Abstract
This work investigates the physical interactions between carbon nanomaterials and tocopheryl polyethylene glycol succinate (TPGS). TPGS is a synthetic amphiphile that undergoes enzymatic cleavage to deliver the lipophilic antioxidant, α-tocopherol (vitamin E) to cell membranes, and is FDA approved as a water-soluble vitamin E nutritional supplement and drug delivery vehicle. Here we show that TPGS 1000 is capable of dispersing multi-wall and single-wall carbon nanotubes in aqueous media, and for multiwall tubes is more effective than the commonly used non-ionic surfactant Triton X-100. TPGS is also capable of solubilizing C60 in aqueous phases by dissolving fullerene in the core of its spherical micelles. Drying of these solutions leads to fullerene/TPGS phase separation and the self-assembly of highly ordered asymmetric nanoparticles, with fullerene nanocrystals attached to the hydrophobic end of crystalline TPGS nanobrushes. The article discusses surface charge, colloidal stability, and the potential applications of TPGS as a safe surfactant for “green” processing of carbon nanomaterials.
Keywords: green chemistry, nanotube functionalization, nanotoxicology, self-assembly
Introduction
The natural hydrophobicity of unfunctionalized carbon nanomaterials is a challenge for their dispersion and processing in aqueous phases. Biological applications typically require aqueous processing, and many non-biological applications can benefit from aqueous processing as a “green” alternative to the use of organic solvents in inks, coatings, thin films, composites, and engineered nanofluids. The importance of nanotube dispersion in aqueous media has led to the exploration of many competing methods including covalent functionalization and non-covalent interaction with amphiphiles that include synthetic surfactants [1], proteins [2,3], polymers [4,5], and the biological polycation, chitosan [6].
Synthetic surfactants have been particularly popular due to their ready availability and low cost. It is not well known in the materials science community, but many synthetic surfactants represent environmental or health hazards upon inhalation or environmental release [7,8,9]. Surfactant toxicity can occur through direct cell membrane damage [10,11,12], and may even be the primary cause of observed toxicity when surfactants are used to disperse nanotubes in nanotoxicology assays [10]. Greater biocompatibility can be achieved by some natural dispersants such as bovine serum albumin (BSA), chitosan, or dipalmitoylphosphatidylcholine (DPPC) [6,13,14], but being biological materials these are subject to bacterial contamination if processed under non-sterile conditions and are not attractive for many non-biological applications. There is significant motivation to identify new synthetic (non-biological), safe surfactants for green nanotube processing.
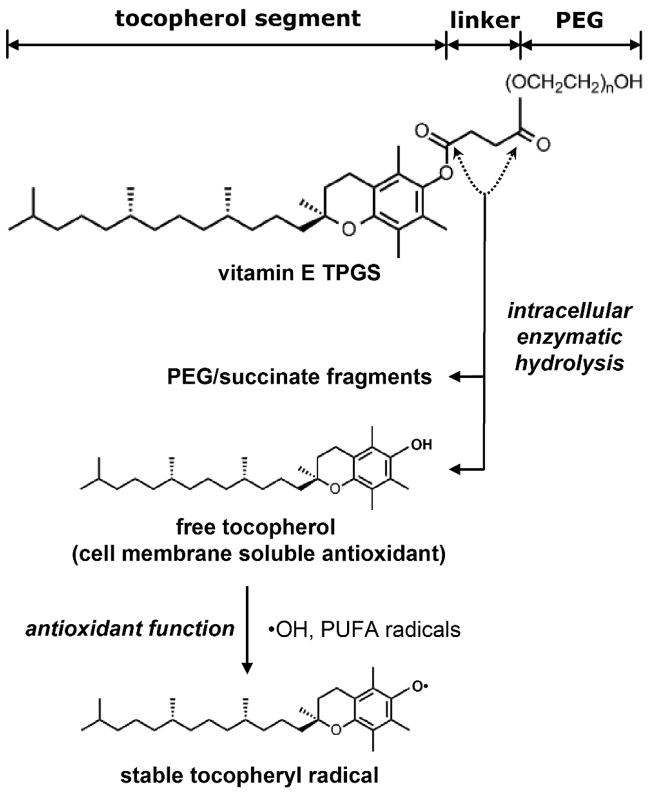
An intriguing commercial material is tocopheryl polyethylene glycol succinate (TPGS) (see Fig 1). TPGS is synthesized from the lipid-soluble antioxidant, α-tocopherol (vitamin E) by grafting to a polyethylene glycol (PEG) oligomer through a succinate diester linker. TPGS is widely used as a water-soluble vitamin E formulation. It is a GRAS (Generally Regarded As Safe)-listed supplement taken orally at long-term doses of 13.4 – 16.8 mg/kg/day or up to 100 mg/kg/day for people with impaired uptake [15,16,17,18]). Further, TPGS 1000 (1000 denoting the PEG chain molecular weight) has been approved by FDA as a drug solubilizer in oral, parenteral, topical, nasal, and rectal/vaginal therapies [19,20]. TPGS has also shown promise as a solublizer for inhalation drug delivery [21,22,23,24].
FIGURE 1.
The structure, hydrolysis, and antioxidant function of TPGS. PUFA are polyunsaturated fatty acids that are vulnerable to peroxidation, leading to free radical propagation reactions and cell membrane damage [27].
To our knowledge TPGS has not been used as a nanomaterial dispersant, but is clearly an amphiphile with a 16-carbon alkyl chain and a PEG oligomer of significant length to impart hydrophilicity (see Fig. 1). There are no published studies on the interactions of TPGS with nanocarbons to aid in their dispersion or processing.
The antioxidant properties of TPGS are based on cellular enzymatic hydrolysis by cytoplasmic esterases that liberate free α-tocopherol, which then localizes in the cell membrane and through free radical quenching protects the membrane from lipid peroxidation and damage (see Fig. 1) [19,25,26]. Culturing fibroblast cells with TPGS for 24 hrs resulted in increased contents of both total and free tocopherol with most of the hydrolytic conversion occurring between 4 and 24 hrs [28]. Oxidized tocopherol can be reduced back to its active state by the water-soluble physiological reductant, ascorbate, to form a continuous cycle [29, 30]. The non-enzymatichydrolysis of TPGS is slow: the manufacturer, Eastman Chemical, reports that less than 20% of TPGS 1000 is hydrolyzed in the first 10 days at 37 °C in the pH range 4 – 10.
The emerging literature on nanotoxicology includes several studies reporting reactive oxygen generation and/or oxidative damage associated with nanocarbons [31,32,33,34]. Sayes et al. [31] report that fullerene toxicity is due to free radical production and lipid peroxidation. Shvedova et al. [32] and Kagan et al. [33] report that transition metal residues in Fe-containing carbon nanotubes may enter into the redox cycle and catalyze oxidative stress within cells. Guo et al. [34] report release of bioavailable, redox-active iron from a range of commercial Fe-containing nanotubes and redox catalysis of free radical production that causes single-strand-breaks in plasmid DNA. Most recently Shvedova et al. [35] report that single-wall nanotubes (SWNTs) induce pulmonary inflammation in mice accompanied by oxidative stress and antioxidant depletion. Further, SWNT-induced antioxidant depletion and acute inflammation were enhanced in mice fed vitamin E-deficient diets relative to mice on vitamin E-sufficient diets [35]. We therefore see an additional motivation for studying TPGS as a nanocarbon dispersant – in some scenarios its antioxidant function [36,37] could provide active protection against nanomaterial-induced free radical damage at the local site of cell/nanomaterial interaction.
In summary, the human health and safety aspects of TPGS along with its known antioxidant function and commercial availability make it potentially attractive as a synthetic surfactant for green processing of carbon nanomaterials. Its behavior in the presence of carbon nanomaterials has not been studied, however, and its effectiveness as dispersant and colloidal stabilizer on those systems is unknown. Here we take the first step to explore this potential of TPGS by investigating its physical interactions with single-wall nanotubes, multi-wall nanotubes, and C60, focusing on aqueous dispersion, surface charge, colloidal stability, and the morphology of the self-assembled nanostructures.
Materials and Methods
Food grade TPGS 1000 (M.W. ~ 1,513 or 22 ethylene glycol repeat units) was a gift from Eastman Chemical Company. Triton X-100 (100%) (M.W. ~ 624) was obtained from Alfa Aesar and used as a common reference surfactant. Purified multi-walled carbon nanotubes (MWNTs) were purchased from MER Corporation (Tucson, AZ), while purified SWNTs with low functionality were obtained from Carbon Solutions, Inc. (Riverside, CA). Commercial high-purity fullerene (99.5%, reagent) were produced by SES Research (Houston, TX). Our aqueous suspensions were filtered through a Corning disposable filter system equipped with 0.22 um cellulose nitrate filter (Fisher Scientific). Nanopure water (conductivity = 1.83 MΩ·cm) was obtained through purification of tap water by a MilliQ water system.
Solution preparation
TPGS aqueous solutions (2 wt%) were made by melting 1.0 g of TPGS (yellow wax; m.p. = 37 – 41 °C) on a hot plate and hydrating the melt with 49 mL of hot nanopure water under continuous magnetic stirring. The mixture was stirred for two more hours until cool (room temperature). The resulting TPGS aqueous solution was colorless and stable at room temperature for months. It was further diluted by nanopure water to 0.05 wt% before use. For the reference surfactant, Triton, 0.05 wt% aqueous solutions were prepared at room temperature by mixing 1.0 g of Triton X-100 (oil-like liquid; m.p. = 6 °C) with 49 mL of nanopure water under continuous magnetic stirring. The mixture was stirred for two additional hours until clear. The resulting Triton aqueous solution was colorless and stable at room temperature for months. It was further diluted by nanopure water to 0.05 wt% before use.
Nanotube dispersion
TPGS solutions of 0.05 wt% (20 – 160 uL) were added to MWNTs (~0.2 mg) and then diluted with nanopure water to 15 ug-C/mL. The resulting mixtures were sonicated for two hours and digital photos taken after 15-hour sitting. The SWNTs were mixed with 0.05 wt% TPGS solutions (0.12 – 6 mL) and then treated similarly. The reference Triton suspensions were made by adding 0.05 wt% Triton solutions (0.2 – 2 mL) to MWNTs (~0.2 mg) and then diluted with nanopure water to 15 ug-C/mL. The resulting mixtures were sonicated for two hours and photos taken after 15-hour sitting. SWNTs were mixed with 0.05 wt% Triton solutions (0.12 – 6 mL) and then treated similarly.
Fullerene dispersion
Excess fullerene powder was added to TPGS melt in a water bath. After 2 hours the brown mixture was hydrated with hot nanopure water under continuous magnetic stirring and cooled. For the Triton reference surfactant, excess fullerene powder was added to Triton liquid, and after 2 hours the brown mixture hydrated with nanopure water under continuous magnetic stirring until the mixture became clear. After 0.22 um filtration both the C60/TPGS and C60/Triton solutions are clear orange and stable at room temperature for months.
Characterization
High-resolution transmission electron microscope (HRTEM) images and electron diffraction patterns were recorded on samples at various stages during the drying process at 200 kV on a JEOL 2010. Dynamic light scattering (DLS) and zeta potential experiments were performed on a Zetasizer Nano-ZS 900. UV-vis light absorption spectra were recorded on a PerkinElmer Lambda 35 UV/VIS Spectrometer in the range 300 – 600 nm.
Results and Discussion
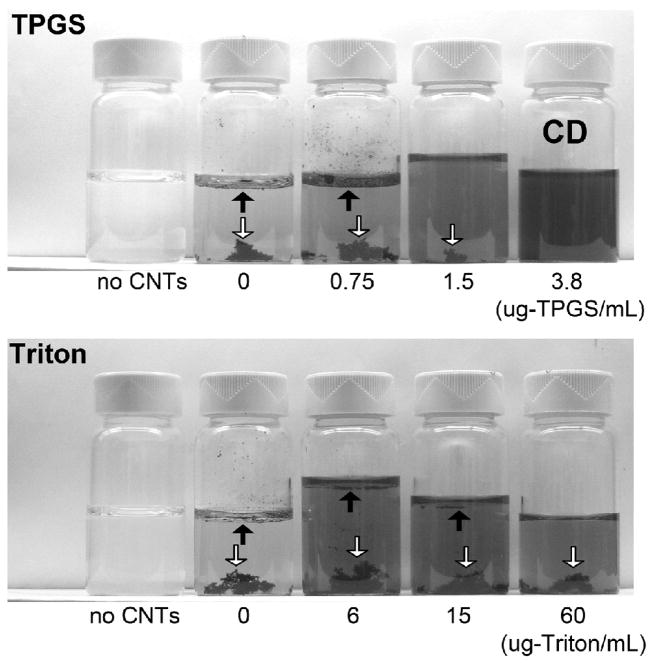
Figure 2 shows the relative ability of TPGS and Triton to disperse multiwall nanotubes at a fixed nanotube concentration of 15 ug/ml. The nanotubes are successfully dispersed at TPGS concentrations of 3.8 ug/ml and above, while lower concentrations leave a film of undispersed solid at the top and bottom liquid/gas interface. At even higher TPGS concentrations, stable gas bubbles collect at the top surface indicating excess TPGS. Though Triton is commonly used for nanotube dispersion [5,38,39], it is less successful here, leaving excess solid at the top liquid/gas interface at concentrations up to 15 or even 60 ug/ml. The polyaromatic content of TPGS may be responsible for its effectiveness in nanotube dispersion based on the known affinity of polyaromatic structures for carbon surfaces [40] and trends seen in other surfactant systems [5].
FIGURE 2.
Effectiveness of TPGS and Triton in dispersing MWNTs. Digital photos of 15 ug/mL MWNT suspensions after 2-hour sonication and 15-hour settling. TPGS studied achieves complete dispersion (“CD”) above 3.8 ug-TPGS/mL. Triton leaves some MWNTs undispersed and visible at the top and bottom surfaces at all concentrations studied (see arrows). Note that 3.8 ug-TPGS/ml is 2.5 uM, while 6 ug-Triton/ml is 10 uM.
Figure 2 indicates dispersion stability after 15 hr of passive settling. We further tested the stability of the suspensions to centrifugation at a fixed surfactant concentration of 25 uM. The MWNTs were removed from the Triton solution after 30 min centrifugation at 1380×G, while an equivalent separation from 25 uM TPGS solution required 4 hr centrifugation at 1380×G. In the case of SWNTs, the two surfactants performed more similarly, dispersing SWNTs well at concentrations above about 6 ug/mL.
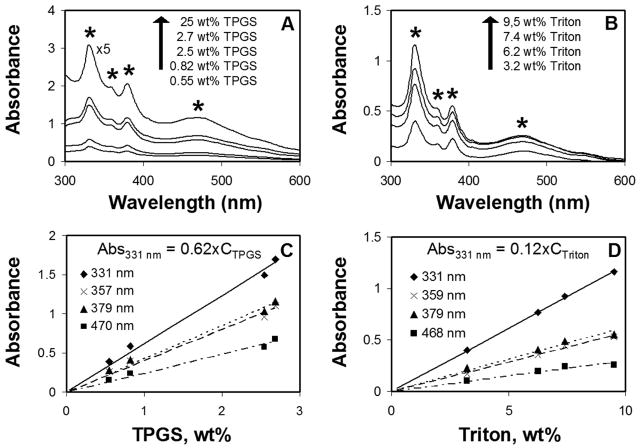
Fullerene C60 is extremely insoluble in water (~1 ng/mL without the use of co-solvents, oxidation, or prolonged stirring [31,41,42]. Figure 3 gives the results for C60 dispersion with TPGS compared with Triton as a reference material using UV/visible spectroscopy as a dispersion metric. Note that in contrast to nanotube dispersion, this is a solubilization process in which molten pure TPGS is saturated with fullerene prior to introduction of water. The UV-vis light spectra in Figs. 3A,C demonstrate the existence of C60, with fullerene characteristic peaks present at 331, 358, 379, and 469 nm [43]. Figures 3C,D give quantitative absorption data for these peaks that can be used to estimate C60 concentration. As an example, we estimate 210 ug-C/mL in 25 wt% TPGS solution using Beer’s Law and ε = 5.19×104 for the absorption peak at 331 nm in water [44]. TPGS is more effective than Triton at dispersing fullerenes as clearly seen by direct comparison of the spectra (A vs. B) or the quantitative absorption measurements (C vs. D). The solubility of C60 is linearly dependent of the surfactant concentration, which is consistent with previous work using commercial TPGS 1000 as drug solubilizer [19]. It is also consistent with our preparation method, in which saturated solutions with a fixed TPGS/C60 ratio are first prepared and then hydrated. Dynamic light scattering experiments show monodisperse nanoparticles of hydrodynamic size 11 nm, which are believed to be fullerene-containing TPGS micelles, and are similar to the pure TPGS micelles with a mean size of 12.7 nm measured by the same technique.
FIGURE 3.
Solubility of C60 in surfactant solutions probed by UV-Vis light absorption. A,B UV-Vis absorption spectra: (A) in TPGS solutions (note: 25 wt-% solution was diluted by 5x prior to spectral measurement); (B) in Triton solutions. C,D: Quantitative optical absorbance at various characteristic wavelengths for C60 absorption as a function of surfactant concentration in aqueous solution. Stars mark characteristic fullerene peaks at 331, 358, 379, and 469 nm [43].
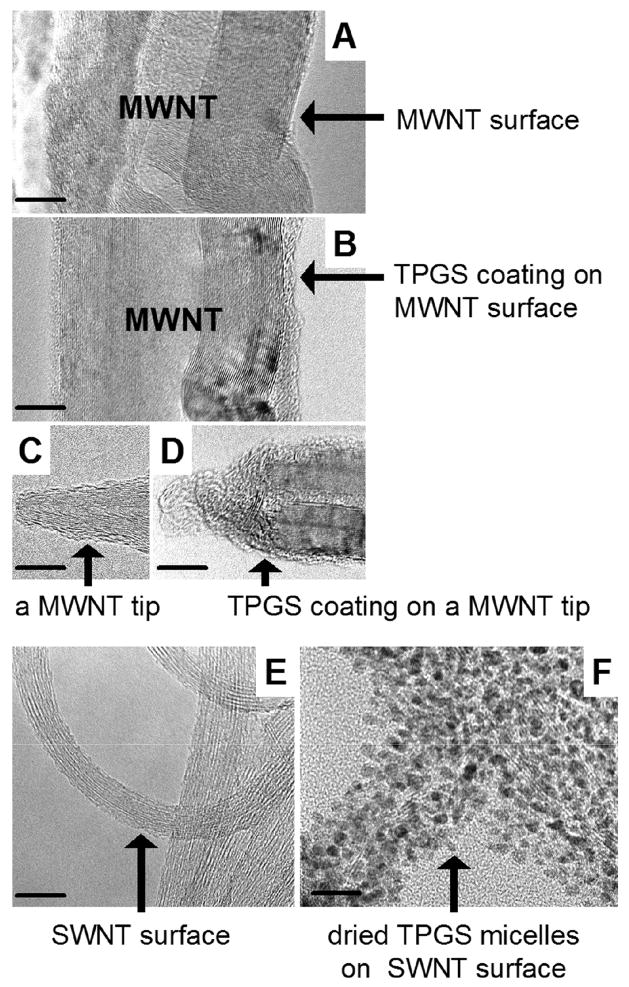
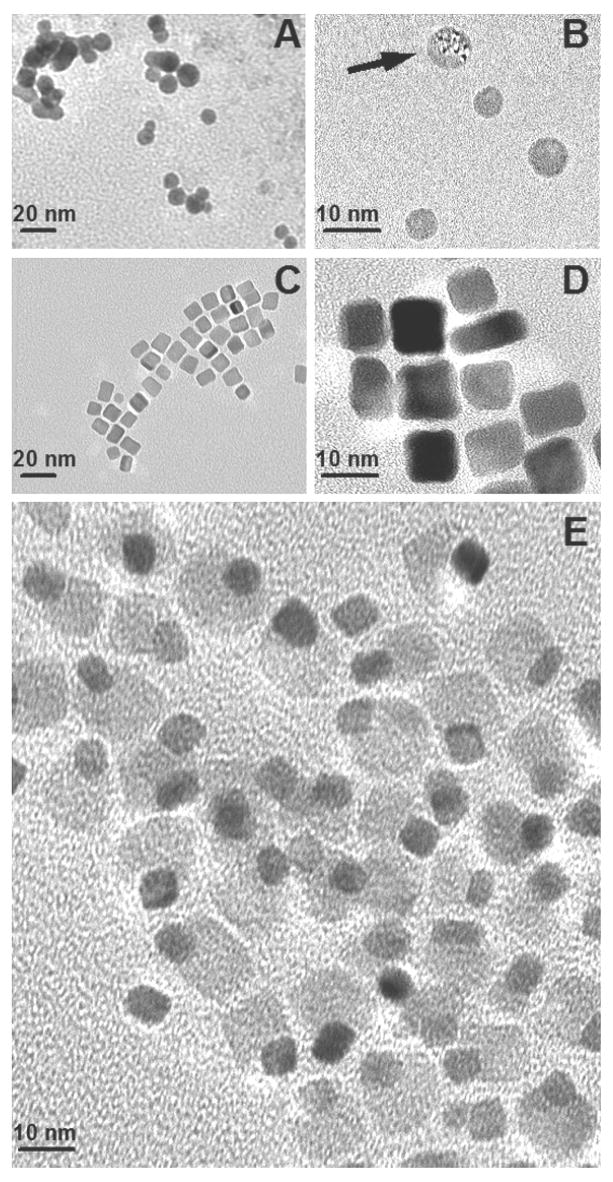
Figure 4 shows the morphology of TPGS/nanotube assemblies by HRTEM. An amorphous TPGS coating is seen on the MWNT surfaces dried from TPGS solution, but not the as-received MWNTs. This tube-by-tube TPGS coating is the most likely colloidal stabilization mechanism. There appears to be an amorphous film on the SWNT bundles as well, though it is not as pronounced in TEM. Neither TEM nor UV-Vis spectroscopy gives evidence for individual, fully unbundled SWNTs in the TPGS or Triton solutions under our conditions [45]. It is possible that these surfactants do not have a favorable geometry for stabilization of the very small 1–2 nm structures as does single-stranded DNA [46]. Figure 4F shows SWNTs dried from concentrated TPGS solutions (15 ug/ml). Under these conditions the excess TPGS associates with the SWNT bundles as clearly visible, intact micelles.
FIGURE 4.
HRTEM images of carbon nanotubes in TPGS solutions after 2-hour sonication. (A–D) 15 ug/mL MWNTs (A,C) in water or (B,D) in 3.8 ug/mL TPGS solution. Scale bar: 5 nm. (E,F) 15 ug/mL SWNTs (E) in water, (F) in 15 ug/mL TPGS solution. Scale bar: 20 nm.
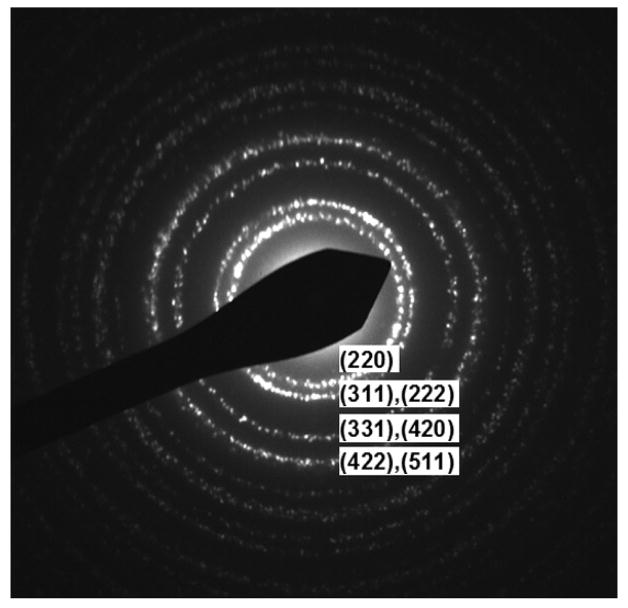
Figure 5 gives the morphologies of TPGS/C60 assemblies at various stages of drying. Initially spherical structures are seen (Fig. 5A), which in size and morphology are consistent with pure TPGS micelles. Under the action of the electron beam we observed the appearance of high-electron-density regions within the spheres that suggests phase separation (see arrow in B). During longer air drying at room temperature (3 days), the spherical nanostructures develop facets and transform into nanocubes, some of which have asymmetric electron density (Fig. 5C,D). Finally after 1 week of drying, the sample transforms into a uniform collection of highly ordered asymmetric particles that appear to be fullerene nanocrystals (nC60) adhered to globular or cubic nanostructures of lower electron density. These cubic structures are equiaxed and about 10 nm in dimension, similar to the length of the TPGS molecule. The ED pattern in Fig. 6 confirms the formation of nC60 during air drying, showing a face-centred cubic (fcc) structure with a crystal parameter of 1.4 – 1.5 nm [43].
FIGURE 5.
HRTEM images of unique TPGS/C60 nanostructures following various degrees of drying from 3.8 ug/mL TPGS/C60 solutions. A,B: 20-hour air drying; (arrow shows appearance of fullerene nanocrystals upon e-beam exposure), C,D: faceted cubic particles after 3-day air drying; and (E) unique asymmetric nanostructures after 1-week air drying.
FIGURE 6.
The ED pattern of TPGS/C60 nanostructures after 3-day air drying. The rings are assigned to fcc-structure of nC60, but the (111) ring is not observed due to its very large d value, 0.82 nm.
We interpret these results as follows. Fullerene is dissolved at the molecular level in TPGS micelles, likely concentrated in the hydrophobic α-tocopheryl cores. Because the mass ratio of fullerene to TPGS is low (~ 1:1000) these micelles are similar in size and structure to those in pure TPGS solutions. The TPGS micelles remain amorphous unless dried extensively in air or under the electron beam, at which point the C60 phase separates and the dehydrated TPGS crystallizes. When the amphiphile TPGS crystallizes at the nanoscale, the result are amphiliphilic nanoparticles with one hydrophobic end (tocopheryl) and one hydrophilic end (PEG). In the presence of C60 this produces a set of unique asymmetric particles with fullerene nanocrystals attached at one face to TPGS nanobrushes. The uniform and highly ordered nature of these particles suggests a significant driving force for their formation and some significant stabilizing mechanism, which we believe is the attachment of fullerene crystals to the hydrophobic end of the TPGS nanobrushes, thus stabilizing the structure by tocopheryl/C60 hydrophobic interaction.
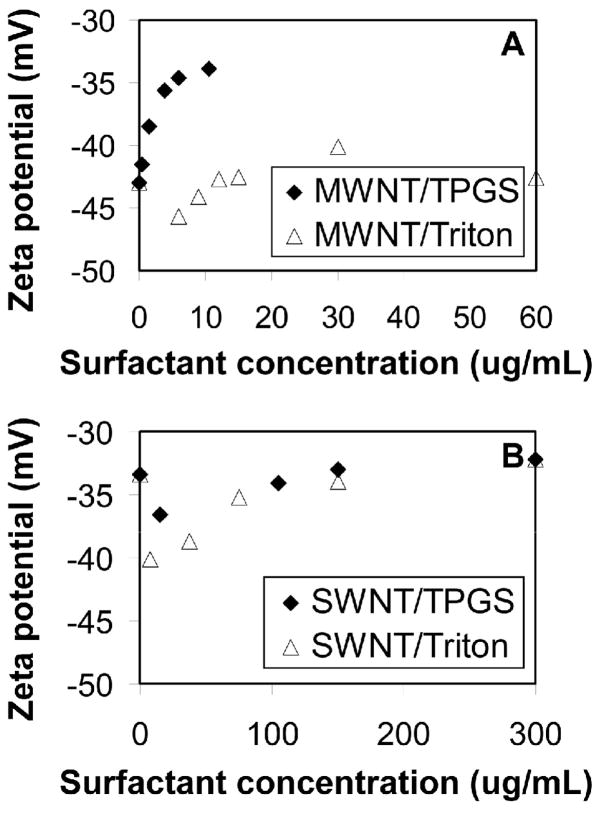
Figure 7 shows zeta potentials of nanotube/surfactant suspensions measured at room temperature. Increasing surfactant concentration decreases the magnitude of the zeta potentials as expected for these non-ionic surfactants, which preserve the nanotube surface charge but increase the hydrodynamic particle size. The zeta potential of aqueous C60 in surfactant solutions was −5.0±1.2 mV, and the pure TPGS and Triton micelles did not have a measurable zeta potential. Compared to other methods of preparing aqueous C60 dispersions, the present method has the advantage of avoiding cosolvents or covalent fullerene modification. The fullerene/TPGS solutions are stable indefinitely and non-enzymatic hydrolysis at physiological pH is extremely slow. A disadvantage is the large TPGS/C60 mass ratio required (~ 1000:1), which in turn is limited by the inherent solubility of C60 in molten TPGS in our preparation method. This is in contract to TPGS/MWNT suspensions, where the minimum ratio for good dispersion is only 1:4 (3.8:15 as seen in Fig. 2).
FIGURE 7.
Zeta potentials of nanotube/surfactant suspensions at: 15 ug-CNT/mL; A: MWNT; B: SWNT.
Conclusions
Overall, TPGS is a promising surfactant for “green” processing of carbon nanomaterials due to its low human health risk, commercial availability, and effectiveness as a dispersant and stabilizer. TPGS is especially promising for multi-wall nanotube processing, where it is an effective dispersant at mass ratios (TPGS:C) of 1:4 or greater and is more effective than the commonly used synthetic non-ionic surfactant Triton. In light of the present results on the physical interactions of TPGS with carbon nanomaterials, detailed biological studies are justified to determine if and under what conditions the antioxidant properties of TPGS may serve to actively mitigate oxidative stress induced by nanomaterial exposure.
Acknowledgments
The authors are grateful for financial support from the NSF (NIRT grant 0506661), the NIEHS Superfund Based Research Program (P42 ES013660) and EPA (STAR grant RD-83171901-0). Although the research described in the article was funded in part by EPA and NIEHS, it does not necessarily reflect the views of either agency. Technical contributions or sample donations from Anthony McCormick, Zhichuan Xu, Indrek Külaots, Yuming Gao, Xinyuan Liu, Lin Guo, and Love Sarin are also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vaisman L, Wagner HD, Marom G. The role of surfactants in dispersion of carbon nanotubes. Adv Colloid Interface Sci. 2006;128–130:37–46. doi: 10.1016/j.cis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Balavoine F, Schultz P, Richard C, Mallouh V, Ebbesen TW, Mioskowski C. Helical crystallization of proteins on carbon nanotubes: a first step towards the development of new biosensors. Angew Chem Int Ed. 1999;38(13–14):1912–5. doi: 10.1002/(SICI)1521-3773(19990712)38:13/14<1912::AID-ANIE1912>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Karajanagi SS, Yang HC, Asuri P, Sellitto E, Dordick JS, Kane RS. Protein-assisted solubilization of single-walled carbon nanotubes. Langmuir. 2006;22(4):1392–5. doi: 10.1021/la0528201. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell MJ, Boul P, Ericson LM, Huffman C, Wang Y, Haroz E, et al. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem Phys Lett. 2001;342:265–71. [Google Scholar]
- 5.Islam MF, Rojas E, Bergey DM, Johnson AT, Yodh AG. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003;3(2):269–73. [Google Scholar]
- 6.Zhang J, Wang Q, Wang L, Wang A. Manipulated dispersion of carbon nanotubes with derivatives of chitosan. Carbon. 2007;45(9):1917–20. [Google Scholar]
- 7.Zelenak JP, Yves Alarie Y, Weyel DA. Assessment of the cough reflex caused by inhalation of sodium lauryl sulfate and citric acid aerosols. Fundam Appl Toxicol. 1982;2(4):177–80. doi: 10.1016/s0272-0590(82)80043-2. [DOI] [PubMed] [Google Scholar]
- 8.Hida S, Yasuda H. Alveolar injury by inhalation of surfactant Triton X-100. Nippon Kaimen Igakkai Zasshi. 1998;29(1–2):79–80. [Google Scholar]
- 9.Lewis MA. The effects of mixtures and other environmental modifying factors on the toxicities of surfactants to fresh-water and marine life. Water Res. 1992;26(8):1013–23. [Google Scholar]
- 10.Monteiro-Riviere NA, Inman AO, Wang YY, Nemanich RJ. Surfactant effects on carbon nanotube interactions with human keratinocytes. Nanomedicine. 2005;1(4):293–9. doi: 10.1016/j.nano.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Van Hamme JD, Singh A, Ward OP. Physiological aspects Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol Adv. 2006;24(6):604–20. doi: 10.1016/j.biotechadv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Arechabala B, Coiffard C, Rivalland P, Coiffard LJM, de Roeck-Holtzhauer Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J Appl Toxicol. 1999;19:163–165. doi: 10.1002/(sici)1099-1263(199905/06)19:3<163::aid-jat561>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura K, Saito T, Okazaki T, Ohshima S, Yumura M, Iijima S. Selectivity of water-soluble proteins in single-walled carbon nanotube dispersions. Chem Phys Lett. 2006;429(4–6):497–502. [Google Scholar]
- 14.Chow AHL, Tong HHY, Chattopadhyay P, Shekunov BY. Particle engineering for pulmonary drug delivery. Pharm Res. 2007;24(3):411–37. doi: 10.1007/s11095-006-9174-3. [DOI] [PubMed] [Google Scholar]
- 15.Traber MG, Kayden HJ, Green JB, Green MH. Absorption of water-miscible forms of vitamin E in a patient with cholestasis and in thoracic duct-cannulated rats. Am J Clin Nutr. 1986;44(6):914–23. doi: 10.1093/ajcn/44.6.914. [DOI] [PubMed] [Google Scholar]
- 16.Traber MG, Schiano TD, Steephen AC, Kayden HJ, Shike M. Efficacy of water-soluble vitamin E in the treatment of vitamin E malabsorption in short-bowel syndrome. Am J Clin Nutr. 1994;59:1270–4. doi: 10.1093/ajcn/59.6.1270. [DOI] [PubMed] [Google Scholar]
- 17.Sokol RJ, Heubi JE, Butler-Simon N, McClung HJ, Lilly JR, Silverman A. Treatment of vitamin E deficiency during chronic childhood cholestasis with oral d-alpha-tocopheryl polyethylene glycol-1000 succinate. Gastroenterology. 1987;93(5):975–85. doi: 10.1016/0016-5085(87)90559-2. [DOI] [PubMed] [Google Scholar]
- 18.Sokol RJ, Butler-Simon N, Conner C, Heubi JE, Sinatra FR, Suchy FJ, et al. Multicenter trial of d-alpha-tocopheryl polyethylene glycol 1000 succinate for treatment of vitamin E deficiency in children with chronic cholestasis. Gastroenterology. 1993;104(6):1727–35. doi: 10.1016/0016-5085(93)90652-s. [DOI] [PubMed] [Google Scholar]
- 19.Constantinides PP, Han J, Davis SS. Advances in the use of tocols as drug delivery vehicles. Pharm Res. 2006;23(2):243–55. doi: 10.1007/s11095-005-9262-9. [DOI] [PubMed] [Google Scholar]
- 20.Varma MV, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005;25(4–5):445–53. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Hidiroglou N, McDowell LR, Batra TR, Papas AM. Tissue alpha-tocopherol concentrations following supplementation with various forms of vitamin E in sheep. Reprod Nutr Dev. 1994;34(3):273–8. doi: 10.1051/rnd:19940309. [DOI] [PubMed] [Google Scholar]
- 22.Feng SS, Mu L, Win KY, Huang GF. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr Med Chem. 2004;11(4):413–24. doi: 10.2174/0929867043455909. [DOI] [PubMed] [Google Scholar]
- 23.Youk HJ, Lee E, Choi MK, Lee YJ, Chung JH, Kim SH, et al. Enhanced anticancer efficacy of alpha-tocopheryl succinate by conjugation with polyethylene glycol. J Controlled Release. 2005;107(1):43–52. doi: 10.1016/j.jconrel.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Fulzele SV, Chatterjee A, Shaik MS, Jackson T, Singh M. Inhalation delivery and anti-tumor Activity of celecoxib in human orthotopic non-small cell lung cancer xenograft model. Pharm Res. 2006;23(9):2094–106. doi: 10.1007/s11095-006-9074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arkhipenko IuV, Dobrina SK, Kagan VE, Kozlov IuP, Nadirov NK, Pisarev VA, et al. Mechanisms of stabilizing effect of vitamin E against lipid peroxidation in biological membranes. Biokhimii a (Moscow, Russia) 1977;42(8):1525–31. [PubMed] [Google Scholar]
- 26.Wagner BA, Buettner GR, Burns CP. Vitamin E slows the rate of free radical-mediated lipid peroxidation in cells. Arch Biochem Biophys. 1996;334(2):261–7. doi: 10.1006/abbi.1996.0454. [DOI] [PubMed] [Google Scholar]
- 27.Majno G, Joris I. Chapter 5: Cell Injury and Cell Death. 2. Oxford University Press; New York: 2004. Cells, Tissues, and Disease: Principles of General Pathology; pp. 196–8. [Google Scholar]
- 28.Traber MG, Thellman CA, Rindler MJ, Kayden HJ. Uptake of intact TPGS (d-a-tocopheryl polyethylene glycol 1000 succinate) a water-miscible form of vitamin E by human cells in vitro. Am J Clin Nutr. 1988;48:605–11. doi: 10.1093/ajcn/48.3.605. [DOI] [PubMed] [Google Scholar]
- 29.McCay PB. Vitamin E: interactions with free radicals and ascorbate. Ann Rev Nutr. 1985;5:323–40. doi: 10.1146/annurev.nu.05.070185.001543. [DOI] [PubMed] [Google Scholar]
- 30.Miki M, Tamai H, Mino M, Yamamoto Y, Niki E. Free-radical chain oxidation of rat red blood cells by molecular oxygen and its inhibition by -tocopherol. Arch Biochem Biophys. 1987;258:373–80. doi: 10.1016/0003-9861(87)90358-4. [DOI] [PubMed] [Google Scholar]
- 31.Sayes CM, Gobin AM, Ausman KD, Mendez J, West JL, Colvin VL. Nano-C60 cytotoxicity is due to lipid peroxidation. Biomaterials. 2005;26:7587–95. doi: 10.1016/j.biomaterials.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 32.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 33.Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264. 7 macrophages. Role of iron Toxicol Lett. 2006;165(1):88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Guo L, Morris DG, Liu X, Vaslet C, Hurt RH, Kane AB. Iron bioavailability and redox activity in diverse carbon nanotube samples. Chem Mater. 2007;19(14):3472–8. [Google Scholar]
- 35.Shvedova AA, Kisin ER, Murray AR, Gorelik O, Arepalli S, Castranova V, et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol Appl Pharmacol. 2007;221(3):339–48. doi: 10.1016/j.taap.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowley MM, Zhang F, Koleng JJ, McGinity JW. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials. 2002;23(21):4241–8. doi: 10.1016/s0142-9612(02)00187-4. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen TK, Nilakantan V, Felix CC, Khanna AK, Pieper GM. Beneficial effect of a-tocopheryl succinate in rat cardiac transplants. J Heart Lung Transplantation. 2006;25(6):707–15. doi: 10.1016/j.healun.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Nagahara L, Amlani I, Lewenstein J, Tsui RK. Directed placement of suspended carbon nanotubes for nanometer-scale assembly. Appl Phys Lett. 2002;80(20):3826–8. [Google Scholar]
- 39.Lewenstein JC, Burgin TP, Ribayrol A, Nagahara LA, Tsui RK. High-yield selective placement of carbon nanotubes on pre-patterned electrodes. Nano Lett. 2002;2(5):443–6. [Google Scholar]
- 40.Hurt RH, Krammer G, Crawford G, Jian K, Rulison C. Polyaromatic assembly mechanisms and structure selection in carbon materials. Chem Mater. 2002;14:4558–65. [Google Scholar]
- 41.Bezmel’nitsyn VN, Eletskii AV, Okun MV. Fullerenes in solutions. Physics - Uspekhi. 1998;41(11):1091–114. [Google Scholar]
- 42.Hotze EM, Badireddy AR, Chellam S, Wiesner MR. Singlet oxygen and superoxide production by three types of aqueous fullerene suspensions. Abstracts of Papers, 233rd ACS National Meeting; Chicago, IL, United States. March 25–29, 2007. [Google Scholar]
- 43.Kroto HW, Allaf AW, Balm SP. C60: Buckminsterfullerene. Chem Rev. 1991;91:1213–35. [Google Scholar]
- 44.Zhang Y, Liu W, Gao X, Zhao Y, Zheng M, Li F. The first synthesis of a water-soluble α-cyclodextrin/C60 supramolecular complex using anionic C60 as a building block. Tetrahedron Lett. 2006;47(48):8571–4. [Google Scholar]
- 45.O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297(5581):593–6. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 46.Zheng M, Jagota A, Strano MS, Santos AP, Barone P, Chou SG, et al. Structure-based carbon nanotubes sorting by sequence-dependent DNA assembly. Science. 2003;302(5650):1545–8. doi: 10.1126/science.1091911. [DOI] [PubMed] [Google Scholar]