Abstract
In the growing apex of Arabidopsis thaliana primary roots, cells proceed through four distinct phases of cellular activities. These zones and their boundaries can be well defined based on their characteristic cellular activities. The meristematic zone comprises, and is limited to, all cells that undergo mitotic divisions. Detailed in vivo analysis of transgenic lines reveals that, in the Columbia-0 ecotype, the meristem stretches up to 200 µm away from the junction between root and root cap (RCJ). In the transition zone, 200 to about 520 µm away from the RCJ, cells undergo physiological changes as they prepare for their fast elongation. Upon entering the transition zone, they progressively develop a central vacuole, polarize the cytoskeleton and remodel their cell walls. Cells grow slowly during this transition: it takes ten hours to triplicate cell length from 8.5 to about 35 µm in the trichoblast cell files. In the fast elongation zone, which covers the zone from 520 to about 850 µm from the RCJ, cell length quadruplicates to about 140 µm in only two hours. This is accompanied by drastic and specific cell wall alterations. Finally, root hairs fully develop in the growth terminating zone, where root cells undergo a minor elongation to reach their mature lengths.
Key words: Arabidopsis, cytoskeleton, development, differentiation zone, elongation zone, growth, growth terminating zone, meristem, root apex, transition zone
Introduction
During their lifetime, plant cells progress through a series of distinct developmental phases. Most of them originate from apical meristems,1 where mother cells continuously generate new cells. Each cell undergoes several mitotic cycles before leaving the meristem and embarking for irreversible postmitotic expansion. Initially, this cellular expansion is slow and accomplished in the three dimensions. Soon afterwards, however, cells effectively polarize their growth and start fast anisotropic cell growth, commonly known as cell elongation along the apical-basal polarity axis.2
A long-held view is that cells initiate cell elongation immediately after leaving the apical meristem. This view is based primarily on static pictures of longitudinal root sections and the accompanying statements are perpetuated in textbooks for more than a century.3,4 In fact relatively little attention has been paid to those changes cells undergo while leaving the meristem and heading for the more mature parts of the root apex.
In the Maize Root Apex, The Transition Zone is Located between the Meristem and the Region of Fast Cell Elongation
This dogmatic idea in plant biology was questioned in 1990 by demonstrations that cells of maize root apices undergo slow isotropic-like growth after leaving the meristem.5 This root apex zone was initially named the post-mitotic ‘isodiametric’ growth zone,5–7 and later renamed into the ‘distal elongation zone’ (DEZ) by Ishikawa and Evans.8 Nevertheless, it is noteworthy that cells in this zone still do not elongate at high rates and, in fact, resemble meristematic cells in many aspects.2,7,9 In this zone, cells undergo a series of fundamental changes in their cytoarchitecture and physiology, and accomplish dramatic rearrangements of the actin cytoskeleton.7,10,11 For this reason, the term transition zone was coined to describe this unique part of the root apex.2,10,12
Arabidopsis root apices are very well-described regarding division patterns and radial organisation.13–15 In sharp contrast, there is a lack of solid data regarding the longitudinal (axial) zonation of Arabidopsis root apices. As a consequence, the traditional anatomical view on the longitudinal zonation of the root apex, inherited from early anatomists of the 19th century,3,4 segregated into meristematic, elongation, and differentiation zones, is preserved up to the present date (reviewed in ref. 13 and 16). Although some authors acknowledge the transition zone (often termed DEZ) in root apices of Arabidopsis,17–20, and some even describe cell wall markers for this zone,21 the overwhelming majority adhere to the classical text-book model. Typically, the transition zone (or DEZ) is overlooked and considered part of the meristem.15,22–30
To address these controversial issues, we have analyzed in detail steadily growing root apices of Arabidopsis with special attention to the level of individual epidermal cells. Here, we provide evidence from in vivo experiments for the existence of three clear-cut zones Arabidopsis roots, namely division, transition, and of fast elongation, similar to those described for maize root apices (see below). In this treatise, we do not discuss the nongrowing differentiation zone, as this has been done elsewhere.31,32 Published data corroborate the fact that the early postmitotic root cells first traverse the transition zone, and only then they are released into the rapid cell elongation zone. In the transition zone, root cells acquire unique physiological properties, the most relevant being their high sensitivity to diverse environmental factors such as gravity, humidity, light, and oxygen all of which have fundamental consequences for both the signal-mediated tropisms as well as morphogenesis of roots.
Especially authors who used kinematic approaches to study root surfaces, but not cells directly, preferred to call this root region the distal elongation zone (DEZ).6,7,8,18,33–39 The cellular concept of the transition zone12 is based on findings that postmitotic root cells leaving the meristem show more similarities to interphase cells of the meristem than to elongating cells.2,5,7,12 In fact, cells leaving the meristem are not able to start elongating immediately. Cells become competent for fast elongation only after spending some time within the transition zone, acquiring the necessary cytoarchitecture and metabolic properties.2,12 For instance, cells leaving the apical meristem do not have the necessary vacuoles that would drive the extremely fast cell elongation and do not have the necessary mechanical properties of cell walls which would allow their rapid yielding.
In postmitotic maize root cells leaving the meristem, the cortical microtubules are not completely ordered in transverse arrays,40 while their actin filaments are not organized into longitudinal bundles yet.10,41 Importantly, all postmitotic cells in the transition zone perform a drastic reorganization of their cytoskeletal elements.2,10,40 This extensive rearrangement of the cytoskeleton is essential for the developmental switch into rapidly elongating root cells which expand strictly uniaxially.2,9,10,42
Arabidopsis Root Apices have Four Distinct Zones of Growth Activities
Small size and simple anatomy is useful.
Arabidopsis root apices were analyzed in vivo and in vitro against a background of the above the maize information. As guidance for the information to follow, a representative root apex of a five-day-old Col-0 Arabidopsis seedling is shown in Figure 1, in which an epidermal trichoblast cell file is highlighted. This confocal micrograph of a live root stained with propidium iodide clearly shows the cell walls. In plant tissues, there is no movement of cells relative to each other. Therefore, cell growth measured for one cell layer is by definition also indicative for the other neighboring cell layers of the organ, unless cell division occurs which is not the case here. In earlier work,43 we have introduced the stage of root hair bulging in trichoblasts as a new useful marker for time-related cellular development. Cells passing this critical developmental stage are extremely sensitive to both auxin44 and ethylene.43,45
Figure 1.
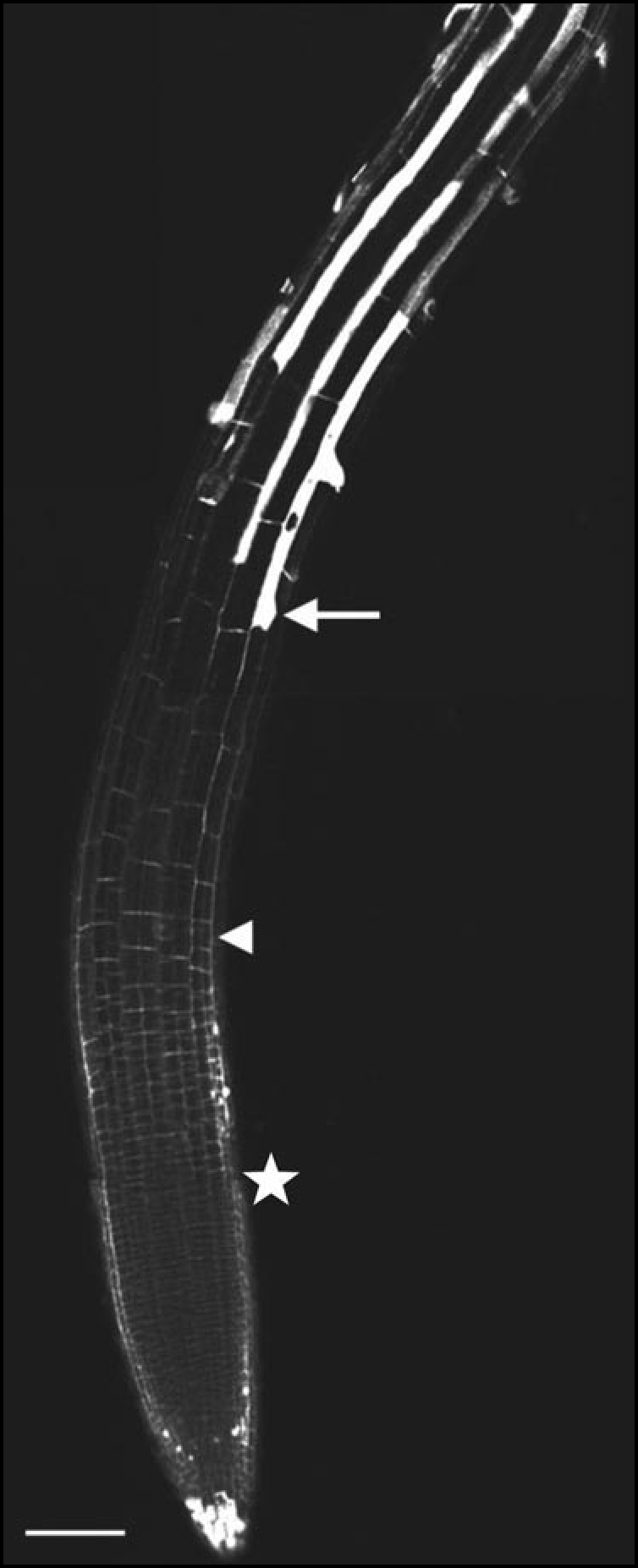
Confocal picture of a propidium iodide staining of a five-day-old Arabidopsis root (Col-0). This in vivo staining marks cell walls of plant cells. The star marks the basal limit of meristem, the arrow marks the youngest trichoblast showing root hair bulging while the arrowhead points to the onset of the fast elongation zone. Scale bar = 100 µm.
During steady-state growth of Arabidopsis roots used in our experiments, a new cell reaches this stage of root hair bulging in each thrichoblast cell row every 30 minutes (see the video sequence at: http://webhost.ua.ac.be/fymo/Root.avi). Knowing the regular timing of development in the trichoblast cell file, it is easy to measure and calculate the course of cells throughout root apices. Obviously, the small size and simple anatomy of Arabidopsis roots makes this model object extremely useful for the detailed in vivo characterization of individual root zones of cellular activities as well as for their easy localization in root apices (Table 1).
Table 1.
Growth zones in the Arabidopsis root apex in glance
| Meristem. Zone of active cell divisions. All cells of this zone express strongly cyclin B1 and are passing through the cell cycle. In the steady-state growing roots of the Columbia-0 ecotype, the meristem stretches up to 200 µm away from the root cap junction (RCJ). |
| Transition Zone. Zone of slow cell growth in both length and width. In the distal portions of the transition zone, few cells end division while most cells keep competence for cell division due to the expression of cdc2. In the proximal part of the transition zone, cells develop competence for the rapid onset of cell elongation. There are several cytological and anatomical features allowing easy recognition of this zone. Nuclei are, similarly like in meristem, positioned in the centre of cells. Cells contain small vacuoles and cell shapes are approximately isodiametric as cell lengths do not exceed significantly cell widths. In the steady-state growing roots of the Columbia-0 ecotype (at 23°C, 16h/8h light/dark cycle, grown on agarose for 5 days), the transition zone covers the distance from about 200 up to 520 µm away from the RCJ. |
| Elongation Zone. Zone of very fast cell elongation without growth in cell width. There are several cytological and anatomical features allowing easy recognition of this zone. Nuclei are pushed to the side cell walls due to very rapid formation of large central vacuoles. Cell shapes are elongated as cell lengths exceed cell widths and increase rapidly. The onset of rapid cell elongation is marked also by bulging of root hairs from the outer apical portions of trichoblasts. In the steady-state growing roots, elongation zone covers the distance from about 520 up to 850 µm away from the RCJ. |
| Growth Terminating Zone. Cells progressively slow down their elongation and finally undergo only minor cell elongation to reach their mature lengths. This root apex zone is characterized with very active tip growth of root hairs and extends from about 850 up to about 1500 µm away from the RCJ. |
Cell division is limited to the meristematic zone.
There are almost no reports that describe the basal (proximal) limit of the apical meristem in root apices of Arabidopsis. Classical cytological studies using pulse-labeled (usually with tritiated thymidine) samples did not include Arabidopsis as object of interest.46 This species only acquired the status of model plant more recently.1,13,14,47 Fortunately, current molecular biology techniques allow a direct approach to answer the question of meristem length. One can easily visualize the expression of proteins which are exclusively confined to dividing cells, such as cyclin B1 using (CYCB1;1) promoter-GUS lines.48–50 Hauser and Bauer30 used this rather elegant technique and reported that the proximal (basal) limit of the meristematic zone is situated at about 160 µm from the RCJ, and that the zone contained an average of about eight mitotic cells per cell file. There are several other reports, all of which did not specifically focus on the size of the meristem, but that did make use of the same CYCB1;1 transgenic line. All these data reveal invariably that the most proximal cell divisions are localized somewhere between 100 and 200 µm from the RCJ, depending on growth conditions and seedling age.29,51–55 During our studies of the cytoskeleton in Arabidopsis roots,45 we have performed a detailed analysis of the occurrence of mitotic and cytokinetic figures along the apex (Le J, unpublished data). Our observations are in accordance with the data published with the CYCB1;1 line, as we never detected preprophase bands, mitotic spindles or phragmoplasts farther than 200 µm from the RCJ.
Our values observed in vivo are in contrast to those indirectly computed from kinematic analysis of Arabidopsis root growth, localizing the proximal limit of the meristem between 488 and 713 µm from the RCJ, encompassing some 43 to 70 meristematic cells for one cortical cell file.24,25,56,57 On the contrary, calculations performed on longitudinal root sections correspond well with data from work with the cyclin reporter gene constructs. Using the longitudinal section through the Arabidopsis root apex (Fig. 2) published by Ishikawa and Evans,35 one can approximately calculate the number of cells in a cell file. In the left epidermal file, which is intact throughout this section, there are about 22 cells in the meristem (the first 200 µm from the RCJ) and about 20 cells in the transition zone (between 200–400 µm from the RCJ; for the determination of the basal limit of the transition zone see below). Kidner et al15 calculated the average number of dividing cells from longitudinal sections of 3 days old Arabidopsis root apices. These authors counted the number of cells within particular cell files from the initial cells towards the nondividing cells. They reported that the number of cells forming the apical root meristem varies between 18 (stele, atrichoblast) up to 35 (trichoblast) cells. From these data, it is clear that the indirect kinematic technique2,24,25,57 shifts the basal border of the meristem into the transition zone or even beyond (see later). The problems arising with such kind of indirect analysis might be the consequence of noise-rich smoothening and curve-fitting procedures.
Figure 2.
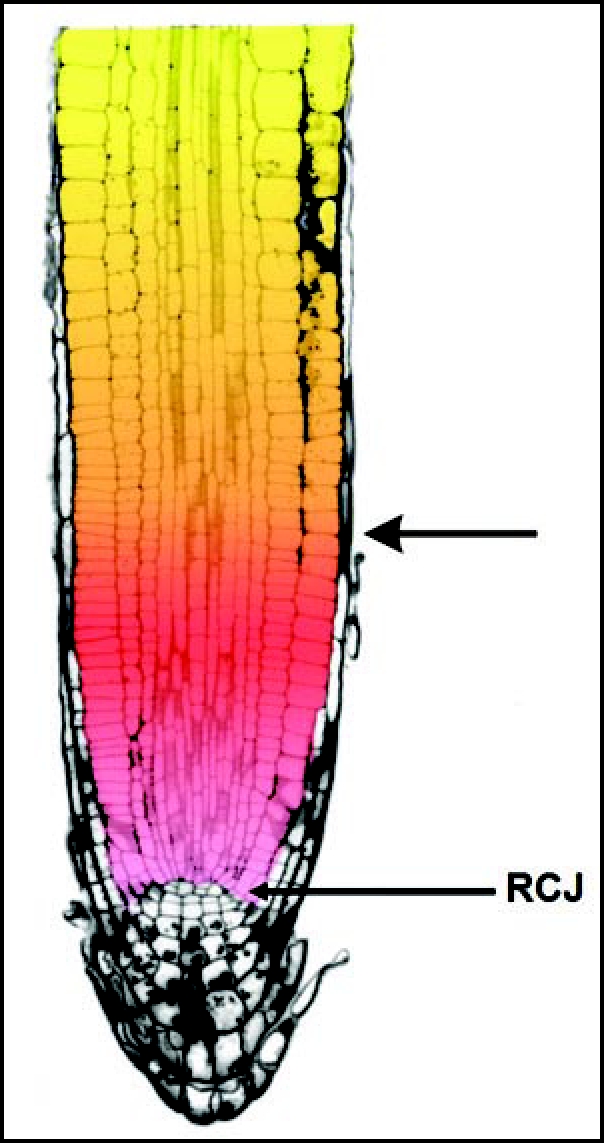
Picture is taken and adapted from the Ishikawa ands Evans (1997).35 It shows a nice longitudinal median section through an Arabidopsis root. The border between the apical meristem (in red) and the transition zone (in yellow) is marked with an arrow. The root cap junction (RCJ) is marked as well. Note that the lateral root cap cell layer covers both the meristem and the transition zone which ends close to the basal border of this root apex section.
Thus, root cells leave the meristem somewhere between 150–200 µm from the RCJ (Fig. 1) in roots of Arabidopsis thaliana ecotype Col-0 seedlings grown in vitro at 23°C, 16 h/8 h light/dark cycle, on agarose for about five days. However, this value is not absolute for all Arabidopsis roots grown under other conditions, as the size of the meristem varies according to factors regulating plant development. During plant growth, the increase in the rate of root growth is accompanied by an enlargement of the apical meristem.24 Also, it is known that apical root cells are monitoring signals arriving from older more proximally located cells.58 Among these signals, sucrose and auxin are the most likely candidates. For instance, addition of 4.5% sucrose to the medium of growing Arabidopsis roots increased the number of dividing cells and enlarged the size of the apical meristem, shifting its proximal limit from about 162 to about 300 µm from the RCJ.30
Similar to sucrose, auxin increased the size of root meristems, while addition of cytokinin resulted in a decrease in the size of apical meristems of Arabidopsis roots.25 In accordance with this effect of cytokinin, genetically engineered tobacco plants with a reduced cytokinin content due to expression of cytokinin oxidase genes from Arabidopsis, showed substantially enlarged root meristems.59,60 A more recent report confirmed that cytokinins control the exit of cells from the root meristem in Arabidopsis.61 Besides these signal-mediated changes in the size of the apical meristem, ectopically expressed mitotic cyclins are known to increase the size of the root meristem48 while dominant-negative forms of Cdc2a kinase lower the population of dividing cells in Arabidopsis root apices.62 Therefore, these core cell cycle molecules appear to act as apparent targets for signal-transduction cascades regulating the cell cycle during growth and development of plant roots. In fact, tight correlations were reported between cell production rates and activities of cyclin-dependent kinases between roots of several Arabidopsis ecotypes.21
In spite of the wide availability and usage if the CYCB1;1 line, it is rather surprising that the basal border of the Arabidopsis root meristem is still an controversial issue in the current literature. Many authors simply ignore the transition (or the DEZ) zone and consider the onset of rapid cell elongation as the basal limit of the root meristem.63–65 On the basis of the CYCB1;1 line, as well as of the careful analysis of the MAP4-GFP line (Le J, unpublished data), the proximal (basal) limit of the meristem is at about 200 µm from the RCJ. The subsequent growth zone, about 320 µm long, corresponds to the transition zone.
In the transition zone, the growth rate is very low and transformation from early post-mitotic to preelongation cells takes many hours.
At about 520 µm from the root cap junction, cell length increases noticeably (Fig. 1). This zone of rapid cell elongation will be discussed later. The cells distal to the zone of fast cell elongation (approximately between 200 to 520 µm from the RCJ) belong to the transition zone.
At 200 µm from the RCJ, cells enter the transition zone with an average cell length of 8 µm. During the subsequent 10 hours, their length increase is negligable reaching only 9 µm at 280 µm from the RCJ, although cell width increases from 14 to 16 µm. This section of the trichoblast cell file is not within the focus plane in the micrograph (Fig. 1). However, in the same cell file, a row of 17 cells covering the distance from 280 to 520 µm from the RCJ (distal to the arrowhead mark) can clearly be discerned: in that row, the elongation from 9 µm to 30 µm takes 8.5 hours. Furthermore, elongation is not homogeneously spread. In the the youngest six cells (three hours of development), no increase in cell length can be measured, while in the oldest six cells the growth in length is much more substantial. It is precisely in the latter part of the transition zone that trichoblast cells develop a central vacuole (upper asterisk in Fig. 3), while this feature is completely absent in the more distal part (younger cells, lower asterisk in Fig. 3) of the transition zone. In Figure 3, the arrowhead marks the end of the transition zone and the arrow the end of the elongation zone. This allows direct comparison with the (Fig. 1).
Figure 3.
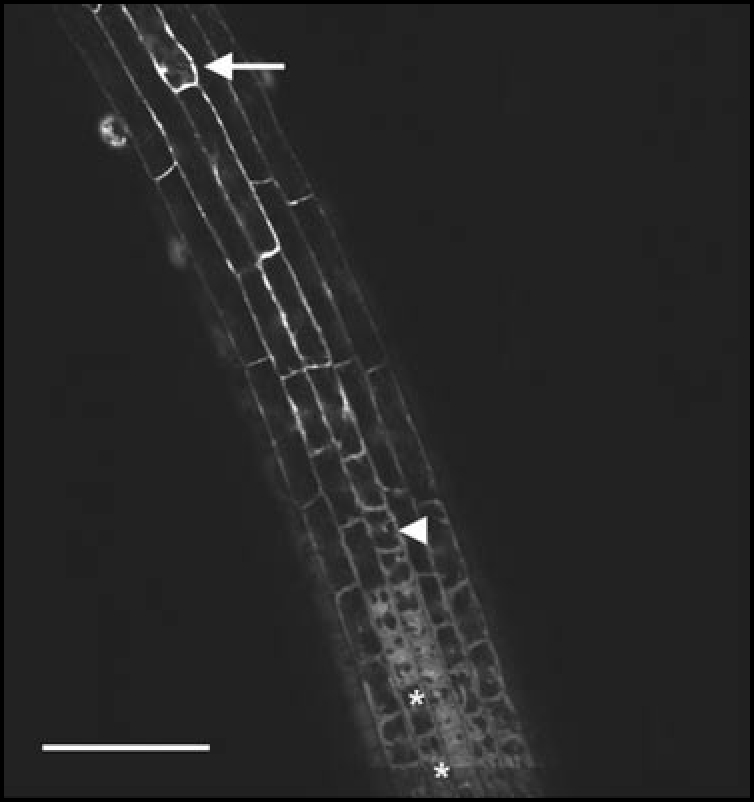
Confocal picture of a fluorescein diacetate (FDA) staining of a five-day-old Arabidopsis root. The vacuoles (black) are detected by staining the cytoplasm with this viability-stain. The lower asterisk marks cells in the distal end of the transition zone, void of vacuoles, the upper asterisk points to a cell with clearly visible but not expanded vacuoles. The arrow marks the youngest trichoblast showing root hair bulging while the arrowhead indicates the onset of the fast elongation zone. Scale bar = 100 µm.
Cells in the proximal part of the transition zone are competent for the onset of fast cell elongation while cells in the distal part are competent to return back to the cell cycle activity. This finding coincides with the expression of cdc2 also in cells of the apical part of the transition zone (see Fig. 2F in ref. 66), suggesting that cells of the distal part of the transition zone maintain competence for cell division. All this renders the transition zone a kind of dynamic reservoir of developmentally plastic cells allowing rapid adjustment of both growth speed as well as direction of root growth according to demands of the actual environmental challenges.
As mentioned earlier, the development of trichoblast cell files mirrors somehow the development occurring in other cell types. Atrichoblasts are longer than trichoblasts (in general about 15%). This feature is seen throughout the transition zone. Vacuolization in this cell-type, as well as in the cortex cells, starts closer to the RCJ than in trichoblast cells, as illustrated in Figure 2. Cortex cells, on the other hand, are good markers for following increases in cell widths that occur in the transition zone. The final width of the cortex cells is only reached at the proximal end of the transition zone at about 520 µm from the RCJ. This is fully in accordance with the situation in maize root apices7 and can serve as another indication of the limit of the transition zone.
Based on kinematographic analysis of the root surface extension, several authors have defined the border between slow cytoplasmic growth and fast cell elongation arbitrarily as the point where the relative elemental growth rate reached 30% of its maximum value.18,35,37,38 Incidentally, this indirect approach fits well with results of our in vivo cytological analysis reported here.
As a matter of fact, the concept of slow cell growth preceding the abrupt acceleration driving the fast cell elongation was confirmed also for Arabidopsis hypocotyl cells.67 Here, cells elongate synchronously during the first 48 hours at a low rate, before the first cells at the base of the hypocotyl start their fast elongation. During the first 48 hours, these slowly elongating cells can be compared with cells passing through the transition zone. Thus, in the root apex transition zone, cell expansion is slow and occurs not only in the longitudinal (axial), but also to a certain extent in the transverse direction. At the basal limit of the transition zone, cells abruptly enter the phase of fast cell elongation and stop their widening. This corresponds exactly to the situation in maize root apices.5 The onset of rapid cell elongation is accompanied by impressive changes in structure and function of vacuoles and cell walls.
Transition-zone cells are flooded with sucrose unloaded from mature protophloem elements and undergo striking cell wall alterations.
In the growing root apex, maturation of the protophloem elements occurs within the transition zone, approximately at 250 µm from the root cap junction,68,69 allowing local unloading of phloem-transported sugars into this growth zone.68,69 As illustrated before, the central vacuole of trichoblasts develops only in the proximal part of the transition zone. This morphological characteristic is corroborated by the finding that the expression of the tonoplast aquaporin γ-TIP starts abruptly at the proximal border of the transition zone.70
Concomitant with these changes in the cytoplasm, the cell wall undergoes specific adaptations. In vivo localization of XET action highlighted its maximal values at the proximal end of the transition zone.71 Xyloglucan endotransglucosylase/hydrolase (XTH) is an enzyme that modifies the cellulose-xyloglucan network, the load-bearing structure in plant cell walls. XTHs can cleave xyloglucans and subsequently rejoin the newly formed ends to available xyloglucan chains or oligosaccharides.72 This activity creates the opportunity for a turgor-pressure driven increase of the distance between two adjacent cellulose microfibrils, leading to growth. Cells within the transition zone are characterized by a progressive increase of the XET activity in their cell walls.71 This XET activity reaches its maximum values at the end of cell growth in three dimensions, at the basal limit of the transition zone, and heralds the onset of strictly polarized rapid cell elongation (no further cell expansion in width).71 In fact, the XET activity can be taken as a physiological marker of the proximal part of the transition zone in root apices of Arabidopsis. McCartney et al.21 reported the occurrence of a cell wall pectic (1 → 4)-β-D-galactan in the transition zone of Arabidopsis roots. This points again to fundamental changes in cell wall properties occurring at the border between the transition zone and the zone of fast cell elongation. In order to loosen cell walls effectively, the cellulose and xyloglucan network is also affected by expansins which are expressed and localized to cells of the transition zone too.73,74 The idea of structural changes occuring in the cell wall at this ‘no return’ developmental point is further stressed by the detection of an increase in cellulose, xyloglucan and methylesterified pectins at the onset of fast cell elongation using FT-IR (De Cnodder T et al., unpublished results).
In a recent study, we have shown that cortical microtubules in the epidermis of Arabidopsis roots have a strictly transverse orientation at the basal limit of the transition zone.45 It was reported that the cessation of radial expansion in postmitotic root cells of Arabidopsis was closely associated with the strict alignment of cortical microtubules into transverse arrays.75 When cortical microtubules do not accomplish this redistribution, postmitotic cells fail to restrict their radial expansion and increase in width even throughout the elongation region as was described in bot1 and fra2 mutants of Arabidopsis.75,76 Similar links between cortical microtubules and cell polarity were indicated by several other mutants of Arabidopsis.77–79 However, other mutants showed a reduced cell polarity, with thicker and shorter root cells, although their cortical microtubules were arranged in highly ordered transverse arrays. In this regard, we can mention CORE mutants,80 the kor mutant75 and rsw4/rsw7 mutants.81 Evidently, besides ordered cortical microtubules, also the correct in muro localization of wall metabolic events is critical. In this respect, the cell wall has a pivotal role.75,76,83–86
At the plasma membrane-cell wall interface,87 COBRA could potentially be involved in establishing polarity of cell growth of postmitotic root cells.88 COBRA is a member of glycosylphosphatidylinositol (GPI) anchored proteins, which are anchored to the outer plasma membrane leaflet and with putative interactions with cell wall molecules. COBRA mRNA levels were shown to be dramatically upregulated in cells located around 200 µm (see Fig. 4D and E in ref. 88) from the RCJ interpreted by the authors as rapidly elongating cells. Both, from distances from the RCJ as well as from the shapes of these cells (see Fig. 4D and E in ref. 88) it is obvious that COBRA mRNAs and proteins are located abundantly within the transition zone. Root cells of the cobra mutant have a reduced content of cellulose and fail the polarization of cell expansion as they expand too much in width and do not achieve proper elongation.88 Nevertheless, mutant root cells reach normal volumes. This phenotype suggests that COBRA could be important for the acquisition and maintenance of the nongrowing status of cross-walls which is the most critical event in the polarization of postmitotic root cells during their switch into the rapid cell elongation.42
Figure 4.
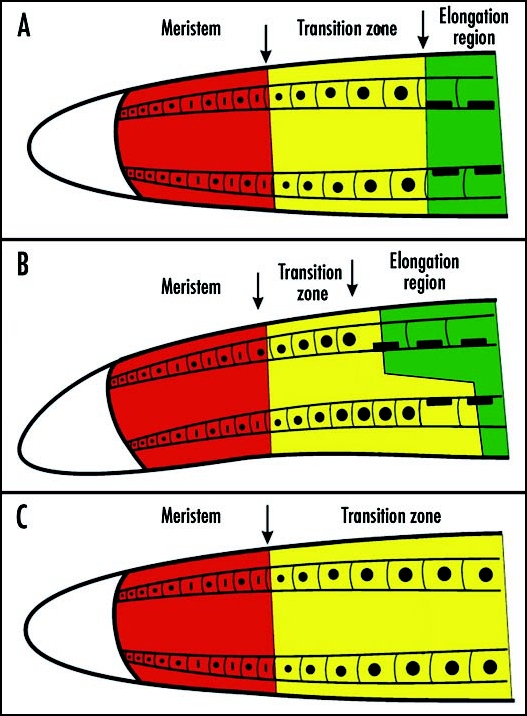
Schematic depiction of growth zones in control root apex (A), gravistimulated root apex (B) and root apex exposed to the F-actin depolymerizing agent latrunculin B (C) for several hours. The transition zone responds differentially in the gravistimulated root apex when it gets shorter in the upper part and longer at the lower part of the root apex. In F-actin devoid root apex, cells at the basal border fail to enter the zone of fast cell elongation but new cells are supplied from the apical meristem. Roots are growing very slowly and the transition zone expands basally. For more details see references 11 and 95.
In the zone of fast cell elongation, cell length increases by 300% in less than three hours.
In the Arabidopsis root, fast cell elongation starts when cells leave the transition zone and slows down when trichoblasts initiate outgrowths of root hairs.45 In the example shown in Figure 1, cells increase their length from about 35 µm (arrowhead) to 135 µm (arrow) in two hours. There is some variability in the elongation rate, but the whole process is always terminated in less than three hours as can be seen in other cell files (Fig. 1). Along the root axis, the end of the fast elongation is situated at about 900 µm from the RCJ. In normal growing conditions, additional increase in cell length occurs up to about 1500 µm from the RCJ. Further on, the relative elemental growth rate of the root is not statistically different from zero.45
Fast cell elongation is based on, and accomplished by, processes clearly different from the slow cell growth in the transition zone. In situ analysis of cell wall composition by FT-IR spectroscopy reveals a unique character for this part of the root (De Cnodder T et al., unpublished results). The β-glucosyl Yariv reagent was found to inhibit specifically the fast cell elongation.89 It affects fucosylated arabinogalactan-proteins which are required for full cell elongation in root apices as deduced from the mur1 phenotype of Arabidopsis.90 In the epidermis wall, the cellulose fibrils are oriented parallel and strictly transverse to the root axis.91 The cortical microtubules mirror this organisation and are aligned strictly parallel and transverse to the root axis.45 The rate of cell elongation is inversely related to the endogenous ethylene concentration.43 Under saturating conditions, the fast cell elongation is completely abolished, as is the residual elongation in the growth-ceasing zone, and cells never elongate beyond the length reached at the basal limit of the transition zone. For trichoblasts this is 35 µm, a length also reported for dwarfed phenotypes like the ctr1-1 mutant that expresses a constitutive ethylene response. On the contrary, it was found that cells have the potential to elongate beyond their normal length as trichoblasts elongated up to 200 µm in the absence of ethylene, a cell size also found in ein2-1, an ethylene insensitive mutant.43 Fast elongation is also very sensitive to other hormone signals. In the root apices of the stunted plant 1 mutant of Arabidopsis, rapidly elongating root cells were affected while slowly expanding more apical cells were unaffected.25,56 In this particular mutant, cell elongation defects are mediated via cytokinin-based signaling as cytokinin-treated wild type seedlings truly phenocopied this mutant.25 Moreover, auxin is also known to preferentially affect the fast cell elongation.8,44,92
The basal limit of the fast cell elongation zone is marked by the disturbance of the transverse arrays of the cortical microtubules.45 This feature is evident when the fast elongation is blocked by a high dose of ethylene (for maize roots see ref. 92). However, the microtubule reorientation is not causally linked to the observed stop in cell elongation. Primary are events in the cell wall, including a rise in apoplastic pH, callose deposition and cross-linking events steered by reactive oxygen species.93 The transit from pure cell elongation to differentiation is highlighted by the expression of a fucose-containing epitope in the cell wall of epidermal cells.90 Furthermore, epidermal cells become symplastically isolated when leaving the fast elongation zone.94 Another marker for the end of rapid elongation is the accumulation of myosin VIII along the cross walls of the epidermal cells exactly where in the trichoblasts root hairs start to develop rapidly (Verbelen et al., unpublished results).
Transition and Fast Elongation Zones Differ in Hormonal and Signaling Status
Intriguingly, cells in the transition zone continue their growth also under osmotic stress, while the growth in the fast elongation region is irreversibly stopped.2,96–98 This reaction is dependent on ABA accumulation and lowering of ethylene production.97,98 Several other hormones differentially affect the two zones. As mentioned before, the endogenous cytokinin level specifically affects cells in the fast elongation zone,25,56 as is the case with ethylene.43 Exogenous auxin inhibits cell growth in the zone of fast elongation, but it can stimulate cell growth within the transition zone upon gravistimulation.2,5,8 Furthermore, the transition zone is the unique site for perception and response to a range of (external) factors. Local application of extracellular calcium inhibited cell growth specifically within the transition zone but exerted only weak responses in the zone of fast elongation.8 Moreover, calcium waves spread through the transition zone of Arabidopsis root apices (Fig. 8 in ref. 99 and Fig. 3D in ref. 100). Differential passage of cells through opposite sides of the transition zone95 allows growing root apices to initiate their curvature culminating in root tropisms in response to gradients of external factors as diverse as gravity, temperature, moisture, salinity, oxygen availability, electric fields, and heavy metals.2,5,39,100 Transition zone cells are also sensitive towards mechanical stimuli6 and to aluminum toxicity,101–103 which is mediated by glutamate receptors.104 Aluminum was shown to inhibit the basipetal auxin transport in the distal part of the transition zone103 and to induce, like the auxin transport inhibitor NPA, cell divisions within the transition zone.105 That aluminum targets specifically cells of the transition zone was also reported in the recent study which showed that aluminum is not only internalized into these cells but it also inhibits endocytosis and vesicle recycling, as well as NO production.106
This unique signalling profile and sensory status is an additional and important characteristic of cells building up the transition zone. This was already shown in maize root apices where cells of the transition zone proved to have unique cytological and metabolical properties allowing them to sense diverse environmental factors and endogenous cues.2,7
The Transition Zone is Specialized for Transcellular Auxin Transport
Since 1993, it has been clear that the transition zone is very special from the standpoint of auxin-mediated cell growth control.8 The authors made the very peculiar observation: exogenous auxin inhibits root growth but induces a burst of cell growth in the cells of the transition zone at the top of the gravistimulated roots, resulting in rapid gravibending of these roots.8 Adaptation of roots to high auxin was well documented in older literature,107–109 but Ishikawa and Evans8 discovered that the transition zone plays a key role in this respect. Such recovery of root growth in a high auxin environment is associated with a complete reconstruction of microtubules.44 Recently, a new technique was introduced which allows in vivo monitoring of auxin influx into maize root apices, revealing that external auxin is preferentially taken up in the transition zone.110 Subsequent application of this technique to roots of Arabidopsis revealed that the distal portion of the transition zone, which has been defined here on the basis of cytological characteristics (150–350 µm), corresponds precisely with the peak of auxin influx.111–113
This observation is in agreement with the situation in maize root apex where the distal portion of the transition zone also shows the highest uptake of external auxin.110 Recent advancements in the understanding of the polar auxin transport in Arabidopsis allowed to indentify unique loops of auxin streams, driven by at least five PINs including PIN1, PIN2, PIN3, PIN4 and PIN7, specifically for the root apex.113,114 Moreover, four of five PINs expressed in root apices (PIN1, PIN2, PIN4, PIN7) localize polarly at the cell peripheries, which are rich in actin,41 preferentially in cells of the transition zone.114–117
Shoot apex lacks such complex auxin flow and uses just PIN1 for the polar auxin transport (reviewed in ref. 118).
Root apex streams are driven by cells of the stele transporting auxin towards the root apex (PIN1, PIN4), and by cells of the lateral root cap and epidermis supporting the basipetal transport stream (PIN2) which then joins the apical one again (PIN4, PIN7) at the basal limit of the transition zone.114 The latter authors interpreted the root apex zone where the basipetal stream loops back into the acropepal stream, as the elongation zone (see the Fig. 5C in ref. 114). However, actual viewing of Figures 2A–H (see especially the Fig. 2A in ref. 114) makes it quite evident that they, are not aware of the precise root apex zonation. This urges for caution in many situations where authors made claims about root apex zones in Arabidopsis.
Conclusions
In root apices of Arabidopsis thaliana, four distinct and successive zones can be clearly discerned: the meristematic zone, the transition zone, the zone of fast cell elongation and the growth terminating zone. Each of these zones is characterized by a specific set of cellular activities as well as by specific responses towards plant hormones and signals. Between the apical meristematic zone and the region of fast cell elongation, a relatively large zone of developmentally plastic cells is located within the transition zone. While the apical (distal) part119 of this region contains cells that optionally can reenter the cell cycle, cells of the basal (proximal) part of this zone are optimized for a sudden signal-mediated entry into the fast cell elongation region. As this developmental passage of cells can be differentially regulated at the opposite root flanks, this unique zone provides the root apices with an effective mechanism to reorientate growth in response to environmental stimuli (Fig. 4). Importantly, the transition zone is easily recognized by simple features such as positions of nuclei, cell shapes, and and organization of vaculoes (Table 1). The definition of these four growth zones in vivo challenges the widespread classic view3,4 of meristematic, elongation, and differentiation zone that is mainly based on the traditional post mortem morphological observations of root apices.
Acknowledgements
Authors acknowledge the financial support of the Research Foundation-Flanders (FWO), grants G0345.02, G0034.97 and G0281.98. K.Vissenberg is a post-doctoral fellow of the Research Foundation-Flanders (FWO). Financial support by grants from the Bundesministerium für Wirtschaft und Technologie (BMWi) via Deutsches Zentrum für Luft und Raumfahrt (DLR, Cologne, Germany; project 50WB 0434), from the European Space Agency (ESA-ESTEC Noordwijk, The Netherlands; MAP project AO-99-098), and from the Ente Cassa di Risparmio di Firenze (Italy) is gratefully acknowledged. F.B. receives partial support from the Slovak Academy of Sciences (Grant Ageny VEGA, Bratislava, Slovakia; project 2/5085/25).
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/abstract.php?id=3511
References
- 1.Weigel D, Jürgens G. Stem cells that make stems. Nature. 2002;415:751–754. doi: 10.1038/415751a. [DOI] [PubMed] [Google Scholar]
- 2.Baluška F, Volkmann D, Barlow PW. A polarity crossroad in the transition growth zone of maize root apices: Cytoskeletal and developmental implications. J Plant Growth Regul. 2001;20:170–181. [Google Scholar]
- 3.Strasburger E, Noll F, Schenck H, Schimper AFW. Lehrbuch der Botanik. 1st ed. Gustav Fischer Verlag; 1894. [Google Scholar]
- 4.Sitte P, Ziegler H, Ehredorfer F, Bresinsky A. Strasburger Lehrbuch der Botanik. 35th ed. Gustav Fischer Verlag; 2002. [Google Scholar]
- 5.Baluška F, Hauskrecht M, Kubica Š. Postmitotic ‘isodiametric’ cell growth in the maize root apex. Planta. 1990;181:269–274. doi: 10.1007/BF00195876. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa H, Evans ML. Induction of curvature in maize roots by calcium or by thigmostimulation. Role of the postmitotic isodiametric growth zone. Plant Physiol. 1992;100:762–768. doi: 10.1104/pp.100.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baluška F, Barlow PW, Kubica Š. Importance of the post-mitotic growth (PIG) region for growth and development of roots. Plant and Soil. 1994;167:31–42. [Google Scholar]
- 8.Ishikawa H, Evans ML. The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol. 1993;102:1203–1210. doi: 10.1104/pp.102.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baluška F, Volkmann D, Hauskrecht M, Barlow PW. Root cap mucilage and extracellular calcium as modulators of cellular growth in post-mitotic growth zones of the maize root apex. Bot Acta. 1996;109:25–34. [Google Scholar]
- 10.Baluška F, Vitha S, Barlow PW, Volkmann D. Rearrangements of F-actin arrays in growing cells of intact maize root apex tissues: A major developmental switch occurs in the postmitotic transition region. Eur J Cell Biol. 1997;72:113–121. [PubMed] [Google Scholar]
- 11.Baluška F, Jásik J, Edelmann HG, Salajová T, Volkmann D. Latrunculin B induced plant dwarfism: Plant cell elongation is F-actin dependent. Dev Biol. 2001;231:113–124. doi: 10.1006/dbio.2000.0115. [DOI] [PubMed] [Google Scholar]
- 12.Baluška F, Volkmann D, Barlow PW. Specialized zones of development in roots: View from the cellular level. Plant Physiol. 1996;112:3–4. doi: 10.1104/pp.112.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan L, Davies J. Cell expansion in roots. Curr Opin Plant Biol. 2003;7:1–7. doi: 10.1016/j.pbi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Scheres B, Wolkenfeldt H, Willemsen V, Terlouw M, Lawson E, Dean C, Weisbeek P. Embryonic origin of Arabidopsis primary root and root meristem initials. Development. 1994;120:2475–2487. [Google Scholar]
- 15.Kidner C, Sundaresan V, Roberts K, Dolan L. Clonal analysis of the Arabidopsis root confirms that position, not lineage, determines cell fate. Planta. 2000;211:191–199. doi: 10.1007/s004250000284. [DOI] [PubMed] [Google Scholar]
- 16.Maloof JN. Plant development: Slowing root growth naturally. Curr Biol. 2004;14:R395–R396. doi: 10.1016/j.cub.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs T. Why do plant cells divide? Plant Cell. 1997;9:1021–1029. doi: 10.1105/tpc.9.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullen JL, Ishikawa H, Evans ML. Analysis of changes in relative elemental growth rate patterns in the elongation zone of Arabidopsis roots upon gravistimulation. Planta. 1998;206:598–603. doi: 10.1007/s004250050437. [DOI] [PubMed] [Google Scholar]
- 19.Truernit E, Siemering KR, Hodge S, Grbic V, Haseloff J. A map of KNAT gene expression in the Arabidopsis root. Plant Mol Biol. 2006;60:1–20. doi: 10.1007/s11103-005-1673-9. [DOI] [PubMed] [Google Scholar]
- 20.Blancaflor EB, Wang YS, Motes CM. Organization and function of the actin cytoskeleton in developing root cells. Int Rev Cytol. 2006;252:219–264. doi: 10.1016/S0074-7696(06)52004-2. [DOI] [PubMed] [Google Scholar]
- 21.McCartney L, Steele-King CG, Jordan E, Knox JP. Cell wall pectic (1 →4)-β-d-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 2003;33:447–454. doi: 10.1046/j.1365-313x.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 22.Beemster GTS, De Vusser K, De Tavernier E, De Bock K, Inzé D. Variation in growth rate between Arabidopsis ecotypes is correlated with cell division and A-type cyclin-dependent kinase activity. Plant Physiol. 2002;129:854–864. doi: 10.1104/pp.002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cramer GR, Jones RL. Osmotic stress and abscisic acid reduce cytosolic calcium activities in roots of Arabidopsis thaliana. Plant Cell Environm. 1996;19:1291–1298. [Google Scholar]
- 24.Beemster GTS, Baskin TI. Analysis of cell division and elongation underlying the developmetal acceleration of root growth in Arabidopsis thaliana. Plant Physiol. 1998;116:1515–1526. doi: 10.1104/pp.116.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beemster GTS, Baskin TI. Stunted plant 1 mediates effects of cytokinin, but not of auxin, on cell division and expansion in the root of Arabidopsis. Plant Physiol. 2000;124:1718–1727. doi: 10.1104/pp.124.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiegle E, Gilliham M, Haseloff J, Tester MA. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 2000;21:225–229. doi: 10.1046/j.1365-313x.2000.00659.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR. Cell-type-specific calcium responses to drought, salt, and cold in the Arabidopsis root. Plant J. 2000;23:267–278. doi: 10.1046/j.1365-313x.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Guan C, Boonsirichai K, Masson PH. Complex physiological and molecular processes underlying root gravitropism. Plant Mol Biol. 2002;49:305–317. [PubMed] [Google Scholar]
- 29.West G, Inzé D, Beemster GTS. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol. 2004;135:1050–1058. doi: 10.1104/pp.104.040022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauser MTH, Bauer E. Histochemical analysis of root meristem activity in Arabidopsis thaliana using a cyclin: GUS (β-glucuronidase) marker line. Plant And Soil. 2000;226:1–10. [Google Scholar]
- 31.Larkin JC, Brown ML, Schiefelbein J. How cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annu Rev Plant Biol. 2003;54:403–430. doi: 10.1146/annurev.arplant.54.031902.134823. [DOI] [PubMed] [Google Scholar]
- 32.Serna L. Epidermal cell patterning and differentiation throughout the apical-basal axis of the seedling. J Exp Bot. 2005;56:1983–1989. doi: 10.1093/jxb/eri213. [DOI] [PubMed] [Google Scholar]
- 33.Evans ML, Ishikawa H. Cellular specificity of the gravitropic motor response in roots. Planta. 1997;203:S115–S122. doi: 10.1007/pl00008099. [DOI] [PubMed] [Google Scholar]
- 34.Ishikawa H, Evans ML. Specialized zones of development in roots. Plant Physiol. 1995;109:725–727. doi: 10.1104/pp.109.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa H, Evans ML. Novel software for analysis of root gravitropism: Comparative response patterns of Arabidopsis wild-type and axr1 seedlings. Plant Cell Environm. 1997;20:919–928. doi: 10.1046/j.1365-3040.1997.d01-129.x. [DOI] [PubMed] [Google Scholar]
- 36.Ishikawa H, Hasenstein KH, Evans ML. Computer based video-digitizer analysis of surface extension in maize roots: Kinetics of growth rate changes during gravitropism. Planta. 1991;183:381–390. doi: 10.1007/BF00197737. [DOI] [PubMed] [Google Scholar]
- 37.Mullen JL, Wolverton C, Ishikawa H, Hangarter RP, Evans ML. Spatial separation of light perception and growth response in maize root phototropism. Plant Cell Environm. 2002;25:1191–1196. doi: 10.1046/j.1365-3040.2002.00899.x. [DOI] [PubMed] [Google Scholar]
- 38.Wolverton C, Mullen JL, Ishikawa H, Evans ML. Two distinct regions of response drive differential growth in Vigna root electrotropism. Plant Cell Environm. 2000;23:1275–1280. doi: 10.1046/j.1365-3040.2000.00629.x. [DOI] [PubMed] [Google Scholar]
- 39.Wolverton C, Ishikawa H, Evans ML. The kinetics of root gravitropism: Dual motors and sensors. J Plant Growth Regul. 2002;21:102–112. doi: 10.1007/s003440010053. [DOI] [PubMed] [Google Scholar]
- 40.Baluška F, Parker JS, Barlow PW. Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.) J Cell Sci. 1992;103:191–200. [Google Scholar]
- 41.Baluška F, Hlavacka A. Plant formins come to age: Something special about cross-walls. New Phytol. 168:499–503. doi: 10.1111/j.1469-8137.2005.01595.x. [DOI] [PubMed] [Google Scholar]
- 42.Baluška F, Wojtaszek P, Volkmann D, Barlow PW. The architecture of polarized cell growth: The unique status of elongating plant cells. BioEssays. 2003;25:569–576. doi: 10.1002/bies.10282. [DOI] [PubMed] [Google Scholar]
- 43.Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. In the early response of Arabidopsis roots to ethylene, cell elongation is up-and down-regulated and uncoupled from differentiation. Plant Physiol. 2001;125:519–522. doi: 10.1104/pp.125.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baluška F, Barlow PW, Volkmann D. Complete disintegration of the microtubular cytoskeleton precedes its auxin-mediated reconstruction in postmitotic maize root cells. Plant Cell Physiol. 1996;37:1013–1021. doi: 10.1093/oxfordjournals.pcp.a029032. [DOI] [PubMed] [Google Scholar]
- 45.Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. Position and cell type-dependent microtubule reorientation characterizes the early response of the Arabidopsis root epidermis to ethylene. Physiol Plant. 2004;121:513–519. [Google Scholar]
- 46.Clowes FAL. Apical meristems of roots. Biol Rev Cambr Phil Soc. 1959;34:501–529. [Google Scholar]
- 47.Somerville C, Koorneef M. A fortunate choice: The history of Arabidopsis as a model plant. Nat Rev Genet. 2002;3:883–889. doi: 10.1038/nrg927. [DOI] [PubMed] [Google Scholar]
- 48.Doerner P, Jørgensen JE, You R, Steppuhn J, Lamb CJ. Control of root growth and development by cyclin expression. Nature. 1996;380:520–523. doi: 10.1038/380520a0. [DOI] [PubMed] [Google Scholar]
- 49.Colon-Carmona A, You R, Haimovitch-Gal T, Doerner P. Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 1999;20:503–508. doi: 10.1046/j.1365-313x.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Potuschak T, Colon-Carmona A, Gutierrez RA, Doerner P. Arabidopsis TCP20 links regulation of growth and cell division control pathways. Proc Natl Acad Sci USA. 2005;102:12978–12983. doi: 10.1073/pnas.0504039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ferreira PCG, Hemerly AS, Engler JDA, Van Montagu M, Engler G, Inzé D. Developmental expression of the Arabidopsis cyclin gene cyc1At. Plant Cell. 1994;6:1763–1774. doi: 10.1105/tpc.6.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the Solitary-Root/IAA14 gene of Arabidopsis. Plant J. 2002;29:153–168. doi: 10.1046/j.0960-7412.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 53.Umeda M, Umeda-Hara C, Uchimiya H. A cyclin-dependent kinase-activating kinase regulates differentiation of root initial cells in Arabidopsis. Proc Natl Acad Sci USA. 2000;97:13396–13400. doi: 10.1073/pnas.240458997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Souter M, Topping J, Pullen M, Friml J, Palme K, Hackett R, Grierson D, Lindsey K. Hydra mutants of Arabidopsis are defective in sterol profiles and auxin and ethylene signaling. Plant Cell. 2002;14:1017–1031. doi: 10.1105/tpc.001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baskin TI, Cork A, Williamson RE, Gorst JR. Stunted plant 1, a gene required for expansion in rapidly elongating but not dividing cells and mediating root growth responses to applied cytokinin. Plant Physiol. 1995;107:233–243. doi: 10.1104/pp.107.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Veylder L, Beemster GTS, Beeckman T, Inzé D. CKS1At overexpression in Arabidopsis thaliana inhibits growth by reducing meristem size and inhibiting cell-cycle progression. Plant J. 2001;25:617–626. doi: 10.1046/j.1365-313x.2001.00996.x. [DOI] [PubMed] [Google Scholar]
- 58.Van den Berg C, Willemsen V, Hage W, Weisbeek P, Scheres B. Determination of cell fate in the Arabidopsis meristem by directional signalling. Nature. 1995;378:62–65. doi: 10.1038/378062a0. [DOI] [PubMed] [Google Scholar]
- 59.Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proc Natl Acad Sci USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmülling T. New insights into the functions of cytokinins in plant development. J Plant Growth Regul. 2002;21:40–49. doi: 10.1007/s003440010046. [DOI] [PubMed] [Google Scholar]
- 61.Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hemerly A, de Almeida Engler J, Bergounioux C, Van Montagu M, Engler G, Inzé D, Ferreira P. Dominant negative mutants of Cdc2 kinase uncouple cell division from iterative plant development. EMBO J. 1995;14:3925–3936. doi: 10.1002/j.1460-2075.1995.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Birnbaum K, Shasha DE, Wang JY, Jung JW, Lambert GM, Galbraith DW, Benfey PN. A gene expression map of the Arabidopsis root. Science. 2003;302:1956–1960. doi: 10.1126/science.1090022. [DOI] [PubMed] [Google Scholar]
- 64.Levesque MP, Vernoux T, Busch W, Cui H, Wang JY, Blilou I, Hassan H, Nakajima N, Matsumoto N, Lohmann JU, Scheres B, Benfey PN. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis. PLOS Biol. 2006;4:e143. doi: 10.1371/journal.pbio.0040143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serralbo O, Perez-Perez JM, Heidstra R, Scheres B. Noncell-autonomous rescue of anaphase-promoting complex function revealed by mosaic analysis of Hobbit, an Arabidopsis CDC27 homolog. Proc Natl Acad Sci USA. 2006;103:13250–13255. doi: 10.1073/pnas.0602410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL, Jr, Inzé D, Van Montagu M, Engler G, Gheysen G. Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell. 1999;11:793–808. doi: 10.1105/tpc.11.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Refrégier G, Pelletier S, Jaillard D, Höfte H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiol. 2004;135:959–968. doi: 10.1104/pp.104.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu T, Lucas WJ, Rost TL. Directional cell-to-cell communication in the Arabidopsis root apical meristem. Ultrastructural and functional analysis. Protoplasma. 1998;203:35–47. [Google Scholar]
- 69.Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, Oparka KJ, Sauer N. Expression of GFP-fusions in Arabidopsis companion cells reveals nonspecific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 2005;41:319–331. doi: 10.1111/j.1365-313X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- 70.Ludevid D, Höfte H, Himelblau E, Chrispeels MJ. The expression pattern of the tonoplast intrinsic protein γ-TIP of Arabidopsis thaliana is correlated with cell enlargement. Plant Physiol. 1992;100:1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vissenberg K, Martinez-Vilchez M, Verbelen JP, Miller JG, Fry SC. In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell. 2000;12:1229–1237. doi: 10.1105/tpc.12.7.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee DK, Ahn JH, Song SK, Choi YD, Lee JS. Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol. 2003;131:985–997. doi: 10.1104/pp.009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang N, Hasenstein KH. Distribution of expansins in graviresponding maize roots. Plant Cell Physiol. 2000;41:1305–1312. doi: 10.1093/pcp/pcd064. [DOI] [PubMed] [Google Scholar]
- 74.Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochem J. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bichet A, Desnos T, Turner S, Grandjean O, Höfte H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 2001;25:137–148. doi: 10.1046/j.1365-313x.2001.00946.x. [DOI] [PubMed] [Google Scholar]
- 76.Burk DH, Liu B, Zhong R, Morrison WH, Ye ZH. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell. 2001;13:807–827. [PMC free article] [PubMed] [Google Scholar]
- 77.Ademe-Onzighi C, Sivaguru M, Judy-March J, Baskin TI, Driouich A. The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan-proteins and the organization of cortical microtubules. Planta. 2002;215:949–958. doi: 10.1007/s00425-002-0836-z. [DOI] [PubMed] [Google Scholar]
- 78.Furutani I, Watanabe Y, Prieto R, Masukawa M, Suzuki K, Naoi K, Thitamadee S, Shikanai T, Hashimoto T. The Spiral genes are required for directional control of cell elongation in Arabidopsis thaliana. Development. 2000;127:4443–4453. doi: 10.1242/dev.127.20.4443. [DOI] [PubMed] [Google Scholar]
- 79.Traas J, Bellini C, Nacry P, Kronenberger J, Bouchez D, Caboche M. Normal differentiation patterns in plants lacking microtubular preprophase bands. Nature. 1995;375:676–677. [Google Scholar]
- 80.Hauser MTH, Morikami A, Benfey PN. Conditional root expansion mutants of Arabidopsis. Development. 1995;121:1237–1252. doi: 10.1242/dev.121.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wiedemeier AMD, Judy-March JE, Hocart CH, Wasteneys GO, Williamson RE, Baskin TI. Mutant alleles of Arabidopsis RADIALLY SWOLLEN 4 and 7 reduce growth anisotropy without altering the transverse orientation of cortical microtubules or cellulose microfibrils. Development. 2002;129:4821–4830. doi: 10.1242/dev.129.20.4821. [DOI] [PubMed] [Google Scholar]
- 82.Fagard M, Desnos T, Desprez T, Goubet F, Refreiger G, Mouille G, McCann M, Rayon C, Vernhettes S, Höfte H. PROCUSTE1 encodes a cellulose synthase required for normal cell elongation specifically in roots and dark-grown hypocotyls of Arabidopsis. Plant Cell. 2000;12:2409–2423. doi: 10.1105/tpc.12.12.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cano-Delgado AI, Metzlaff K, Bevan MW. The eli mutation reveals a link between cell expansion and secondary cell wall formation in Arabidopsis thaliana. Development. 2000;127:3395–3405. doi: 10.1242/dev.127.15.3395. [DOI] [PubMed] [Google Scholar]
- 84.Shevell DE, Kunkel T, Chua NH. Cell wall alterations in the Arabidopsis emb30 mutant. Plant Cell. 2000;12:2047–2059. doi: 10.1105/tpc.12.11.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall Q, Cannon MC. The cell wall hydrocyproline-rich glycoprotein RSH is essential for normal embryo development in Arabidopsis. Plant Cell. 2002;14:1161–1172. doi: 10.1105/tpc.010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pagant S, Bichet A, Sugimoto K, Lerouxel O, Desprez T, McCann M, Lerouge P, Vernhettes S, Höfte H. KOBITO1 encodes a novel plasma membrane protein necessary for normal synthesis of cellulose during cell expansion in Arabidopsis. Plant Cell. 2002;14:2001–2013. doi: 10.1105/tpc.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baluška F, Šamaj J, Wojtaszek P, Volkmann D, Menzel D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol. 2003;133:482–491. doi: 10.1104/pp.103.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001;15:1115–1127. doi: 10.1101/gad.879101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willats WGT, Knox JP. A role for arabinogalactan-proteins in plant cell expansion: Evidence from studies on the interaction of β-glucosyl Yariv reagent with seedlings of Arabidopsis thaliana. Plant J. 1996;9:919–925. doi: 10.1046/j.1365-313x.1996.9060919.x. [DOI] [PubMed] [Google Scholar]
- 90.Van Hengel AJ, Roberts K. Fucosylated arabinogalactan-proteins are required for full root cell elongation in Arabidopsis. Plant J. 2002;32:105–113. doi: 10.1046/j.1365-313x.2002.01406.x. [DOI] [PubMed] [Google Scholar]
- 91.Kerstens S, Verbelen JP. Cellulose orientation at the surface of the Arabidopsis seedling: Implications for the biomechanics in plant development. J Struct Biol. 2003;144:262–270. doi: 10.1016/j.jsb.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 92.Baluška F, Brailsford RW, Hauskrecht M, Jackson MB, Barlow PW. Cellular dimorphism in the maize root cortex: Involvement of microtubules, ethylene and gibberellin in the differentiation of cellular behaviour in post-mitotic growth zones. Bot Acta. 1993;106:394–403. [Google Scholar]
- 93.De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP. Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: A matter of apoplastic reactions. New Phytol. 2005;168:541–550. doi: 10.1111/j.1469-8137.2005.01540.x. [DOI] [PubMed] [Google Scholar]
- 94.Duckett C, Dolan L, Prior DAM, Oparka KJ, Roberts K. Dye coupling in the root epidermis of Arabidopsis is progressively reduced during development. Development. 1994;120:3247–3255. [Google Scholar]
- 95.Baluška F, Hauskrecht M, Barlow PW, Sievers A. Gravitropism of the primary root of maize: A complex pattern of differential cellular growth in the cortex independent of the microtubular cytoskeleton. Planta. 1996;197:310–318. doi: 10.1007/BF00206258. [DOI] [PubMed] [Google Scholar]
- 96.Wu Y, Spollen WG, Sharp RE, Hetherington PR, Fry SC. Root growth maintenance at low water potentials. Increased activity of xyloglucan endotransglycosylase and its possible regulation by abscisic acid. Plant Physiol. 1994;106:607–615. doi: 10.1104/pp.106.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 2000;122:967–976. doi: 10.1104/pp.122.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT. Root growth maintenance during water deficits: Physiology to functional genomics. J Exp Bot. 2004;54:813–824. doi: 10.1093/jxb/erh276. [DOI] [PubMed] [Google Scholar]
- 99.Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 101.Sivaguru M, Horst WJ. The distal part of the transition zone is the most aluminium-sensitive apical root zone of maize. Plant Physiol. 1998;116:155–163. [Google Scholar]
- 102.Sivaguru M, Baluška F, Volkmann D, Felle HH, Horst WJ. Impacts of aluminum on the cytoskeleton of the maize root apex short-term effects on the distal part of the transition zone. Plant Physiol. 1999;119:1073–1082. doi: 10.1104/pp.119.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kollmeier M, Felle HH, Horst WJ. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum. Plant Physiol. 2000;122:945–956. doi: 10.1104/pp.122.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sivaguru M, Pike S, Grassmann W, Baskin TI. Aluminum rapidly depolymerizes cortical micro-tubules and depolarizes the plasma membrane: Evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol. 2003;44:667–675. doi: 10.1093/pcp/pcg094. [DOI] [PubMed] [Google Scholar]
- 105.Doncheva S, Amenós M, Poschenrieder C, Barceló J. Root cell patterning: A primary target for aluminium toxicity in maize. J Exp Bot. 2005;56:1213–1220. doi: 10.1093/jxb/eri115. [DOI] [PubMed] [Google Scholar]
- 106.Illéš P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluška F, Ovecka M. Aluminium toxicity in plants: Internalization of aluminum into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot. 2006 doi: 10.1093/jxb/erl197. In press. [DOI] [PubMed] [Google Scholar]
- 107.Burström H. On the adaptation of roots to β-indolylacetic acid. Physiol Plant. 1957;10:187–197. [Google Scholar]
- 108.Hejnowicz Z. The response of different parts of the cell elongation zone in root to external β-indolylacetic acid. Acta Soc Bot Pol. 1961;30:25–42. [Google Scholar]
- 109.Gougler JA, Evans ML. Adaptation of corn roots to exogenously applied auxin. Physiol Plant. 1981;51:394–398. [Google Scholar]
- 110.Mancuso S, Marras AM, Volker M, Baluška F. Noninvasive and continuous recordings of auxin fluxes in intact root apex with a carbon-nanotube-modified and self-referencing microelectrode. Anal Biochem. 2005;341:344–351. doi: 10.1016/j.ab.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 111.Schlicht M, Strnad M, Scanlon MJ, Mancuso S, Hochholdinger F, Palme K, Volkmann D, Menzel D, Baluška F. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal Behav. 2006;1:122–133. doi: 10.4161/psb.1.3.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Düchtig P, Mancuso S, Martinoia E, Geisler M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005;579:5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 113.Bouchard R, Bailly A, Blakeslee JJ, Vincenzetti V, Paponov I, Palme K, Mancuso S, Murphy AS, Schulz B, Geisler M. Immunophilin-like TWISTED DWARF1 modulates auxin efflux activities of Arabidopsis p-glycoproteins. J Biol Chem. 2006;281:30603–30612. doi: 10.1074/jbc.M604604200. [DOI] [PubMed] [Google Scholar]
- 114.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 115.Kepinski S, Leyser O. Plant development: Auxin in loops. Curr Biol. 2005;15:R208–R210. doi: 10.1016/j.cub.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 116.Friml J, Benkova E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, Palme K. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- 117.Abas L, Benjamins R, Malenica N, Paciorek T, Wisniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 118.Scheres B, Xu J. Polar auxin transport and patterning: Grow with the flow. Gen Dev. 2006;20:922–926. doi: 10.1101/gad.1426606. [DOI] [PubMed] [Google Scholar]
- 119.Baluška F, Barlow PW, Baskin T, Chen R, Feldman L, Forde BG, Geisler M, Jernstedt J, Menzel D, Muday G, Murphy A, Šamaj J, Volkmann D. What is apical and what is basal in plant root development? Trends Plant Sci. 2005;10:409–411. doi: 10.1016/j.tplants.2005.07.004. [DOI] [PubMed] [Google Scholar]