Abstract
We have designed a membrane-anchored form of the Toll-like receptor 5 ligand flagellin, the major proinflammatory determinant of enteropathogenic Salmonella, which was found to be glycosylated and expressed on cell surfaces. A chimeric influenza virus-like particle (cVLP) vaccine candidate containing A/PR8/34 (H1N1) hemagglutinin (HA), matrix protein (M1), and the modified flagellin as a molecular adjuvant was produced. The immunogenicity, including the serum antibody levels and cellular immune responses, and the protective efficacy against homologous and heterologous live virus challenge of the resulting VLPs were tested after intramuscular administration in a mouse model. The results demonstrated that flagellin-containing VLPs elicited higher specific immunoglobulin G (IgG) responses than standard HA and M1 VLPs, indicating the adjuvant effect of flagellin. Enhanced IgG2a and IgG2b but not IgG1 responses were observed with flagellin-containing VLPs, illuminating the activation of Th1 class immunity. The adjuvant effects of flagellin were also reflected by enhanced specific cellular responses revealed by the secretion of cytokines by freshly isolated splenocyte cultures when stimulated with pools of major histocompatibility complex class I or II peptides. When immunized mice were challenged with homologous live PR8 virus, complete protection was observed for both the standard and cVLP groups. However, when a heterosubtypic A/Philippines (H3N2) virus was used for challenge, all of the standard VLP group lost at least 25% of body weight, reaching the experimental endpoint. In contrast, for the cVLP group, 67% of mice survived the challenge infection. These results reveal that cVLPs designed by incorporating flagellin as a membrane-anchored adjuvant induce enhanced cross-protective heterosubtypic immune responses. They also indicate that such cVLP vaccines are a promising new approach for protection against pandemic influenza viruses.
Since the outbreak of a highly pathogenic avian influenza virus (HPAI) H5N1 variant in 1997 in Hong Kong, there have been increased concerns about the threat of a new pandemic that may cause widespread fatal infection in humans. Although the transmission of avian influenza viruses from birds to humans is a rare event, both the continuing increase of infected human cases and the high mortality rates suggest the persisting threat of an H5N1 pandemic (6). There is evidence that the 1918 pandemic virus, which caused an estimated 40 million deaths, was an avian virus directly adapted to humans (53). Although two classes of antiviral drugs targeting the viral matrix protein M2 and neuraminidase, respectively, are available against influenza A viruses, financial and supply limitations as well as frequent drug resistance may limit the ability to utilize these drugs for preventing a new pandemic (6, 36, 38, 66). It is well recognized that an effective vaccine is the primary strategy for protection against an emerging pandemic (37, 51, 61).
Currently, an inactivated influenza vaccine is the dominant form used, although a live attenuated (cold-adapted) influenza virus vaccine was also recently licensed (22). However, the emergence of a new pandemic strain could easily overwhelm the present capacity of vaccine production, which is based on embryonic hens’ eggs. There are additional concerns that biosafety containment facilities may be needed for virus-based vaccine production, and a period of 6 to 9 months would be required. A safe, convenient, and more reliable alternative is needed as a countermeasure to the emerging challenge.
As a new form of vaccine candidate, virus-like particles (VLPs) have been reported to be potent vaccines for a variety of pathogenic viruses (24, 25, 44, 47, 65). VLPs elicit immune responses including both B-cell-mediated antibody and specific T-cell-mediated cellular responses to protect experimental animals against lethal influenza virus challenge (3, 13, 30, 40, 42). However, though VLPs provide an attractive platform for designing vaccines against a possible new influenza virus pandemic strain, they resemble the current vaccines in inducing immune responses that are predominantly subtype specific (23). An important advance would be the development of new vaccines with enhanced breadth of immunity, which could potentially be used to prevent infection by newly emerging variants, including influenza viruses of other subtypes.
To develop a more effective influenza virus vaccine, we have designed a chimeric influenza VLP (cVLP) vaccine candidate by incorporating flagellin, the Toll-like receptor 5 (TLR-5) ligand, into VLPs as a molecular adjuvant. Flagellin is the primary protein component of the highly complex flagellar structures that extend from the outer membrane of gram-negative organisms. It has been well documented that TLR-5 recognizes a conserved site on flagellin (15, 33, 48, 49). As a natural agonist of TLR-5, flagellin is an effective inducer of innate immune effectors such as cytokines and nitric oxide, thereby stimulating the activation of adaptive immune responses (34, 35). Furthermore, flagellin-induced enhancement of adaptive immune responses is known to influence the presentation of antigens and the activation of cellular immune responses (10, 32, 56). In the present study, we designed a modified membrane-anchored form of flagellin for incorporation into influenza VLPs. We determined the immune responses to these VLPs in a mouse model, including their ability to protect against challenge infection with an influenza A virus of a different subtype.
MATERIALS AND METHODS
Cell lines and viruses.
Spodoptera frugiperda Sf9 cells were maintained as suspension cultures in flasks with serum-free SF900 II medium (Gibco-BRL) at 27°C with stirring at a speed of 80 rpm. Madin-Darby canine kidney (MDCK) cells were cultured in Dulbecco's modification of Eagle's medium (DMEM) (Cellgro) plus 10% fetal bovine serum. Influenza A/PR8 (H1N1) virus was grown in and purified from hen egg embryonic fluid as described previously (41). Mouse-adapted PR8 and A/Philippines/2/82/X-79 (H3N2) (A/Philippines) viruses were prepared as described previously (42).
Construction of a membrane-anchored flagellin gene.
A full-length membrane-anchored flagellin encoding gene was generated by fusing a signal peptide (SP) from honeybee mellitin and the transmembrane (TM) and cytoplasmic tail (CT) regions from the influenza A virus PR8 hemagglutinin (HA) to the 5′ and 3′ termini of the flagellin gene in frame, respectively. The mellitin SP-encoding fragment was PCR amplified from the plasmid M-TM.CTMMTV (60) by use of primers 5′-GGTTCTAGAATGAAATTCTTAGTC-3′ and 5′-GTGGGATCCTTTCATGTTGATCGG-3′ (XbaI and BamHI sites are underlined) and cloned into cloning vector pBluescript (−) with XbaI/BamHI sites, resulting in plasmid pBluescript-SP. The Salmonella enterica serovar Typhimurium flagellin gene (fliC; GenBank accession no. D13689) was amplified from plasmid pEM045 pEF6 FliC stop (a kind gift from Alan Aderem, Institute of Systems Biology, Seattle, WA) by using primers 5′-GCAGGATCCATGGCACAAGTCAT-3′ and 5′-CGCGAATTCACGCAGTAAAGAGAG-3′ (underlined sites are BamHI and EcoRI, respectively) and inserted into pBluescript-SP, resulting in pBluescript-SP-FliC. HA TM-CT was amplified from plasmid pc/pS1 containing the full-length HA gene by using primers 5′-GCTAGAATTCCAGATTCTGGCGATC-3′ and 5′-GCTAGGGCCCTTATCAGATGCATATTCT-3′ (underlined sites are EcoRI and ApaI, respectively) and cloned into pBluescript-SP-FliC to produce pBluescript-SP-FliC-HA tail. The full-length membrane-anchored flagellin gene was amplified from pBluescript-SP-FliC-HA tail by using primers 5′-GCTCGTCGACATGAAATTCTTAG-3′ and 5′-GCTACTCGAGTTATCAGATGCATATTC-3′ (SalI and XhoI sites, respectively, are underlined) and cloned into pFastBac 1 under the control of the polyhedrin promoter. The sequence of the membrane-anchored flagellin gene was verified by DNA sequencing.
Generation of rBVs.
A recombinant baculovirus (rBV) expressing membrane-anchored flagellin was derived from the transfer plasmid pFastBac1 constructed as described above by using a Bac-to-Bac expression system (Invitrogen) according to the manufacturer's instructions. rBVs expressing PR8 HA and M1 were described previously (42).
Determination of the cell surface expression of membrane-anchored flagellin.
The presence of the membrane-anchored flagellin on cell surfaces was determined by a cell surface expression assay. Sf9 cells were seeded in six-well plates at 106 cells/well. The infection with the flagellin-expressing rBV and isotopic labeling were performed as described previously (60). The biotinylation of cell surface proteins and immunoprecipitation were carried out (62). Final samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were dried and then used for autoradiography.
Production and characterization of cVLPs.
Flagellin-containing influenza VLPs were produced by infection of Sf9 cells with rBVs expressing PR8 HA, M1, and membrane-anchored flagellin. Standard VLPs containing HA/M1 and control VLPs containing M1 only were produced by infecting Sf9 cell with rBVs expressing PR8 HA and M1 as described previously (42). Cell culture supernatants were collected on day 3 postinfection and cleared by a brief centrifugation (4,000 × g for 20 min at 4°C). VLPs were pelleted by ultracentrifugation at 100,000 × g for 1 h at 4°C. The pellets were resuspended in phosphate-buffered saline (PBS) at 4°C overnight. VLPs were further purified through a 20%-35%-60% discontinuous sucrose gradient at 100,000 × g for 1 h at 4°C. The VLP band between 35% and 60% was collected and then diluted with PBS and pelleted at 100,000 × g for 1 h at 4°C. VLPs were resuspended in PBS overnight at 4°C. The resulting VLPs were characterized by Western blot analysis, hemagglutination activity analysis, and electric microscopic observation. For Western blot analysis, HA and M1 bands were probed by mouse anti-HA or M1 polyclonal antibodies. Membrane-anchored flagellin was detected by rabbit antiflagellin polyclonal antibodies (Provided by Alan Aderem). The flagellin content in cVLPs was estimated by comparison with a standard purified soluble standard flagellin in Western blotting. The hemagglutination activity of VLPs was determined by the capacity to hemagglutinate chicken red blood cells (42). For electron microscopy, VLP samples (5 to 10 μl; 0.1 mg/ml protein) were examined as described previously (60).
Treatment with glycosidases.
Peptides N-glycosidase F (PNGase F) and endoglycosidase H (endo-H) (New England Biolabs) were used to determine the glycosylation of membrane-anchored flagellin by following the manufacturer's instructions. Flagellin-containing VLPs (10 μg in a volume of 10 μl) were mixed with 1 μl of denaturing buffer and heated at 100°C for 10 min. After being cooled in an ice bath for 2 min, samples were mixed with reaction buffer and PNGase F or endo-H and incubated at 37°C for 1 h. The reaction was terminated by adding sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer, and the mixture was heated at 100°C for 5 min. Samples were subjected to Western blotting analysis.
TLR-5-specific bioactivity assay.
A RAW264.7 cell-based assay (31) with modifications was used to determine the bioactivity of membrane-anchored flagellin in VLPs. The RAW264.7 cell line is a mouse macrophage cell line which expresses TLR-2 and -4 but not TLR-5 (64). In brief, 80%-confluent RAW264.7 (TLR-5-negative) cells in a 75-cm2 T flask were transfected with 10 μg of plasmid pUNO-hTLR5 expressing the human TLR-5 (InvivoGen) by use of the transfection reagent Lipofectamine (Invitrogen) by following the manufacturer's instructions. Six hours posttransfection, cells were removed from the T flask with a cell scraper and seeded into 96-well plates by using 5 × 104 cells/well in 100 μl of fresh medium. Nontransfected RAW264.7 cells (TLR-5 negative) were also seeded into 96-well plates for comparison. The TLR-5-positive and -negative cells were incubated with 100 μl of serially diluted purified soluble flagellin, flagellin-containing VLPs, or standard HA/M1 VLPs in DMEM, and supernatants were collected after 24 h. Levels of tumor necrosis factor alpha (TNF-α) production stimulated by soluble flagellin, flagellin-containing VLPs, or standard HA/M1 VLPs in both TLR-5-positive and TLR-5-negative cell cultures were determined by enzyme-linked immunosorbent assay (ELISA). TLR-5 bioactivity was expressed as the level of TNF-α production of TLR-5-positive cells from which was subtracted that of TLR-5-negative cells stimulated by flagellin, flagellin-containing VLPs, or standard HA/M1 VLPs.
Immunization and challenge.
Inbred female BALB/c mice were obtained from Charles River Laboratory. Mouse groups (six mice per group) were immunized twice with 10 μg/mouse of VLPs at 4-week intervals (weeks 1 and 4). For virus challenge, mice were anesthetized with isoflurane and infected with 40 times the 50% lethal dose (40×LD50) (LD50 = 50 PFU/mouse) of mouse-adapted A/PR8 virus (2,000 PFU) or 40×LD50 (LD50 = 25 PFU/mouse) of mouse-adapted A/Philippines virus (1,000 PFU) in 50 μl of PBS per mouse 4 weeks after the boosting immunization. For the determination of lung virus titers, six mice from each group were sacrificed on day 4 postchallenge. Blood samples were collected on weeks 0, 3, and 7 by retro-orbital plexus puncture. After clotting and a brief centrifugation, serum samples were collected and stored at −80°C prior to use for assays.
Antibody titration.
The influenza virus-specific serum antibody endpoint titers, including those for immunoglobulin G (IgG) and subtypes (IgG1, IgG2, and IgG2b), were determined by ELISA as described previously (42). In brief, 96-well microtiter plates (Nunc Life Technologies) were coated with 100 μl/well of inactivated PR8 virus (5 μg/ml) in PBS overnight at 4°C. For serum IgG titers against the heterologous A/Philippines virus (H3N2), plates were coated with 100 μl/well of inactivated A/Philippines virus (5 μg/ml). The serum samples were serially diluted in twofold steps. After being washed and blocked with 1.5% bovine serum albumin, plates were used to bind antibody with the diluted sera. The detection color was developed by binding horseradish peroxidase-labeled goat anti-mouse IgG, IgG1, IgG2a, or IgG2b (Southern Biotechnology) at 37°C for 1 h. After extensive washing, the substrate TMB (Zymed, Invitrogen) was applied. The optical density at 450 nm (OD450) was read using an ELISA reader (model 680; Bio-Rad). The highest dilution which gave an OD450 twice that of the naive group without dilution was designated as the antibody endpoint titer.
Virus neutralization and HI assays.
A neutralization assay was performed using MDCK cells as described previously (42). Hemagglutination inhibition (HI) assays were carried out as described previously (7) with modifications. Briefly, 8 hemagglutination units of PR8 or A/Philippines virus were mixed with serially diluted receptor-destroying enzyme-pretreated serum samples in a total volume of 50 μl and incubated at 37°C for 1 h. An equal volume of chicken blood cells (0.5%) was mixed with the virus-serum mixture and incubated at 25°C for 30 min. HI titers were recorded.
Cytokine assays.
Interleukin-2 (IL-2), gamma interferon (IFN-γ), TNF-α, and IL-4 levels were determined by ELISA (eBioscience, San Diego, CA) according to the manufacturer's instructions. Splenocytes (1 × 106 cells) isolated from immunized mice were seeded into 96-well issue culture plates and stimulated with a mixture of two major histocompatibility complex class I (MHC-I) HA peptides (IYSTVASSL and LYEKVKSQL) or a pool of five MHC-II HA peptides (SFERFEIFPKE, HNTNGVTAACSH, CPKYVRSAKLRM, KLKNSYVNKKGK, and NAYVSVVTSKYN RRF) at a concentration of 10 μg/ml. The plates were incubated at 37°C for 2 days, and cell culture supernatants were collected for cytokine assays.
RESULTS
Construction of the membrane-anchored flagellin coding sequence.
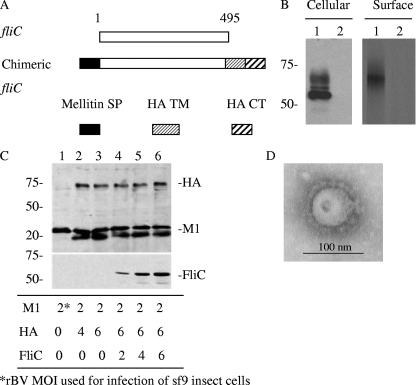
To incorporate flagellin into VLPs as a molecular adjuvant, the gene must be modified to enable membrane translocation, transport, and cell surface expression. As schematized in Fig. 1A, a membrane-anchored flagellin-encoding gene was constructed by merging the coding sequence for the SP from the honeybee protein mellitin at the N terminus and a TM-CT from influenza HA in frame at the C terminus. As a heterologous SP, mellitin SP is known to improve glycoprotein cell surface expression in an insect cell system (60). The TM-CT sequence from HA provides a membrane anchor sequence which would be expected to engage the modified flagellin to assemble into influenza virus matrix protein (M1)-derived VLPs (1). rBV was generated using the resulting membrane-anchored flagellin coding sequence. As shown in Fig. 1B, left, flagellin fused with the HA TM-CT region was expressed well in rBV-infected Sf9 cells. The modified protein showed two major bands in cell lysates by the autoradiography (Fig. 1B, left, lane 1). The lowest band is around 55 kDa, which corresponds to the molecular mass estimated according to its amino acid composition in a nonglycosylated form. The top band is about 6.5 kDa, indicating that glycosylation of this modified protein occurs in the insect cell protein expression system. There are other bands between the two main bands, suggesting differences in glycosylation of the membrane-anchored flagellin. As shown in Fig. 1B, right panel, the surface-expressed flagellin corresponds to the top band with a molecular mass of 65 kDa, suggesting that only glycosylated flagellin was transported to cell surfaces.
FIG. 1.
Construction of membrane-bound flagellin and VLP production. (A) Schematic diagram of the membrane-anchored flagellin construct. The mellitin SP and HA TM-CT coding sequences were fused to the 5′ and 3′ ends of flagellin-encoding gene fliC, respectively, to form the full-length chimeric fliC. (B) Cellular and cell surface expression of membrane-anchored flagellin. Surface expression of the membrane-anchored flagellin was detected by cell surface biotinylation. Lanes: 1, cell lysate from cells infected with rBV expressing membrane-anchored flagellin; 2, mock rBV (rBV expressing human immunodeficiency virus Gag)-infected cells. (C) Optimization of VLP production: Sf9 cells were infected with rBVs expressing HA, M1, and flagellin at different MOI as designated at the bottom. VLPs were prepared as described in Materials and Methods. The resulting VLPs were analyzed by Western blotting. HA and M1 bands were probed with mouse anti-influenza serum. The band below M1 was variable in different VLP preparations and may represent a degradation product. Membrane-anchored flagellin (FliC) was probed with rabbit antiflagellin polyclonal antibody. (D) Electron microscopy of influenza VLPs. Influenza VLPs containing flagellin, HA, and M were negatively stained as described in Materials and Methods.
Production of cVLPs containing flagellin.
As described previously, VLPs were produced in an rBV-derived protein expression system in Sf9 insect cells and purified by gradient centrifugation (42). To optimize the production of cVLPs, rBVs expressing HA, M1, and flagellin at various of multiplicities of infection (MOI) shown in Fig. 1C, bottom, were compared. The results in Fig. 1C, top, demonstrate that standard influenza VLPs with a high HA content resulted from coinfection of HA- and M1-expressing rBVs at MOI of 4 and 2, respectively, and the VLPs (total protein concentration, 1 mg/ml) have an HA titer as high as 2,048 U, as titrated with chicken blood cells. The cVLPs containing flagellin were produced by coinfection of rBVs expressing HA, M1, and flagellin at MOI of 6, 2, and 6, respectively. These cVLPs have HA and M1 contents comparable to those of standard HA and M1 VLPs, as shown by the Western blot results in Fig. 1C, top. The HA titer of the cVLPs (protein concentration, 1 mg/ml) was 2,048 U, the same as that for standard VLPs. When purified recombinant flagellin was used as a standard, a Western blot comparison (not shown) showed that the cVLPs have a flagellin content of about 8 μg/100 μg VLPs. To further confirm the morphology and integrity of these cVLPs, the cVLP samples were examined by electron microscopy after negative staining. As shown in Fig. 1D, enveloped VLPs with projections on the surface were observed, with diameters of about 80 to 100 nm. In addition, the cVLPs have morphological characteristics similar to those of standard HA/M1 VLPs, as described previously (42). These results indicate that membrane-anchored flagellin, together with HA, is incorporated into M1-derived VLPs with a morphology and size similar to those of standard HA/M1 VLPs and influenza virions.
Characterization of the membrane-anchored flagellin in cVLPs.
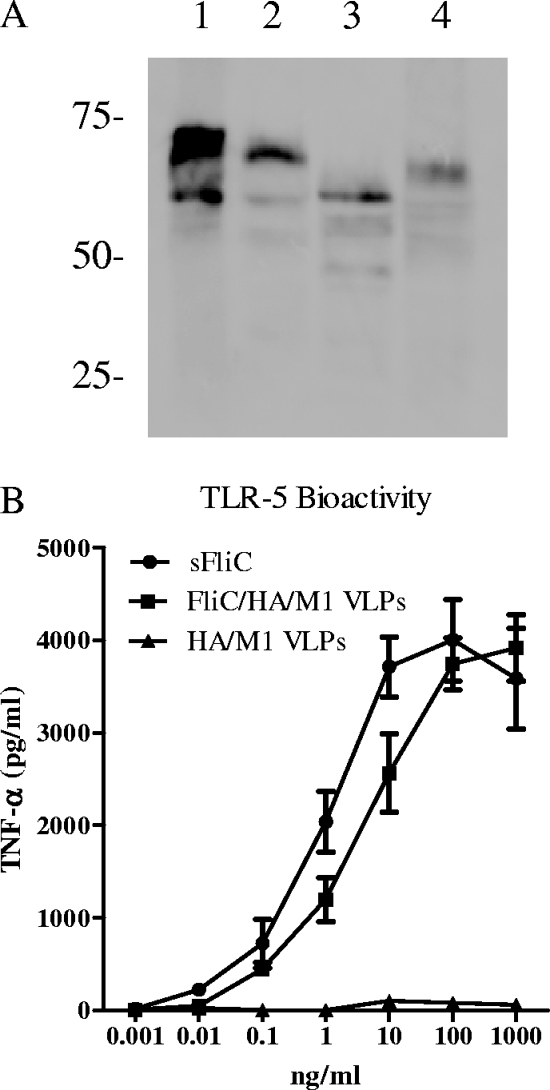
There are six potential N-linked glycosylation sites in the full-length flagellin sequence with an NXT/S motif (Asn19, 101, 200, 346, 446, and 465, respectively), and four of them are located in the TLR-5-recognizing region (Asn19, 101, 446, and 465). To further characterize the possible glycosylation of the membrane-anchored flagellin in cVLPs, VLPs containing flagellin were treated with PNGase F or endo-H. PNGase F is an amidase which can remove N-linked oligosaccharides from glycoproteins, whereas endo-H cleaves the chitobiose core of high-mannose and hybrid oligosaccharides from N-linked glycoproteins (29). As shown in Fig. 2A, most of the modified flagellin in cell lysates is seen as two main portions on the blot with molecular masses of 55 and 65 kDa (lane 1). The flagellin incorporated into cVLPs corresponds to the upper band (65 kDa) (lane 2). After treatment of VLPs with PNGase F, flagellin bands of faster mobility at 55 kDa were observed (Fig. 2A, lane 3), demonstrating that the membrane-anchored flagellin is glycosylated by N-linked oligosaccharides. When the glycosylated flagellin in VLPs was treated by endo-H, an intermediate band (around 60 kDa) was observed (Fig. 2A, lane 4), revealing the partial sensitivity of the flagellin in VLPs to endo-H. These results indicate that at least some of the oligosaccharides are of the high-mannose type. In conclusion, these results indicate that flagellin in VLPs is glycosylated and that the oligosaccharides are linked to the flagellin peptide backbone by N-type glycosidic linkages.
FIG. 2.
Glycosylation and TLR-5 agonist activity of membrane-anchored flagellin. (A) Ten-microgram aliquots of flagellin-containing VLPs were left untreated (lane 2) or were digested by PNGase F (lane 3) or endo-H (lane 4). Lane 1 is cell lysate from cells infected by membrane-anchored flagellin expressing rBV. Samples were resolved by Western blotting. (B) TLR-5-positive and -negative RAW264.7 cells were activated with soluble flagellin (sFliC) and flagellin-containing VLPs (FliC/HA/M1 VLPs), respectively. Standard HA/M1 VLPs were used as controls. Supernatants were collected 24 h after stimulation, and the TNF-α concentration in the supernatant was determined by ELISA as described in Materials and Methods. TLR-5-specific bioactivity was expressed by the production by TNF-α of TRL-5-positive cells, from which was subtracted that of TLR-5-negative cells stimulated by flagellin, flagellin-containing VLPs, or standard HA/M1 VLPs at the same concentration. Data represent means ± standard errors from triplicate repeats.
The adjuvant effect of flagellin is based on its TLR-5-activating activity. To evaluate the ability of the membrane-anchored flagellin in VLPs to function as a TLR-5 ligand, flagellin-containing VLPs were analyzed by a mouse macrophage cell line RAW264.7-based assay (31), and results were compared to those from purified soluble flagellin. As shown in Fig. 2B, flagellin-containing cVLPs stimulated TLR-5-positive RAW264.7 cells to produce TNF-α over a broad concentration spectrum, similar to what was seen with soluble flagellin. The 50% effective concentration (concentration which produces 50% of maximal activity) of flagellin-containing VLPs was around 8 ng/ml, whereas the 50% effective concentration of soluble flagellin was 1 ng/ml. Because the flagellin content in cVLPs is about 8%, the results indicate that the TLR-5 agonist activity of membrane-anchored flagellin in cVLPs is comparable to that of soluble flagellin.
cVLPs containing flagellin induce enhanced humoral immune responses.
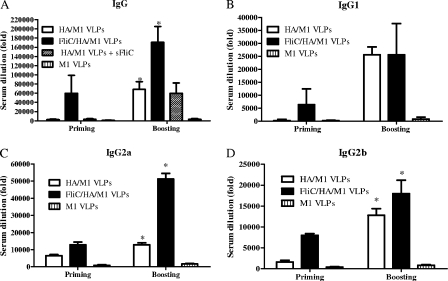
It is well recognized that flagellin in full-length, truncated, or fusion protein forms enhances antigen-specific antibody responses (8, 18, 31). To evaluate the ability of membrane-bound flagellin in influenza cVLPs to function as an adjuvant, the humoral immune response against influenza viral antigen was determined for mice immunized with standard HA/M1 VLPs or flagellin/HA/M1 cVLPs. As shown in Fig. 3A, high levels of serum antigen-specific IgG were promoted by priming or priming plus boosting for mice immunized with flagellin-containing cVLPs. A 2,500-fold higher IgG titer was achieved by the flagellin-containing VLP group after only the priming immunization compared with that of the standard HA/M1 VLP group, and this IgG level was comparable to that of the standard VLP group after two immunizations, demonstrating a significant enhancement of responses promoted by the incorporated flagellin. After two immunizations, the IgG level of the cVLP group remained two times higher than that of the standard VLP group (P < 0.05). In contrast, when mice were immunized with mixtures of HA/M1 VLPs plus soluble recombinant flagellin, no significant difference in antibody response was detected compared to what was seen for HA/M1 VLPs alone. These results indicate that the incorporation of the membrane-anchored flagellin into VLPs is important for its adjuvant effect.
FIG. 3.
Serum IgG and isotype endpoint titers. Serum antibodies specific for influenza A/PR8 virus were determined. The highest serum dilution (n-fold) which gave an OD450 two times higher than that of naive mice was designated as the serum antibody endpoint titer. Representative data are the mean ± standard deviation (SD) of six mice/group and were analyzed by an unpaired t test. A two-tailed P value of <0.05 is designated as a significant difference. (A to D) Serum IgG (*, P < 0.05) (A), IgG1 (B), IgG2a (*, P < 0.05) (C), and IgG2b (*, P < 0.05) (D). sFliC, soluble flagellin.
Previously, we determined that influenza VLP vaccines induce mixed Th1/Th2-type immune responses (42). To further evaluate the serum antibody response induced by flagellin, production levels of IgG subtypes IgG1, IgG2a, and IgG2b were determined. As shown in Fig. 3B, C, and D, both standard VLPs and cVLPs promoted the production of all three IgG subtypes compared to what was seen for the control (M1-only) VLP group, demonstrating that both Th1 and Th2 immune responses were induced by VLP vaccines; this was consistent with our previous observation (42). However, the flagellin-containing VLPs elicited a level of IgG2a (IgG1/IgG2a ratio, 0.5) significantly higher than that seen for standard VLPs (IgG1/IgG2a ratio, 1.5; P < 0.05), but this was not the case for IgG1, demonstrating that Th1-biased type-mixed responses and IgG2a-dominant class switching were effectively promoted by the incorporation of flagellin compared to standard VLPs.
Flagellin stimulates enhanced virus neutralization and HI activity.
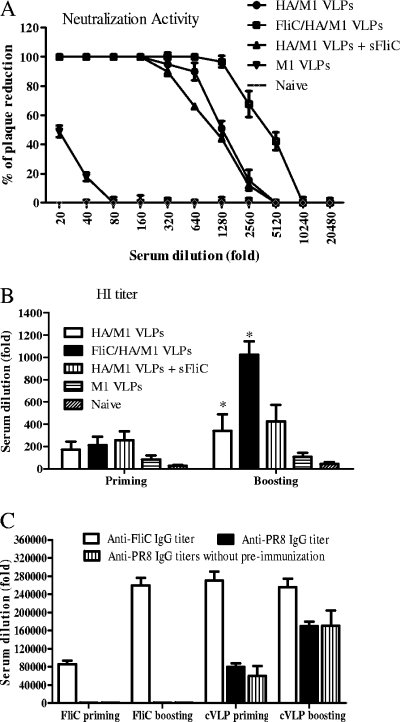
Virus neutralization activity is the most important serological assay to reflect the functional antibodies providing protective immunity. To determine the effects of flagellin on conferring protective humoral responses, sera from mouse groups immunized with HA/M1 VLPs or flagellin-containing HA/M1 VLPs were evaluated for neutralization activities against PR8 virus. As shown in Fig. 4A, sera from standard VLP-immunized mice 3 weeks after the boost immunization showed a neutralization titer (50% plaque reduction) of 1,280. In contrast, the flagellin-containing VLP group showed a virus neutralization titer of 4,000, more than threefold higher, revealing the effectiveness of flagellin incorporated into VLPs as an adjuvant. The enhanced responses were also demonstrated by the HI titers, which are based on blocking the ability of influenza HA to agglutinate erythrocytes by specific antibodies. As shown in Fig. 4B, the flagellin-containing VLP group achieved an HI titer of 1,080, threefold higher than that of the standard VLP group (P < 0.05), which had a mean HI titer of 360. The neutralization activity and HI titers were found to be highly consistent, demonstrating that functional antibodies elicited by influenza VLPs are directed against the HA. Similar to what was found with the serum IgG titers, immune sera from the group immunized with a mixture of soluble flagellin plus HA/M1 VLPs achieved levels of neutralization and HI titers similar to those of the standard HA/M1 VLP group.
FIG. 4.
Neutralization and HI titers against influenza A/PR8 virus, and the effect of preexisting antiflagellin immunity. (A) Neutralization activities were determined using the capacity of sera to neutralize plaque formation by influenza PR8 virus in MDCK cell cultures. Serial dilutions of sera were incubated with influenza PR8 virus (about 100 PFU) at 37°C for 1 h. A standard plaque reduction assay was performed using MDCK cells. (B) HI titers of sera were determined using the capacity of sera to inhibit virus hemagglutination of chicken red blood cells (*, P < 0.05). (C) The preexisting antiflagellin IgG titer was determined with ELISA. A group of six mice was preimmunized twice intramuscularly at a 4-week interval with 10 μg of soluble recombinant flagellin and subsequently immunized twice with 10 μg cVLPs at a 4-week interval. A six-mouse group without preimmunization was used as the control. Serum antiflagellin and anti-inactivated PR8 virus IgG titers were determined by ELISA. For flagellin-specific IgG titers, microplates were coated with 100 μl of recombinant flagellin per well at 5 μg/ml. IgG titer determinations are described in Materials and Methods. Representative data are the mean ± SD from six mice in each group. sFliC, soluble flagellin.
A concern for using a protein component as an adjuvant is the antigenicity of the protein itself, and preexisting immunity against flagellin might block its further function as an adjuvant. To evaluate the effects of preexisting antiflagellin antibody, mice were preimmunized intramuscularly twice with 10 μg of recombinant flagellin. Subsequently, the same group was immunized twice with 10 μg of cVLPs at 4-week intervals. As shown in Fig. 4C, this resulted in a significant mean antiflagellin IgG titer of 2.7 ×105, and this titer was stable for 8 weeks. Interestingly, the PR8-specific IgG titers of flagellin-preimmunized mice rose to levels similar to those of the cVLP control group without flagellin preimmunization, as shown in Fig. 4C. In conclusion, flagellin is an effective adjuvant to promote antigen-specific humoral responses when incorporated into VLPs, and the presence of preexisting flagellin immunity was not found to decrease its adjuvant function.
Because cVLPs induced significantly higher humoral responses as described above, we evaluated whether these immune sera confer a cross-reaction with a heterosubtypic virus. Therefore we determined the serum IgG titer and HI titer against a heterosubtypic A/Philippines virus (H3N2). We found that though the humoral responses against A/Philippines were at low levels compared to those against A/PR8, flagellin-containing cVLPs induced significantly high IgG (2,800) and HI (60) titers against A/Philippines compared to those induced by standard VLPs (all P values were <0.05) (Fig. 5A and B). These results demonstrated the effects of the membrane-anchored flagellin on broadening the spectrum of immunity to confer heterosubtypic immune responses.
FIG. 5.
Serum IgG endpoint and HI titers against the heterosubtypic virus A/Philippines (H3N2). Serum IgG endpoint titer (A) and HI titer (B) were determined as described in Materials and Methods. Data depict the mean ± SD from six mice per group (*, P < 0.05). sFliC, soluble flagellin.
Flagellin promotes antigen-specific T-cell responses.
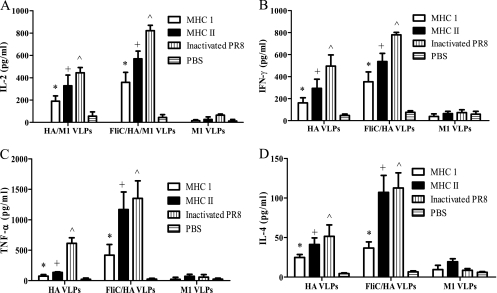
Several microbial products, such as lipopolysaccharide and CpG DNA, have recently been reported to induce dendritic cell (DC) maturation by binding to TLR family molecules, including TLR-5 (16, 20, 32, 52). DCs are found in physical contact with naive T cells in vivo (12, 63). Consequently, DCs recognize microbial products and activate antigen-specific T-cell clonal expansion. To test the effects of membrane-anchored flagellin on enhancing the production of cytokines, cytokine secretions from splenocytes of mice immunized with flagellin-containing VLPs were determined and compared to those of the standard VLP-immunized mice. The results in Fig. 6 show that spleen T cells from mice immunized with flagellin-containing VLP secreted high levels of IL-2, IFN-γ, TNF-α, and IL-4 when stimulated by MHC-I or -II HA peptides or inactivated PR8 virus compared to those for the standard VLP group (all P values were <0.05). MHC-II-recognized HA peptides induced relatively higher levels of secretion of cytokines, demonstrating a CD4-dominant memory T-cell population; both Th1 (IL-2 and IFN-γ)- and Th2 (TNF-α and IL-4)-type cytokine production was observed. Also, we did not observe detectable TNF-α secretion from splenocytes from the standard VLP group upon stimulation with either MHC-I or -II HA peptides, but splenocytes from flagellin-containing VLP-immunized mice produced high levels of TNF-α.
FIG. 6.
Cytokine secretion from immunized mouse splenocytes. Splenocytes were isolated from immunized six-mouse groups 3 weeks after the boosting immunization. Cells (1 × 106) were seeded into 96-well cell culture plates with 200 μl RPMI 1640 medium. The MHC-I- or MHC-II-specific HA peptides of A/PR8 virus were added into cell culture medium, and secreted cytokines were determined as described in Materials and Methods. Data depict the mean ± SD of six mice per group with similar results in triplicate assays (*, +, or ^, P < 0.05). (A) IL-2. (B) IFN-γ. (C) TNF-α. (D) IL-4. FliC, flagellin.
Flagellin-containing VLPs promote protective immunity against lethal virus challenge.
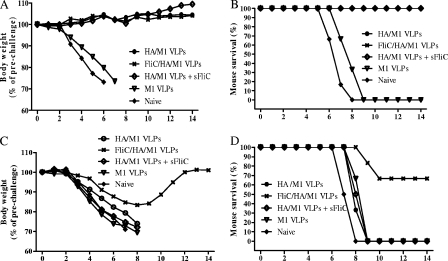
Providing protective immunity to laboratory animals against lethal virus challenge is the most important goal for preclinical vaccine studies. To determine whether the enhanced antibody and T-cell responses observed in our studies conferred protection, immunized mice were challenged intranasally with mouse-adapted PR8 viruses at 40×LD50. As shown in Fig. 7A and B, mice immunized with the standard VLPs, flagellin-incorporating VLPs, or a mixture of standard VLPs plus soluble flagellin retained their healthy status, as measured by body weight. These groups showed 100% protective immunity to lethal PR8 virus challenge. By contrast, no protection was observed using M1 VLPs, though mice immunized with M1 VLPs showed a minor improvement clinical score, as represented by body weight loss and a 1- to 2-day delay in reaching the endpoint upon lethal virus challenge compared to what was seen for the negative control group.
FIG. 7.
Protection from challenge with A/PR8 or A/Philippines virus. Mouse groups containing six mice were challenged with 40×LD50 of PR8 (H1N1) or A/Philippines (H3N2) virus. Mice were monitored daily for 14 days for body weight changes (A) and percentages of survival (B) after PR8 virus challenge or for body weight changes (C) and percentages of survival (D) after A/Philippines virus challenge. sFliC, soluble flagellin.
Because the flagellin-containing VLP group showed much higher antibody levels and enhanced T cellular responses, we tested whether their enhanced immunity provides cross-protection to a heterologous virus challenge. Thus, immunized mice were challenged with an A/Philippines/82 H3N2 virus at 40×LD50 per mouse. Data shown in Fig. 7C demonstrate that all VLP groups lost weight, as did the naive group, but the flagellin-containing VLP-immunized group lost less weight. Though there was a 1-day delay compared to the control group, all mice in the standard VLP group or in the group immunized with the standard VLPs plus soluble flagellin, as well as those of the M1 VLP group, reached the endpoint by day 8 or 9. Notably, in the flagellin-containing VLP-immunized group, mice began to regain body weight from day 9 (Fig. 7C), and 67% of mice survived the challenge with the heterologous A/Philippines virus, as shown in Fig. 7D.
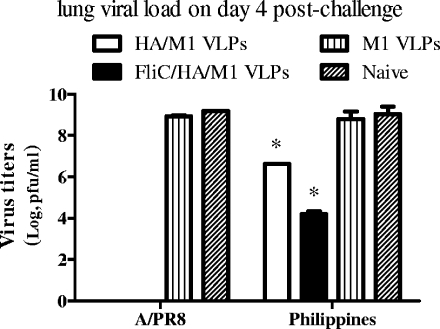
Rapid clearance of virus from the body and low virus loads after infection are important signs of decreased morbidity and mortality after infection. To further evaluate the protective immunity induced by flagellin-containing VLPs, immunized mice were intranasally infected with A/PR8 or A/Philippines virus at the same dose used in the challenge experiment. Mice were sacrificed on day 4, and the virus loads in lungs were determined by plaque assay on MDCK cells. As shown in Fig. 8, virus titers were not detected for either the standard or the flagellin-containing VLP groups in the PR8-infected mice. In contrast, naive mice and mice of the M1 VLP group were carrying high virus loads of 1.5 × 109 and 8.5 × 108 PFU/ml of lung extract, respectively. For A/Philippines virus-infected mice, the standard VLP group showed a lung virus titer of 4.2 × 106 PFU/ml of lung extract, whereas the flagellin-containing VLP group showed 1.6 × 104 PFU/ml of lung extract, a relatively low titer compared to that of standard VLP group (P < 0.05). The naive and M1 VLP groups showed titers of 1.5 × 109 and 8.7 × 108 PFU/ml of lung extract, respectively. These results demonstrate that by incorporating flagellin into VLPs as an adjuvant, influenza VLPs induce enhanced immunity, providing complete protection against a homologous virus challenge and significant cross-protection against a heterosubtypic virus challenge.
FIG. 8.
Lung viral load on day 4 postchallenge. Six mice in each group were challenged with 40×LD50 of PR8 (H1N1) or A/Philippines (H3N2) virus. Mouse lung samples were collected on day 4 postchallenge. Six lungs in each group were pooled, ground, and cleared in 6 ml of DMEM. Lung virus loads were determined using a standard plaque assay with MDCK cells. Bars represent mean virus titers ± standard errors from three independent assays (*, P < 0.05). FliC, flagellin.
DISCUSSION
There is no adjuvant which is known to be effective for enveloped VLP vaccines, and some adjuvants contain emulsive components which would damage the VLP structure. In the present studies, we focused on the development of an effective adjuvant for VLP vaccines by constructing a membrane-anchored form of Salmonella flagellin and incorporating the modified flagellin into VLPs. We observed that by fusion of the SP encoding DNA from honeybee protein mellitin and the influenza HA TM-CT region in frame, the genetically modified flagellin was expressed well in insect cells with a BV-derived protein expression system, and the expressed protein was presented on the cell surface. This membrane-anchored flagellin, together with HA, was found to be incorporated into M1-derived influenza VLPs. Because the adjuvant function of flagellin is derived from its natural capacity for binding TLR-5, one concern in this study was that the fused HA TM-CT might block the folding of flagellin into a correct conformation, which is necessary for TLR-5 recognition. Since several reports have shown that chimeric flagellins fused to antigen in frame at the C terminus retain their TLR-5 binding capacity and promote the immunogenicity of the antigen (8, 19, 31), we introduced the HA membrane anchor at the C terminus of flagellin. The TLR-5 agonist activity of membrane-anchored flagellin in VLPs was verified by a TLR-5 bioactivity assay, and the successful design of the membrane-anchored flagellin as the adjuvant for VLP vaccines was confirmed by its adjuvant function, as shown in our immunization results.
Previously, we have investigated the immune responses induced by influenza VLPs containing HA and M1 (42) and demonstrated that the influenza VLPs induced high levels of antibody responses. In the present study, by incorporation of flagellin into VLP, cVLPs were found to induce antibody responses that were more rapid and higher than those induced by standard VLPs, revealing that membrane-anchored flagellin is a potent adjuvant for promoting humoral immunity. Furthermore, we found that flagellin-containing VLPs activated a Th1-preferred Th1/Th2 profile, demonstrated by a lower IgG1/IgG2a ratio. We observed that standard and flagellin-containing VLPs induced comparable levels of IgG1 but that the flagellin-containing VLPs induced fourfold higher levels of IgG2a production. In this respect, membrane-anchored flagellin is quite different from recombinant soluble flagellin, which induces a Th2 phenotype (10), but is similar to flagellin in its native surface-bound context on live Salmonella (9). Though the mechanism of Ig isotype switching induced by “native flagellin” is not clear, the context in which it is related to immunogens has been recognized to be dominant (9).
We observed that splenocytes from mice immunized with flagellin-containing VLPs produced high levels of IL-2, IFN-γ, TNF-α, and IL-4 when stimulated by HA-specific MHC-I or -II-restricted peptides, indicating the induction of antigen-specific T cells after immunization. Higher levels of cytokine production stimulated by an MHC-II HA-specific peptide demonstrated that flagellin primed a CD4-dominant T-cell response. Though Th1- and Th2-type cytokine secretions were both detected, we did not see a significant IgG1 increase, suggesting that a cytokine-independent mechanism is contributing to IgG2a dominance. Similar Ig isotype switching was also found after infection by mouse mammary tumor virus (28) or inactivated Bordetella pertussis immunization (54). Some reports have implicated a direct role of TLR signaling in B cells that is related to class switching (26). It remains to be determined whether the IgG2a-dominant immunity observed in this study is related to TLR-5 signaling in B cells.
Remarkably, we observed that flagellin-containing VLPs primed a high level of TNF-α secretion, but the TNF-α secretion of splenocytes induced by standard VLPs was at the background level. TNF-α is well recognized for its roles both in mediating innate immune responses and in inducing undesirable systemic effects (46). In antiadenoviral responses, TNF-α expression contributes to the efficient maturation and migration of DCs (11, 39, 55). It is known that TNF-α is produced by activated macrophages and plays a central role in the defense against intracellular organisms. Flagellin has been reported to stimulate TNF-α production either in vitro or in vivo (4, 5, 17). The high-level production of TNF-α induced by flagellin-containing VLPs and the enhanced specific immunity suggest that TNF-α plays an important role in activating adaptive immunity in flagellin-containing VLP immunization.
Flagellin has been reported to be a powerful immunogen that acts as its own adjuvant (9). We also found that mice preimmunized with soluble recombinant flagellin produced high levels of flagellin-specific IgG responses. Interestingly, such preexisting immunity against flagellin did not block the adjuvant function of membrane-anchored flagellin in VLPs. This observation is consistent with two previous reports (2, 18) and supports the conclusion that flagellin is an effective adjuvant even in the presence of preexisting antiflagellin immunity. It is possible that the preexisting antiflagellin antibody might enhance the targeting of cVLPs to antigen-presenting cells (APCs) by the Fc portion of the cVLP-bound antiflagellin IgG, since APCs express Fcγ receptors (43, 57-59). However, we did not observe significant differences in responses with or without preexisting antiflagellin immunity. Because TLR-5 recognizes flagellin in both terminal regions, an alternative is to eliminate its ectodomain genetically and link the two regions with a hinge sequence to support necessary flexibility. This approach has been used recently to produce a fusion protein vaccine (31). Whether such a truncated flagellin in a membrane-anchored form is stably incorporated into VLPs and functions as an agonist of TLR-5 is being investigated.
Naturally, flagellin is a glycosylated protein with six sites glycosylated by O-linked β-acetylglucosamine (45). Glycosylation has been demonstrated to have roles for both flagellar assembly and biological function (27). In the two termini of Salmonella flagellin, which are recognized by TLR-5, four N-linked glycosylation sites are located at Asn19 and Asn101 in the N-terminal region and at Asn446 and Asn465 in the C-terminal region. For the insect cell-derived membrane-anchored flagellin, though the whole-cell lysate showed two bands at 55 and 65 kDa, the VLPs showed only the higher-molecular-mass form. It is known that insect cells mostly produce simple N-glycans with terminal mannose residues (14). Our results indicate that such glycosylated forms of flagellin retain potent adjuvant activity.
There are some reports that mixtures of soluble flagellin plus antigen do not promote antigen-specific immune responses, and the physical association of an antigen with flagellin (fusion protein of antigen with flagellin) may be necessary for the promotion of the specific immune responses (19, 31). We also found that although membrane-anchored flagellin incorporated into VLPs boosted strong specific immune responses, a mixture of soluble flagellin plus standard VLPs failed to show a similar adjuvant effect. Similar phenomena have been observed with other pathogen-associated molecular patterns related to their corresponding TLRs. Previous studies showed that simian immunodeficiency virus VLPs conjugated with cholera toxin subunit B induced humoral and cellular responses that were stronger than those seen for a mixture of toxin with VLPs (21). It was also reported that when CpG-DNA is linked to ovalbumin, the protein uptake is facilitated by a receptor-mediated mechanism (50). Because TLR-5 is expressed on the surfaces of APCs, the association with flagellin may result in the TLR-5-mediated uptake of antigens and consequent processing and presentation.
The goal of vaccines is to elicit protection against pathogens. An effective adjuvant should extend this protective effect by promoting the immunogenicity of specific antigens in vaccines, increasing the magnitude and the duration of immunity. The results in this study demonstrate that flagellin-containing VLPs induced complete protection against homologous virus challenge and partial cross-protection against heterologous virus challenge, a broader-spectrum immunity. The incorporation of flagellin into cVLPs thus represents a new direction for the development of efficient vaccines against viral pathogens, including pandemic influenza viruses.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (AI068003).
We thank Alan Aderem (Institute for Systems Biology, Seattle, WA) for suggesting the use of flagellin as an adjuvant in chimeric VLPs and Alan Aderem and Andrew Gewirtz (Emory University, Atlanta, GA) for providing the fliC gene and antiflagellin antibody. We thank Erin-Joi Collins for her valuable assistance in the preparation of the manuscript. Also, we thank Huan Nguyen for the mouse-adapted influenza virus strains A/PR8/34 and A/Philippines.
Baozhong Wang, Sang-Moo Kang, Jadranka Bozja, Ioanna Skountzou, Richard W. Compans, and Emory University have a financial interest in Zetra Biologicals, which is developing virus-like particles as vaccines.
Footnotes
Published ahead of print on 10 September 2008.
REFERENCES
- 1.Ali, A., R. T. Avalos, E. Ponimaskin, and D. P. Nayak. 2000. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J. Virol. 748709-8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Yedidia, T., and R. Arnon. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 649-15. [DOI] [PubMed] [Google Scholar]
- 3.Bright, R. A., D. M. Carter, S. Daniluk, F. R. Toapanta, A. Ahmad, V. Gavrilov, M. Massare, P. Pushko, N. Mytle, T. Rowe, G. Smith, and T. M. Ross. 2007. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 253871-3878. [DOI] [PubMed] [Google Scholar]
- 4.Ciacci-Woolwine, F., I. C. Blomfield, S. H. Richardson, and S. B. Mizel. 1998. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect. Immun. 661127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciacci-Woolwine, F., P. F. McDermott, and S. B. Mizel. 1999. Induction of cytokine synthesis by flagella from gram-negative bacteria may be dependent on the activation or differentiation state of human monocytes. Infect. Immun. 675176-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cinatl, J., Jr., M. Michaelis, and H. W. Doerr. 2007. The threat of avian influenza A (H5N1). Part IV. Development of vaccines. Med. Microbiol. Immunol. 196213-225. [DOI] [PubMed] [Google Scholar]
- 7.Compans, R. W. 1974. Hemagglutination-inhibition: rapid assay for neuraminic acid-containing viruses. J. Virol. 141307-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuadros, C., F. J. Lopez-Hernandez, A. L. Dominguez, M. McClelland, and J. Lustgarten. 2004. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect. Immun. 722810-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham, A. F., M. Khan, J. Ball, K. M. Toellner, K. Serre, E. Mohr, and I. C. MacLennan. 2004. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur. J. Immunol. 342986-2995. [DOI] [PubMed] [Google Scholar]
- 10.Didierlaurent, A., I. Ferrero, L. A. Otten, B. Dubois, M. Reinhardt, H. Carlsen, R. Blomhoff, S. Akira, J. P. Kraehenbuhl, and J. C. Sirard. 2004. Flagellin promotes myeloid differentiation factor 88-dependent development of Th2-type response. J. Immunol. 1726922-6930. [DOI] [PubMed] [Google Scholar]
- 11.Elkon, K. B., C. C. Liu, J. G. Gall, J. Trevejo, M. W. Marino, K. A. Abrahamsen, X. Song, J. L. Zhou, L. J. Old, R. G. Crystal, and E. Falck-Pedersen. 1997. Tumor necrosis factor alpha plays a central role in immune-mediated clearance of adenoviral vectors. Proc. Natl. Acad. Sci. USA 949814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer, U. B., E. L. Jacovetty, R. B. Medeiros, B. D. Goudy, T. Zell, J. B. Swanson, E. Lorenz, Y. Shimizu, M. J. Miller, A. Khoruts, and E. Ingulli. 2007. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. Proc. Natl. Acad. Sci. USA 1047181-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galarza, J. M., T. Latham, and A. Cupo. 2005. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 18244-251. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, R. L., and D. L. Jarvis. 2006. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv. Virus Res. 68159-191. [DOI] [PubMed] [Google Scholar]
- 15.Hawn, T. R., A. Verbon, K. D. Lettinga, L. P. Zhao, S. S. Li, R. J. Laws, S. J. Skerrett, B. Beutler, L. Schroeder, A. Nachman, A. Ozinsky, K. D. Smith, and A. Aderem. 2003. A common dominant TLR5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to Legionnaires' disease. J. Exp. Med. 1981563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 17.Honko, A. N., and S. B. Mizel. 2004. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect. Immun. 726676-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honko, A. N., N. Sriranganathan, C. J. Lees, and S. B. Mizel. 2006. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect. Immun. 741113-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huleatt, J. W., V. Nakaar, P. Desai, Y. Huang, D. Hewitt, A. Jacobs, J. Tang, W. McDonald, L. Song, R. K. Evans, S. Umlauf, L. Tussey, and T. J. Powell. 2008. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine 26201-214. [DOI] [PubMed] [Google Scholar]
- 20.Kaisho, T., O. Takeuchi, T. Kawai, K. Hoshino, and S. Akira. 2001. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J. Immunol. 1665688-5694. [DOI] [PubMed] [Google Scholar]
- 21.Kang, S. M., Q. Yao, L. Guo, and R. W. Compans. 2003. Mucosal immunization with virus-like particles of simian immunodeficiency virus conjugated with cholera toxin subunit B. J. Virol. 779823-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemble, G., and H. Greenberg. 2003. Novel generations of influenza vaccines. Vaccine 211789-1795. [DOI] [PubMed] [Google Scholar]
- 23.Kodihalli, S., D. L. Kobasa, and R. G. Webster. 2000. Strategies for inducing protection against avian influenza A virus subtypes with DNA vaccines. Vaccine 182592-2599. [DOI] [PubMed] [Google Scholar]
- 24.Koutsky, L. A., K. A. Ault, C. M. Wheeler, D. R. Brown, E. Barr, F. B. Alvarez, L. M. Chiacchierini, and K. U. Jansen. 2002. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 3471645-1651. [DOI] [PubMed] [Google Scholar]
- 25.Leclerc, D., D. Beauseigle, J. Denis, H. Morin, C. Pare, A. Lamarre, and R. Lapointe. 2007. Proteasome-independent major histocompatibility complex class I cross-presentation mediated by papaya mosaic virus-like particles leads to expansion of specific human T cells. J. Virol. 811319-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, L., A. J. Gerth, and S. L. Peng. 2004. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur. J. Immunol. 341483-1487. [DOI] [PubMed] [Google Scholar]
- 27.Logan, S. M. 2006. Flagellar glycosylation—a new component of the motility repertoire? Microbiology 1521249-1262. [DOI] [PubMed] [Google Scholar]
- 28.Luther, S. A., A. Gulbranson-Judge, H. Acha-Orbea, and I. C. MacLennan. 1997. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J. Exp. Med. 185551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maley, F., R. B. Trimble, A. L. Tarentino, and T. H. Plummer, Jr. 1989. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal. Biochem. 180195-204. [DOI] [PubMed] [Google Scholar]
- 30.Matassov, D., A. Cupo, and J. M. Galarza. 2007. A novel intranasal virus-like particle (VLP) vaccine designed to protect against the pandemic 1918 influenza A virus (H1N1). Viral Immunol. 20441-452. [DOI] [PubMed] [Google Scholar]
- 31.McDonald, W. F., J. W. Huleatt, H. G. Foellmer, D. Hewitt, J. Tang, P. Desai, A. Price, A. Jacobs, V. N. Takahashi, Y. Huang, V. Nakaar, L. Alexopoulou, E. Fikrig, and T. J. Powell. 2007. A West Nile virus recombinant protein vaccine that coactivates innate and adaptive immunity. J. Infect. Dis. 1951607-1617. [DOI] [PubMed] [Google Scholar]
- 32.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 1693914-3919. [DOI] [PubMed] [Google Scholar]
- 33.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 1705165-5175. [DOI] [PubMed] [Google Scholar]
- 34.Mizel, S. B., A. N. Honko, M. A. Moors, P. S. Smith, and A. P. West. 2003. Induction of macrophage nitric oxide production by gram-negative flagellin involves signaling via heteromeric Toll-like receptor 5/Toll-like receptor 4 complexes. J. Immunol. 1706217-6223. [DOI] [PubMed] [Google Scholar]
- 35.Moors, M. A., L. Li, and S. B. Mizel. 2001. Activation of interleukin-1 receptor-associated kinase by gram-negative flagellin. Infect. Immun. 694424-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palese, P. 2004. Influenza: old and new threats. Nat. Med. 10S82-S87. [DOI] [PubMed] [Google Scholar]
- 37.Palese, P. 2006. Making better influenza virus vaccines? Emerg. Infect. Dis. 1261-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palese, P., and R. W. Compans. 1976. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J. Gen. Virol. 33159-163. [DOI] [PubMed] [Google Scholar]
- 39.Peng, Y., J. Trevejo, J. Zhou, M. W. Marino, R. G. Crystal, E. Falck-Pedersen, and K. B. Elkon. 1999. Inhibition of tumor necrosis factor alpha by an adenovirus-encoded soluble fusion protein extends transgene expression in the liver and lung. J. Virol. 735098-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushko, P., T. M. Tumpey, F. Bu, J. Knell, R. Robinson, and G. Smith. 2005. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 235751-5759. [DOI] [PubMed] [Google Scholar]
- 41.Quan, F. S., R. W. Compans, H. H. Nguyen, and S. M. Kang. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 821350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quan, F. S., C. Huang, R. W. Compans, and S. M. Kang. 2007. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J. Virol. 813514-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Revaz, V., R. Zurbriggen, C. Moser, J. T. Schiller, F. Ponci, M. Bobst, and D. Nardelli-Haefliger. 2007. Humoral and cellular immune responses to airway immunization of mice with human papillomavirus type 16 virus-like particles and mucosal adjuvants. Antivir. Res. 7675-85. [DOI] [PubMed] [Google Scholar]
- 45.Schirm, M., S. K. Arora, A. Verma, E. Vinogradov, P. Thibault, R. Ramphal, and S. M. Logan. 2004. Structural and genetic characterization of glycosylation of type a flagellin in Pseudomonas aeruginosa. J. Bacteriol. 1862523-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sethi, G., B. Sung, and B. B. Aggarwal. 2008. TNF: a master switch for inflammation to cancer. Front. Biosci. 135094-5107. [DOI] [PubMed] [Google Scholar]
- 47.Skountzou, I., F. S. Quan, S. Gangadhara, L. Ye, A. Vzorov, P. Selvaraj, J. Jacob, R. W. Compans, and S. M. Kang. 2007. Incorporation of glycosylphosphatidylinositol-anchored granulocyte-macrophage colony-stimulating factor or CD40 ligand enhances immunogenicity of chimeric simian immunodeficiency virus-like particles. J. Virol. 811083-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 41247-1253. [DOI] [PubMed] [Google Scholar]
- 49.Smith, K. D., and A. Ozinsky. 2002. Toll-like receptor-5 and the innate immune response to bacterial flagellin. Curr. Top. Microbiol. Immunol. 27093-108. [DOI] [PubMed] [Google Scholar]
- 50.Sparwasser, T., R. M. Vabulas, B. Villmow, G. B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur. J. Immunol. 303591-3597. [DOI] [PubMed] [Google Scholar]
- 51.Subbarao, K., and T. Joseph. 2007. Scientific barriers to developing vaccines against avian influenza viruses. Nat. Rev. Immunol. 7267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11443-451. [DOI] [PubMed] [Google Scholar]
- 53.Taubenberger, J. K., A. L. Reid, R. M. Lourens, M. Wang, G. Jin, and T. G. Fanning. 2005. Characterization of the 1918 influenza virus polymerase genes. Nature 437889-893. [DOI] [PubMed] [Google Scholar]
- 54.Toellner, K. M., S. A. Luther, D. M. Sze, R. K. Choy, D. R. Taylor, I. C. MacLennan, and H. Acha-Orbea. 1998. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J. Exp. Med. 1871193-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trevejo, J. M., M. W. Marino, N. Philpott, R. Josien, E. C. Richards, K. B. Elkon, and E. Falck-Pedersen. 2001. TNF-alpha-dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc. Natl. Acad. Sci. USA 9812162-12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsujimoto, H., T. Uchida, P. A. Efron, P. O. Scumpia, A. Verma, T. Matsumoto, S. K. Tschoeke, R. F. Ungaro, S. Ono, S. Seki, M. J. Clare-Salzler, H. V. Baker, H. Mochizuki, R. Ramphal, and L. L. Moldawer. 2005. Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J. Leukoc. Biol. 78888-897. [DOI] [PubMed] [Google Scholar]
- 57.Unkeless, J. C. 1989. Function and heterogeneity of human Fc receptors for immunoglobulin G. J. Clin. Investig. 83355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Unkeless, J. C. 1989. Human Fc gamma receptors. Curr. Opin. Immunol. 263-67. [DOI] [PubMed] [Google Scholar]
- 59.Unkeless, J. C. 1989. Human Fc receptors for IgG. Int. Rev. Immunol. 5165-171. [DOI] [PubMed] [Google Scholar]
- 60.Wang, B. Z., W. Liu, S. M. Kang, M. Alam, C. Huang, L. Ye, Y. Sun, Y. Li, D. L. Kothe, P. Pushko, T. Dokland, B. F. Haynes, G. Smith, B. H. Hahn, and R. W. Compans. 2007. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J. Virol. 8110869-10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Webster, R. G., R. J. Webby, E. Hoffmann, J. Rodenberg, M. Kumar, H. J. Chu, P. Seiler, S. Krauss, and T. Songserm. 2006. The immunogenicity and efficacy against H5N1 challenge of reverse genetics-derived H5N3 influenza vaccine in ducks and chickens. Virology 351303-311. [DOI] [PubMed] [Google Scholar]
- 62.Yang, C., and R. W. Compans. 1996. Palmitoylation of the murine leukemia virus envelope glycoprotein transmembrane subunits. Virology 22187-97. [DOI] [PubMed] [Google Scholar]
- 63.Zell, T., A. Khoruts, E. Ingulli, J. L. Bonnevier, D. L. Mueller, and M. K. Jenkins. 2001. Single-cell analysis of signal transduction in CD4 T cells stimulated by antigen in vivo. Proc. Natl. Acad. Sci. USA 9810805-10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang, D., G. Zhang, M. S. Hayden, M. B. Greenblatt, C. Bussey, R. A. Flavell, and S. Ghosh. 2004. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 3031522-1526. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, Y. L., Y. J. Guo, K. Y. Wang, K. Lu, K. Li, Y. Zhu, and S. H. Sun. 2007. Enhanced immunogenicity of modified hepatitis B virus core particle fused with multiepitopes of foot-and-mouth disease virus. Scand. J. Immunol. 65320-328. [DOI] [PubMed] [Google Scholar]
- 66.Zuckerman, M., J. Oxford, J. Wood, and J. Taylor. 1990. Influenza A (H3N2) component of recommended vaccine induces antibody to current virus. Lancet 335179-180. [DOI] [PubMed] [Google Scholar]