Abstract
Purpose
The signal transducer and activator of transcription 3 (STAT3) is frequently overexpressed in most cancers, propagates tumorigenesis and is a key regulator of immune suppression in cancer patients. We sought to determine the incidence of phosphorylated STAT3 (p-STAT3) expression in malignant gliomas of different pathologic types, whether p-STAT3 expression is a negative prognostic factor, and whether p-STAT3 expression influences the inflammatory response within gliomas.
Methods
Using immunohistochemical analysis, we measured the incidence of p-STAT3 expression in 129 patients with gliomas of various pathologic types in a glioma tissue microarray. We categorized our results according to the total number of p-STAT3–expressing cells within the gliomas and correlated this number with the number of infiltrating T cells and T regulatory cells (Tregs). We then evaluated the association between p-STAT3 expression and median survival time using univariate and multivariate analyses.
Results
We did not detect p-STAT3 expression in normal brain tissues or low-grade astrocytomas. We observed significant differences in the incidence of p-STAT3 expression between the different grades of astrocytomas and different pathologic glioma types. p-STAT3 expression was associated with the population of tumor-infiltrating immune cells but not with that of Tregs. On univariate analysis, we found that p-STAT3 expression within anaplastic astrocytomas was a negative prognostic factor.
Conclusions
p-STAT3 expression is common within gliomas of both the astrocytic and oligodendroglial lineages and portends poor survival in patients with anaplastic astrocytomas. p-STAT3 expression differs significantly between gliomas of different pathologic types and grades and correlated with the degree of immune infiltration.
Keywords: gliomas, p-STAT3, T lymphocytes, T regulatory cells, prognosis
Introduction
A key transcription factor, signal transducer and activator of transcription (STAT)3, has been shown to drive the fundamental components of tumorigenesis and metastasis by preventing apoptosis (by increasing survivin, BCL-XL, and MCL1 expression) and enhancing proliferation (by increasing c-Myc and cyclin D1/D2 expression) (1), angiogenesis (by increasing vascular endothelial growth factor and hypoxia-inducible factor-1α expression), invasion (by increasing matrix metalloproteinase-2 and -9 expression), and metastasis (2,3). Growth factors and cytokines, including interleukin (IL)-6, can activate Janus kinase 2 (Jak2), which subsequently activates STAT3 by phosphorylating the tyrosine residue in the STAT3 transactivation domain (4). Phosphorylated STAT3 (p-STAT3) then translocates into the nucleus and induces the expression of a variety of target genes. IL-6, which is expressed in the central nervous system (CNS) under a variety of conditions, such as hypoxia (5), traumatic and metabolic injury (6), and inflammation (7) has been shown to attract T cells to the CNS (8). Specifically, IL-6 signaling by means of STAT3 is tightly linked to the homing and migrational capacity of T cells (8).
STAT3 is constitutively overexpressed in a variety of cancers; for instance, in melanoma, 81% of CNS metastases express activated STAT3 (9). Preclinical studies using decoy antisense STAT3 oligonucleotides, dominant-negative vectors, and small-molecule inhibitors have provided convincing evidence that STAT3 is highly relevant to the growth and survival of many tumor types (10–20), including gliomas (14), in vitro and in vivo.
STAT3 is also a key regulator of immune suppression; it is believed to regulate anti-inflammatory responses by suppressing macrophage activation (21–23) and limiting inflammatory responses (24). STAT3 also becomes constitutively active in diverse tumor-infiltrating immune cells. STAT3 activity within natural killer (NK) cells and neutrophils directly reduces their cytotoxicity, whereas STAT3 activity in dendritic cells reduces the expression of MHC II, CD80, CD86, and IL-12, rendering the dendritic cells unable to stimulate T cells and generate antitumor immunity (25). By ablating STAT3 in hematopoietic cells in tumor-bearing mice, Kortylewski et al., showed that there was marked enhancement of activated and functional T cells, NK cells, and dendritic cells. STAT3 ablation in only the hematopoietic cells resulted in marked antitumor effects in vivo, indicating that STAT3 expression in immune cells restrains the immune cell eradication of the tumor (25). In addition, the tumor microenvironment induced STAT3 activity in tumor-associated immune cells (26,27). Furthermore, IL-2 has been shown to regulate FoxP3 expression in human CD4+CD25+ T regulatory cells (Tregs) by inducing STAT3 binding of the first intron of the FoxP3 gene (28). We have previously shown that STAT3 blockade within immune cells restores immune responses (29) and inhibits Treg induction (30).
We previously examined whether the glioma-infiltrating Treg population acted as a prognostic marker in patients with gliomas (31). However, we did not see a significant relationship for glioma cell infiltration, which was likely due to the presence of multiple redundant immunosuppressive mechanisms, including Tregs. Recent data has emerged that p-STAT3 may be a key component to the overall immune suppression seen in cancer patients. Thus, the purpose of this study was to determine the incidence of p-STAT3 expression among patients with gliomas of various pathologic types and grades and to determine if p-STAT3 expression was a prognostic marker, especially since p-STAT3 confers properties of enhanced tumorgenesis. We hypothesized that the presence of p-STAT3 would be associated with a poor prognosis within specific glioma pathologies. Finally, we sought to determine whether p-STAT3 expression was correlated with enhanced infiltration of glioma by T cells and, specifically, Tregs.
Materials and Methods
Glioma tissue microarray slides
We used a previously described (32) glioma tissue microarray (TMA) that included tissue sections from 53 patients with WHO grade IV glioblastomas (GBMs), 17 with WHO grade III anaplastic astrocytomas (AAs), 3 with WHO grade II low-grade astrocytomas (LGAs), 16 with WHO grade II oligodendrogliomas, 15 with WHO grade III anaplastic oligodendrogliomas (AOs), 6 with WHO grade II mixed oligoastrocytomas (MOAs), 12 with WHO grade III anaplastic mixed oligoastrocytomas (AMOAs), and 7 with WHO grade IV gliosarcomas. The study neuropathologist (GNF) gathered the tissue sections and confirmed the tumor pathology based on the archived paraffin-blocked tissue sections. The TMA also contained normal brain tissues (white matter, cortex, and cerebellum), which were obtained from surgical specimens, excluding autopsy specimens, containing parenchyma that overlaid deep metastases but that were not themselves involved by the neoplasm. We conducted our study under the institutional review board–approved protocol LAB03-0228 at The University of Texas M. D. Anderson Cancer Center.
Immunohistochemical analysis of p-STAT3 expression, CD3+ T cells, CD8+ T cells, and Tregs
Formalin-fixed, paraffin-embedded sections of the glioma TMA were first deparaffinized in xylene and rehydrated in ethanol. We blocked the endogenous peroxidase with 0.3% hydrogen peroxide/methanol for 10 min at room temperature before beginning antigen retrieval. We performed antigen retrieval for p-STAT3 by immersing the sections in a citrate-buffered solution (pH 6.0) and heating the sections in a microwave for 20 min. The sections were then cooled to room temperature for 40 min. For antigen retrieval of FoxP3 staining, we autoclaved the sections in 10 mM citrate buffer (pH 6.0) for 10 min at 121°C. We performed antigen retrieval for CD3 and CD8 expression by placing the sections in an electric kitchen pot filled with approximately 800 mL of 0.05% citraconic anhydride solution (pH 7.4; Immunosaver; Nisshin EM Co. Ltd., Tokyo, Japan) for 35 min at 100°C. The sections were then cooled to room temperature for 20 min and washed 6 times in PBS solution. After blocking with a protein block serum-free solution (DAKO, Carpinteria, CA), we added diluted anti-p-STAT3 (tyrosine705) antibody (1:50; Cell Signaling Technology, Danvers, MA), a primary antibody to CD3 (clone SK7 8–11, 1:100; DAKO) or CD8 (clone 144B, 1:20; DAKO), or an antibody to FoxP3 (1:20; provided by Dr. Nobuyoshi Hiraoka) (32) to the tissue arrays and incubated the specimens overnight in a humidified box at 4°C. We subjected the slides to biotin-labeled secondary antibody staining (biotinylated link universal solution; DAKO) for 60 min at room temperature. Finally, we added streptavidin-horseradish peroxidase (DAKO) and incubated the slides for 30 min at room temperature. We used diaminobenzidine (DAKO) as the chromogen, and we stopped color development by gently dipping slides in distilled water. The nuclei were then counterstained with hematoxylin. We used a human melanoma TMA (9) as a positive control for p-STAT3 staining, and we used MDACC banked human lymph nodes and tonsils as a positive control for CD3, CD8, and FoxP3 staining. Omitting the primary antibody from the immunohistochemical (IHC) analysis and replacing it with protein block serum-free solution (DAKO) acted as the negative control for CD3 and CD8 staining. For p-STAT3 staining, we used normal goat serum (Santa Cruz Technology, Santa Cruz, CA) as a negative isotype control.
Three independent observers (MA-G, DSY, GNF) quantitatively evaluated p-STAT3 expression and lymphocyte infiltration by analyzing the cores using high-power fields (max: x40 objective and x10 eyepiece) of each specimen. The observers examined each sample in duplicate from different areas of the same tumor in a blinded fashion. Each observer recorded the absolute number of cells with positive staining for p-STAT3 seen per 1-mm diameter core. The duplicate numbers were then averaged for the final number of p-STAT3–expressing cells and lymphocytes per surgical specimen. If there were discrepancies between the recorded numbers, the observers recounted the number of cells with positive staining in each specimen, and the neuropathologist (GNF) conducted the final arbitration. We minimized potential mismatching of the data by staining an intact microarray with hematoxylin and eosin and identifying the correct location of each tissue core by visually matching the tumors based on their unique histologic elements.
Statistical analysis
We conducted an equal proportion examination of tumor grade, pathologic tumor type, and glial lineage (astrocytic versus oligodendroglial) (33). We calculated the Kaplan-Meier product-limit overall survival (OS) probability estimates (34) and performed log-rank tests (35) to determine whether there was an association between OS and p-STAT3 expression (versus none), tumor grade, astrocytic and oligodendroglial lineage, and age. For each fitted OS regression model, we eliminated the nonsignificant variables in a step-down fashion using a P value cut-off of P = 0.10. A P value of less than 0.05 was considered significant.
Results
Study population
Tissue sections from 129 patients with gliomas were included in the TMA. The median age of the patients was 44 years (range, 4–91 years). Most patients (97%) had a Karnofsky performance score (KPS) ≥ 70, with a median KPS of 90 at the time of diagnosis (range, 50–100). Table 1 summarizes the patients’ demographic characteristics and is stratified according to the patients’ pathologic diagnoses, age, KPS, and OS time. The patients’ demographic characteristics in our study did not differ significantly from the demographic characteristics of patients with gliomas in previous studies examining prognostic markers (36–38). Of the GBM and AA cases, 19 (36%) and 6 (35%) were recurrent, respectively. Of the patients with GBM, nine (17%) had received prior chemotherapy and 11 (21%) had received prior radiation therapy. Table 2 summarizes the overall composition of the glioma TMA, and Figure 1 shows the IHC staining of p-STAT3 and CD3+ T cell infiltration.
Table 1.
Demographic characteristics of patients with glioma stratified according to pathology.
| Age (years) | KPS | Median survival time (months) * | |||||
|---|---|---|---|---|---|---|---|
| Pathology | Median | Minimum | Maximum | Median | Minimum | Maximum | |
| O | 38.5 | 7.0 | 55.0 | 100 | 80 | 100 | 99.8 |
| MOA | 42.5 | 24.0 | 52.0 | 100 | 70 | 100 | _† |
| AO | 40.0 | 25.0 | 59.0 | 90 | 70 | 100 | _† |
| AMOA | 35.5 | 22.0 | 47.0 | 100 | 80 | 100 | 89.2 |
| LGA | 33.0 | 4.0 | 44.0 | 95 | 90 | 100 | 166.7 |
| AA | 49.0 | 24.0 | 91.0 | 90 | 90 | 100 | 27.7 |
| GS | 51.0 | 23.0 | 68.0 | 80 | 60 | 100 | 4.4 |
| GBM | 56.0 | 17.0 | 77.0 | 90 | 50 | 100 | 13.8 |
Abbreviations: AA, anaplastic astrocytoma; AMOA, anaplastic mixed oligoastrocytoma; AO, anaplastic oligodendroglioma; GBM, glioblastoma multiforme; GS, gliosarcoma; KPS, Karnofsky performance score; LGA, low-grade astrocytoma; MOA, mixed oligoastrocytoma.
Based on Kaplan-Meier estimates.
Not analyzed due to rarity.
Table 2.
Composition of the glioma tissue microarray.
| Lineage | Pathology | Number of patients (%) |
|---|---|---|
| Oligodendroglial (n = 49) | O | 16 (12.4%) |
| MOA | 6 (4.7%) | |
| AO | 15 (11.6%) | |
| AMOA | 12 (9.3%) | |
|
| ||
| Astrocytic (n = 80) | LGA | 3 (2.3%) |
| AA | 17 (13.2%) | |
| GS | 7 (5.4%) | |
| GBM | 53 (41.1%) | |
Abbreviations: AA, anaplastic astrocytoma; AMOA, anaplastic mixed oligoastrocytoma; AO, anaplastic oligodendroglioma; GS, gliosarcoma; LGA, low-grade astrocytoma; O, oligodendroglioma; MOA, mixed oligoastrocytoma.
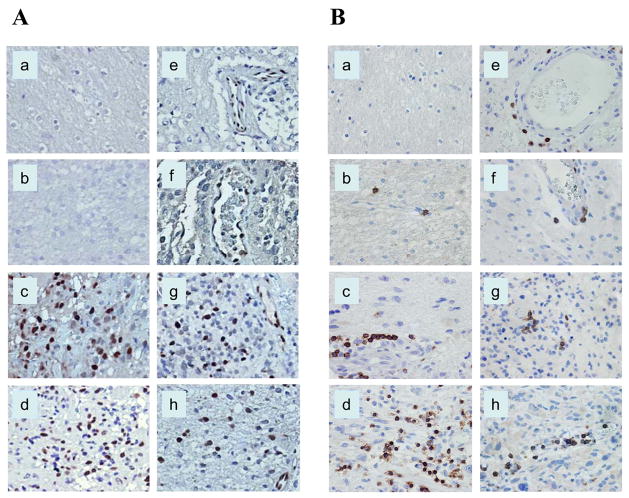
Figure 1.
Immunohistochemical staining of human glioma tissue sections demonstrating p-STAT3 and CD3+ lymphoid cells. The p-STAT3 staining was confined to the nucleus, whereas CD3 staining was noted on the cell surface. A, The number of p-STAT3–expressing cells was more evident in astrocytic, higher-grade gliomas. B, CD3 staining demonstrated high numbers of infiltrative CD3+ T cells, also within higher-glioma grades. All images were taken at a magnification of x400. Normal brain (a), low-grade astrocytoma (b), anaplastic astrocytoma (c), glioblastoma multiforme (d), oligodendroglioma (e), mixed oligoastrocytoma (f), anaplastic oligodendroglioma (g), and anaplastic mixed oligoastrocytoma (h).
Incidence of p-STAT3 expression varies according to tumor grade in astrocytomas
To determine whether oligodendrogliomas and astrocytomas expressed p-STAT3, we stained the tumor specimens with an anti-p-STAT3 antibody and determined the number of cells with positive nuclear staining. We did not observe p-STAT3 expression in the normal brain tissue specimens (n = 5) or in the patients with WHO grade II LGAs (n = 3; Table 3 and Figure 1). In patients with WHO grade III AAs (n = 17), 9 (53%) expressed p-STAT3, and in patients with WHO grade IV GBMs or gliosarcomas (n = 60), 32 (53%) expressed p-STAT3.
Table 3.
Proportion of p-STAT3 positive cases according to pathology and WHO tumor grade.
| Pathology | p-STAT3>0/Total (%) | * Mean (SD) | (Min, Max) |
|---|---|---|---|
| O | 6/16 (38%) | 4.3 (7.7) | (0.0, 23.5) |
| MOA | 6/6 (100%) | 67.9 (55.2) | (9.0, 136.0) |
| AO | 6/15 (40%) | 17.5 (41.0) | (0.0,153.0) |
| AMOA | 7/12 (58%) | 13.3 (27.0) | (0.0, 85.5) |
| LGA | 0/3 (0%) | 0 | 0 |
| AA | 9/17 (53%) | 5.6 (7.6) | (0.0, 23.0) |
| GS | 5/7 (71%) | 23.1 (34.1) | (0.0, 91.0) |
| GBM | 27/53 (51%) | 11.7 (24.4) | (0.0, 133.5) |
Abbreviations: AA, anaplastic astrocytoma; AMOA, anaplastic mixed oligoastrocytoma; AO, anaplastic oligodendroglioma; GS, gliosarcoma; LGA, low-grade astrocytoma; O, oligodendroglioma; MOA, mixed oligoastrocytoma.
The mean calculation was based on the mean p-STAT3 value.
We did not observe an increase in p-STAT3 expression corresponding with an increase in tumor grade in either the oligodendrogliomas or MOAs. Specifically, 38% of the patients with WHO grade II oligodendrogliomas (n = 16) had p-STAT3 expression, and 40% of the patients with WHO grade II AOs (n = 15) had p-STAT3 expression, indicating the incidence of p-STAT3 expression did not increase with increasing tumor grade in oligodendrogliomas. This trend is further supported by our finding that 100% of the patients with WHO grade II MOAs (n = 6) had p-STAT3 expression, while only 58% of the patients with WHO grade III AMOAs (n = 12) had p-STAT3 expression.
Incidence of p-STAT 3 expression varies according to tumor pathology
We observed significant differences in p-STAT3 expression according to the pathologic type of the tumor. Specifically, the presence of any p-STAT3 staining was most often observed in MOAs (100%), followed by GBMs (53%) and AAs (53%; Table 3 and Figure 1). The pathologic types with the lowest incidence of p-STAT3 staining were LGAs (0%) followed by oligodendrogliomas (38%; Table 3).
Number of p-STAT3–expressing cells varies according to tumor grade in astrocytomas and oligodendrogliomas
Although the incidence of p-STAT3 expression was not significantly different between patients with WHO grade III AAs and patients with WHO grade IV GBMs, the number of cells within the gliomas that expressed p-STAT3 increased. Specifically, in patients with WHO grade II LGAs, there were no p-STAT3–expressing cells per core, which increased to a mean of 5.6 p-STAT3–expressing cells per core (SD 7.6 [range, 0–23.0]) for patients with WHO grade III AAs and 11.7 cells per core (SD 24.4 [range, 0–133.5]) for patients with WHO grade IV GBMs. Similarly, among patients with WHO grade II oligodendrogliomas, there was a mean of 4.3 cells per core (SD 7.7 [range, 0–23.5]), which increased to 17.5 cells per core (SD 41.0 [range, 0–153.0]) for patients with WHO grade III A0s. This propensity did not hold true for the patients with WHO grade II MOAs, in whom the mean was 67.9 cells per core (SD 55.2 [range, 9.0–136.0]), when compared to patients with WHO grade III AMOAs, in whom the mean was 13.3 cells per core (SD 27.0 [range, 0–85.5]).
Correlation of p-STAT3 expression and T cell infiltration
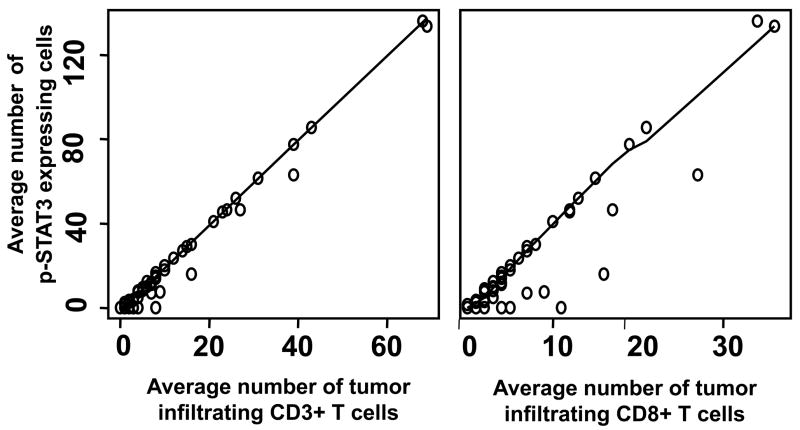
Across all tumor pathologies and tumor grades, increased p-STAT3 expression was associated with an increase in glioma-infiltrating T cells. In the pair-wise scatter plots with Loess smooth curves examining the relationship between p-STAT3 expression and CD3+ and CD8+ T cell infiltration, an almost straight trend indicated that there was a strong linear correlation between CD3+ and CD8+ T cell infiltration and p-STAT3 expression (P < 0.001 for both; Figure 2).
Figure 2.
Pair-wise scatter plots between p-STAT3, CD3, and CD8 across all tumor pathologies and tumor grades with Loess smooth curves added. The almost straight trend of the Loess curves indicates that both CD3 and CD8 had a strong linear correlation with p-STAT3 expression. Specifically, the correlation of the number of p-STAT3 expressing cells with the number of tumor infiltrating CD3+ T cells was 0.99 (P < 0.001) and with the number of tumor infiltrating CD8+ T cells was 0.94 (P < 0.001).
Lack of correlation between p-STAT3 expression and Treg infiltration
To determine if there was an association between p-STAT3 expression and the presence of Tregs, we stained the glioma TMA with FoxP3 (31). Within the GBM specimens that expressed p-STAT3, 59% (16/27) had an infiltrating Treg population. However, the level of p-STAT3 expression was not associated with the number of infiltrating Tregs. In the MOA, AMOA, and AO specimens, in which there was a high incidence of p-STAT3–expressing cells, there was no correlating Treg glioma-infiltrating population. Thus, the presence of p-STAT3 within the tumor does not appear to be a key regulator of the presence or degree of the infiltrating Treg population.
p-STAT-3 expression level is a prognostic marker for survival times
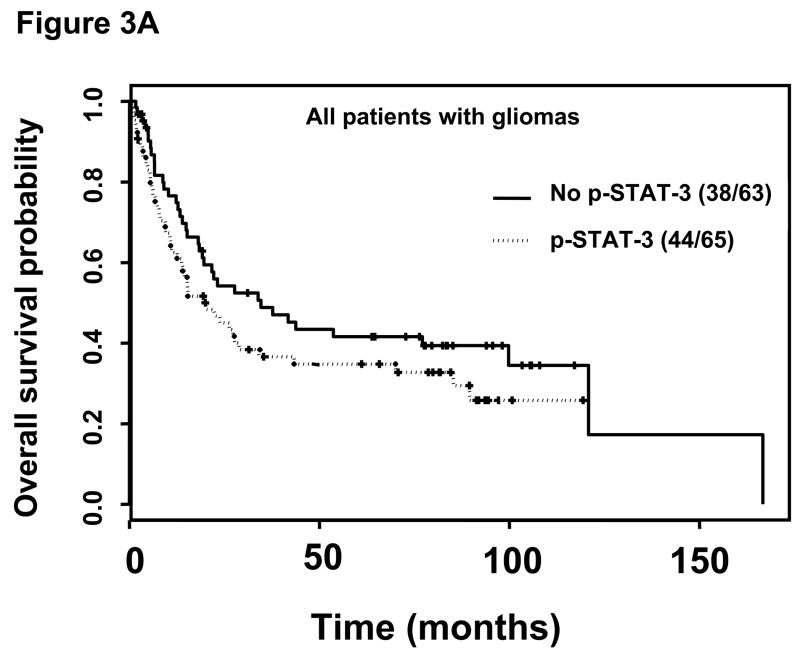
Among the patients with gliomas with no p-STAT3 expression, regardless of the specific tumor pathology, the median survival time was 34.6 months (95% confidence interval [CI], 19.2 months-NA). In contrast, for patients with gliomas with p-STAT3 expression, the median survival time was 20.1 months (95% CI, 13.8–43.0 months); however, this was not statistically significant (Figure 3A).
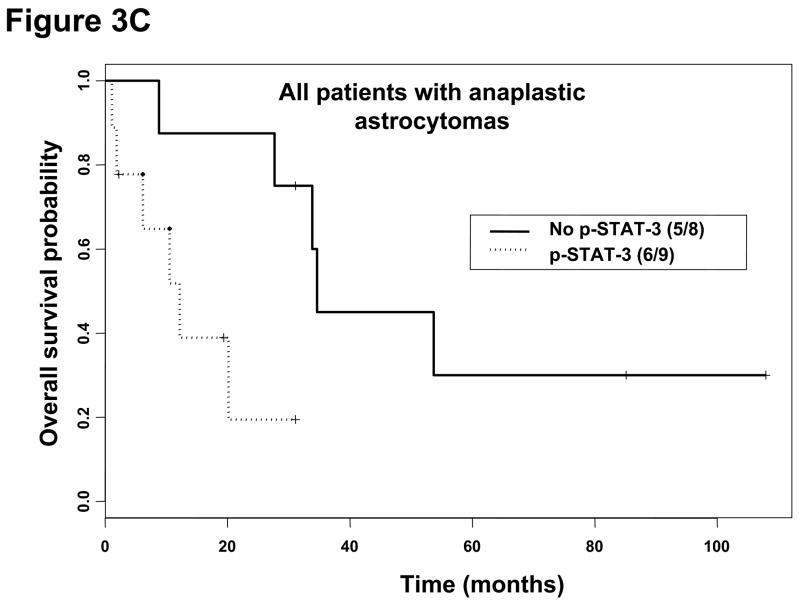
Figure 3.
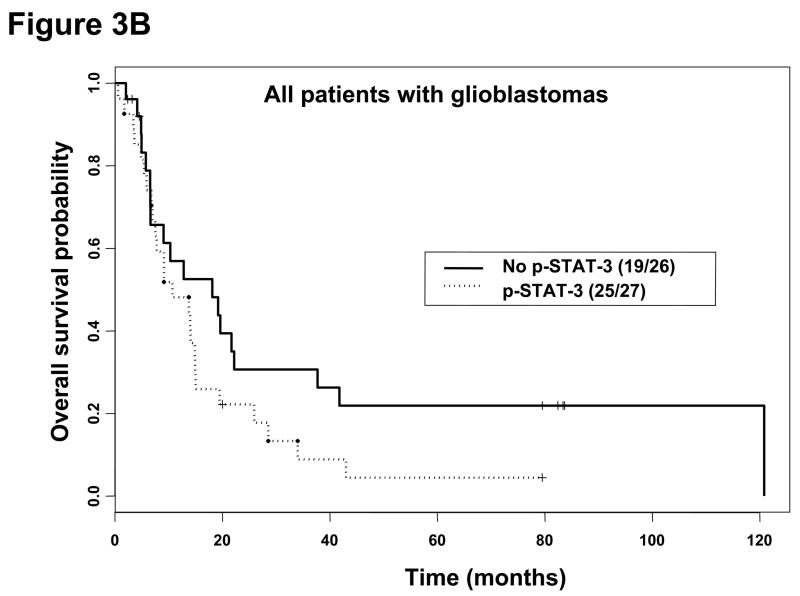
Kaplan-Meier survival estimates stratified by the presence or absence of p-STAT3 immunohistochemical staining within all patients with gliomas, patients with glioblastoma multiforme, and patients with anaplastic astrocytoma. A, Among all patients with gliomas without p-STAT3 expression, the median survival time was 34.6 months (95% CI, 19.2, months-A) and 20.1 months (95% CI, 13.8–43.0 months) in patients with gliomas with p-STAT3 expression (P = 0.16). B, In patients with glioblastoma multiforme without p-STAT3 expression, the median survival time was 18.1 months (95% CI, 6.6–41.8 months) and 10.7 months (95% CI, 7.5–15.0 months) in patients with glioblastoma multiforme with p-STAT3 expression (P = 0.12). C, In patients with anaplastic astrocytoma patients without p-STAT3 expression, the median survival time was 34.6 months (95% CI, 33.9 months-NA) and 12.2 months (95% CI, 6.2 months-NA) in patients with anaplastic astrocytoma with p-STAT3 expression (P = 0.02).
For patients with GBMs and p-STAT3 expression, the median survival time was 10.7 months (95% CI, 7.5–15.0 months), while the median survival time was 18.1 months (95% CI, 6.6–41.8 months) for patients with GBMs without p-STAT3 expression; however, this was not statistically significant (Figure 3B). In patients with AAs expressing p-STAT3, the median survival time was 12.2 months (95% CI, 6.2 months-NA), while the median survival time was 34.6 months (95% CI, 33.9 months-NA) for patients with AAs that lacked p-STAT3 expression (P = 0.02; Figure 3C). On univariate analysis, the presence of p-STAT3 (P = 0.04; hazard ratio [HR] 5.94) in patients with AAs was a prognostic factor. However, after adjusting for age in the multivariate analysis, the presence of p-STAT3 was only marginally significant (P = 0.06; HR 5.32), which could be due to our limited sample size. All AA patients underwent combinational radiation and chemotherapy. The absolute number of p-STAT3–expressing cells did not have a prognostic impact for patients with AAs on either univariate or multivariate analyses. In addition, p-STAT3 expression did not appear to be a prognostic factor in patients with either AOs or oligodendrogliomas. Because there was no p-STAT3 expression in low-grade gliomas and all MOAs expressed p-STAT3, no prognostic significance for survival can be ascertained within these pathologic subtypes. Removing GBMs and AAs with prior radiotherapy or chemotherapy (i.e. recurrent) from our analysis did not have a statistically significant effect on our results.
Discussion
In this study, we found that p-STAT3 is commonly expressed in a variety of gliomas. Furthermore, as astrocytomas become more malignant, the number of p-STAT3–expressing cells increases. We frequently observed p-STAT3–expressing cells in GBMs, gliosarcomas, and AAs, but we did not see significant p-STAT3 expression in normal brain tissues or low-grade gliomas. Because we employed a glioma TMA that contains only a small proportion of the overall tumor we cannot completely exclude the possibility that p-STAT3–expressing cells were present in the low-grade tumors and normal tissue. However, in an attempt to negate this as a possibility, we used duplicate tissue cores from different areas of the tumors and normal tissue from various CNS anatomical positions in the TMA.
Investigators have examined p-STAT3 expression in other malignancies, such as gastric (39), renal (40), ovarian (41), squamous (42,43), and hepatocellular carcinomas (44) and anaplastic large-cell lymphoma (45,46), and have determined that p-STAT3 expression was associated with poor prognosis. Other studies have also shown that p-STAT3 expression correlated with lymph node spread of colorectal cancer (47) and the depth of tumor invasion (48). However, some studies found no relationship between p-STAT3 expression and prognosis in patients with non-small cell lung cancer (49,50). While these studies addressed p-STAT3 expression at the tyrosine705 location, similar to our current study, another group found that p-STAT3 at the serine727 location correlated with the degree of cervical intraepithelial neoplasia (51). Similar to most studies examining the prognostic significance of p-STAT3 expression, we found on univariate analysis that in AAs, p-STAT3 expression was a negative prognostic factor.
Our study is in stark contrast, however, to a previous study in which a similar glioma microarray was utilized, which found that less than 10% of p-STAT3 expression at tyrosine705 occurred in gliomas of all grades and pathologic types (52). The potential reasons for the difference between this previous study and our current study may include our process for antigen retrieval and the titration of the primary antibody. Because very little p-STAT3 expression was seen in the previous study, the authors did not attempt to correlate p-STAT3 expression with prognosis. However, another IHC study of p-STAT3 expression in just GBMs and AAs found an almost identical incidence of p-STAT3 expression (i.e., 55.6% in AAs and 56.4% in GBMs (53)) as in our current study. In this latter study, the authors saw a trend that activated STAT3 conferred a survival advantage, but this was not statistically significant. The differences between the results of our current study and the previous study’s results may be a matter of the level of p-STAT3 expression deemed positive for the analysis of prognosis. In our current study, if any cell was found that expressed p-STAT3, the specimen was categorized as being positive. In the study by Mizoguchi et al, approximately 20% of the tumor cells had to express p-STAT3 in order to be classified as positive (C. L Nutt, personal correspondence, April 18, 2008). This study also demonstrated a correlation between STAT3 activation and EGFR status that was attributed exclusively to EGFRvIII expression.
We also found that in gliomas with p-STAT3 expression, that there was a statistically significant corresponding influx of CD3+ T cells. Since IL-6 is expressed in the CNS under a wide variety of conditions and has been shown to attract T cells to the CNS, it was not surprising that the amount of p-STAT3 expression directly correlated with the number of CD3+ glioma– infiltrating T cells. One needs to bear in mind, however, that the presence of infiltrating T cells does not correlate with their having functional activity (54). The tumor microenvironment induces STAT3 activity in tumor-associated immune cells (25,27), and the induced p-STAT3 in the tumor-infiltrating immune cells resulted in their functional down modulation (21–25,29). Thus, while STAT3 expression in the tumor correlates with the degree of immune infiltration in the tumor microenvironment, it has also likely resulted in their immune suppression. This may partially resolve the divergent findings of various studies that have examined the prognostic effects of glioma infiltrating T cell populations on prognosis (55–57).
Investigators have reported the presence FoxP3+ Tregs in a variety of cancers, including hepatocellular carcinoma, colorectal cancer, ovarian cancer, and others (32,58–62). Studies have shown that the presence of FoxP3+ Tregs in ovarian cancer is not only a predictor of poor prognosis but also an independent predictor of OS and progression-free survival times (58,59). However, in anal squamous cell carcinoma, the presence of Tregs did not have a prognostic influence (63). We examined FoxP3 expression in gliomas and did not find it to be an independent negative prognostic factor for median survival time (31). Within some pathologies, such as oligodendrogliomas, we did not observe the presence of FoxP3 despite the presence of p-STAT3 expression. Conversely, we identified Treg infiltration in tumors without p-STAT3 expression. Thus, although p-STAT3 may be a transcriptional factor related to the induction of FoxP3 expression, it may not be the only factor that influences Treg generation.
Since p-STAT3 is frequently observed in gliomas it represents a viable target for a variety of small-molecule inhibitors of p-STAT3, such as WP1066 (14,29), JSI-124 (cucurbitacin I) (64), and S3I-201 (65), which are in various stages of preclinical development. However, the variability of p-STAT3 expression within the various glioma pathologies suggests that not all patients may uniformly benefit from treatment with these types of anti-p-STAT3 approaches. Future studies will be directed at evaluating the in vivo effects of small molecule inhibitors of p-STAT3 in preclinical models of intracerebral gliomas with variable expression levels of p-STAT3. In conclusion, p-STAT3 is frequently expressed among patients with high grade gliomas, MOA and AMOA, has a prognostic influence within patients with AAs, and its expression correlates with enhanced T cell infiltration within gliomas.
Acknowledgments
We thank Alyson Todd for editorial assistance.
Grant support: The Brain Tumor Society, The Rose Foundation grant, the Anthony D. Bullock III Foundation grant, and National Institutes of Health grant RO1 CA120813-01A1 to A. Heimberger
Footnotes
Statement of Clinical Relevance: Signal transducer and activator of transcription (STAT3) has been found to play a significant and varied role within the tumor microenvironment, involving not only tumor cells, but also the immune system through dendritic cells, T regulatory cells and others as well. Our hypothesis was that p-STAT3 expression in human gliomas influences inflammatory responses and is an independent negative prognostic indicator for patient survival. This manuscript details that p-STAT-3 expression differs between tumor pathology, is greatest in astrocytic tumors and is predictive of shortened overall survival in patients with anaplastic astrocytoma. In addition, p-STAT-3 expression closely correlates with the degree of intratumoral T cell infiltration. In conclusion, p-STAT-3 is frequently observed in gliomas and represents a viable therapeutic target for a variety of small molecule inhibitors of p-STAT-3 that are in preclinical development.
References
- 1.Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Activation of JAK-STAT pathway is required for platelet-derived growth factor-induced proliferation of pancreatic stellate cells. World J Gastroenterol. 2005;11:3385–91. doi: 10.3748/wjg.v11.i22.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 2007;13:1362–6. doi: 10.1158/1078-0432.CCR-06-2313. [DOI] [PubMed] [Google Scholar]
- 3.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 4.Li B, Chang CM, Yuan M, McKenna WG, Shu HK. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res. 2003;63:7443–50. [PubMed] [Google Scholar]
- 5.Tarkowski E, Rosengren L, Blomstrand C, et al. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995;26:1393–8. doi: 10.1161/01.str.26.8.1393. [DOI] [PubMed] [Google Scholar]
- 6.Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–9. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- 7.De Simoni MG, Del Bo R, De Luigi A, Simard S, Forloni G. Central endotoxin induces different patterns of interleukin (IL)-1 beta and IL-6 messenger ribonucleic acid expression and IL-6 secretion in the brain and periphery. Endocrinology. 1995;136:897–902. doi: 10.1210/endo.136.3.7867598. [DOI] [PubMed] [Google Scholar]
- 8.McLoughlin RM, Jenkins BJ, Grail D, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9589–94. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie TX, Huang FJ, Aldape KD, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–96. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 10.Bao JJ, Fokt I, Szymanski S, Priebe W. Inhibition of constitutively active STAT3 by WP1066 suppresses proliferation and induces apoptosis in pancreatic cancer cells. Clin Cancer Res. 2005;11:9026S–7S. [Google Scholar]
- 11.Chakraborty A, Guha S, Helgason T, et al. A novel Jak2/STAT3 pathway inhibitor promotes apoptosis and blocks growth of bladder cancer cells. 98th American Association of Cancer Research Annual Meeting; Los Angeles, CA. 2007. [Google Scholar]
- 12.Chan KS, Sano S, Kiguchi K, et al. Disruption of Stat3 reveals a critical role in both the initiation and the promotion stages of epithelial carcinogenesis. J Clin Invest. 2004;114:720–8. doi: 10.1172/JCI21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guha S, Chakraborty A, Szymanski S, et al. WP1066, a potent inhibitor of Jak2/STAT3 pathway inhibits pancreatic tumor growth both in vitro and in vivo. 98th American Association of Cancer Research Annual Meeting; Los Angeles, CA. 2007. [Google Scholar]
- 14.Iwamaru A, Szymanski S, Iwado E, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2006;26:2435–44. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 15.Kong L-Y, Kapuria V, Bartholomeusz G, Priebe W, Talpaz M, Donato N. Antitumor activity and mechanism of action of a novel Stat3 inhibitor, WP1066, against human B-cell non-Hodgkin’s lymphoma and multiple myeloma. Blood. 2005;106:429A. 1489 Part 1. [Google Scholar]
- 16.Kupferman ME, Zhou G, Zhao M, et al. A novel inhibitor of STAT3 signaling in head and neck squamous cell carcinoma. 97th American Association of Cancer Research Annual Meeting; Washington, DC. 2006. [Google Scholar]
- 17.Leong PL, Andrews GA, Johnson DE, et al. Targeted inhibition of Stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003;100:4138–43. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samanta A, Kantarjian H, Priebe W, Arlinghaus R. Cross talk between Jak2 and Lyn in Bcr-Abl signaling pathway in cells from Imatinib-sensitive and resistant chronic myelogenous leukemia (CML). 98th American Association of Cancer Research Annual Meeting; Los Angeles, CA. 2007. [Google Scholar]
- 19.Tang GS, Cai JM, Ni J, et al. Effects of STAT3 antisense oligodeoxynucleotides on apoptosis and proliferation of mouse melanoma cell line B16. Ai Zheng. 2006;25:269–74. [PubMed] [Google Scholar]
- 20.Xi S, Gooding WE, Grandis JR. In vivo antitumor efficacy of STAT3 blockade using a transcription factor decoy approach: implications for cancer therapy. Oncogene. 2005;24:970–9. doi: 10.1038/sj.onc.1208316. [DOI] [PubMed] [Google Scholar]
- 21.Lang R, Patel D, Morris J, Rutschman R, Murray P. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 22.O’Farrell AM, Liu YW, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda K, Clausen B, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Bost K. STAT3 activation in macrophages following infection with Salmonella. Biochem Biophys Res Commun. 2004;321:828–34. doi: 10.1016/j.bbrc.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 26.Kortylewski M, Yu H. Stat3 as a potential target for cancer immunotherapy. J Immunother (1997) 2007;30:131–9. doi: 10.1097/01.cji.0000211327.76266.65. [DOI] [PubMed] [Google Scholar]
- 27.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 28.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain SF, Kong L-Y, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67:9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 30.Kong LY, Abou-Ghazal MK, Wei J, et al. A novel inhibitor of STAT3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0377. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heimberger AB, Reina-Ortiz C, Yang DS, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008 doi: 10.1158/1078-0432.CCR-08-0320. (In press) [DOI] [PubMed] [Google Scholar]
- 32.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 33.Snedecor GW, Cochran WG. Statistical Methods. 7. Ames: Iowa State University Press; 1980. [Google Scholar]
- 34.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 35.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 36.Barnett JA, Urbauer DL, Murray GI, Fuller GN, Heimberger AB. CYP1B1 Expression in Glial Cell Tumors: An Immunotherapeutic Target. Clin Cancer Res. 2007:13. doi: 10.1158/1078-0432.CCR-06-2430. [DOI] [PubMed] [Google Scholar]
- 37.Heimberger AB, Hlatky R, Suki D, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–6. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 38.Heimberger AB, McGary E, Suki D, et al. Loss of the AP-2alpha transcription factor is associated with the grade of human gliomas. Clin Cancer Res. 2005;11:267–72. [PubMed] [Google Scholar]
- 39.Gong W, Wang L, Yao J, et al. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin Cancer Res. 2005;11:1386–93. doi: 10.1158/1078-0432.CCR-04-0487. [DOI] [PubMed] [Google Scholar]
- 40.Horiguchi A, Oya M, Shimada T, Uchida A, Marumo K, Murai M. Activation of signal transducer and activator of transcription 3 in renal cell carcinoma: A study of indicence and its association with pathological features and clinical outcome. J Urology. 2002;168:762–5. [PubMed] [Google Scholar]
- 41.Meinhold-Heerlein I, Bauerschlag D, Hilpert F, et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene. 2005;24:1053–65. doi: 10.1038/sj.onc.1208298. [DOI] [PubMed] [Google Scholar]
- 42.Masuda M, Suzui M, Yasumatu R. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–5. [PubMed] [Google Scholar]
- 43.Shah NG, Trivedi TI, Tankshali RA. STAT3 expression in oral squamous cell carcinoma: association with clinicopathological parameters and survival. Int J Biol Markers. 2006;21:175–83. doi: 10.1177/172460080602100307. [DOI] [PubMed] [Google Scholar]
- 44.Yang S, Wang S, Wu C, et al. Altered p-STAT3 (tyr705) expression is associated with histological grading and intratumour microvessel density in hepatocellular carcinoma. J Clin Pathol. 2007;60:642–8. doi: 10.1136/jcp.2006.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoury JD, Medeiros LJ, Rassidakis G, et al. Differential expression and clinical significance of tyrosine-phosphorylated STAT3 in ALK+ and ALK- anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:3692–9. [PubMed] [Google Scholar]
- 46.Schlette E, Medeiros LJ, Goy A, Lai R, Rassidakis G. Survivin expression predicts poorer prognosis in anaplastic large-cell lymphoma. J Clin Oncol. 2004;22:1682–8. doi: 10.1200/JCO.2004.10.172. [DOI] [PubMed] [Google Scholar]
- 47.Lassmann S, Schuster I, Walch A, et al. STAT3 mRNA and protein expression in colorectal cancer: effects on STAT3-inducible targets linked to cell survival and proliferation. J Clin Pathol. 2007;60:173–9. doi: 10.1136/jcp.2005.035113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kusaba T, Nakayama T, Yamazumi K, et al. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J Clin Pathol. 2005;58:833–8. doi: 10.1136/jcp.2004.023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortas T, Eisenberg R, Fu P, Kern J, Patrick L, Dowlati A. Activation state EGFR and STAT-3 as prognostic markers in resected non-small lung cancer. Lung Cancer. 2007;55:349–55. doi: 10.1016/j.lungcan.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–94. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 51.Yang S, Yuan S, Yeh Y, et al. The role of p-STAT3 (ser727) revealed by its association with Ki-67 in cervical intraepithelial neoplasia. Gynecologic Oncol. 2005;98:446–52. doi: 10.1016/j.ygyno.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Wang H, Zhang W, Huang H, Liao W, Fuller GN. Analysis of the activation status of Akt, NFkB, and STAT3 in human diffuse gliomas. Lab Invest. 2004;84:941–51. doi: 10.1038/labinvest.3700123. [DOI] [PubMed] [Google Scholar]
- 53.Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65:1181–8. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 54.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks WH, Markesbery WR, Gupta GD, Roszman TL. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol. 1978;4:219–24. doi: 10.1002/ana.410040305. [DOI] [PubMed] [Google Scholar]
- 56.von Hanwehr RI, Hofman FM, Taylor CR, Apuzzo ML. Mononuclear lymphoid populations infiltrating the microenvironment of primary CNS tumors. Characterization of cell subsets with monoclonal antibodies. J Neurosurg. 1984;60:1138–47. doi: 10.3171/jns.1984.60.6.1138. [DOI] [PubMed] [Google Scholar]
- 57.Strik HM, Stoll M, Meyermann R. Immune cell infiltration of intrinsic and metastatic intracranial tumours. Anticancer Res. 2004;24:37–42. [PubMed] [Google Scholar]
- 58.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 59.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–11. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 61.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–93. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 62.Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res. 2007;13:2075–81. doi: 10.1158/1078-0432.CCR-06-2139. [DOI] [PubMed] [Google Scholar]
- 63.Grabenbauer GG, Lahmer G, Distel L, Niedobitek G. Tumor-infiltrating cytotoxic T cells but not regulatory T cells predict outcome in anal squamous cell carcinoma. Clin Cancer Res. 2006;12:3355–60. doi: 10.1158/1078-0432.CCR-05-2434. [DOI] [PubMed] [Google Scholar]
- 64.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- 65.Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of STAT3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–6. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]