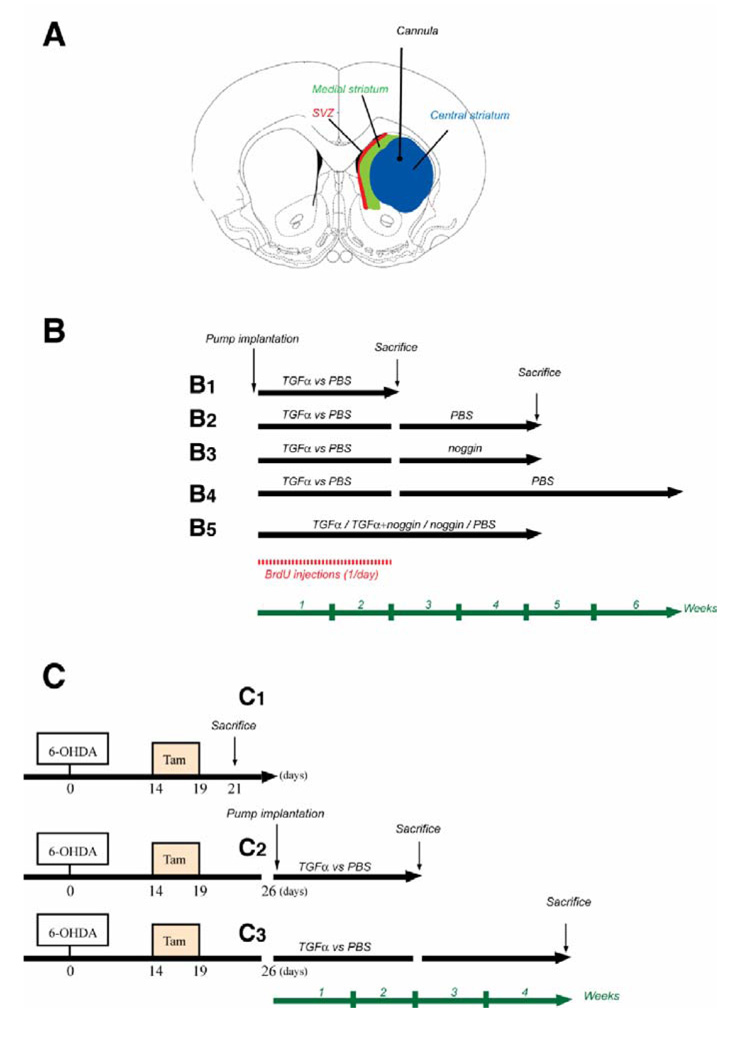
Figure 1. Schematic of experimental paradigms.
(A) Schematic of the rat brain at the level of the striatum, showing the infusion site and tissue regions examined. (B) BrdU was administered daily during the 2 first weeks of infusion (shown in red). Adult 6-OHDA lesioned rats were sacrificed either on day 14 during TGFα or PBS infusion (B1), on day 28 after infusion of the second agent (PBS or noggin) for a further 14 days (B2, B3), on day 41 after TGFα then PBS infusion (B4), or on day 28 during infusion of PBS, TGFα, TGFα+noggin or noggin (B5). (C) Tamoxifen was administered to young adult nestin-CreERT2/R26R-YFP mice daily between days 14 and 19 after 6-OHDA injection. (C1) A first group of mice was sacrificed 2 days after the end of tamoxifen regimen. For the 2 other groups, pumps were implanted 1 week after the last tamoxifen dose. Nestin-CreERT2/R26R-YFP mice were sacrificed either on day 14 during TGFα or PBS infusion (C2), or on day 28 after infusion of PBS for a further 14 days period (C3).
BrdU administration
Rats were administered BrdU (Sigma, St. Louis, MO) i.p. once per day from days 3 to 14 of the first TGFα/PBS infusion at a dose of 50 mg/kg body weight (Fig. 1B). We waited 3 days after pump implantation before starting BrdU injections in order to avoid BrdU incorporation by reactive astroglia around the cannula site.
Histological procedures
Animals were terminally anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused with intracardial heparin saline (0.1% heparin in 0.9% saline; 100 ml/rat) and paraformaldehyde (4% in PBS; 200 ml/rat). The brains were post-fixed and sectioned (40 µm) as described [18].
Immunohistochemistry
Routine indirect immunofluorescence was performed on randomly selected series of sections that represented 1/12th of the total brain [18] (see Supplemental Methods for primary antibody details). For BrdU immunostaining, the sections were pretreated with 0.2% Triton X-100 for 2 h and denatured with 2 N HCl for 40 min at 37 °C. Primary antibodies were kept overnight at room temperature, except for EGFR, Mash1 and Dlx2 antibodies that were kept 48–72 hours at 4 °C. For double and triple labeling with BrdU, cell markers were detected before BrdU immunostaining. Primary antibodies were visualized using fluorescent secondary antibodies (AlexaFluor, Molecular Probes). For EGFR, Mash1 and Dlx2 immunostainings, sections were treated for 2 h at room temperature with a biotinylated secondary antibody (1:300, Jackson Laboratories) and then visualized using the tyramide signal amplification kit (TSA Plus System, PerkinElmer Life Science, Waltham, MA).
TUNEL detected apoptosis-related DNA fragmentation using the manufacturer’s protocol (Neurotacs II, R&D Systems).
Cell Counts
Cells were counted blind to experimental conditions. For striatal counts, a 100–300 µm diameter circle with its center at the infusion site was excluded in order to avoid contamination of analyses by reactive astroglia. Sections were analyzed using either a standard microscope (BX51, Olympus or Axioskop 2+, Carl Zeiss) or a confocal microscope (LSM 510/Meta, Carl Zeiss, Thornwood, NY). Cell counts and distances were measured using a stereology workstation (Stereoinvestigator, Microbrightfield, Williston, VT) with an integrated epifluorescence microscope (Axioskop 2+, Carl Zeiss). BrdU+ cells colabeled with phenotypic markers were identified from orthogonal projections to confirm colocalization. For each marker and per animal, we analyzed 130 to 280 BrdU+ cells per section on a total of 4–6 striatal sections spaced by 480 µm (once every 12th section). For nestin expression, we identified high expression when signal was detectable using the following parameters on the confocal microscope: pinhole: 85 µm, Detector gain: 600, Amplifier Offset: −0.02 V. We considered nestin expression to be low when emission fluorescence was not detected using the above parameters but was detected using the following parameters: pinhole: 89 µm, Detector gain: 814, Amplifier Offset: −0.02 V.
Behavioral testing
Unilaterally lesioned rats were tested for rotational behavior in response to amphetamine (4mg/kg i.p.) before infusion and 3 days prior to sacrifice. Animals were randomized and placed into automated rotometer bowls and left and right full-body turns were monitored by a computerized activity monitor system as described [18].
Statistical Analysis
Data are presented as mean ± SEM. One way ANOVA and Student’s unpaired t test were employed to assess differences between data groups using the software (SAS Institute, Cary, NC). Differences were considered statistically significant when p<0.05.
RESULTS
Phenotypic characterization of newly generated cells in the DA-depleted striatum during TGFα infusion
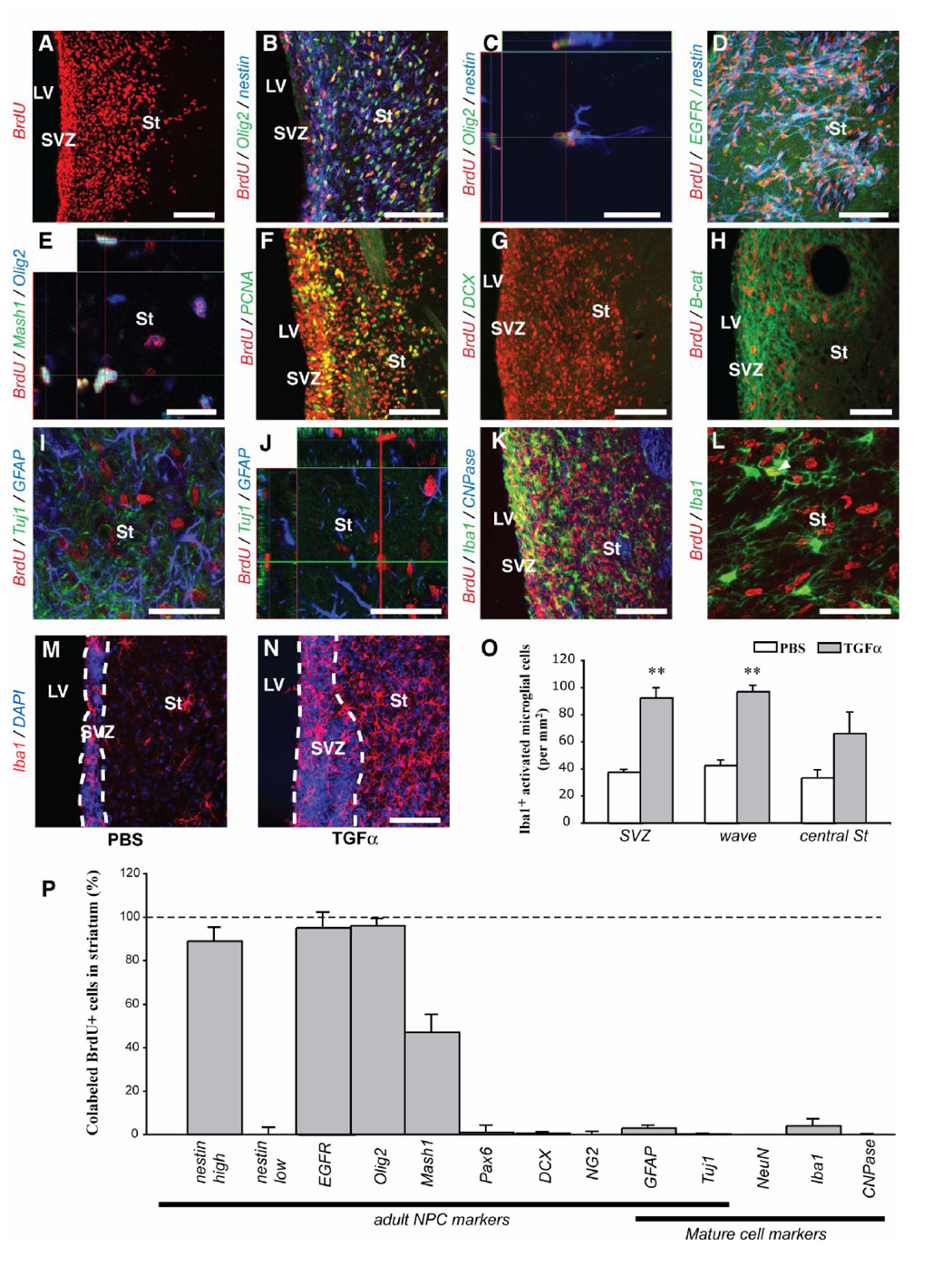
To determine the precise phenotype of the striatal nestin+/PCNA+ cells in 6-OHDA lesioned rats [1, 16], we investigated NPC marker expression by BrdU+ cells in the ipsilateral striatum after 2 weeks of TGFα infusion (Fig. 1B1). BrdU was injected throughout the infusion period to label cells that were in S-phase (Fig. 1B). The majority (> 89 %) of BrdU+ cells in the TGFα-induced ipsilateral striatal and septal waves coexpressed nestin, EGFR and Olig2, indicative of a “C-like” phenotype [13, 21] (Suppl. Fig. 1 and Fig. 2A–D, P). We also found that 47 ± 8 % of the BrdU+ cells expressed Mash1 (Fig. 2E, P), another marker of an SVZ C cell subpopulation [22]. Newborn cells in the TGFα-activated contralateral, septal wave [1] and proliferative nodules also displayed this “C-like cell” phenotype (Suppl. Fig. 1). However, BrdU+ “C-like” cells in the waves differed from normal SVZ C cells in that they did not express Dlx2 (data not shown) [21] and had an elongated morphology (Fig. 2C). Almost no BrdU+ cell in the striatum expressed the mature neural cell markers GFAP, Tuj1, NeuN, CNPase or Iba1 (Fig. 2I–L and P), confirming that new cells did not differentiate during TGFα infusion. Of note, TGFα infusion increased the number of Iba1+ microglial cells near where BrdU+ C-like cells were proliferating (both SVZ and striatal wave) compared to PBS infusion (Fig. 2M–O), although few BrdU+ cells expressed Iba1 (4 ± 3 %) and few Iba1+ cells expressed BrdU (2 ± 3 %, Fig. 2K, L and P).
Figure 2. Distribution and phenotypes of ectopic striatal cells during intrastriatal TGFα infusion in adult 6-OHDA lesioned rats.
(A) Distribution of BrdU+ cells in the ipsilateral hemisphere (coronal section at the level of the striatum). (B) Nestin (blue) and Olig2 (green) were co-expressed in the majority of BrdU+ cells (red) in the ipsilateral striatum. (C) Orthogonal projection showing a BrdU+/nestin+/Olig2+ cell. EGFR (green, D) and Mash1 (green, E) were also expressed by striatal BrdU+/Olig2+ cells. (F) Some, but not all, BrdU+ cells in the ipsilateral striatal wave and SVZ expressed the endogenous proliferation marker PCNA. (G) BrdU+ (red) / DCX+ (green) cells were virtually absent in TGFα infused rats, and DCX expression was downregulated in SVZ (control not shown). (H) β-catenin (green) was upregulated in the ipsilateral SVZ and striatal wave. (I, J) No BrdU+ cells (red) expressed either Tuj1 (green) or GFAP (blue) in the striatal wave (orthogonal projection in J). (K, L) Few striatal BrdU+ cells (red) were Iba1+ microglial cells (green) and none were CNPase+ oligodendrocytes (blue). Only one BrdU+ cell expresses Iba1 in (L). (M, N) Representative images of Iba1+ microglia in the SVZ and ipsilateral striatum during PBS or TGFα infusion. (O) Upon quantification, a higher Iba1+ microglial density was found in the ipsilateral SVZ and striatum during TGFα administration (grey) than during PBS infusion (white). (P) Histogram showing the percentage of marker-positive cells among striatal BrdU+ cells. Scale bars: A, B, F, G, K, M, N, 100 µm; C, E, 20 µm; D, H, 50 µm; I, J, L, 40 µm.
Distribution and phenotypes of SVZ-derived cells after TGFα withdrawal
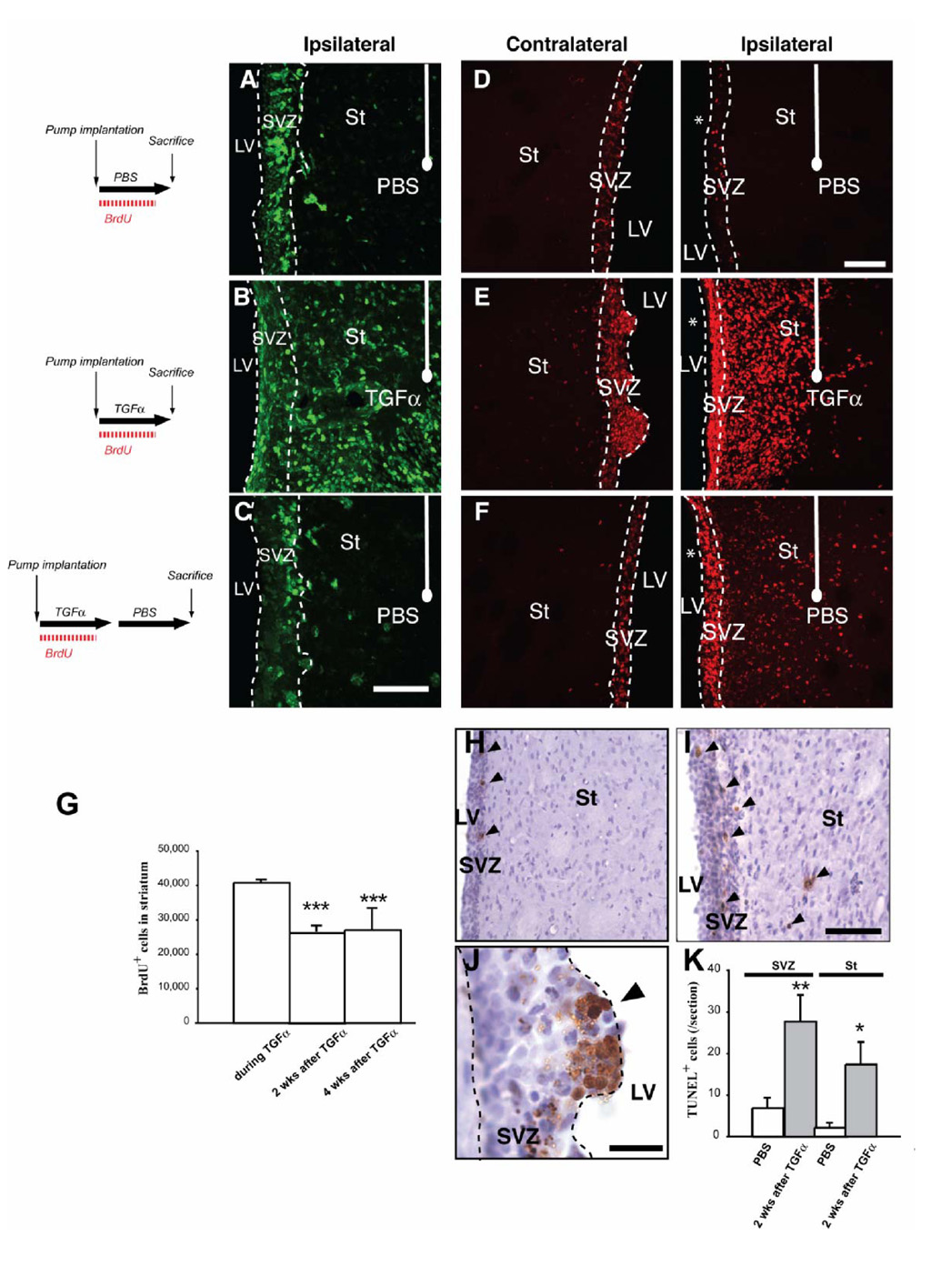
Next, we examined the response of the C-like cells in the ipsilateral striatal wave after TGFα withdrawal. Unilaterally 6-OHDA lesioned rats received TGFα for 2 weeks followed by a 2 week PBS infusion (Fig. 1B2). In these rats, the number of striatal PCNA+ dividing cells had markedly decreased compared to during TGFα infusion (Fig. 3A–C), indicating that striatal mitotic activity was reduced. The number and distribution of BrdU+ cells in the ipsilateral striatum during, or after TGFα infusion were also determined (Fig. 3D–G). During TGFα infusion, BrdU+ cells were found in septal and striatal cellular waves (Fig. 3E). After TGFα withdrawal, fewer BrdU+ cells remained in the striatal wave (Fig. 3F) (40,767 ± 814 BrdU+ cells vs 28,109 ± 2622 BrdU+ cells for TGFα and TGFα/PBS infusion conditions, respectively; 34 % reduction, unpaired t test, p<0.05; Fig. 3G). This reduced number of newborn striatal cells was due, at least in part, to apoptotic mechanisms because more TUNEL+ profiles were observed in the ipsilateral SVZ and striatum after TGFα withdrawal than in rats infused with PBS (Fig. 3H–K; p<0.05). Hypercellular nodules seen during TGFα infusion were virtually absent 2 weeks after TGFα withdrawal (Fig. 3E, F), and their remnants were undergoing apoptotic cell death (Fig. 3J). Importantly, the number of striatal BrdU+ cells was similar at 2 and 4 weeks post-TGFα (Fig. 1B4 and 3G; 26,684 ± 1,534 BrdU+ cells vs 27,003 ± 2,340 BrdU+ cells at 2 weeks and 4 weeks post- TGFα withdrawal, respectively), indicating that cell death was negligible from 2 weeks after TGFα withdrawal.
Figure 3. Distribution of immature dividing cells during PBS or during and after TGF-alpha withdrawal.
(A, C) Distribution of immature dividing cells in the ipsilateral striatum. (A) During PBS infusion in the DA-depleted striatum, PCNA+ (green) dividing cells were present in the ipsilateral SVZ, but rarely detected in the adjacent striatum. (B) TGFα striatal infusion for 2 weeks induced a large population of PCNA+ cells in the ipsilateral DA-depleted striatum. (C) Two weeks after withdrawal of the TGFα infusing pump, the distribution of PCNA+ cells resembled that of PBS infused rats. (D–F) Distribution of BrdU+ cells. (D) During PBS infusion, BrdU+ cells (red) were confined to the ipsilateral and contralateral SVZs. (E) During TGFα infusion, BrdU+ cells formed a striatal wave in the ipsilateral striatum (right panel), while in the contralateral hemisphere they formed hyper-proliferative nodules protruding from the SVZ towards the source of TGFα (left panel). (F) Two weeks after TGFα withdrawal, contralateral ventricular BrdU+ hyper-proliferative nodules were not found (left panel). BrdU+ cells were observed in the ipsilateral striatum (right), although their distribution pattern (C, right) and number had changed. Note the ~ 35% reduction in total BrdU+ cell number after TGFα withdrawal (G). (H–K) Programmed cell death is induced by TGFα treatment. (H, I) TUNEL+ cells in ipsilateral SVZ and striatum of rats infused with PBS (H) or TGFα (I), 2 weeks after its withdrawal. Arrowheads show TUNEL+ cells. The number of TUNEL+ cells was significantly higher in rats sacrificed after TGFα infusion than in their control counterparts (K). (J) In one rat that died 5 days after TGFα withdrawal, ventricular nodules were found and contained a high proportion of TUNEL+ cells. Scale bars: A–B, 50 µm; D–F, 100 µm; H, I, 200 µm; J, 50 µm.
Next we investigated the phenotype(s) of the remaining ~ 28,000 BrdU+ striatal wave cells per rat (Fig. 4A), 2 weeks after TGFα pump withdrawal. The majority of these BrdU+ cells (> 92%, Fig. 4E) did not express Olig2, Mash1 or EGFR (Fig. 4B and data not shown), indicating that the C-like cells had differentiated. However, 79 ± 8 % of BrdU+ striatal cells still expressed nestin, although to a lower level than during TGFα infusion (compare Fig. 4B, C to Fig. 2B, C and see Materials and Methods). These data demonstrate that TGFα withdrawal did not result in full differentiation of many SVZ-derived striatal cells by 2 weeks. Importantly however, we found that 35 ± 4% of striatal BrdU+ cells were GFAP+ astrocytes (Fig. 4D, E), 70 ± 10 % of which co-expressed low levels of nestin (data not shown). These BrdU+/GFAP+ astrocytes did not express SSEA-1 or PCNA (data not shown), suggesting that they were post-mitotic cells and not B-like cells. Importantly, 540 ± 12 BrdU+ cells per DA-depleted striatum co-expressed DCX, PSANCAM and nestin (demonstrated using staining on adjacent sections), which represented ~ 2 % of the striatal BrdU+ population (Fig. 4F, G). In contrast, the number of ectopic striatal DCX+ neuroblasts was approximately 15 times larger (7,606 ± 2,131 cells per ipsilateral striatum, Fig. 4H, M). Striatal DCX+/PSANCAM+ cells were proliferating after TGFα withdrawal because 82 ± 6 % of them expressed the endogenous proliferation marker PCNA (Fig. 4I). Therefore, absence of BrdU labeling in ectopic striatal neuroblasts was probably due to BrdU dilution following a large number of divisions during the 2 weeks PBS infusion period (i.e. “washing period”) post TGFα withdrawal. Seventy-five percent of such DCX+ cells expressed Tuj1 (Fig. 4J), confirming a neuroblast phenotype. However, none of the DCX+ or PSANCAM+ cells in the striatum expressed Pax6, while SVZ neuroblasts were Pax6+ in the same tissue section (data not shown). Interestingly, most ectopic striatal neuroblasts formed clusters that were in close contact with GFAP+ processes of astrocytes that had their end feet on blood vessels (Fig. 4K, L). Furthermore, no BrdU+ cells were CNPase+ or O4+ oligodendrocytes, and only few were Iba1+ microglial cells at this time (1.5 ± 2 %, Fig. 4E). Finally, we found similar phenotypes of BrdU+ cells at 4 weeks post-TGFα-withdrawal (data not shown). Strikingly however, the number of PSANCAM+ neuroblasts was increased at this later time point (Fig. 4M, Suppl. Fig. 2; 7,607 ± 2,031 PSANCAM+ cells vs 13,827 ± 1980 PSANCAM+ cells at 2 weeks and 4 weeks post-TGFα withdrawal, respectively), further demonstrating the mitotic activity of striatal neuroblasts.
Figure 4. Phenotypes of newborn striatal cells in the rat striatum 2 weeks after TGFα withdrawal.
(A) Numerous BrdU+ cells remained in the ipsilateral striatum 2 weeks after TGFα osmotic pump withdrawal and its replacement by a PBS containing pump. (B) These striatal BrdU+ cells (red) did not express Olig2 (green) at this time, but most of them expressed nestin (blue). (C) Higher magnification of the ipsilateral DA-depleted striatum showing BrdU and nestin expression. (D) High magnification confocal scan of a BrdU+/GFAP+ cell in the ipsilateral striatum. (E) Histogram comparing the percentage of marker-positive cells among striatal BrdU+ cells. (F–M) Large number of ectopic striatal neuroblasts were found in the ipsilateral striatum 2 weeks after TGFα withdrawal. (F–H) The ipsilateral SVZ and striatum stained for BrdU and DCX. Some of the neuroblast clusters contained BrdU+ cells (F), but mostly the neuroblast clusters were BrdU− (G). (H) Clusters of DCX+ neuroblasts were numerous and distributed throughout the striatum. (I) Most DCX+ cells inside striatal neuroblast clusters expressed PCNA, demonstrating a mitotic state. (J) Neuroblasts in striatal clusters coexpressed PSANCAM (green) and the neuronal marker Tuj1 (red). Arrowheads show striatal PSANCAM+/Tuj1+ cells. (K, L) Most PSANCAM+ neuroblast clusters (red) were directly apposed to blood vessels and astrocyte processes (green). (M) Histogram showing an increased number of striatal neuroblasts 2 weeks after TGFα withdrawal as compared to control, PBS infused rats. Scale bars: A, 100 µm; B, 50 µm; C, 25 µm; D, 20 µm, F, G, I–K, 25 µm; H, 50 µm.
Distribution and phenotypes of SVZ-derived cells after TGFα followed by noggin infusions
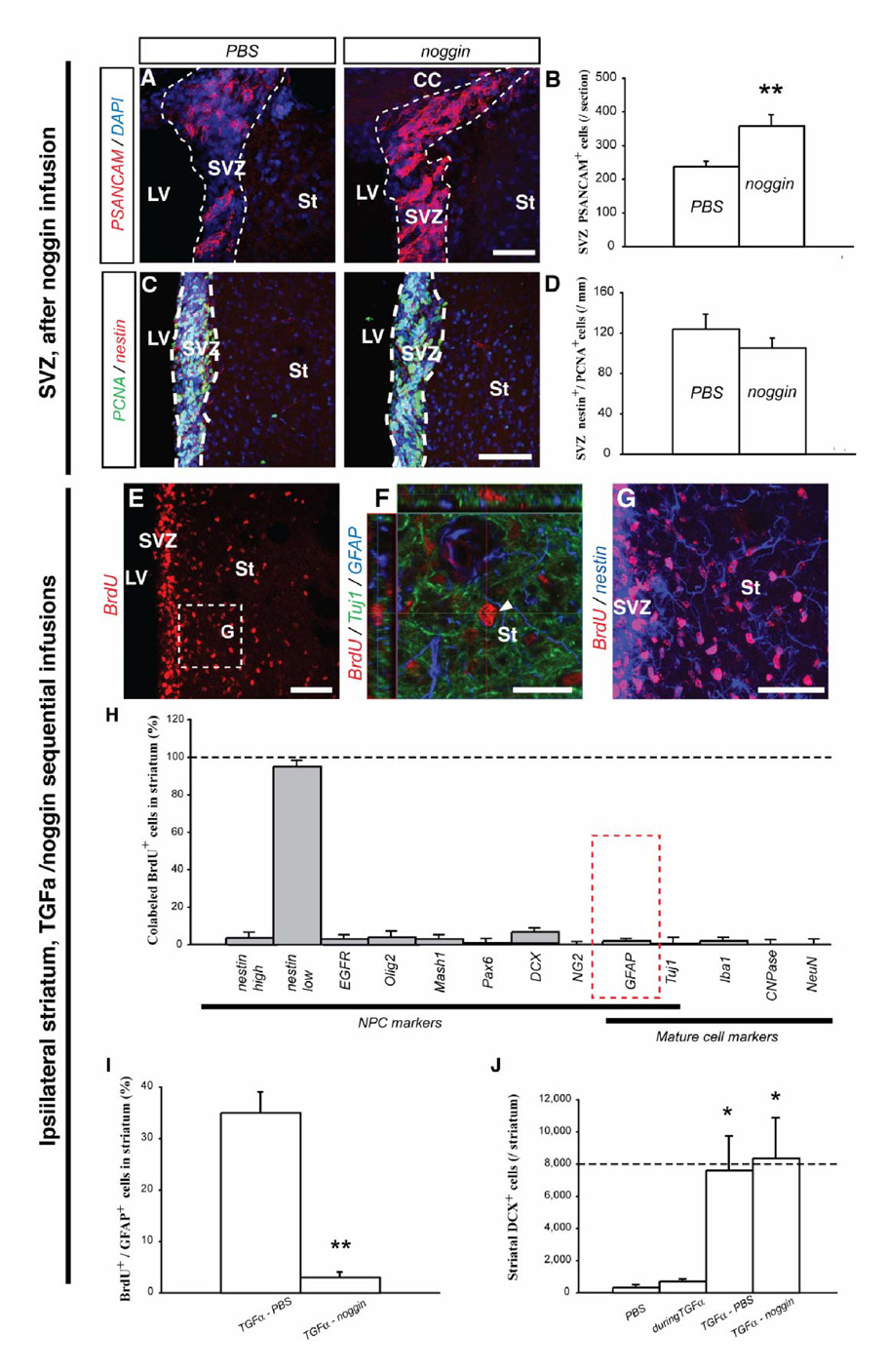
In an attempt to block astroglial differentiation and to enhance neuronal differentiation of TGFα-stimulated cells in the DA-depleted striatum, we used the BMP antagonist noggin [23]. Noggin was first infused alone in the rat DA-depleted striatum for 4 weeks, in order to verify previous data indicating that noggin was pro-neuronal for SVZ NPCs in vivo [17]. Interestingly, noggin expanded the PSANCAM+ neuroblast population in the ipsilateral SVZ, when compared to PBS infusion (358 ± 33 cells versus 238 ± 15 cells per SVZ section for noggin infused and PBS infused rats, respectively; unpaired t test, p<0.01; Fig. 5A, B). Importantly, noggin infusion did not alter the number of nestin+/PCNA+ NPCs in the ipsilateral SVZ or striatum, relative to PBS administration alone (Fig. 5C, D), indicating that proliferation, migration and survival of NPCs were unaltered. These data demonstrate diffusion over long distances and the induction of a pro-neuronal effect on SVZ cells by noggin.
Figure 5. Phenotypes of striatal newborn cells in the rat striatum after TGFα and noggin sequential infusions.
(A–D) Increased SVZ neurogenesis after noggin infusion in the DA-depleted rat striatum. (A) PSANCAM+ cells in the SVZ of PBS (left panel) and noggin infused rats (right panel). (B) Cell counts revealed significantly more SVZ neuroblasts in rats treated with noggin than in rats treated with PBS (unpaired t test, p < 0.01). (C) PCNA+ and nestin+ profiles in PBS (left) and noggin infused rats (right). (D) Cell counts revealed similar numbers of nestin+/PCNA+ cells in the SVZ after PBS or noggin infusion. (E–J) Striatal fate of newborn cells after TGFα/noggin consecutive infusions. (E) Numerous BrdU+ cells (red) were detected in the ipsilateral striatum after TGFα and noggin sequential infusions. (F) Orthogonal projection of a striatal BrdU+ cell (red) that neither expressed Tuj1 (green), nor GFAP (blue). (G) Higher magnification image of the region in (E) showed that most striatal BrdU+ cells expressed nestin (blue). (H) Histogram comparing the percentage of marker-positive cells among striatal BrdU+ cells. (I) Histogram comparing the proportion of BrdU+ cells colabeled with GFAP, in rats sequentially infused with TGFα and then PBS, or TGFα and then noggin. (J) Mean striatal number of DCX+ neuroblasts per ipsilateral striatum in the 4 conditions. Scale bars: A, G, 50 µm; C, E, 100 µm; F, 25 µm.
To test the effect of noggin on TGFα-activated BrdU+ cells recruited in the striatum, rats were administered TGFα for 2 weeks, followed by 2-weeks of noggin infusion (Fig. 1B3). The total number of striatal BrdU+ cells was unchanged compared to rats with TGFα/PBS sequential infusions (compare Fig. 5E to Fig. 4A; 28,109 ± 2622 BrdU+ cells vs 26,109 ± 2980 BrdU+ cells for TGFα/PBS and TGFα/noggin infusion conditions, respectively), confirming that noggin did not alter proliferation, survival or migration. This TGFα/noggin sequential infusion paradigm virtually blocked the generation of BrdU+/GFAP+ astrocytes in the DA-depleted striatum (Fig. 5F, H–I; 3 ± 1 % versus 35 ± 4 % of astrocytes among new cells, for TGFα/noggin sequential infusion and TGFα/PBS sequential infusion, respectively; unpaired t test, p < 0.01). In contrast, neuronal commitment, as measured by DCX expression, was unchanged compared to TGFα/PBS sequentially infused rats (Fig. 5H, J; 7,606 ± 2,131 versus 8,345 ± 2,512 DCX+ cells for TGFα/PBS and TGFα/noggin infused rats, respectively). The percentage of immature BrdU+ cells (nestin+) tended to increase when compared to TGFα/PBS sequentially infused rats (Fig. 5G, H; p = 0.079), suggesting that BrdU+ cells that did not differentiate into astrocytes stayed in a nestin+ immature state rather than differentiated.
We also determined the effect of TGFα and noggin co-infusion for 4 weeks (Fig. 1B5). In this condition, most BrdU+ striatal cells were EGFR+/Olig2+/nestin+ “C-like” cells (Suppl. Fig. 2), which was similar to the TGFα only condition. This data shows that the TGFα effect was predominant and blocked the noggin effect.
Nestin-CreERT2/R26R-YFP mice demonstrate that TGFα-induced striatal cells originate from SVZ nestin+ precursors
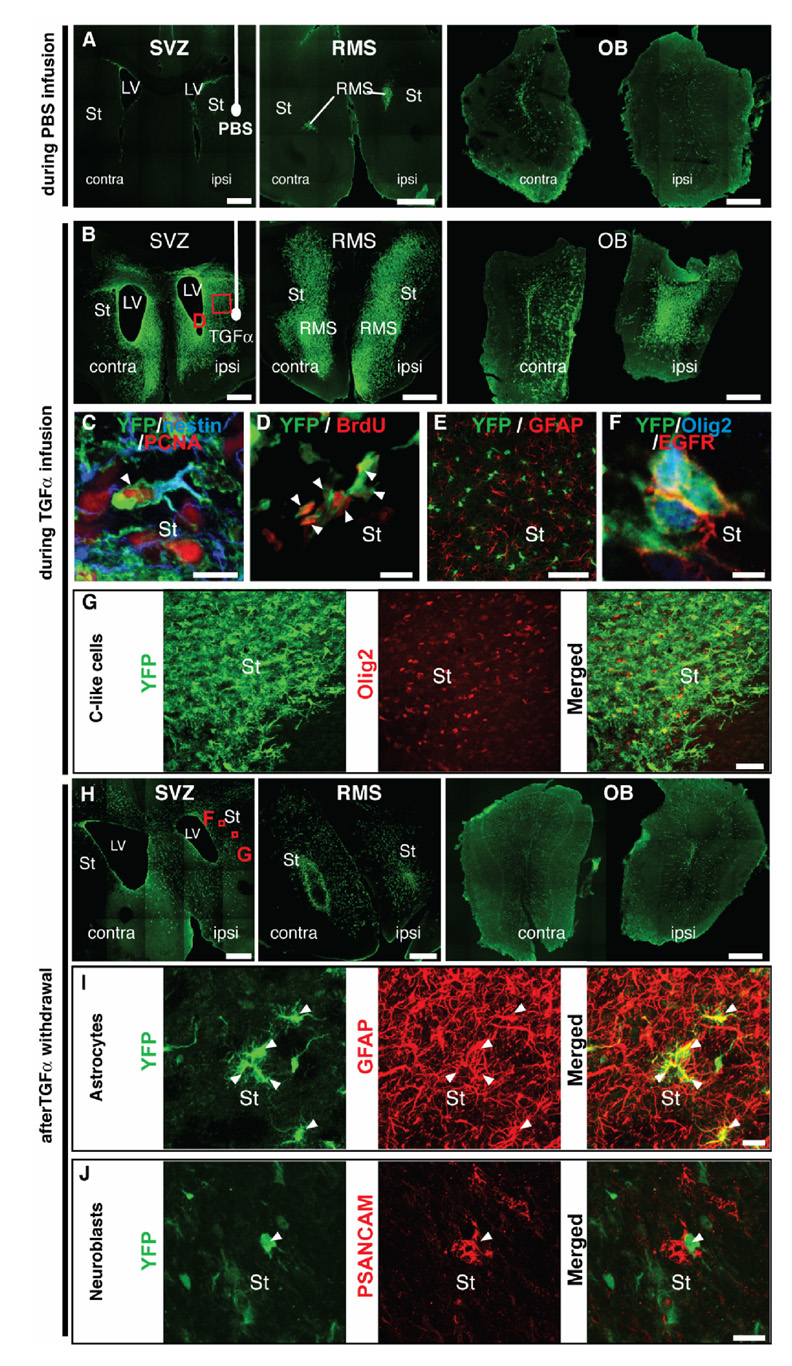
An important basic biological question was to determine whether the striatal wave of C-like cells observed during TGFα infusion originated from nestin+ SVZ precursors. For this purpose, we used inducible nestin-CreERT2/R26R-YFP mice. Upon tamoxifen administration, YFP expression in SVZ and SGZ precursors [19] allows genetic fate-mapping of adult neurogenesis. We administered tamoxifen to 5–8 week-old transgenic mice that had received a unilateral 6-OHDA injection 2 weeks before (Fig. 1C). Two days after tamoxifen treatment (Fig. 1C1), YFP+ cells were only observed in the SVZ, RMS and dentate gyrus of hippocampus (data not shown). In mice sacrificed during PBS infusion (Fig. 1C2), forebrain YFP+ cells were restricted to the same regions, except that their distribution had changed, with many YFP+ cells in the OB (Fig. 6A). In contrast, in mice sacrificed during TGFα infusion (Fig. 1C2), YFP+ SVZ cells had expanded and colonized the ipsilateral medial striatum, septum, corpus callosum and deep layers of the neocortex (Fig. 6B). The majority of these YFP+ cells expressed nestin and PCNA (Fig. 6C), as well as BrdU (Fig. 6D). These data unambiguously show that striatal nestin+/PCNA+ precursors described by Cooper and Isacson [1] originated from SVZ nestin+ precursors. They also indicate that tamoxifen did not substantially interfere with TGFα signaling [24]. YFP cells did not express GFAP at this time (Fig. 6E), indicating that we were not measuring reactive gliosis at this point.
Figure 6. 6-OHDA lesioned nestin-CreERT2/R26R-YFP mice show that TGFα-induced C-like cells and their progeny arise from SVZ nestin+ cells.
(A) Mice that received an intrastriatal infusion of PBS for 2 weeks showed YFP+ cells (green) that were restricted to SVZs (left panel), RMS (middle panel) and olfactory bulbs (right panel). (B) Strikingly, mice that received an intrastriatal infusion of TGFα for 14 days exhibited an increased number of YFP+ cells (green) in SVZs (left), RMS (middle) and olfactory bulbs (right). Such cells contributed to YFP+ cellular waves in the ipsilateral striatum, septum as well as corpus callosum and deep cortical layers (see left and middle panels). (C) YFP+ cells (green) in the ipsilateral striatum coexpressed PCNA (red) and nestin (blue). (D) Most ipsilateral striatal YFP+ cells (green) expressed BrdU (red), thus confirming a proliferative state. (E) Single plan confocal scan showing that no YFP+ cells (green) were GFAP+ astrocytes (red) in mice sacrificed during TGFα infusion. (F) YFP+ cells (green) coexpressing EGFR (red) and Olig2 (blue) in the DA-depleted striatum. (G) At a lower magnification, most ipsilateral striatal YFP+ cells (green) coexpressed Olig2 (red). (G–I) Distribution and phenotypes of SVZ-derived NPCs 2 weeks after TGFα withdrawal. (G) Numerous YFP+ cells (green) were observed in the SVZs, ipsilateral striatum, septum (left), RMS (middle) and olfactory bulbs (right). (H) Numerous ipsilateral striatal YFP+ cells (green, left) coexpressed GFAP (red, middle). Right panel depicts a merged image. (I) Other striatal YFP+ cells (green, left) coexpressed PSANCAM (red, middle), indicating a neuroblast phenotype (see right panel for a merged image). Scale bars: A, B, H, 500 µm; C, D, 10 µm, E, 400 µm; F, 5 µm; G, 50 µm, I, J, 20 µm.
The C-like cell phenotype of ectopic striatal YFP+ SVZ cell progeny was confirmed by coexpression with EGFR and Olig2 (Fig. 6F, G). The transgenic mouse model also allowed us to determine the fine morphology of striatal C-like cells: YFP+/Olig2+/nestin+/PCNA+ cells had a complex morphology, with numerous processes oriented towards the source of TGFα (Fig. 6C, G).
Two weeks after TGFα withdrawal (Fig. 1C3), we observed a decrease in striatal, septal and olfactory bulb YFP+ cells, as well as a dispersion of YFP+ cells in the ipsilateral striatum (compare Fig. 6H with Fig. 6B), which corroborated the rat data. Also, examining the fate of YFP+ cells in nestin-CreERT2/R26R-YFP mice 2 weeks after TGFα pump withdrawal showed that some striatal YFP+ cells were GFAP+ astrocytes, while others were PSANCAM+ neuroblasts (Fig. 6I, J), again confirming the rat data. We also observed striatal YFP+ cells that were nestin+/GFAP−/PSANCAM− undifferentiated cells, with a morphology reminiscent of astrocytes (data not shown).
Differential fate of striatal and olfactory bulb C-like cells after TGFα withdrawal
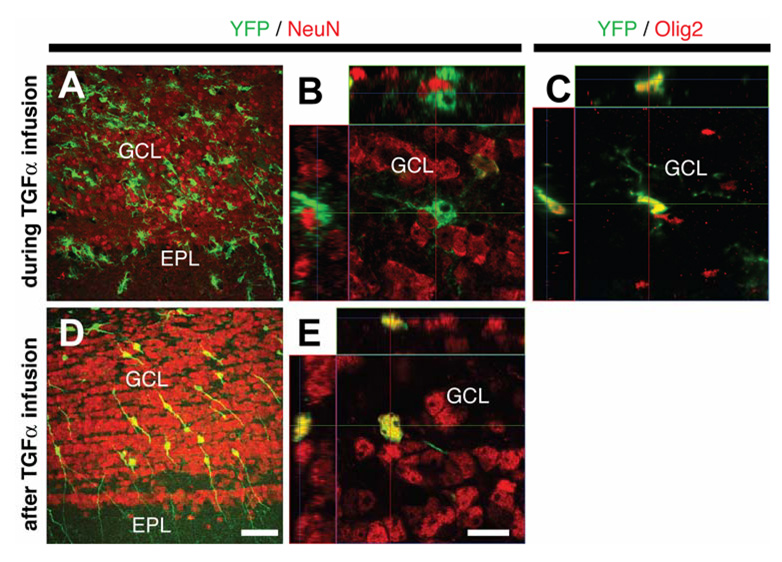
In the olfactory bulb of 6-OHDA lesioned nestin-CreERT2/R26R-YFP mice during PBS infusion, YFP+ cells were either granular or periglomerular interneurons (data not shown), consistent with the normal fate of SVZ NPCs [25]. In contrast, TGFα infusion generated olfactory bulb YFP+/NeuN−/Olig2+ cells, reminiscent of the C-like phenotype observed in the striatum (Fig. 7A–C). The number of YFP+ cells in the olfactory bulb was also greatly enhanced by TGFα infusion (compare Fig. 6A and Fig. 6B). Importantly, upon TGFα withdrawal, most YFP+ cells were granular or periglomerular interneurons (NeuN expression with typical neuronal morphology, Fig. 7D, E), and were much more numerous than during PBS infusion (Suppl. Fig. 3). This indicates that olfactory bulb C-like cells, as opposed to their striatal counterparts, had differentiated into mature neurons in the olfactory bulb upon TGFα withdrawal, thus highlighting a decisive influence of the microenvironment – striatal versus bulbar – on the fate of the C-like cells.
Figure 7. Phenotypes of SVZ-derived cells in the olfactory bulb of 6-OHDA lesioned nestin-CreERT2/R26R-YFP mice during and after TGFα infusion.
(A–C) During TGFα infusion, olfactory bulb YFP+ cells were morphologically similar to striatal C-like cells (A) and did not express NeuN (B). (C) Orthogonal projection of a YFP+ cell that coexpressed Olig2 in the olfactory bulb. (D–E) Two weeks after TGFα withdrawal, most olfactory bulb YFP+ cells had the typical morphology of granular neurons (D) and expressed NeuN (E). EPL: external plexiform layer, GCL, granule cell layer. Scale bars: A, D, 50 µm; B, C, E, 10 µm.
Absence of new TH+ neurons and behavioral improvement
The ability of TGFα, noggin or TGFα̣ and noggin co- and sequential intrastriatal infusions to restore motor function in rat groups with a unilateral 6-OHDA lesion was examined. Amphetamine-induced rotations were counted before and after the 2–4 weeks infusion period [26]. No significant reductions in rotation scores for any infusion groups were found (Suppl. Fig. 4). Consistent with these findings, not a single TH+ cell that had incorporated BrdU or YFP was seen in the DA-depleted striatum in all rat and mouse groups, respectively (data not shown).
DISCUSSION
In this study, we first characterized the phenotype of striatal nestin+/PCNA+ cells generated by TGFα infusion in the rat DA-depleted striatum [1]. These BrdU+ cells coexpressed Olig2, EGFR, nestin and Mash1, consistent with the phenotype of multipotent C cells [21]. Secondly, we determined the fate of these cells upon TGFα withdrawal. We observed large numbers of new striatal neuroblasts and astrocytes, confirming the in vivo multipotency of the striatal “C-like” cells while some nestin+ SVZ derived striatal cells remained undifferentiated at this stage. Thirdly, we increased the neuroblast to astrocyte ratio in the DA-depleted striatum by administering the BMP antagonist noggin after TGFα withdrawal. Interestingly, in our 2 weeks infusion paradigm noggin treatment blocked glial differentiation but neuroblast differentiation was not increased. Finally, using nestin-CreERT2/R26R-YFP mice, we showed that striatal neurogenic C-like cells originate from the SVZ and confirmed the phenotype and neuronal/glial fate of such rat cells after TGFα withdrawal. We also determined that, in contrast to their striatal counterparts, olfactory bulb C-like cells give rise to mature neurons, emphasizing an environmental effect on the fate of C-like cells. The generation of a large number of NPCs with neurogenic potential in the DA-depleted striatum is of therapeutic interest, and our evidence suggests that such C-like cells require further midbrain or forebrain cues [13, 27–29] to differentiate into mature DA neurons suitable to improve function in PD. A longer period post-infusion may also be required to completely understand the differentiation potential of these newly generated cells.
Phenotype of SVZ-derived cells and striatal response during TGFα infusion
In vivo, EGFR ligands increase C cell proliferation and colonization of the striatum by their cellular progeny [1, 21]. However, the progeny phenotype of these striatal C cells has not been determined, making it difficult to determine whether such cells can be directed for neural repair in PD. In this study, we found that during TGFα infusion, most SVZ derived cells recruited to the striatum displayed features of C cells, such as Olig2, nestin, EGFR, and Mash1 expression [1, 8, 13, 16, 21, 22]. However, such cells were different from SVZ C cells due to an elongated and complex morphology (while SVZ C cells have a simple, globular morphology), expressed nestin more strongly and did not express Dlx2 [7, 21].
TGFα-induced striatal “C-like” cells expressed markers of mitotic activity (i.e. BrdU and PCNA), as well as β-catenin, EGFR and Olig2, which are known markers of SVZ C cells and glioma-forming cells [30–33]. Hence, our data emphasize the roles of EGFR signaling, the Wnt/β-catenin signaling pathway, and the transcriptional repressor activity of Olig2 in inducing adult NPCs to adopt a high replication state.
Another novel finding in this field is that TGFα̣ infusion elevated the number of microglial cells in both ipsilateral SVZ and striatum. This was not due to a direct mitogenic effect of TGFα on microglia since they rarely incorporated BrdU (2 ± 3 %). Rather, existing microglia were probably recruited from other brain regions [34, 35]. Such microglial recruitment towards the region of cell proliferation was not likely due to a direct action of TGFα, since TGFα overexpression in transgenic mice does not modify microglia number or behavior [36]. Instead, microglial cells may have been attracted by signals released by cells undergoing apoptosis, as discussed below. Recent reports indicate that microglia influence adult neurogenesis [37–40]. For example, microglia activated by cytokines associated with T-helper cells may stimulate neurogenesis [41], while inflammation-associated microglia reduces neurogenesis [42, 43]. Whether the microglia affected neurogenesis in the present paradigm remains to be elucidated.
TGFα withdrawal induces a large population of neuroblasts in the DA-depleted striatum
In the second phase of the investigation, we examined the effect of TGFα withdrawal on the fate of striatal C-like cells. Two weeks after TGFα withdrawal, ~ 34 % fewer BrdU+ cells were present. This was at least partly due to apoptotic cell death, because TUNEL+ cells were found in the striatal cellular wave. The surviving new cells in the striatum were composed of GFAP+ astrocytes, DCX+/PSANCAM+/nestin+ neuroblasts, and immature cells that expressed nestin and/or PCNA only. The BrdU+/GFAP+ astrocytes often expressed low levels of nestin but did not express SSEA-1 or PCNA. Therefore, these cells likely represent an immature astrocyte population rather than B-like “neural stem” cells [44]. Among the striatal neuroblast population, ~ 82 % of the cells expressed PCNA while only ~ 7 % expressed BrdU. This, along with YFP/PSANCAM coexpression in the striatum of nestin-CreERT2/R26R-YFP mice, is consistent with the progressive loss of BrdU by dilution over numerous cell divisions [45]. Because PCNA was almost restricted to DCX+ cells 2 weeks after TGFα withdrawal, any disappearance of BrdU by dilution is likely to have preferentially affected this population. The absence of YFP+/NeuN+ cells in the DA-depleted striatum of nestin-CreERT2/R26R-YFP mice indicates that mature neurons were not generated by TGFα in this region.
Another surprising finding is that, in contrast to SVZ and RMS neuroblasts, ectopic striatal neuroblasts did not express the transcription factor Pax6. This difference suggests that ectopic striatal neuroblasts would differentiate into olfactory bulb GABAergic interneurons, rather than A16 DA neurons without further stimulation [13, 14].
We also observed the presence of newly generated neuroblast clusters along blood vessels and in contact with astrocytes, similar to their normal SVZ niche [5]. The astrocytes surrounding the neuroblast clusters likely did not arise from the SVZ given the absence of BrdU, PCNA or nestin coexpression.
Besides the astrocyte and neuroblast populations, the majority of remaining striatal BrdU+ cells were immature because they expressed nestin, without Olig2, EGFR or Mash1 expression at this time. The long-term fate of those BrdU+/nestin+ cells is uncertain: they may retain an immature phenotype, or ultimately differentiate or die due to lack of fate specification and/or trophic supply.
Interestingly, no BrdU+ cells in the striatum were CNPase+, O4+ oligodendrocytes. This result was surprising because, in the presence of TGFα, a high proportion of BrdU+ cells expressed Olig2 and Mash1, which are transcription factors involved in oligodendrocyte specification in the embryo and postnatal SVZ [46, 47]. Nevertheless, the absence of NG2 expression by the Olig2+/Mash1+ cells confirmed that they were not typical oligodendrocyte precursors [48]. A recent study using CNP-hEGFR overexpressing mice indicates that EGFR signaling activation is involved in oligodendrogenesis and remyelination in demyelinating conditions [49]. Therefore, it is possible that such demyelinating conditions are required for TGFα-activated NPCs to undergo oligodendrogenesis [50].
Effects of TGFα and noggin sequential infusions on the striatal neuroblast to astrocyte ratio
We examined whether administration of the BMP antagonist noggin after TGFα withdrawal would influence the neuroblast to astrocyte ratio by blocking gliogenesis and/or inducing neurogenesis [51]. We found that striatal infusion of noggin without TGFα in 6-OHDA lesioned rats significantly increased SVZ neurogenesis. This is in line with published data [17] and demonstrates that the noggin protein diffuses over long distances (about 2.7 mm) and remains functional when chronically infused in vivo. In contrast to SVZ, the normal striatum is gliogenic for SVZ cells [52], probably due to the absence or too low levels of noggin and other BMP antagonists such as follistatin or chordin [53]. However, virally delivered noggin reverses this effect and induces neurogenesis from SVZ cells recruited to the striatum, either after transplantation or following BDNF-induced recruitment [17, 54]. Strikingly, in our in vivo DA depletion paradigm noggin did not increase striatal neurogenesis, but only blocked gliogenesis. This difference with previous literature [17, 54] may be due to paradigm differences. The present data suggest that noggin is primarily anti-gliogenic, while other factors confined to SVZ induce neurogenesis when gliogenesis is blocked.
Comparisons with other paradigms inducing adult SVZ neurogenesis
As mentioned, we found ~ 8,000 newly generated striatal neuroblasts 2 weeks after the EGFR ligand withdrawal. This cell number is similar to that found by Arvidsson et al. [55] 2 weeks after striatal stroke induction (6,312 DCX+ neuroblasts), but it is ~ 4 times larger than the 1,600 BrdU+/Tuj1+ cells found after adenoviral co-administration of BDNF and noggin [54]. In our paradigm, striatal neuroblasts formed tight clusters throughout the medial striatum, whereas they were individualized in the studies from Arvidsson et al. and Chmielnicki et al. [54, 55]. Furthermore, in our paradigm 82% of neuroblasts continued to divide 2 weeks after growth factor withdrawal, but this remains unknown in the other paradigms [54, 55].
There are both analogies and differences between our in vivo paradigm and published in vitro neurosphere culture assays [56]. In neurosphere cultures, SVZ cells grow as clonal spheres in the presence of EGF [57]. Notably, neurosphere cells rapidly upregulate Olig2 and downregulate Pax6 in these proliferative conditions [58], and withdrawing EGF is required for differentiation into neurons and glia after plating [21], as shown here in vivo. However, the fact that SVZ cells do not differentiate into oligodendrocytes in vivo in the present study while some do in vitro [56] suggests that the striatal microenvironment influences the fate of SVZ cells.
CONCLUSION
We demonstrated that striatal TGFα infusion recruits a large population of SVZ-derived multipotent “C-like” cells to the DA-depleted striatum. After TGFα withdrawal, astrocytes, neuroblasts and undifferentiated cells, but no oligodendrocytes or differentiated neurons, are generated. Noggin infusion after TGFα blocks astrocyte generation in favor of an undifferentiated phenotype, thus increasing the neuroblast to astrocyte ratio. The findings that striatal neural precursors with neurogenic potential are generated in large numbers in the adult DA-depleted striatum provides a unique opportunity to differentiate such cells to functional DA neurons for PD.
Supplementary Material
ACKNOWLEDGMENTS
Supported by NIH/NINDS P50 NS39793, the Orchard Foundation, Anti-Aging Foundation, Michael K. Stern Foundation, Harold and Ronna Cooper Family (OI); NIDA K02 DA023555, R01 DA16765, NARSAD Young Investigator Award (AJE); Canadian Institute of Health Research (DCL).
REFERENCES
- 1.Cooper O, Isacson O. Intrastriatal transforming growth factor alpha delivery to a model of Parkinson's disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons. J Neurosci. 2004;24:8924–8931. doi: 10.1523/JNEUROSCI.2344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 3.Isacson O. The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol. 2003;2:417–424. doi: 10.1016/s1474-4422(03)00437-x. [DOI] [PubMed] [Google Scholar]
- 4.Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003;53 Suppl 3:S135–S146. doi: 10.1002/ana.10482. discussion S146-138. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Verdugo JM, Doetsch F, Wichterle H, et al. Architecture and cell types of the adult subventricular zone: in search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 9.Belluzzi O, Benedusi M, Ackman J, et al. Electrophysiological differentiation of new neurons in the olfactory bulb. J Neurosci. 2003;23:10411–10418. doi: 10.1523/JNEUROSCI.23-32-10411.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puopolo M, Bean BP, Raviola E. Spontaneous activity of isolated dopaminergic periglomerular cells of the main olfactory bulb. Journal of neurophysiology. 2005;94:3618–3627. doi: 10.1152/jn.00225.2005. [DOI] [PubMed] [Google Scholar]
- 11.Menn B, Garcia-Verdugo JM, Yaschine C, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freundlieb N, Francois C, Tande D, et al. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hack MA, Saghatelyan A, de Chevigny A, et al. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- 14.Kohwi M, Osumi N, Rubenstein JL, et al. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winner B, Geyer M, Couillard-Despres S, et al. Striatal deafferentation increases dopaminergic neurogenesis in the adult olfactory bulb. Exp Neurol. 2006;197:113–121. doi: 10.1016/j.expneurol.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 16.Fallon J, Reid S, Kinyamu R, et al. In vivo induction of massive proliferation, directed migration, and differentiation of neural cells in the adult mammalian brain. Proc Natl Acad Sci U S A. 2000;97:14686–14691. doi: 10.1073/pnas.97.26.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim DA, Tramontin AD, Trevejo JM, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 18.Bjorklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci U S A. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagace DC, Whitman MC, Noonan MA, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ries V, Henchcliffe C, Kareva T, et al. Oncoprotein Akt/PKB induces trophic effects in murine models of Parkinson's disease. Proc Natl Acad Sci U S A. 2006;103:18757–18762. doi: 10.1073/pnas.0606401103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doetsch F, Petreanu L, Caille I, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 22.Parras CM, Galli R, Britz O, et al. Mash1 specifies neurons and oligodendrocytes in the postnatal brain. The EMBO journal. 2004;23:4495–4505. doi: 10.1038/sj.emboj.7600447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 24.Johnston SR, Dowsett M, Smith IE. Towards a molecular basis for tamoxifen resistance in breast cancer. Ann Oncol. 1992;3:503–511. doi: 10.1093/oxfordjournals.annonc.a058251. [DOI] [PubMed] [Google Scholar]
- 25.Carleton A, Petreanu LT, Lansford R, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 26.Moore AE, Cicchetti F, Hennen J, et al. Parkinsonian motor deficits are reflected by proportional A9/A10 dopamine neuron degeneration in the rat. Exp Neurol. 2001;172:363–376. doi: 10.1006/exnr.2001.7823. [DOI] [PubMed] [Google Scholar]
- 27.Shim JW, Park CH, Bae YC, et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells. 2007;25:1252–1262. doi: 10.1634/stemcells.2006-0274. [DOI] [PubMed] [Google Scholar]
- 28.Papanikolaou T, Lennington JB, Betz A, et al. In vitro generation of dopaminergic neurons from adult subventricular zone neural progenitor cells. Stem Cells Dev. 2008;17:157–172. doi: 10.1089/scd.2007.0090. [DOI] [PubMed] [Google Scholar]
- 29.Hermann A, Maisel M, Wegner F, et al. Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells. 2006;24:949–964. doi: 10.1634/stemcells.2005-0192. [DOI] [PubMed] [Google Scholar]
- 30.Caricasole A, Bakker A, Copani A, et al. Two sides of the same coin: Wnt signaling in neurodegeneration and neuro-oncology. Biosci Rep. 2005;25:309–327. doi: 10.1007/s10540-005-2893-6. [DOI] [PubMed] [Google Scholar]
- 31.Holland EC. Progenitor cells and glioma formation. Curr Opin Neurol. 2001;14:683–688. doi: 10.1097/00019052-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholas MK, Lukas RV, Jafri NF, et al. Epidermal growth factor receptor - mediated signal transduction in the development and therapy of gliomas. Clin Cancer Res. 2006;12:7261–7270. doi: 10.1158/1078-0432.CCR-06-0874. [DOI] [PubMed] [Google Scholar]
- 34.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 35.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 36.Rabchevsky AG, Weinitz JM, Coulpier M, et al. A role for transforming growth factor alpha as an inducer of astrogliosis. J Neurosci. 1998;18:10541–10552. doi: 10.1523/JNEUROSCI.18-24-10541.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walton NM, Sutter BM, Laywell ED, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 38.Ziv Y, Ron N, Butovsky O, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 39.Ziv Y, Avidan H, Pluchino S, et al. Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury. Proc Natl Acad Sci U S A. 2006;103:13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goings GE, Kozlowski DA, Szele FG. Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia. 2006;54:329–342. doi: 10.1002/glia.20381. [DOI] [PubMed] [Google Scholar]
- 41.Butovsky O, Ziv Y, Schwartz A, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Ekdahl CT, Claasen JH, Bonde S, et al. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 44.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 45.Dayer AG, Ford AA, Cleaver KM, et al. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- 46.Kondo T, Raff M. Basic helix-loop-helix proteins and the timing of oligodendrocyte differentiation. Development. 2000;127:2989–2998. doi: 10.1242/dev.127.14.2989. [DOI] [PubMed] [Google Scholar]
- 47.Lu QR, Yuk D, Alberta JA, et al. Sonic hedgehog--regulated oligodendrocyte lineage genes encoding bHLH proteins in the mammalian central nervous system. Neuron. 2000;25:317–329. doi: 10.1016/s0896-6273(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 48.Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguirre A, Dupree JL, Mangin JM, et al. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 50.Chandran S, Compston A. Neural stem cells as a potential source of oligodendrocytes for myelin repair. Journal of the neurological sciences. 2005;233:179–181. doi: 10.1016/j.jns.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Sonntag KC, Pruszak J, Yoshizaki T, et al. Enhanced yield of neuroepithelial precursors and midbrain-like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells. 2007;25:411–418. doi: 10.1634/stemcells.2006-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann Neurol. 1999;46:867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 53.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. Journal of medical genetics. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chmielnicki E, Benraiss A, Economides AN, et al. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 56.Doetsch F, Caille I, Lim DA, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 57.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. Proc Natl Acad Sci U S A. 1999;96:11619–11624. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hack MA, Sugimori M, Lundberg C, et al. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci. 2004;25:664–678. doi: 10.1016/j.mcn.2003.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.