Abstract
Background
Ly-6Chi monocytes are key contributors to atherosclerosis in mice. However, how Ly-6Chi monocytes selectively accumulate in atherosclerotic lesions is largely unknown. Monocyte homing to sites of atherosclerosis is primarily initiated by rolling on P- and E-selectin expressed on endothelium. We hypothesize that P-selectin glycoprotein ligand-1 (PSGL-1), the common ligand of P- and E-selectin on leukocytes, contributes to the preferential homing of Ly-6Chi monocytes to atherosclerotic lesions.
Methods and Results
To test this hypothesis, we examined the expression and function of PSGL-1 on Ly-6Chi and Ly-6Clo monocytes from wild-type mice, ApoE-/- mice, and mice lacking both ApoE and PSGL-1 genes (ApoE-/-/PSGL-1-/-). We found that Ly-6Chi monocytes expressed a higher level of PSGL-1, and had enhanced binding to fluid-phase P- and E-selectin, compared to Ly-6Clo monocytes. Under in vitro flow conditions, more Ly-6Chi monocytes rolled on P-, E-, and L-selectin at slower velocities than Ly-6Clo cells. In an ex vivo perfused carotid artery model, Ly-6Chi monocytes interacted preferentially with atherosclerotic endothelium compared with Ly-6Clo monocytes in a PSGL-1-dependent manner. In vivo, ApoE-/- mice lacking PSGL-1 had impaired Ly-6Chi monocyte recruitment to atherosclerotic lesions. Moreover, ApoE-/-/PSGL-1-/- mice exhibited significantly reduced monocyte infiltration in wire injury-induced neointima and in atherosclerotic lesions. ApoE-/-/PSGL-1-/- mice also developed smaller neointima and atherosclerotic plaques.
Conclusions
These data indicate that PSGL-1 is a new marker for Ly-6Chi monocytes and a major determinant for Ly-6Chi cell recruitment to sites of atherosclerosis in mice.
Keywords: atherosclerosis, leukocytes, endothelium, cell adhesion molecules
Atherosclerosis is characterized by inflammatory cell infiltration of the arterial wall.1, 2 Early atherosclerotic lesions are largely composed of lipid-laden macrophages known as foam cells, which are derived from circulating monocytes.1, 2 Thus, the recruitment of monocytes into the arterial wall plays decisive roles in the initiation and progression of atherosclerosis.
In mice, monocytes are divided into Ly-6Chi and Ly-6Clo subsets. Ly-6Chi monocytes are considered short-lived “inflammatory” cells that are actively recruited to inflamed tissues, whereas Ly-6Clo monocytes have a longer half-life and home to non-inflamed tissues to differentiate into resident macrophages.3 Ly-6Chi monocytes are preferentially recruited to atherosclerotic plagues and give rise to lipid-laden macrophages in apoE-deficient (ApoE-/-) mice.4 Ly-6Chi monocytes are recognized as the key monocyte subset in the development of atherosclerosis,4, 5 but how these monocytes selectively accumulate in atherosclerotic lesions are largely unknown.
During inflammation, monocyte recruitment follows the well-defined leukocyte trafficking cascade.6-8 Circulating leukocytes first tether to and roll on the activated endothelium, then firmly adhere and eventually transmigrate into the underlying tissues. These steps are regulated by adhesion molecules and chemokines. Murine Ly-6Chi and Ly-6Clo monocytes differ with regard to their expression of chemokine receptors and adhesion molecules. Ly-6Chi monocytes are CCR2+, CX3CR1lo, L-selectin+, CD44+, LFA1+, and VLA4+, whereas Ly-6Clo cells are CCR2-, CX3CR1hi, L-selectin-, CD44+, LFA1++, and VLA4+.3 Interestingly, only CCR2 and L-selectin are differentially expressed on Ly-6Chi cells.3, 4 Although CCR2 is important for monocytes to enter atherosclerotic lesions,5, 9 it does not mediate the early adhesion steps such as tethering and initial rolling.5, 10 In blood flow conditions, especially arterial flow conditions, the initial steps serve as an anchoring system to capture flowing leukocytes and initiate rolling on the endothelium for subsequent firm adhesion and transmigration. The initial steps are primarily mediated by P-, E-, and L-selectin and their common ligand on leukocytes, P-selectin glycoprotein ligand-1 (PSGL-1).7 E-selectin and P-selectin are expressed on activated endothelial cells and/or platelets, whereas L-selectin is expressed on leukocytes. PSGL-1 binds to selectins with exceptionally high on- and off-rates, which favors interactions under flow. Moreover, shear stress actually strengthens the interactions of PSGL-1 with P- and L-selectin.7, 11 Ly-6Chi cells exhibit an advantage over the Ly-6Clo subset with regard to homing to atherosclerotic plagues.4, 5 This preferential homing could be a result of differential expression or function of PSGL-1. In addition, L-selectin-mediated rolling may also contribute to this difference because L-selectin is expressed on Ly-6Chi but not on Ly-6Clo monocytes.
To test this hypothesis, we examined the surface expression and function of PSGL-1 on Ly-6Chi and Ly-6Clo monocytes from wild-type and ApoE-/- mice. We also investigated PSGL-1-dependent interactions of Ly-6Chi or Ly-6Clo monocytes with P-, E-, and L-selectin under in vitro flow conditions, and with early atherosclerotic endothelium using ex vivo perfusion of carotid arteries. To evaluate the role of PSGL-1 in the development of atherosclerosis, we studied atherosclerosis and wire injury-induced neointimal formation in the carotid artery in ApoE-/- mice and in mice lacking both ApoE and PSGL-1 genes (ApoE-/-/PSGL-1-/-). Our results demonstrate that PSGL-1 is highly expressed on Ly-6Chi monocytes and is a major determinant for Ly-6Chi monocyte recruitment to atherosclerotic lesions.
Methods
A full description of all Methods can be found in the online-only Data Supplement.
Mice
ApoE-/-/PSGL-1-/--deficient mice (C57BL/6J background) were generated after crossing PSGL-1-/- mice12 with ApoE-/- mice. Wild-type C57BL/6J (WT) and ApoE-/- mice were from the Jackson Laboratory (Bar Harbor, ME). Mice were kept in a specific pathogen-free facility. All mouse experiments were approved by the Institutional Animal Care and Use Committees of the Oklahoma Medical Research Foundation and the University of Minnesota.
Flow cytometry
All antibodies were obtained from BD Biosciences (San Diego, CA) unless specified. Mouse peripheral blood was used for monocyte analysis. Leukocytes that were positive for myeloid marker CD11b but negative for the rest of the lineage markers were defined as monocytes.4 Anti-Ly-6C mAb (AL-21) was used to classify monocytes into subsets with either high expression of Ly-6C (Ly-6Chi) or low expression of Ly-6C (Ly-6Clo).4 A rat anti-mouse PSGL-1 mAb (4RA10) was used to measure PSGL-1 expression.
The chemokine receptor CX3CR1 was originally used to classify monocytes.3 In mice, the CX3CR1loCD11b+ monocyte subset is the equivalent of Ly-6Chi monocytes.3 To examine PSGL-1 expression and P- and E-selectin binding of CX3CR1loCD11b+ monocytes, C57BL/6 CX3CR1GFP/+ mice were used. In CX3CR1GFP/+ mice, one CX3CR1 allele was replaced with the gene encoding green fluorescent protein (GFP).13
Human monocytes have CD14+CD16- and CD14-CD16+ subsets, which resemble murine Ly-6ChiCX3CR1lo and Ly-6CloCX3CR1hi subsets, respectively.3 Human leukocytes that were HLA-DR and CD14 double-positive, or HLA-DR and CD16 double-positive were analyzed for PSGL-1 expression using a mAb to human PSGL-1 (KPL1). The protocol was approved by the IRB committee of the University of Minnesota.
Flow cytometry was performed on a FACSCalibur (BD Biosciences). Data were analyzed using Summit Software v4.3 (Dako, Carpinteria, CA).
In vitro flow chamber assay
To obtain sufficient cells, Ly-6Chi and Ly-6Clo monocytes from murine spleens were used. The spleen-derived monocytes are surrogates for circulating monocytes.4 Ly-6Chi and Ly-6Clo monocytes were sorted from the monocyte population using the inFlux V-GS Cytometer Work Bench (Cytopeia, Seattle, WA).
Flow chamber experiments were carried out as described.11 Briefly, murine P-selectin-IgM, E-selectin-IgM, control CD45-IgM, biotinylated 2-glycosulfopeptide-6 (2-GSP-6, gift from Dr. Richard Cummings, Emory University), or human L-selectin IgG chimera was captured on the dishes. 2-GSP-6 is modeled after the NH2-terminal selectin-binding region of PSGL-1.14 Human L-selectin is the equivalent of murine L-selectin because they share the same binding activity to murine PSGL-1. Sorted Ly-6Chi or Ly-6Clo monocytes (0.5 × 106/ml in HBSS with 0.5% HSA) were perfused over P-selectin, E-selectin, control CD45-IgM, 2-GSP-6, or L-selectin in dishes mounted in a parallel-plate flow chamber. Rolling cells were analyzed using a Silicon Graphics workstation (Silicon Graphics, Sunnyvale, CA).
Ex vivo perfusion of murine carotid arteries
WT or PSGL-1-/- Ly-6Chi or Ly-6Clo monocytes were labeled with calcein AM (Molecular Probes, Eugene, Oregon), and were infused into the ApoE-/- carotid arteries at a rate of 10 ml/min (1 × 106 cells/ml), resulting in a wall shear stress of 3.0 ± 0.1 dyne/cm2.10 Cell rolling and adhesion were recorded on videotape by stroboscopic epifluorescence illumination with an intravital microscope (Axioskop, 10× water immersion objective, NA 0.5, Carl Zeiss, Thornwood, NY).
Atherosclerosis and carotid artery wire injury models
To induce atherosclerosis, ApoE-/-/PSGL-1-/- or ApoE-/- mice at 5 weeks of age were fed the Western diet for 12 weeks.5 Atherosclerotic lesions stained with Oil red O were quantified by en face analysis of the whole aorta and by cross-sectional analysis of the proximal aorta.15 For the carotid artery wire injury models, 8-week-old male ApoE-/- mice were fed the Western diet for 2 weeks and carotid artery wire injury was performed.16 For quantification of neointimas, ten 5-μm aortic sections that were stained with Movat pentachrome (Sigma, St. Louis, MO) were analyzed for each mouse. Images were analyzed using the NIH Image software.10
Immunostaining
Cryosections of aortas from ApoE-/- and ApoE-/-/PSGL-1-/- mice were stained with anti-F4/80 mAb (MOMA-2) (Accurate Chemical, Westbury, NY).17 Eight sections every 40 μm from each aorta root were examined. Digital images were used to quantify macrophages with NIH Image J software.
Ly-6Chi monocytes in cryosections of ApoE-/- and ApoE-/-/PSGL-1-/- carotid arteries were examined using an FITC-conjugated anti-mouse Ly-6C mAb (AL-21, 1:100 dilution, BD Biosciences) as described.4 Peripheral Ly-6Chi or Ly-6Clo monocytes, which were cytocentrifuged on glass slides, were stained with the same FITC-conjugated mAb and used as controls for the identification of Ly-6Chi cells. Fluorescence was detected using a fluorescence microscope (4× objective, NA 0.3, Olympus America, Center Valley, PA).
Statistical analyses
Mean and SEM are reported where appropriate. Data were analyzed by the Student’s t test. P < 0.05 was considered significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Murine Ly-6ChiCX3CR1lo monocytes and human CD14+CD16- monocytes express high levels of PSGL-1
We examined PSGL-1 expression on mouse Ly-6Chi and Ly-6Clo monocytes. We defined monocytes as CD11b+CD90loB220loCD49bloNK1.1loLy-6Glo cells (Figure 1A).4 Within this monocyte population, cells were classified into Ly-6Chi and Ly-6Clo subsets based on their differential expression of Ly-6C (Figure 1B). The overall monocytes were not significantly different among ApoE-/- and ApoE-/-/PSGL-1-/- mice fed the Western diet for 12 weeks and ApoE-/- mice fed a standard chow (Figure 1A and data not shown). However, ApoE-/- mice fed the Western diet had an elevated number of Ly-6Chi monocytes compared to the number of Ly-6Chi monocytes from ApoE-/- mice fed chow (Figure 1B and C), which is consistent with published results.4, 5 The percentage of Ly-6Chi monocytes was similar in ApoE-/- and in ApoE-/-/PSGL-1-/- mice fed the high-fat diet (Figure 1C). Remarkably, PSGL-1 was expressed at a much higher level on Ly-6Chi than on Ly-6Clo monocytes from ApoE-/- mice fed either chow or the Western diet (Figure 1D and E). The increase in PSGL-1 expression was not caused by the ApoE deficiency because Ly-6Chi monocytes from WT mice also expressed more PSGL-1 (Figure 1E). Real-time PCR demonstrated that PSGL-1 mRNA transcripts in Ly-6Chi monocytes were also expressed at a higher level than the transcripts in Ly-6Clo monocytes (Figure 1F).
Figure 1.
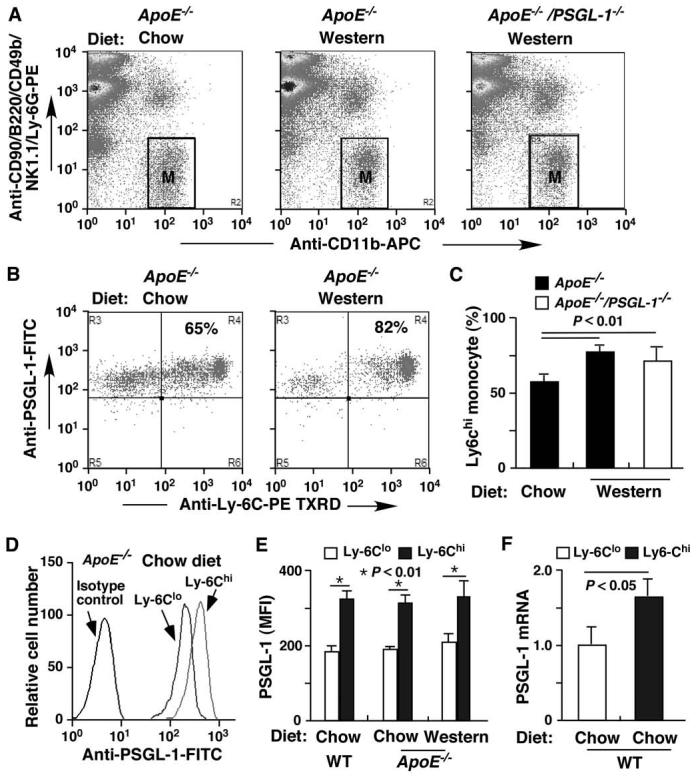
Ly-6Chi monocytes have significantly increased PSGL-1 expression. A. Peripheral murine leukocytes were stained with mAbs to CD90, B220, CD49b, NK1.1, Ly-6G, and CD11b. Cells in gate M were defined as monocytes. B. Representative dot plots showing expression of Ly-6C and PSGL-1 in the total monocytes (gate M). Percentages in the upper right panels represent Ly-6ChiPSGL-1hi monocytes. C. Comparisons of the number of Ly-6Chi monocytes in ApoE-/- and ApoE-/-/PSGL-1-/- mice on the Western diet with ApoE-/- mice fed the chow diet. D. Representative PSGL-1 expression on Ly-6Chi and Ly-6Clo monocytes from an ApoE-/- mouse fed the chow diet. E. Quantification of PSGL-1 expression (MFI, mean fluorescence intensity) on Ly-6Chi and Ly-6Clo monocytes from WT and ApoE-/- mice. F. Real-time PCR quantification (fold changes) of PSGL-1 transcripts of Ly-6Chi and Ly-6Clo monocyte subsets from WT mice. The fluorescent intensities of both axes (A and B) or x axis (D) are on a log scale. Data are means ± SEM (A-E: n = 17 mice/group; F, n = 9 mice/group).
We confirmed that PSGL-1 was also highly expressed on CX3CR1loCD11b+ monocytes compared to its expression on CX3CR1hiCD11b+ monocytes (Figure 2A and B). In addition, CX3CR1loCD11b+ monocytes exhibited greater binding to P- and E-selectin (Figure 2C and D) compared with CX3CR1hiCD11b+ monocytes.
Figure 2.
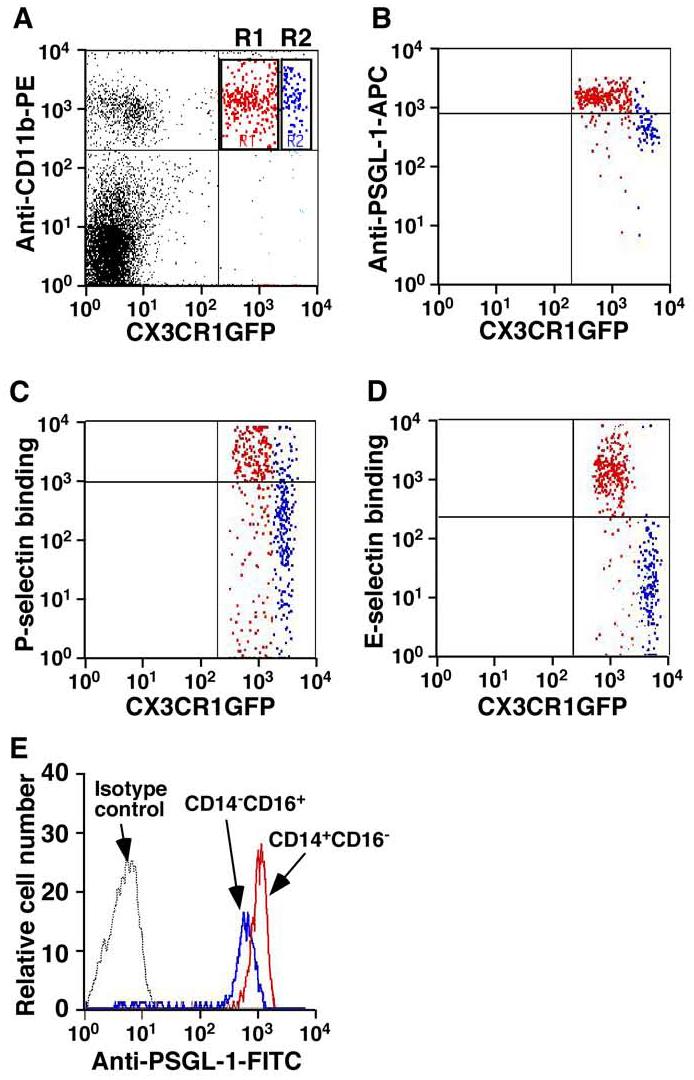
PSGL-1 is highly expressed on murine CX3CR1loCD11b+ monocytes as well as on human CD14+CD16- monocytes. A. Representative dot plot showing CX3CR1loCD11b+ (R1 region, red) and CX3CR1hiCD11b+ (R2 region, blue) monocytes. Cells in the gated R1 and R2 regions were examined for expression of PSGL-1 (B) and for interactions with P-selectin (C) and E-selectin (D). Labels on both axes are on a log scale. The data represent at least three experiments. E. Representative histogram showing PSGL-1 expression on human CD14+CD16- monocytes. Labels on x axis is on a log scale. Shown are the results of three independent experiments.
We also investigated PSGL-1 level on human monocytes. Interestingly, consistent with the expression patterns of PSGL-1 on murine monocytes, PSGL-1 level was also higher on human CD14+CD16- monocytes than on CD14-CD16+ monocytes (Figure 2E, n = 3, P < 0.01).
Ly-6Chi monocytes interact preferentially with P-, E-, and L-selectin as well as 2-GSP-6 under flow
To evaluate the function of PSGL-1 on Ly-6Chi monocytes, we first tested fluid-phase binding of P- and E-selectin to WT monocytes using flow cytometry. Compared with Ly-6Clo monocytes, Ly-6Chi monocytes had greater binding to P- and E-selectin (Figure 3A-B). The interaction between PSGL-1 and P-selectin was PSGL-1-dependent because 4RA10, a mAb that blocks PSGL-1 function, eliminated this interaction (Figure 3A). 4RA10 substantially reduced but did not abolish the PSGL-1 interaction with E-selectin (Figure 3B), which reflects E-selectin ligand activities other than PSGL-1 on Ly-6Chi monocytes.18 Ly-6Chi monocytes from ApoE-/- mice fed the Western diet for 12 weeks had similar P- and E-selectin binding profiles (data not shown), suggesting that the 12-week high-fat diet did not significantly alter selectin ligand activities on monocytes. Similar levels of mRNAs of α1,3-fucosyltransferase VII (FTVII) and core 2 β1,6-glucosaminyltransferase-I (C2GlcNAcT-I), the protein products of which are essential for PSGL-1 function, were detected in Ly-6Chi and Ly-6Clo monocytes (Figure S1).
Figure 3.

Ly-6Chi monocytes exhibit enhanced rolling on P-, E- and L-selectin under flow, and increased PSGL-1-dependent interactions with atherosclerotic endothelium. A and B. Representative histograms compare PSGL-1-dependent P-selectin and E-selectin binding activities of Ly-6Chi and Ly-6Clo monocytes from WT mice fed chow. 4RA10 is a blocking mAb to PSGL-1. Shaded histograms represent isotype controls. The fluorescent intensity of x axis is on a log scale. C, D and E. Accumulation of rolling Ly-6Chi and Ly-6Clo monocytes over P-selectin (P-sel) or E-selectin (E-sel), or L-selectin (L-sel). Shown are the mean ± SEM of three independent experiments. F and G. Rolling and adhesion of WT Ly-6Chi and Ly-6Clo as well as PSGL-1-/- Ly-6Chi monocytes on atherosclerotic endothelium in an ex vivo carotid artery model. Shown are the mean ± SEM of three independent experiments.
We then compared the rolling of Ly-6Chi and Ly-6Clo monocytes, respectively, on immobilized P-, E-, or L-selectin under flow conditions. Comparatively more Ly-6Chi than Ly-6Clo monocytes rolled on P-, E-, and L-selectin at low shear stress (0.5-1 dyn/cm2) (Figure 3C, D, and E). Ly-6Chi cells rolled more stably on P- and L-selectin as manifested by their slow rolling velocities (μm/s, 1.37 ± 0.48 for Ly-6Chi vs. 3.55 ± 1.22 for Ly-6Clo on P-selectin; 8.9 ± 3.07 for Ly-6Chi and 117.9 ± 4.38 for Ly-6Clo on L-selectin, at 0.5 dyn/cm2, P < 0.01, n = 15). There was no significant difference in rolling velocities on E-selectin between Ly-6Chi and Ly-6Clo cells, which is consistent with previous studies that PSGL-1 is important for tethering to E-selectin and other E-selectin ligand(s) such as CD44 other than PSGL-1 contributes to the slow rolling on E-selectin.12, 18 Rolling was PSGL-1- and selectin-dependent because mAbs to PSGL-1, P-, E-, or L-selectin reduced the number of rolling cells to the basal levels observed on the surfaces coated with control reagents (data not shown). Significantly, only Ly-6Chi monocytes rolled on P- or E-, and L-selectin at relative high shear stress (>2 dyn/cm2) (Figure 3C, D, and E).
Because L-selectin was preferentially expressed on Ly-6Chi monocytes (Figure 4), we also compared rolling of Ly-6Chi and Ly-6Clo monocytes on immobilized 2-GSP-6, which is modeled after the NH2-terminal L-selectin-binding region of PSGL-1.13 At all shear stress tested, Ly-6Chi cells rolled on 2-GSP-6 (32 ± 4 cells/mm2 at 1 dyn/cm2) (Figure S2A). Rolling was PSGL-1-dependent as it was blocked by mAb 4RA10 to PSGL-1 (Figure S2A). In contrast, almost no Ly-6Clo rolling on 2-GSP-6 was observed (Figure S2A and B). This experiment suggests that L-selectin may contribute to the selective homing of Ly-6Chi cells to atherosclerotic lesions.
Figure 4.
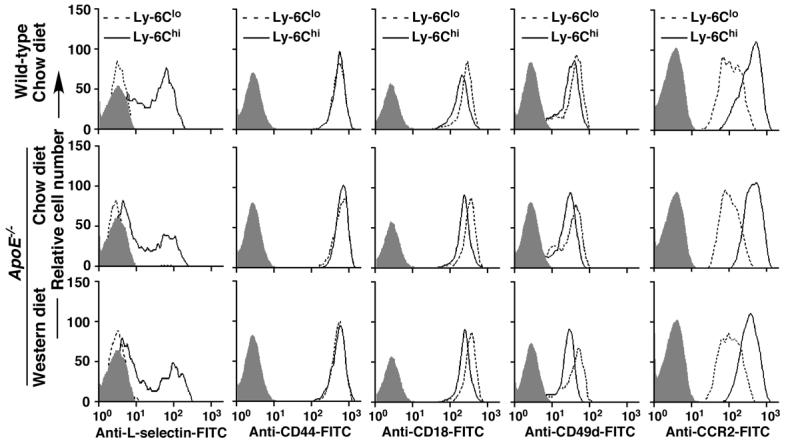
The Western diet does not change the expression profiles of other adhesion molecules or the chemokine receptor CCR2 on Ly-6Chi and Ly-6Clo monocytes. Representative histograms compare expression of L-selectin, CD44, CD18, CD49d, and CCR2 on both Ly-6Chi and Ly-6Clo monocytes from WT or ApoE-/- mice. Shaded histograms represent isotype controls. The fluorescent intensity of x axis of each histogram is on a log scale. Shown are the results of three independent experiments.
Ly-6Chi monocytes roll preferentially on early atherosclerotic endothelium in a PSGL-1-dependent manner
To test whether Ly-6Chi monocytes have increased capacity to interact with early atherosclerotic endothelium, we used the ex vivo perfusion of ApoE-/- carotid artery model.10 In this model, rolling of monocytes on early atherosclerotic endothelium of ApoE-/- mice is primarily mediated by P-selectin and vascular cell adhesion molecule 1 (VCAM-1).10, 19 Compared with Ly-6Clo monocytes, more Ly-6Chi monocytes rolled on atherosclerotic endothelium at 3 dyn/cm2 (Figure 3F). Consequently, the number of adherent Ly-6Chi monocytes was increased at all time points compared to Ly-6Clo monocytes (Figure 3G). PSGL-1-/- Ly-6Chi monocytes had dramatically reduced rolling and adhesion on atherosclerotic endothelium, indicating primarily PSGL-1-dependent interactions. The observed residual rolling of PSGL-1-/- Ly-6Chi monocytes on endothelium may be from interactions between integrin VLA4 and VCAM-1.20
High-fat diet does not change the expression profile of other adhesion molecules or the chemokine receptor CCR2 on Ly-6Chi monocytes
To exclude the possibility that the increased recruitment of Ly-6Chi monocytes into atherosclerotic lesions is a result of altered expression of other molecules resulting from consumption of the Western diet, we compared the expression of L-selectin, CD44, CD18 (b2 integrin), CD49d (VLA4), and CCR2 on both Ly-6Chi and Ly-6Clo monocytes. Consistent with previous reports,3, 5 expression of L-selectin and CCR2 was higher on Ly-6Chi than on Ly-6Clo monocytes, whereas CD18 and CD49d were lower on Ly-6Chi than on Ly-6Clo monocytes (Figure 4). Both Ly-6Chi and Ly-6Clo cells expressed a similar level of CD44 (Figure 4). Ly-6Chi or Ly-6Clo monocytes from WT or from ApoE-/- mice fed either chow or the Western diet for 12 weeks express similar levels of these molecules (Figure 4), suggesting that diet does not affect the expression of these molecules.
ApoE-/-/PSGL-1-/- mice have reduced monocyte/macrophage accumulation in atherosclerotic lesions and develop smaller atherosclerotic plaques
Ly-6Chi monocytes are key contributors to atherosclerosis.4 To examine whether PSGL-1 contributes to monocyte recruitment in atherosclerotic lesions and to the development of atherosclerosis, ApoE-/- and ApoE-/-/PSGL-1-/- mice were fed the Western diet for 12 weeks. Compared to ApoE-/- mice, ApoE-/-/PSGL-1-/- mice exhibited less monocyte/macrophage infiltration in the lesions (Figure 5A and B). In addition, the lesions in ApoE-/-/PSGL-1-/- aortas were significantly smaller than lesions in ApoE-/- aortas (Figure 5C, D, E, and F). There was no significant difference between ApoE-/- and ApoE-/-/PSGL-1-/- mice in their plasma lipid levels (Figure S3). These data support the important role of PSGL-1 in mediating Ly-6Chi monocyte recruitment into atherosclerotic lesions and in the development of early atherosclerotic plaques.
Figure 5.
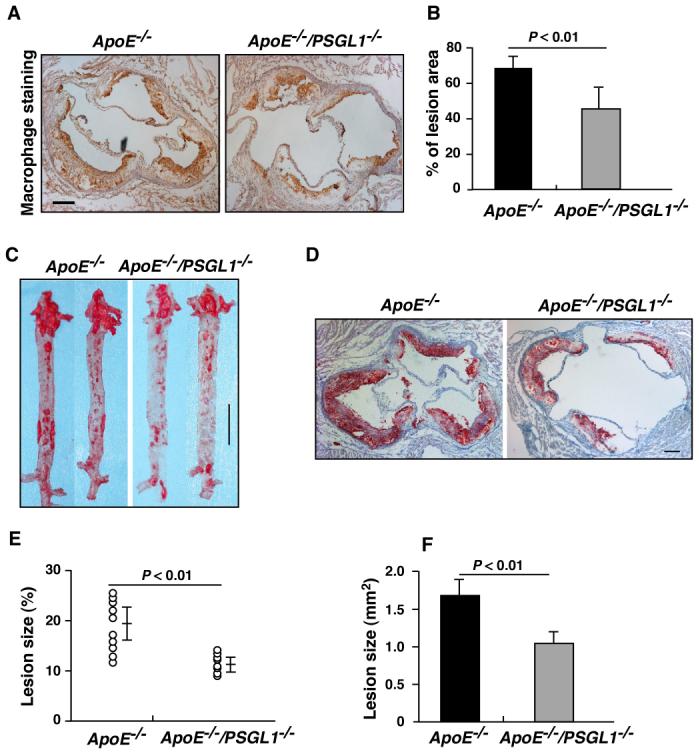
ApoE-/-/PSGL-1-/- mice have reduced macrophage accumulation in atherosclerotic lesions and develop smaller atherosclerotic plaques. A. Macrophage staining (light brown color) in aortic root cross-sections of atherosclerotic lesions in ApoE-/- and ApoE-/-/PSGL-1-/- mice that were fed the Western diet for 12 weeks. B. Quantitative analysis of the macrophages (n = 8 mice/group). Data are means ± SEM. C and D. Oil-Red O-stained (red color) en face preparations of aortas (C), and aortic root cross-sections (D) from atherosclerotic lesions in ApoE-/- and ApoE-/-/PSGL-1-/- mice that were fed the Western diet for 12 weeks. E and F. Quantitative analysis of atherosclerotic lesion area in the entire aorta (E, n = 10 mice/group) and aortic root cross-sections (F, 8 sections/mouse, n = 12 mice/group) using NIH Image J software. Data are means ± SEM. Scale bars: A and D = 100 mm; C = 1 cm.
Lack of PSGL-1 results in impaired Ly-6Chi monocyte recruitment to injured arterial walls and reduces formation of neointimal lesions
Monocytes are critical in arterial neointimal formation.21 We examined whether PSGL-1 deficiency reduces monocyte infiltration in neointima and the size of the lesions. Our experiments showed that ApoE-/-/PSGL-1-/- mice had less monocyte/macrophage infiltration in the neointima of carotid arteries after wire injury compared to ApoE-/- mice (Figure 6A and B). To examine Ly-6Chi monocyte homing to injured arteries, cryosections of ApoE-/- and ApoE-/-/PSGL-1-/- carotid arteries, which were harvested 7 days after wire-induced injury, were stained using an anti-murine Ly-6C mAb as described.4 In this model, P-selectin expressed by regenerating endothelial cells and adherent platelets contributes to the monocyte recruitment into injured carotid arterial wall.22 We found that ApoE-/- carotid arteries had substantial accumulation of Ly-6Chi monocytes after wire-induced injury (Figure 6C). In contrast, Ly-6Chi monocytes were rarely observed in wire-injured ApoE-/-/PSGL-1-/- carotid arterial sections (Figure 6D). These data suggest the critical contribution of PSGL-1 to the recruitment of Ly-6Chi monocytes to neointimal lesions.
Figure 6.
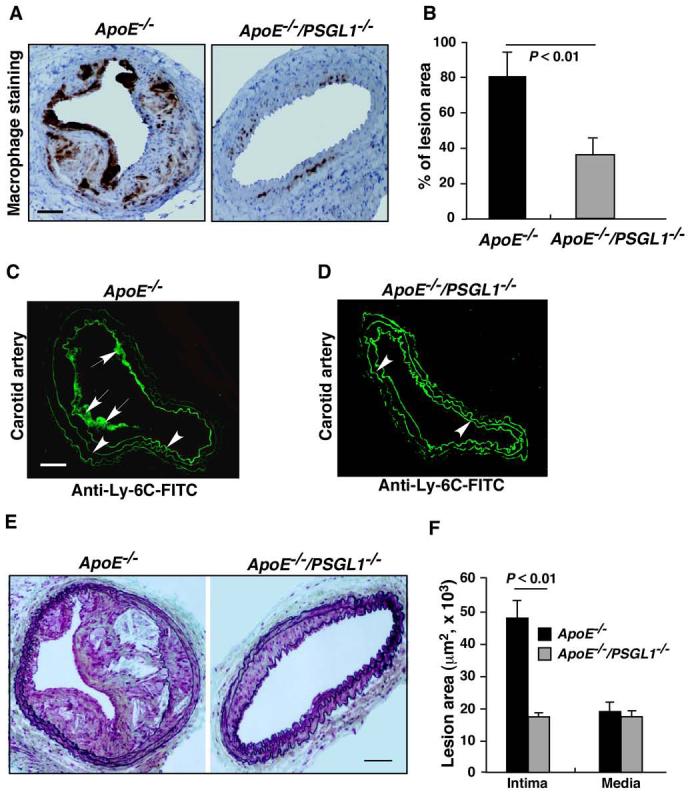
Lack of PSGL-1 results in reduced monocyte infiltration in neointima, impaired Ly-6C-positive monocyte recruitment to injured arterial walls and reduced formation of neointimal lesions. A. Macrophage staining (dark brown color) in neointima of carotid arteries of ApoE-/- and ApoE-/-/PSGL-1-/- mice after wire-induced injury. B. Quantification of the macrophage staining of neointima. Data are means ± SEM (n = 8 mice/group). C and D. Representative immunofluorescence staining of Ly-6C-positive monocytes (arrows) of ApoE-/- (C) or ApoE-/-/PSGL-1-/- (D) carotid arterial cryosections after wire-induced injury. Arrowheads indicate autofluorescence of vascular elastic membranes. E. Movat stained neointimas in ApoE-/- and ApoE-/-/PSGL-1-/- carotid arteries after wire injury. F. Quantification of the sizes of neointima and media (n = 8). Scale bars: A, C, and E = 100 mm.
We also used this model to investigate the role of PSGL-1 in neointima formation. The average size of neointima in the carotid arteries of ApoE-/-/PSGL-1-/- mice after wire injury was dramatically reduced compared to that in ApoE-/- mice (Figure 6E and F), indicating an important role for PSGL-1 in this pathology.
Discussion
Our results demonstrate that PSGL-1 is highly expressed on both murine Ly-6Chi and human CD14+CD16- monocytes, and, therefore, is a new marker for this subset of inflammatory monocytes. PSGL-1 on Ly-6Chi monocytes interacts with all three selectins and contributes significantly to the selective homing of Ly-6Chi monocytes to atherosclerotic lesions.
Selective leukocyte homing to a particular tissue is primarily regulated by unique combinations of adhesion molecules and chemokines.6, 23 Regulated expression of functional PSGL-1 has been recognized as a primary mechanism for the tissue-specific homing of activated T cells.23 All T cells express a similar level of PSGL-1, yet naïve T cells do not interact with P-selectin because their PSGL-1 is not appropriately glycosylated and thus not functional. During activation, T cells acquire functional PSGL-1 upon induced expression of glycosyltransferases FT VII and C2GlcNAcT-I, which add selectin-interacting carbohydrate groups to PSGL-1.7, 23-26 We sought to examine whether a similar mechanism regulates selective homing of Ly-6Chi monocytes. Surprisingly, Ly-6Chi monocytes expressed a higher level of PSGL-1, which interacted with all three selectins, compared with Ly-6Clo monocytes. However, there was no difference in the level of FTVII and C2GlcNAcT-I transcripts in both Ly-6Chi and Ly-6Clo monocytes. Thus, unlike activated T cells that gain function of PSGL-1 as a result of upregulation of glycosyltransferases, Ly-6Chi monocytes express a higher level of fully modified and functional PSGL-1 than Ly-6Clo monocytes, which provides a molecular basis for their preferential homing to atherosclerotic plaques.
PSGL-1 is critical for leukocyte homing; it is a well-characterized ligand for all three selectins and enables circulating leukocytes to roll on activated endothelium under physiological flow conditions.7 In humans, P- and E-selectin have been detected on the luminal surface of arteries with nascent or established atherosclerotic lesions 27. In mouse models, P- and E-selectin are closely correlated with monocyte/macrophage infiltration and lesion formation.27, 28 Leukocytes can initiate rolling on endothelial cells in three ways.7, 8, 23 First, leukocytes can directly contact the endothelium when entering venules from capillaries. Alternatively, leukocytes can be captured by the endothelium through PSGL-1-dependent tethering to P- and E-selectins, a process called primary tethering (Figure 7A, left panel).6-8 Primary tethering is considered particularly important for leukocytes to initiate rolling in larger venules and arterial vessels because these vessels do not have upstream capillaries. The recruitment of Ly-6Chi monocytes occurs in arteries during early atherosclerosis. Thus, PSGL-1-mediated primary tethering may be the key to Ly-6Chi monocyte homing. PSGL-1 on Ly-6Chi monocytes may interact with P-selectin on either activated endothelial cells or on activated platelets adhered to injured arterial wall.27 PSGL-1 may also mediate tethering to and rolling on E-selectin on activated endothelium, either independently or in cooperation with other leukocyte E-selectin ligand(s).7, 12, 18 Thirdly, Ly-6Chi monocytes may interact with the endothelium through secondary tethering in which a freely flowing leukocyte transiently interacts with a rolling or adherent leukocyte or adherent leukocyte fragments, and subsequently rolls on the endothelium (Figure 7A, right panel).29-31. Interestingly, Ly-6Chi monocytes express L-selectin, whereas Ly-6Clo monocytes do not. Ly-6Chi monocyte L-selectin interacted well with 2-GSP-6, the functional domain of PSGL-1, under flow conditions. Reciprocal interactions between PSGL-1 and L-selectin on leukocytes are important for leukocyte adhesion to atherosclerotic lesions.29 Limited by the availability of Ly-6Chi and Ly-6Clo monocytes, we were unable to analyze L-selectin-dependent secondary rolling. Nevertheless, secondary tethering may serve as another important mechanism for selective Ly-6Chi monocyte homing. In vivo, the secondary tethering of a flowing Ly-6Chi monocyte may occur at much higher frequencies on rolling or adherent neutrophils, which are known to interact with atherosclerotic lesions, than on rolling or adherent monocytes due to the dramatic difference in their number in circulation.29 Our data demonstrate that Ly-6Chi monocytes, which are PSGL-1hi and L-selectin+, use PSGL-1-dependent primary and possibly secondary capturing mechanisms to selectively home to atherosclerotic sites (Figure 7A), whereas Ly-6Clo monocytes, which are PSGL-1lo and L-selectin-, are impaired in these homing mechanisms (Figure 7B).
Figure 7.
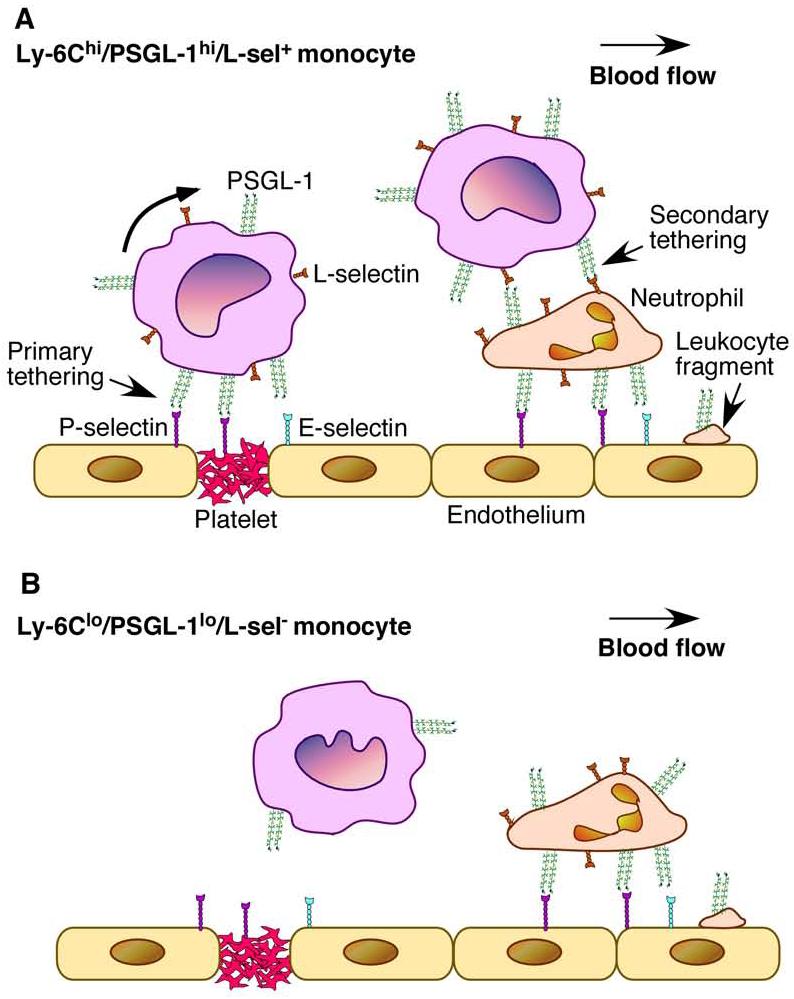
Model for PSGL-1-mediated selective homing of Ly-6Chi monocytes. A. Ly-6Chi monocytes that are also PSGL-1hi and L-selectin+ preferentially interact with P- and E-selectin on activated endothelium/adherent platelets (primary tethering) or with L-selectin on a rolling/adherent leukocyte or a leukocyte fragment (a neutrophil in this case, secondary tethering) under flow. B. Ly-6Clo monocytes that are also PSGL-1lo and L-selectin- cannot efficiently interact with activated endothelium under flow because of low-level surface expression of PSGL-1, and, possibly, lack of L-selectin.
Ly-6Chi monocytes use the chemokine receptors CCR2 and CX3CR1 to enter atherosclerotic plaques.5 However, CCR2 was found not to be involved in the early steps of monocyte adhesion,10 and CX3CR1 is expressed at a lower level on Ly-6Chi monocytes than on Ly-6Clo monocytes.3, 5 Therefore, these chemokine receptors are less likely to contribute to the homing advantage of Ly-6Chi monocytes. Other adhesion molecules such as ICAM-1 and VCAM-1 may not be key factors for this selective Ly-6Chi cell homing process because their ligands are expressed at lower levels on Ly-6Chi monocytes than on Ly-6Clo monocytes.3 Nevertheless, these chemokine receptors and adhesion molecules are important for later processes such as firm adhesion and transmigration once Ly-6Chi monocytes initiate rolling on the endothelium.5, 8, 20
In our study, Ly-6Chi monocytes exhibit PSGL-1-dependent rolling ability on selectins under relative high shear stress (2-4 dyn/cm2), whereas almost no Ly-6Clo monocytes rolled under such conditions. Wall shear stress in vivo is higher in arteries than in veins. Atherosclerotic lesions are prone to develop at bifurcations, branchings, and curvatures where the shear stress ranges from 1-3 dyn/cm2.32, 33 Neointimal lesions after wire injury develop at locations where shear stress can be much higher because of angioplasty procedure.32, 33 Thus, the high level of functional PSGL-1 may give Ly-6Chi monocytes a unique homing advantage under arterial flow conditions. Indeed, lack of PSGL-1 protected ApoE-/- mice from developing severe wire injury-induced neointimal and atherosclerotic plaques, which may be attributable to diminished Ly-6Chi monocyte recruitment. Significantly, lack of PSGL-1 provided ApoE-/- mice more protection from developing neointimas than atherosclerotic plaques, which supports the contention that PSGL-1 plays a more important role in the recruitment of monocytes in acute lesions than in chronic lesions. Our data indicate that PSGL-1 is also highly expressed on human CD14+CD16- monocytes. Thus, these results support the potential for PSGL-1 blockade as a therapeutic approach for the treatment of restenosis after angioplasty.
Supplementary Material
Acknowledgments
We thank Rodger McEver and Charles Esmon for critically reading the manuscript.
Sources of Funding
This study was supported by NIH HL 085607 and RR 018758 to L. Xia, and AHA 0430151N, NIH HL78679 and HL080569 to Y. Huo.
Footnotes
Disclosures
None
CLINICAL PERSPECTIVE
The recruitment of monocytes into the arterial wall plays decisive roles in the initiation and progression of atherosclerosis. In mice, monocytes are divided into Ly-6Chi and Ly-6Clo subsets. Ly-6Chi monocytes are recognized as the key monocyte subset in the development of atherosclerosis, but the mechanisms by which these monocytes selectively accumulate in atherosclerotic lesions are largely unknown. In the present study, we identified the Ly-6Chi monocytes expressed a higher level of P-selectin glycoprotein ligand-1 (PSGL-1), and had enhanced binding to P-, E-, and L-selectin, compared to Ly-6Clo monocytes under physiological conditions. ApoE-/- mice lacking PSGL-1 (ApoE-/-/PSGL-1-/-) had impaired Ly-6C hi monocyte recruitment to arterial injuries and exhibited significantly reduced monocyte infiltration in wire injury-induced neointima and in atherosclerotic lesions. Moreover, ApoE-/-/PSGL-1-/- mice also developed smaller neointima and atherosclerotic plaques. These results indicate that PSGL-1 is a new marker for Ly-6Chi monocytes and a major determinant for Ly-6Chi cell recruitment to sites of atherosclerosis in mice.
References
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 4.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 7.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 8.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 9.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 10.Huo Y, Weber C, Forlow SB, Sperandio M, Thatte J, Mack M, Jung S, Littman DR, Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 2001;108:1307–1314. doi: 10.1172/JCI12877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C. Transport governs flow-enhanced cell tethering through L-selectin at threshold shear. Biophys J. 2007;92:330–342. doi: 10.1529/biophysj.106.090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia L, Sperandio M, Yago T, McDaniel JM, Cummings RD, Pearson-White S, Ley K, McEver RP. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109:939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leppanen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- 15.Nunnari JJ, Zand T, Joris I, Majno G. Quantitation of oil red O staining of the aorta in hypercholesterolemic rats. Experimental and molecular pathology. 1989;51:1–8. doi: 10.1016/0014-4800(89)90002-6. [DOI] [PubMed] [Google Scholar]
- 16.Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106:1523–1529. doi: 10.1161/01.cir.0000028590.02477.6f. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Tahara Y, Matsumoto M, Iguchi M, Sano H, Murayama T, Arai H, Oida H, Yurugi-Kobayashi T, Yamashita JK, Katagiri H, Majima M, Yokode M, Kita T, Narumiya S. Roles of thromboxane A(2) and prostacyclin in the development of atherosclerosis in apoE-deficient mice. J Clin Invest. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K. Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res. 1999;84:1237–1244. doi: 10.1161/01.res.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 20.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schober A, Zernecke A, Liehn EA, von Hundelshausen P, Knarren S, Kuziel WA, Weber C. Crucial role of the CCL2/CCR2 axis in neointimal hyperplasia after arterial injury in hyperlipidemic mice involves early monocyte recruitment and CCL2 presentation on platelets. Circ Res. 2004;95:1125–1133. doi: 10.1161/01.RES.0000149518.86865.3e. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Sanders JM, Phan ET, Ley K, Sarembock IJ. Arterial macrophages and regenerating endothelial cells express P-selectin in atherosclerosis-prone apolipoprotein E-deficient mice. Am J Pathol. 2005;167:1511–1518. doi: 10.1016/S0002-9440(10)61237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nature reviews. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 24.Knibbs RN, Craig RA, Maly P, Smith PL, Wolber FM, Faulkner NE, Lowe JB, Stoolman LM. Alpha(1,3)-fucosyltransferase VII-dependent synthesis of P- and E-selectin ligands on cultured T lymphoblasts. J Immunol. 1998;161:6305–6315. [PubMed] [Google Scholar]
- 25.Ellies LG, Tsuboi S, Petryniak B, Lowe JB, Fukuda M, Marth JD. Core 2 oligosaccharide biosynthesis distinguishes between selectin ligands essential for leukocyte homing and inflammation. Immunity. 1998;9:881–890. doi: 10.1016/s1074-7613(00)80653-6. [DOI] [PubMed] [Google Scholar]
- 26.Lim YC, Xie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW. IL-12, STAT4-dependent up-regulation of CD4(+) T cell core 2 beta-1,6-n-acetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J Immunol. 2001;167:4476–4484. doi: 10.4049/jimmunol.167.8.4476. [DOI] [PubMed] [Google Scholar]
- 27.Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta physiologica Scandinavica. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 28.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J Exp Med. 2001;194:205–218. doi: 10.1084/jem.194.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alon R, Fuhlbrigge RC, Finger EB, Springer TA. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol. 1996;135:849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim YC, Snapp K, Kansas GS, Camphausen R, Ding H, Luscinskas FW. Important contributions of P-selectin glycoprotein ligand-1-mediated secondary capture to human monocyte adhesion to P-selectin, E-selectin, and TNF-alpha-activated endothelium under flow in vitro. J Immunol. 1998;161:2501–2508. [PubMed] [Google Scholar]
- 32.Pantos I, Patatoukas G, Efstathopoulos EP, Katritsis D. In vivo wall shear stress measurements using phase-contrast MRI. Expert review of cardiovascular therapy. 2007;5:927–938. doi: 10.1586/14779072.5.5.927. [DOI] [PubMed] [Google Scholar]
- 33.Feldman CL, Stone PH. Intravascular hemodynamic factors responsible for progression of coronary atherosclerosis and development of vulnerable plaque. Current opinion in cardiology. 2000;15:430–440. doi: 10.1097/00001573-200011000-00010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


