Abstract
The most widely studied stimuli for ion channel activation are changes in membrane voltage and binding of a chemical ligand in a pocket of the channel protein. While modulation by redox potential has also been appreciated our study shows previously unrecognised channel activation via electron donation from the extracellular redox protein thioredoxin (TRX)1. The ion channel type involved is a member of the Transient Receptor Potential (TRP) family. Activation by TRX led us to consider the relevance of TRP channels to the inflammatory condition of rheumatoid arthritis, where functions of ion channels are relatively unknown and TRX concentrations are high. TRP channel activation was found to be inhibitory for secretion of matrix metalloproteinases, suggesting activation by TRX may have a protective role against disease. Here we expand on our original article and discuss the potential wider implications of the findings in terms of concepts for channel activation and relevance to other ion channel types and systems.
Keywords: Cation channel, thioredoxin, arthritis, inflammation, matrix metalloproteinases, secretion
THIOREDOXIN (TRX)
Redox, the balance between gain (reduction) and loss (oxidation) of electrons, is a widespread feature of biological systems. With oxygenation of the planet and evolution of respiration presumably came increasing need for control of redox and associated free radicals, with their reactive unpaired electrons. Oxidative stress and free radicals can damage cells and may contribute to major diseases2. For this reason, dietary antioxidants are suggested anti-disease therapies3. However, although free radicals have potential to cause damage, cells also evolved to use free radicals in immune defence and as physiological signaling molecules. Therefore, physiologically and pathologically cells use and need to cope with variations in extracellular and intracellular redox4. In some cases it is advantageous to our health if the cells cope, in others it is not. Tumour cells are a notable example of cells that actively protect themselves against free radicals, making their protective mechanisms attractive targets for novel therapeutic agents5.
Cells have developed protein systems to manage redox6. The two main systems are the TRX and glutaredoxin systems. Both were first discovered in bacteria but have since been found widely distributed in mammalian organs and cells. Mechanistically both operate similarly using the physiologically reversible redox control of cysteine pairs, which either couple through a disulphide bridge when oxidized, or separate as sulphydryl groups when reduced. The system of particular relevance to this article is the TRX system, which comprises the protein components TRX, TRX reductase and TRX-binding protein and requires the presence of an electron donor such as NADPH. TRX-2 is a mitochondrial protein, while TRX-1 is cytoplasmic, nuclear and subject to secretion in response to oxidative stress. TRX-1 is thus not only important for intracellular redox control but also for extracellular redox. It is not only protective, but also regulatory. Intriguingly, TRX-1 is suggested to regulate other proteins through direct binding as well as exchange of electrons. It is also a multifunctional protein, with anti- inflammatory, apoptotic and oxidative effects. In a wide range of diseases high concentrations of TRX-1 occur in the plasma or at the focus of disease (e.g. rheumatoid arthritic joint, atherosclerotic plaque) and it is a biomarker of diseases characterized by oxidative stress7,8.
TRANSIENT RECEPTOR POTENTIAL (TRP) CHANNELS
As cells evolved with redox so they also evolved in wet and salty environments, leading to ion transport mechanisms such as ion channels. While retaining key roles in survival of varying salt intensity these mechanisms also enable electric rhythms, electrical communication and Ca2+-signaling. The ion channels that influence Ca2+ have expanded in number with the emergence of higher species. As such, the subtleties conferred by Ca2+ signaling may have been instrumental in the building of complex systems.
One class of Ca2+-permeable (not Ca2+-selective) channel that is considerable in number in mammals is the transient receptor potential (TRP) channels9. The channels were named because of the transient nature of the light response in Drosophila melanogaster photoreceptors that lack functional TRP. In mammals there are 28 TRP homologues encoded by distinct multi-exon genes. They are sub-classified according to amino acid sequence into seven TRPCs, six TRPVs, eight TRPMs, three TRPPs, one TRPA and three mucolipins. Each TRP protein shows broad structural similarity to voltage-gated potassium channel subunits like KV1.2, for which a crystallographic structure exists10. Each TRP channel is considered to arise from four TRP proteins coming together as a homo- or hetero- tetramer. An example of a heteromultimeric channel is TRPC1 with TRPC4 or TRPC5.
Although some TRP channels are voltage-sensitive it seems that none is voltage-activated (i.e. change in membrane voltage is not the signal that activates TRP channels). Instead an emerging concept is of TRP channels as polymodal sensors of other factors – such as heat and chemicals. Some TRP channels are constitutively closed and so need to be activated, where as others are constitutively partially or fully activate, so the physiological regulator may be an inhibitor or enhancer. The question arises as to the identities of the factors. Of relevance in this article is that redox factors are candidates; for example, hydrogen peroxide activates TRPM2 and redox reagents sensitize TRPV111,12.
TURRETS AS EXTRACELLULAR TRANSDUCTION SITES
A feature in the KV1.2- like structures is the turret, an extracellular loop between the fifth membrane-spanning segment (S5) and the amino acids determining ion selectivity (Figure 1)10,13. Many other channels of this type, including TRP channels, are predicted to have a similar feature; a region also referred to, generically, as E3 (the third extracellular loop)14. One concept for the turret is that it is purely structural, ensuring correct positioning of the selectivity filter. Alternatively, or in addition, the turret may be a site for chemical regulation; that is, provide an agonist or reverse agonist interaction site, as in ligand-gated ion channels. There have been few data to validate such a function but emerging evidence is persuasive
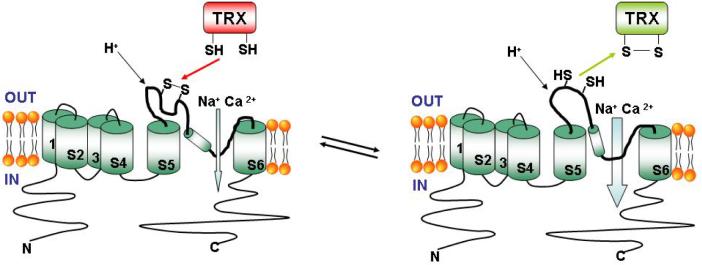
FIGURE 1.
Regulation of TRPC5 cation channel via its predicted turret. Two states of TRPC5 are indicated. On the left: the turret disulphide bridge is formed, the channel has low activity and reduced TRX is approaching the channel. On the right: the cysteine residues are reduced, the channel has enhanced activity and the TRX has oxidized. TRX and proton effects are described1,24. Only one TRPC5 is drawn, but four such proteins are thought to be required to form one ion channel.
Lanthanides (e.g. lanthanum and gadolinium) block many ion channels. It is therefore intriguing to find that lanthanides are, conversely, powerful activators of TRPC4 or TRPC515. Importantly, a critical amino acid for lanthanide activation is in the predicted turret. The effect seems relatively unimportant because humans contain only trace amounts of lanthanides that are not thought to have physiological or pathological relevance. We and others have, however, wondered if the lanthanides mimic another, endogenous, signaling factor. A clue to the identity of such a factor was a pair of conserved cysteine residues in TRPC1, 4 and 5. We found that chemical reducing agents activate the channels and that mutation of these cysteine residues to alanine leads to constitutively active, lanthanum-insensitive, channels1. Based on this and other evidence we hypothesised that the turret-like structure of TRPC5 contains an inhibitory disulphide bridge (Figure 1). Again, this was not especially interesting because disulphide bridges are quite common in membrane proteins and chemical reducing agents are not endogenous factors.
TRX LINK TO TRP
A common assumption is that disulphide bridges occur, and are stable, in the extracellular oxidizing environment, and are lacking in the intracellular reducing environment. However, dynamics of disulphide bridges are an important intracellular regulatory mechanism, and it is plausible that similar dynamics exist extracellularly3,16. We were therefore interested to learn that TRX is a secreted protein and that major diseases are associated with high extracellular TRX concentrations7,17.
Importantly, extracellular application of reduced recombinant TRX (bacterial or human) led to strong stimulation of TRPC5 or TRPC5 in heteromultimeric assembly with TRPC11. Oxidised TRX had no effect, suggesting that the reducing capability of TRX is necessary for channel activation, and that binding of TRX to the channel (if it occurs) is not. TRX can therefore donate electrons to the cysteine residues in the predicted turret of TRPC5. A previously unrecognized type of ion channel activation mechanism is implied, whereby the channel activates as it receives electrons from an endogenous extracellular protein that does not, or does not need to, bind the channel (Figure 1). Relevance to endogenous TRX and TRP is suggested by the concentration of human TRX necessary to activate the channels and the similar effects of TRX on native TRP channels.
RHEUMATOID ARTHRITIS
TRPC channel expression occurs in many different cell types throughout the body and TRX is ubiquitous6,18. There is therefore potential for TRP regulation by TRX to have wide implications. However, as a first-pass, we were struck by the particularly high concentrations of TRX in samples from patients with rheumatoid arthritis, evidence that reduced TRX and TRX reductase are presented in arthritic joints, and by the paucity of information on ion channels of the synovial joints that are affected by this important disease1,19. Rheumatoid arthritis is a complex autoimmune disease of unknown cause, but one of its characteristics is neutrophil invasion with associated free radical production and high TRX concentrations.
Ion channel investigation has often focused on excitable cells but crucial roles exist in many kinds of additional cell types, including cancer cells. In the spirit of addressing these wider roles of ion channels we explored whether TRPC1 and TRPC5 proteins occur in synovial cells of biopsies obtained from the knees of patients suffering from rheumatoid arthritis. Notably, they occur in these cells, including in the identified CD55-positive fibroblast-like synoviocytes that normally secrete synovial fluid and are inflamed in rheumatoid arthritis. Such cells show ionic current in response to reduced TRX that is mediated substantially by TRPC1/5-containing channels. The effective concentrations of TRX are comparable with those detected in synovium of rheumatoid arthritic joints1.
In excitable cells, non-selective cationic channels such as TRPC1/5 would be expected to be excitatory, but in non-excitable cells the functional implications are difficult to predict. Given the few studies of ion channels in synovial cells (especially native cells rather than cell-lines) there is much work to do to understand the functional relevance in joints. However, an aspect that attracted our attention was the cells' capacity to secrete matrix metalloproteinases (MMPs), which are important in remodeling and destruction of cartilage and bone20-22. Strikingly, antibodies that inhibit TRPC1 or TRPC5 stimulate MMP secretion, suggesting constitutive TRPC1/5 activity that inhibits MMP secretion. Exogenous reducing TRX further inhibited MMP secretion in a TRPC5-dependent manner. Therefore, there is a relationship between TRP and MMP secretion where the Ca2+- and Na+-permeable TRPC1/5 seems able to suppress secretion and have a potentially protective role against disease progression. There may be broader effects of the channel on secretion because we also found effects on interleukin-6 (Jairaman A, Sukumar P & Beech D J unpublished).
POLYMODALITY
TRPC5 and TRPC1/5 are not only regulated by TRX. As with other members of the TRP family, and other types of ion channel, there is polymodality; responsiveness to more than one factor – a property also referred to as multiplicity of activation23. Therefore, the net channel activity in a living animal or human will depend on the balance of multiple inputs, which presumably shifts with context and disease. TRPC5 or TRPC1/5 can also be stimulated by G-protein coupled receptor agonists, certain phospholipids and acidosis18,24. There are conflicting results, but effects of hydrogen peroxide and nitric oxide have been suggested1,25. Elevated intracellular Ca2+ concentrations modulate (positively or negatively) TRPC1 and TRPC5, but the channels are not considered to be Ca2+-activated18.
CONCLUSIONS
Our study1 draws attention to the possibility that the turret structure of this family of ion channels has greater importance than previously recognised; it may not only have structural purpose but also confer capacity for regulation or activation by extracellular proteins and other factors. For TRPC5, there is evidence for two physiological regulators acting at the turret – thioredoxin and protons (Figure 1). Modulation via the turret has also been suggested for TRPV1 and toxins interact in this region12,13. Some other members of this ion channel family have cysteine pairs in their predicted turrets, so might be regulated like TRPC5. In other cases there may be other, as yet unappreciated, endogenous “turret modulators”.
The idea that a redox protein activates an ion channel by breaking an extracellular disulphide bridge is perhaps surprising. However, there could be several contexts in which it is important; most obviously in conditions of redox stress caused by inflammation or disease. For example, there are parallel lines of discovery suggesting functional roles of TRX and TRPC channels in cancers and occlusive vascular diseases5,26-28. It would seem worth investigating whether TRX and TRP come together as parts of integrated systems in these contexts also.
ACKNOWLEDGEMENTS
Funded by the Wellcome Trust and an Overseas Research Scholarship and University Studentship to P. Sukumar.
Footnotes
ADDENDUM TO: TRPC channel activation by extracellular thioredoxin. Xu, S.Z., Sukumar, P., Zeng, F., Li, J., Jairaman, A., English, A., Naylor, J., Ciurtin, C., Majeed, Y., Milligan, C.J., Bahnasi, Y.M., AL-Shawaf, E., Porter, K.E., Jiang, L.H., Emery, P., Sivaprasadarao, A. & Beech, D.J. Nature 2008; 451:69-72.
REFERENCES
- 1.Xu SZ, Sukumar P, Zeng F, Li J, Jairaman A, English A, Naylor J, Ciurtin C, Majeed Y, Milligan CJ, Bahnasi YM, Al-Shawaf E, Porter KE, Jiang LH, Emery P, Sivaprasadarao A, Beech DJ. TRPC channel activation by extracellular thioredoxin. Nature. 2008;451:69–72. doi: 10.1038/nature06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winyard PG, Moody CJ, Jacob C. Oxidative activation of antioxidant defence. Trends Biochem Sci. 2005;30:453–61. doi: 10.1016/j.tibs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 4.Darley-Usmar V, Halliwell B. Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system. Pharm Res. 1996;13:649–62. doi: 10.1023/a:1016079012214. [DOI] [PubMed] [Google Scholar]
- 5.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–7. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Berndt C, Lillig CH, Holmgren A. Thiol-based mechanisms of the thioredoxin and glutaredoxin systems: implications for diseases in the cardiovascular system. Am J Physiol. 2007;292:H1227–36. doi: 10.1152/ajpheart.01162.2006. [DOI] [PubMed] [Google Scholar]
- 7.Burke-Gaffney A, Callister ME, Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26:398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Jikimoto T, Nishikubo Y, Koshiba M, Kanagawa S, Morinobu S, Morinobu A, Saura R, Mizuno K, Kondo S, Toyokuni S, Nakamura H, Yodoi J, Kumagai S. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol Immunol. 2002;38:765–72. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 9.Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–8. doi: 10.1126/science.1116270. 5. [DOI] [PubMed] [Google Scholar]
- 11.Hara Y, Wakamori M, Ishii M, Maeno E, Nishida M, Yoshida T, Yamada H, Shimizu S, Mori E, Kudoh J, Shimizu N, Kurose H, Okada Y, Imoto K, Mori Y. LTRPC2 Ca2+-permeable channel activated by changes in redox status confers susceptibility to cell death. Mol Cell. 2002;9:163–73. doi: 10.1016/s1097-2765(01)00438-5. [DOI] [PubMed] [Google Scholar]
- 12.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol. 2006;70:383–94. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 13.Xu CQ, Zhu SY, Chi CW, Tytgat J. Turret and pore block of K+ channels: what is the difference? Trends Pharmacol Sci. 2003;24:446–8. doi: 10.1016/S0165-6147(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 14.Xu SZ, Zeng F, Lei M, Li J, Gao B, Xiong C, Sivaprasadarao A, Beech DJ. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol. 2005;23:1289–93. doi: 10.1038/nbt1148. [DOI] [PubMed] [Google Scholar]
- 15.Jung S, Mühle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–71. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- 16.Linke K, Jakob U. Not every disulfide lasts forever: disulfide bond formation as a redox switch. Antioxid Redox Signal. 2003;5:425–34. doi: 10.1089/152308603768295168. [DOI] [PubMed] [Google Scholar]
- 17.Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–4. [PubMed] [Google Scholar]
- 18.Beech DJ. Canonical transient receptor potential 5. Handb Exp Pharmacol. 2007;(179):109–23. doi: 10.1007/978-3-540-34891-7_6. [DOI] [PubMed] [Google Scholar]
- 19.Maurice MM, Nakamura H, Gringhuis S, Okamoto T, Yoshida S, Kullmann F, Lechner S, van der Voort EA, Leow A, Versendaal J, Muller-Ladner U, Yodoi J, Tak PP, Breedveld FC, Verweij CL. Expression of the thioredoxin-thioredoxin reductase system in the inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2430–9. doi: 10.1002/1529-0131(199911)42:11<2430::AID-ANR22>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–74. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 22.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–43. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 23.Zeng F, Xu SZ, Jackson PK, McHugh D, Kumar B, Fountain SJ, Beech DJ. Human TRPC5 channel activated by a multiplicity of signals in a single cell. J Physiol. 2004;559:739–50. doi: 10.1113/jphysiol.2004.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–78. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol. 2006;2:596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 26.El Hiani Y, Ahidouch A, Roudbaraki M, Guenin S, Brûlé G, Ouadid-Ahidouch H. Calcium-sensing receptor stimulation induces nonselective cation channel activation in breast cancer cells. J Membr Biol. 2006;211:127–37. doi: 10.1007/s00232-006-0017-2. [DOI] [PubMed] [Google Scholar]
- 27.Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, Naylor J, Forte A, Cipollaro M, McHugh D, Kingston PA, Heagerty AM, Munsch CM, Bergdahl A, Hultgårdh-Nilsson A, Gomez MF, Porter KE, Hellstrand P, Beech DJ. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–63. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamawaki H, Haendeler J, Berk BC. Thioredoxin: a key regulator of cardiovascular homeostasis. Circ Res. 2003;93:1029–33. doi: 10.1161/01.RES.0000102869.39150.23. [DOI] [PubMed] [Google Scholar]