Abstract
Antibodies that neutralize cytomegalovirus (CMV) entry into fibroblasts are predominantly directed against epitopes within virion glycoproteins that are required for attachment and entry. However, the mechanism of CMV entry into epithelial and endothelial cells differs from fibroblast entry. Using assays that simultaneously measured neutralizing activities against CMV entry into fibroblasts and epithelial cells, we found that human immune sera and CMV-hyperimmuneglobulins have on average 48-fold higher neutralizing activities against epithelial cell entry compared to fibroblast entry, suggesting that natural CMV infections elicit neutralizing antibodies that are epithelial entry-specific. This activity could not be adsorbed with recombinant gB. The Towne vaccine and the gB/MF59 subunit vaccine induced epithelial entry-specific neutralizing activities that were on average 28-fold (Towne) or 15-fold (gB/MF59) lower than those observed following natural infection. These results suggest that CMV vaccine efficacy may be enhanced by induction of epithelial entry-specific neutralizing antibodies.
Keywords: cytomegalovirus, vaccines, neutralizing antibody
1. Introduction
Congenital cytomegalovirus (CMV) infections cause sensorineural hearing loss and are the major infectious cause of congenital neurological disease among infants. Each year in the US 40,000 infants are born shedding CMV, and of these, 8000 are born with symptoms and/or develop severe handicaps [1]. This disease burden could be prevented by an effective vaccine and indeed development of a CMV vaccine is a national priority [2].
Effective viral vaccines need to induce antibodies that neutralize viral entry into host cells. Entry of laboratory strains of CMV into fibroblasts has served as the in vitro model for studying viral entry and the antibodies that block it. Such studies revealed that major neutralizing epitopes reside in glycoproteins gB, gH, and gM/gN [3–5] and further suggested that epitopes within gB can comprise up to half the neutralizing activity of human immune sera [3].That the live attenuated Towne vaccine and the gB-based gB/MF59 protein subunit vaccine elicit neutralizing titers in human subjects similar to natural CMV infections [6,7] was encouraging that these vaccines might provide protection similar to that associated with natural seropositivity.
Recent findings, however, demonstrate that CMV enters different cell types using distinct mechanisms. Fibroblast entry occurs at the cell membrane and is pH-independent, while entry into endothelial and epithelial cells requires a complex of gH and gL with three additional viral proteins that are unnecessary for fibroblast entry, UL128, UL130, and UL131 [8–10]. These results suggested that neutralizing assays using fibroblasts would not measure the contributions made by antibodies to viral proteins such as UL128, UL130, or UL131 that specifically mediate epithelial or endothelial cell entry. This was confirmed when mouse or rabbit antibodies to UL128, UL130, or UL131 were found to neutralize viral entry into endothelial or epithelial cells without impairing fibroblast entry [8,11,12]. However, the extent to which human immune sera contain epithelial- or endothelial-specific neutralizing antibodies remained unknown. Here we show that the exclusive use of fibroblasts in neutralizing assays vastly underestimates the capacity of human immune sera to neutralize viral entry. In the course of completing these studies Gerna et al. reported findings that are entirely consistent with those presented here; they found that neutralizing activities of human immune sera are substantially higher when measured using either endothelial or epithelial cells as compared to fibroblasts [12].
Hence, the abilities of CMV vaccine candidates to induce neutralizing activities comparable to natural infection were again uncertain, as they had not been evaluated using assays able to detect antibodies that specifically block epithelial or endothelial cell entry. While the gB/MF59 vaccine would not be expected to elicit antibodies to UL128-131, antibodies to certain epitopes within gB could selectively inhibit epithelial or endothelial cell entry, perhaps by blocking interactions between gB and UL128-131 that have been proposed to induce a conformational change in gB necessary for membrane fusion during endothelial cell entry [13]. Similarly, although the viruses that comprise the Towne vaccine contain a mutation in UL130 [9,14] that renders the UL130 protein unstable and very poorly expressed [15], expression of UL128 and UL131 by Towne may be unaffected. Hence, the Towne vaccine could potentially elicit antibodies to epitopes within UL128 or UL131 that specifically neutralize epithelial or endothelial cell entry.
To resolve these questions, we developed an assay to simultaneously quantitate the ability of human serum antibodies to neutralize CMV entry into fibroblasts versus epithelial cells, then used this assay to evaluate fibroblast and epithelial entry neutralizing activities induced by natural CMV infection and by Towne or gB/MF59 vaccination.
2. Materials and methods
2.1 Human sera and hyperimmune antibodies
Serum samples were obtained from individuals vaccinated with either Towne or recombinant gB adjuvanted with MF59 (gB/MF59) vaccines [6,16]. All subjects were seronegative immediately prior to vaccination. Towne-recipients received 2×103.47 pfu of Towne vaccine. Sera were analyzed immediately before and 2 months after administration, and additional sera were obtained at various times up to 24 months after administration. Recipients of the gB/MF59 vaccine received three intramuscular injections at 0, 1, and 6 months of either 5 µg, 30 µg, or 100 µg per dose of gB/MF59. Sera were analyzed immediately before and 7 months after the first dose. Antibody responses were independent of the vaccine dose [16]. Informed consent was obtained from all subjects and studies were conducted in accordance with human experimentation guidelines of the US Department of Health and Human Services. Anonymous blood donor sera were provided by Virginia Blood Services. Sera were determined to be CMV seronegative or seropositive by ELISA as described previously [17]. Titers of gB-specific antibodies were determined using a gB-based ELISA assay [16]. CMV-hyperimmuneglobulin products CytoGam® (CSL Behring, King of Prussia, PA) and Cytotect CP (Biotest, Dreieich Germany) were purchased from the manufacturers.
2.2 Cells and virus
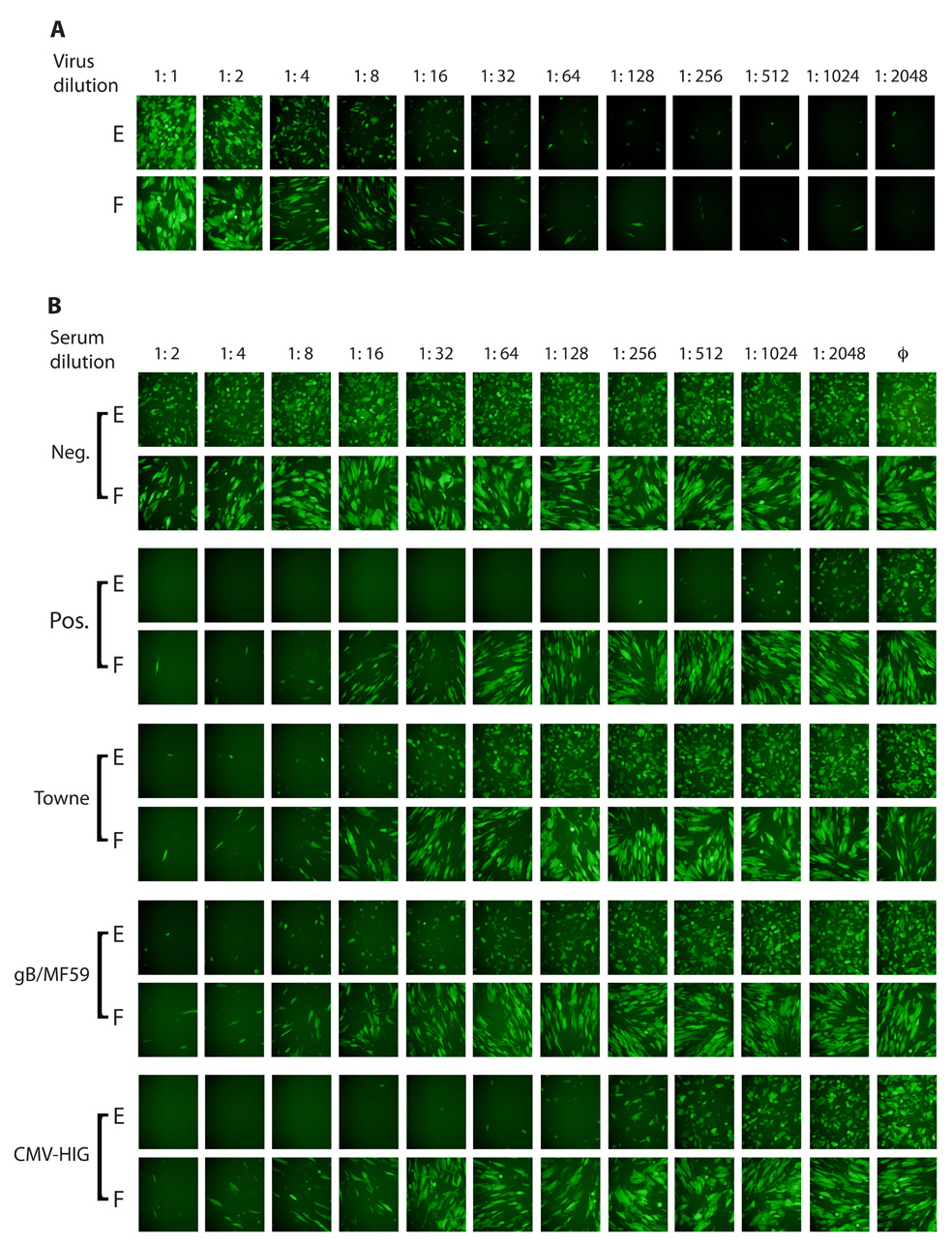
MRC-5 human fetal lung fibroblasts (ATCC CCL-171) and human ARPE-19 retinal pigment epithelial cells (ATCC CRL-2302) were propagated in high glucose Dulbecco’s modified Eagle medium (Gibco-BRL) supplemented with 10% fetal calf serum (HyClone Laboratories), 10,000 IU/L penicillin, 10 mg/L streptomycin (Gibco-BRL) (DMEM). CMV strain BADrUL131-Y4 was derived from a bacterial artificial chromosome (BAC) clone of the CMV strain AD169 genome that had been modified in E. coli by Wang and Shenk to (1) contain a green fluorescent protein (GFP) reporter cassette to permit efficient detection and quantitation of viral infection [18], and (2) to express a functional UL131 protein, which permits efficient entry and replication in either ARPE-19 or MRC-5 cells [19]. BADrUL131-Y4 was reconstituted by transfection of BAC DNA into ARPE-19 cells as described [20] and amplified by passage exclusively in ARPE-19 cells. Tissue culture supernatants (BADrUL131-Y4) were clarified by centrifugation, adjusted to 0.1 M sucrose, aliquoted, and stored at −80 °C. Viral titers of 1×105 (BADrUL131-Y4) were determined by limiting dilution in 96-well plates [21] using MRC-5s. Stocks of BADrUL131-Y4 were capable of entering ARPE-19 and MRC-5 cells with equal efficiency (Figure 1A).
Figure 1.
A GFP-based virus neutralization assay. (A) A stock of BADrUL131-Y4 was two-fold serially diluted and equal aliquots of each dilution were added to ARPE-19 retinal pigmented epithelial cells (E) or MRC-5 fibroblasts (F). (B) Two-fold serial dilutions of human sera or CMV-hyperimmuneglobulin were incubated with a fixed amount of virus for one hour, then replicate volumes were used to infect the two cell types. Samples include a seronegative serum (Neg.) and a convalescent serum (Pos.) obtained 11 years after seroconversion, representative sera from Towne and gB/MF59 vaccine recipients, and CytoGam® pre-diluted 20-fold. Negative controls (ϕ) contained virus but no sera. Representative micrographs were taken with a fixed exposure three days post infection.
2.3 Neutralization assays
Two-fold serial dilutions of sera (125 µl) in DMEM were prepared in triplicate in 96-well plates and mixed with 125 µl (2500 pfu) BADrUL131-Y4 in DMEM per well. After incubation at 37 °C for 1 h replicate 100 µl aliquots (1000 pfu) from each well were transferred to white-walled clear/flat-bottomed 96-well plates (Costar) that contained confluent MRC-5 or ARPE-19 cells in 150 µl DMEM. Plates were incubated at 37 °C with 5% CO2. Representative photographs were taken three days post infection using a Nikon Diaphoto 300 microscope equipped with a 470–525 nm UV filter. GFP fluorescence was measured four (MRC-5) or five (ARPE-19) days post infection using a PerkinElmer Victor2 1420 Multilable Counter. The same procedure was used for IE-based assays except that neutralization was quantitated by counting immediate early (IE) antigen-positive cells in each well 19 hours post infection by the method of Abai et al. [22]. Fifty percent inhibitory concentration (IC50) values were calculated by plotting the means of triplicate GFP values or IE-positive cell counts for each serum dilution against log2 serum concentration, using the Solver function of Microsoft Excel to calculate the best fit four-parameter equation for the data, and interpolating the serum dilution at the mid-point of the curve as the IC50 neutralizing titer.
2.3 Adsorption
Adsorption was achieved by incubating 100 µl serum with PBS or various amounts of recombinant gB (identical to that used in the gB/MF59 vaccine; a gift from Sanofi Pasteur) diluted to a final volume of 200 µl with DMEM for 1 h at room temperature prior to use in the neutralization assay. Aliquots removed after adsorption were assayed by gB-specific ELISA [16] to monitor adsorption efficiency.
2.4 Statistical analyses
Logarithmic means were calculated by taking the arithmetic mean of Log2 transformed IC50 values or E/F ratios, then back-transforming by taking the inverse Log2 of the resulting means. All statistical analyses of IC50 values or E/F ratios were conducted on Log2 transformed data. Log4 transformation was used both for logarithmic means and statistical analyses of gB-ELISA titers as they are reported in 4-fold increments. Two-tailed t-tests were used to compare fibroblast and epithelial neutralizing IC50 values of natural seropositives and epithelial neutralizing IC50 values, E/F ratios, and gB-ELISA titers between the four groups (natural seropositives, CMV-hyperimmuneglobulins, Towne-recipients, and gB/MF59 recipients).
3. Results
3.1 Neutralizing titers induced by natural infection
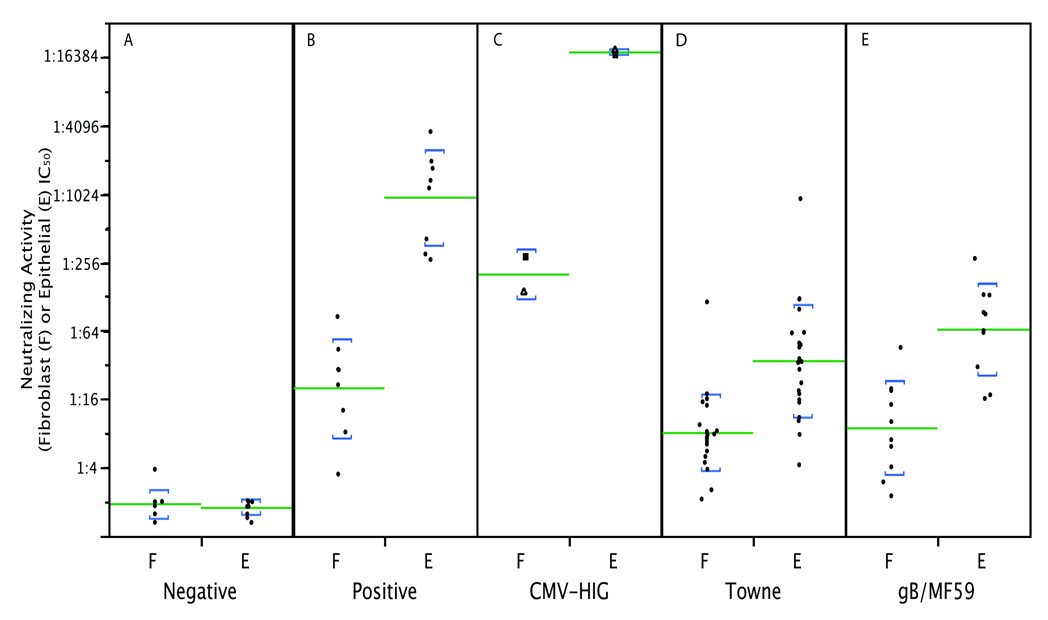
To initially determine if neutralizing activities differ when measured with epithelial cells versus fibroblasts, one seronegative serum and one seropositive convalescent serum obtained 11 years after the subject seroconverted were evaluated. The seronegative serum did not neutralize CMV infection of either cell type (Figure 1B). The seropositive convalescent serum neutralized both fibroblast and epithelial cell entry, but the titer of neutralizing activity was higher when epithelial cells were used (Figure 1B). Ten additional seronegative and eight seropositive sera were obtained from random blood donors and were assayed for neutralizing activities. Neutralizing titers determined as fifty percent inhibitory concentrations (IC50) obtained using fibroblasts or epithelial cells are shown in Fig. 2. Neutralizing titers for all ten seronegative sera were below 1:4 regardless of target cell type. The seropositive sera neutralized fibroblast entry with a logarithmic mean titer of 1:20, but when epithelial cells were used as targets the logarithmic mean titer was 1:944 (t = 7.9, df = 14, p < 0.0001).
Figure 2.
Fibroblast and epithelial neutralizing titers compared between different subject groups. The GFP-based assay shown in Fig. 1 was used to measure fibroblast (F) and epithelial (E) IC50 neutralizing titers for sera from human subjects or two CMV-hyperimmuneglobulin (CMV-HIG) products. (A) Ten seronegative random blood donor sera (note: four sera having no neutralizing activity were assigned a value of 1:2 to permit presentation on the graph); (B) eight naturally seropositive random blood donor sera; (C) CytoGam® (squares) and Cytotect CP (triangles); (D) sera from 23 Towne vaccine recipients; (E) sera from ten gB/MF59 vaccine recipients. Lines indicate logarithmic means; brackets represent one standard deviation above and below means.
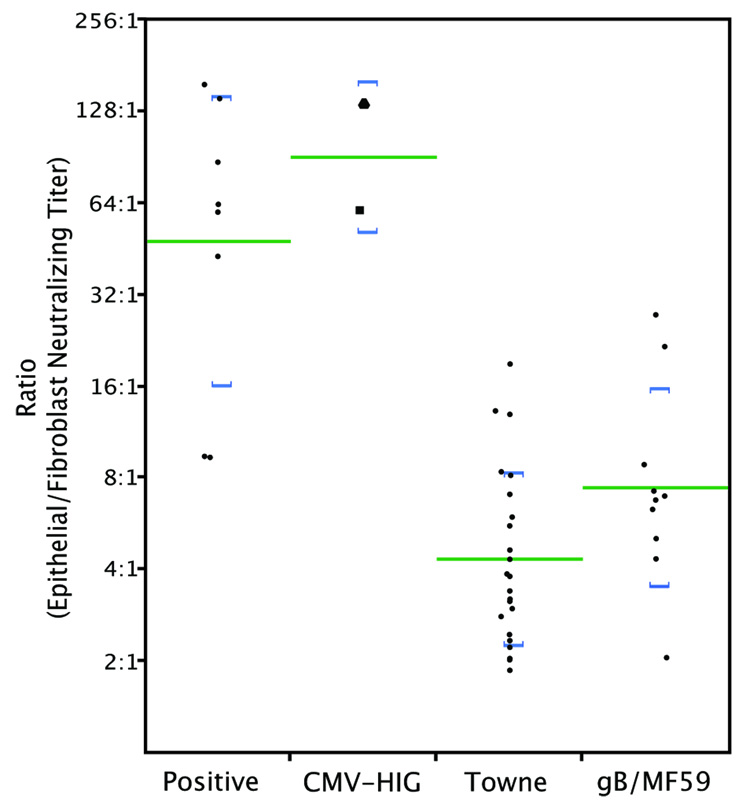
For each serum the reciprocal neutralizing titer for epithelial cells was divided by the reciprocal titer for fibroblasts. These ratios (Figure 3) ranged from 9:1 to 155:1. The logarithmic mean ratio of 48:1 suggests that on average immune sera are 48-fold more active in neutralizing epithelial cell entry than fibroblast entry.
Figure 3.
Ratios of reciprocal epithelial to fibroblast neutralizing titers were calculated from data shown in Fig. 2. CytoGam® (square); Cytotect CP (triangle). Lines indicate logarithmic means; brackets represent one standard deviation above and below means.
3.2 Neutralizing titers induced by experimental vaccines
To determine if vaccines induce epithelial-neutralizing antibodies, sera from 23 recipients of the Towne live attenuated vaccine and from ten recipients of the gB/MF59 vaccine were tested. Sera from gB/MF59 recipients were obtained 7 months after administration of the first dose. Towne recipient sera were obtained between 1 and 24 months after vaccination. For some Towne recipients multiple sera obtained at different times after vaccination were tested; however, only one serum representing the maximal activity for that subject was included in the data analysis.
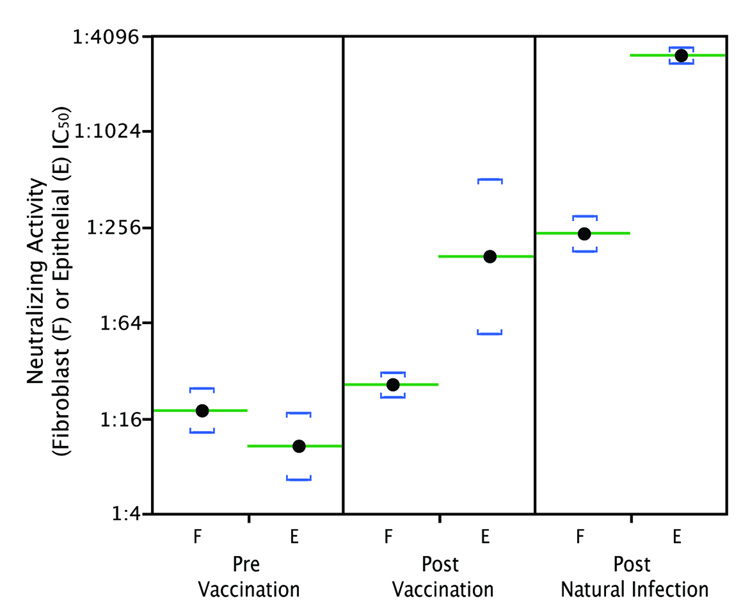
Figure 1B shows micrographs of neutralization assays conducted with sera from one representative Towne and one representative gB/MF59 recipient. IC50 values for sera from both groups are shown in Figure 2. Fibroblast-neutralizing titers of Towne vaccine recipients were slightly lower than those of sera from naturally infected individuals (Fig. 2) (t = 2.3, df = 10, p = 0.0418); however, epithelial-neutralizing titers of sera from Towne recipients were much lower than those from naturally infected subjects (logarithmic mean 1:34 for Towne versus 1:944 for naturally infected subjects; (t = 8.0, df = 14, p < 0.0001) (Fig. 2). Also available were sera from thirteen recipients of the Towne vaccine who became naturally infected after vaccination. Initial antibody titers were typical for Towne recipients, but after these subjects became naturally infected epithelial neutralizing titers increased to titers comparable to those of natural seropositives. Representative data from one subject are shown in Figure. 4.
Figure 4.
Fibroblast and epithelial IC50 neutralizing titers were measured for serial sera from a subject that underwent natural CMV infection after receiving the Towne vaccine. The pre-vaccination sample was seronegative, post-vaccination sample was drawn 2 months after Towne vaccination, and the post-natural infection sample was drawn 12 months post vaccination. A urine sample collected concurrent with the 12 month serum was culture positive for wild type CMV. Lines indicate logarithmic means of triplicate assays on each serum; brackets represent one standard deviation above and below means.
For gB/MF59 recipients, fibroblast-neutralizing titers were similar to those of naturally infected subjects (t = 1.75, df = 15, p = 0.1007) and Towne recipients (t = 0.29, df = 15, p = 0.61). Epithelial-neutralizing titers of gB/MF59 recipients were higher than but not statistically different from those of Towne recipients (t = 1.6, df = 38, p = 0.1113) but were still well below those of natural seropositives (logarithmic mean of 1:65 for gB/MF59 versus 1:944 for those naturally infected; t = 6.0, df = 15, p < 0.0001) (Fig. 2).
3.3 Neutralizing titers in CMV-hyperimmuneglobulin
Two human CMV-hyperimmuneglobulin products, CytoGam® and Cytotect CP, were evaluated for neutralizing activities. Both products had very high epithelial-neutralizing titers (1:17121 and 1:18660, respectively) and high fibroblast-neutralizing titers (1:285 and 1:139, respectively) (Fig. 1B and Fig. 2). When adjusted for IgG concentration (20-fold higher in hyperimmuneglobulin versus serum) these titers are similar to those of human immune sera. The ratios of epithelial to fibroblast titers (60:1 for CytoGam®, 134:1 for Cytotect CP) were also not statistically different from convalescent sera (t = 1.0, df = 39, p = 0.3022) (Fig. 3).
3.4 Evaluation by a neutralization assay that detects immediate early antigen
Measuring viral entry by GFP is indirect and requires 4–5 days of viral amplification before adequate signal strength is obtained. To determine if similar results occurred with a direct measure of viral entry, neutralizing assays were repeated using a monoclonal antibody to CMV immediate early (IE) antigen to stain infected-cell nuclei 19 h post infection [22]. Four samples were evaluated: the convalescent serum shown in Fig. 1B, CytoGam®, one representative serum from a Towne recipient, and one representative serum from a gB/MF59 recipient. The results are summarized in Table 1. While the titers obtained from the IE-based assay differed somewhat from those obtained from the GFP-based assay, the results confirmed the conclusions that substantial epithelial-specific neutralizing activities are induced by natural infection but not by vaccination.
Table 1.
Neutralizing titers obtained using GFP-based versus IE-based assays
| Sample | GFP-based | IE-based | ||||
|---|---|---|---|---|---|---|
| fibroblast | epithelial | ratio a | fibroblast | epithelial | ratioa | |
| natural seropositiveb | 1:32 | 1:2341 | 73 | 1:18 | 1:1045 | 58 |
| CytoGam® | 1:285 | 1:17121 | 60 | 1:131 | 1:1590 | 12 |
| Townec | 1:11 | 1:94 | 9 | 1:6 | 1:4 | 0.7 |
| gB/MF59d | 1:10 | 1:276 | 28 | 1:5 | 1:4 | 0.8 |
reciprocal epithelial titers divided by reciprocal fibroblast titers
serum from a natural seropositive obtained 11 years after seroconversion
serum from a representative Towne-recipient obtained two months after vaccination
serum from a representative gB/MF59-recipient that received the three five µg doses
3.5 Anti-gB antibody titers
Anti-gB antibody titers for all sera were assayed by gB-specific ELISA. The logarithmic mean gB-ELISA titer for Towne vaccine recipients, 1:14011, was not statistically different from the logarithmic mean titer of 1:21526 for natural seropositives (t = 1.0, df = 38, p = 0.3226). However, the logarithmic mean titer for gB/MF59 recipients, 1:166349, was significantly higher than those for natural seropositives (t = 4.1, df = 38, p = 0.0002) or Towne recipients (t = 6.3, df = 38, p < 0.0001).
3.6 Pre-adsorption with recombinant gB
To determine the extent to which epitopes within gB contribute to serum neutralizing activities, the 11 year convalescent serum shown in Fig. 1B and Table 1 was assayed for neutralizing activity after gB-specific antibodies were adsorbed by preincubation with recombinant gB. Adsorption with gB removed gB-specific antibodies from the serum and lowered the fibroblast neutralizing titer by almost two-fold (Table 2); however, gB adsorption did not affect neutralizing titers against epithelial cell entry (Table 2). In contrast, adsorption of a representative gB/MF59 recipient’s serum removed the majority of gB-specific antibodies and reduced both fibroblast and epithelial neutralizing titers to levels comparable to those of seronegative sera (Table 2).
Table 2.
Adsorption of antibodies with recombinant gB
| serum | Adsorptionc | gB-ELISAd | Neutralizing Titers | |
|---|---|---|---|---|
| fibroblast | epithelial | |||
| natural infectiona | buffer | 1.07 | 1:11 | 1:1418 |
| 15 µg gB | 0.16 | 1:6.3 | 1:2183 | |
| 30 µg gB | 0.01 | 1:8.1 | 1:1492 | |
| gB/MF59 vaccineb | buffer | 2.543 | 1:5 | 1:40 |
| 10 µg gB | 2.071 | 1:3 | 1:19 | |
| 20 µg gB | 1.155 | 1:2 | 1:14 | |
| 40 µg gB | 0.193 | 1:2 | 1:5 | |
serum from a natural seropositive obtained 11 years after seroconversion.
serum obtained 7 months after the first dose of the gB/MF59 vaccine.
sera were incubated with buffer or the indicated amounts of recombinant gB for one hour prior to ELISA or neutralizing assay.
optical densities.
4. Discussion
Neutralizing antibodies usually prevent cells from becoming infected by binding to virion epitopes necessary for viral attachment and entry. For nearly all viral vaccines the induction of neutralizing antibodies is necessary for vaccine efficacy. With respect to CMV, maternal antibodies protect premature infants and passive immunization is effective in preventing CMV-associated disease in transplant patients [23,24]. Also, as predicted by animal models, administration of CMV hyperimmuneglobulin to women with primary CMV infections during pregnancy has been reported to protect the fetus from infection and effectively treats infants infected in utero [25].
CMV neutralizing epitopes have been extensively studied. Antibodies binding to epitopes within gB comprise up to one-half of the neutralizing activity in human sera [3], although neutralizing epitopes also exist in gH [4] and gM/gN [5]. These studies, however, used fibroblasts as the target cells and fibroblast-adapted virus strains that lack the ability to infect endothelial and epithelial cells. Consequently, prior studies of CMV-neutralizing antibodies have focused on epitopes that are important for fibroblast entry. The discovery that CMV enters epithelial and endothelial cells by a distinct, endocytic mechanism suggested that previously unrecognized neutralizing epitopes may exist.
The results of Gerna et al. [12] as well as those presented here reveal not only that such epitopes exist, but in naturally infected individuals antibodies to these epitopes comprise epithelial-neutralizing responses that far exceed, in titer, fibroblast-neutralizing responses. Our data, which used GFP fluorescence to accurately quantitate IC50 values, indicate that epithelial neutralizing titers are on average 48-fold higher than fibroblast neutralizing titers.
The epitopes that are targeted by the antibodies that comprise this substantial epithelial- or endothelial-specific neutralizing activity of human immune sera remain unknown. Antibodies induced by the gB/MF59 vaccine did neutralize epithelial entry and titers were on average seven times higher against epithelial cell entry than fibroblast entry. In virions, gB is presumed to exist in a “prefusion” conformation that, upon triggering, undergoes a structural transformation that initiates membrane fusion. In fibroblast entry the trigger occurs at neutral pH and probably involves the interaction of gB with a cell surface receptor. This interaction may also involve the gH/gL/gO complex, which is important for fibroblast entry [8,26,27]. In contrast, epithelial cell entry requires a gH/gL/UL128-131 complex [8,28]. Studies of viral entry into endothelial cells suggest that UL128-131 interacts with and induces a conformational change in gB which promotes a transient gB-gH interaction that facilitates membrane fusion [13]. Other studies observed a pH dependence for epithelial cell entry [10] but this appears to be influenced by cell type of origin of the virus used [29]. Thus, the enhanced ability of anti-gB antibodies to neutralize epithelial entry versus fibroblast entry may derive from antibodies reactive with epitopes within gB that are specifically involved in pH-dependent triggering and/or with interactions between gB and the gH/gL/UL128-131 complex.
Although we found that Towne vaccination elicits gB-specific antibody titers comparable to titers induced by natural infection, epithelial-neutralizing titers of Towne sera were on average 28-fold lower than natural infection sera. This suggests that antibodies directed against gB epitopes, even the native gB expressed by Towne, do not provide high titer epithelial-specific neutralizing activity. Consistent with this, adsorption with the recombinant gB used in the gB/MF59 vaccine failed to reduce the epithelial-neutralizing titer of a convalescent serum. For this serum at least, the majority of epithelial-specific neutralizing antibodies are specific to epitopes that are not represented by the vaccine gB. However, because the gB represented in the gB/MF59 vaccine is derived from Towne, it is possible that epithelial neutralizing titers induced by both vaccines are low because epithelial-specific epitopes within gB are strain-specific and differ between strains Towne and AD169. Preliminary studies, however, do not suggest that the virus strain makes a significant difference: using an epithelial-adapted Towne virus the epithelial neutralizing titers of sera from two Towne recipients were still well below those of two natural seropositives (not shown).
The endocytic entry mediators UL128, UL130, and UL131 are strong potential candidates for antigens containing epithelial neutralizing epitopes. Animal antibodies raised against epitopes in each of these antigens neutralize viral entry into epithelial or endothelial cells without affecting fibroblast entry [8,11,12]. Due to a frame-shift mutation the Towne vaccine expresses a mutant UL130 that is unstable, poorly expressed, and unable to support endothelial [15] or epithelial cell entry. This implies that Towne is unlikely to induce potent antibody responses to UL130. The mutation may further preclude presentation of conformational epitopes found only within the intact gH/gL/UL128-131 complex as this complex does not form without UL130 [28]. Hence, the inability of Towne to express UL130 may result in a defect in the induction of antibodies that neutralize by targeting conformational epitopes within the gH/gL/UL128-131 complex. Thus, induction of antibodies to the gH/gL/UL128-131 complex, perhaps in concert with epitopes in gB, may be needed to elicit epithelial-neutralizing activities.
The ability to neutralize viral entry into mucosal epithelial cells is highly relevant to vaccine efficacy. While our data reflect only antibodies that neutralize viral entry into retinal pigment epithelial cells, data from Wang and Shenk indicate that an intact UL128-131 locus also promotes viral entry into epithelial cell lines derived from breast, cervix, lung, and colon [19]. Moreover, CMV entry into dendritic cells [30] and a variety of endothelial cells, including lung macrovascular [19], microvascular [11], and umbilical vein [8,9,15], also depend on UL128-131. Therefore, although details of the entry mechanism(s) remain controversial and may differ between cell types [10,13,29,31], the common requirement for UL128-131 suggests that neutralizing data obtained with retinal pigment epithelial cells will likely apply to mucosal epithelial cells. Consistent with this prediction, Gerna et al. observed a general concordance between neutralizing titers obtained with retinal pigment epithelial cells and umbilical vein endothelial cells, although a few sera were discordant [12].
Induction of neutralizing antibodies targeting UL128-131-mediated entry may be important for the efficacy of a CMV vaccine. Despite inducing high titer anti-gB antibodies, the Towne vaccine does not elicit high titer epithelial neutralizing responses. It also does not protect renal transplant recipients from CMV infection post transplantation, although it does reduce CMV-associated disease [32]. And, when tested at low dose, Towne did not protect immunocompetent mothers from acquiring CMV infections from their children [33]. In contrast, antibodies induced by natural infection have high epithelial neutralizing titers and, whether naturally or passively acquired, appear to be protective against child-to-mother transmission, congenital transmission, and fetal disease [25,33]. The gB/MF59 vaccine falls in between, inducing higher levels of epithelial-neutralizing antibodies than Towne but still 15-fold lower than natural infection. That gB/MF59 vaccine recipients are more likely to remain uninfected than placebo recipients [34] suggests that the vaccine has some efficacy, but the extent of this efficacy remains uncertain. Thus, induction of epithelial-neutralizing antibodies comparable to those induced by natural infection may be necessary for an effective CMV vaccine. If present within secretions such antibodies could be effective in blocking viral transmission by preventing entry of inoculum virus into epithelial cells of the oral or genital mucosa. Therefore, it will be important to identify the viral epitopes involved in neutralization of epithelial cell entry.
Acknowledgements
We are grateful to Dai Wang and Thomas Shenk for providing the BADrUL131-Y4 BAC, Sanofi Pasteur for providing recombinant gB, John Grider for use of the Nikon Diaphoto 300 microscope and PerkinElmer Victor2 1420 Multilable Counter, and Kimber White and Chris Sheth for technical assistance and use of the CTL ImmunoSpot Analyzer. We also thank Julie McVoy for advice on data analysis and for providing the Excel program used for curve fitting and IC50 determinations. This work was supported by grants from then National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326(10):663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 2.Stratton KR, Durch SJ, Lawrence RS, editors. Vaccines for the 21st Century: A Tool for Decisionmaking. Washingto D.C: National Academy Press; 2000. [PubMed] [Google Scholar]
- 3.Marshall GS, Rabalais GP, Stout GG, Waldeyer SL. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J Infect Dis. 1992;165(2):381–384. doi: 10.1093/infdis/165.2.381. [DOI] [PubMed] [Google Scholar]
- 4.Urban M, Klein M, Britt WJ, Hassfurther E, Mach M. Glycoprotein H of human cytomegalovirus is a major antigen for the neutralizing humoral immune response. J Gen Virol. 1996;77(Pt 7):1537–1547. doi: 10.1099/0022-1317-77-7-1537. [DOI] [PubMed] [Google Scholar]
- 5.Shimamura M, Mach M, Britt WJ. Human cytomegalovirus infection elicits a glycoprotein M (gM)/gN-specific virus-neutralizing antibody response. J Virol. 2006;80(9):4591–4600. doi: 10.1128/JVI.80.9.4591-4600.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler SP, Hempfling SH, Starr SE, Plotkin SA, Riddell S. Safety and immunogenicity of the Towne strain cytomegalovirus vaccine. Pediatr Infect Dis J. 1998;17(3):200–206. doi: 10.1097/00006454-199803000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Pass RF, Duliege AM, Boppana S, et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis. 1999;180(4):970–975. doi: 10.1086/315022. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci U S A. 2005;102(50):18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hahn G, Revello MG, Patrone M, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78(18):10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryckman BJ, Jarvis MA, Drummond DD, Nelson JA, Johnson DC. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J Virol. 2006;80(2):710–722. doi: 10.1128/JVI.80.2.710-722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler B, Scrivano L, Ruzcics Z, Rupp B, Sinzger C, Koszinowski U. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J Gen Virol. 2006;87(Pt 9):2451–2460. doi: 10.1099/vir.0.81921-0. [DOI] [PubMed] [Google Scholar]
- 12.Gerna G, Sarasini A, Patrone M, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89(Pt 4):853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 13.Patrone M, Secchi M, Bonaparte E, Milanesi G, Gallina A. Cytomegalovirus UL131-128 products promote gB conformational transition and gB-gH interaction during entry into endothelial cells. J Virol. 2007;81(20):11479–11488. doi: 10.1128/JVI.00788-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dolan A, Cunningham C, Hector RD, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85(Pt 5):1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 15.Patrone M, Secchi M, Fiorina L, Ierardi M, Milanesi G, Gallina A. Human cytomegalovirus UL130 protein promotes endothelial cell infection through a producer cell modification of the virion. J Virol. 2005;79(13):8361–8373. doi: 10.1128/JVI.79.13.8361-8373.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang JB, Adler SP, Hempfling S, et al. Mucosal antibodies to human cytomegalovirus glycoprotein B occur following both natural infection and immunization with human cytomegalovirus vaccines. J Infect Dis. 1996;174(2):387–392. doi: 10.1093/infdis/174.2.387. [DOI] [PubMed] [Google Scholar]
- 17.Adler SP, McVoy M. Detection of cytomegalovirus antibody by enzyme immunoassay and lack of evidence for an effect resulting from strain heterogeneity. J Clin Microbiol. 1986;24(5):870–872. doi: 10.1128/jcm.24.5.870-872.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Bresnahan W, Shenk T. Human cytomegalovirus encodes a highly specific RANTES decoy receptor. Proc Natl Acad Sci U S A. 2004;101(47):16642–16647. doi: 10.1073/pnas.0407233101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Shenk T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol. 2005;79(16):10330–10338. doi: 10.1128/JVI.79.16.10330-10338.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hahn G, Jarosch M, Wang JB, Berbes C, McVoy MA. Tn7-mediated introduction of DNA sequences into bacmid-cloned cytomegalovirus genomes for rapid recombinant virus construction. J Virol Methods. 2003;107(2):185–194. doi: 10.1016/s0166-0934(02)00232-x. [DOI] [PubMed] [Google Scholar]
- 21.Cui X, McGregor A, Schleiss MR, McVoy MA. Cloning the complete guinea pig cytomegalovirus genome as an infectious bacterial artificial chromosome with excisable origin of replication. J Virol Methods. 2008;149(2):231–239. doi: 10.1016/j.jviromet.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abai AM, Smith LR, Wloch MK. Novel microneutralization assay for HCMV using automated data collection and analysis. J Immunol Methods. 2007;322(1–2):82–93. doi: 10.1016/j.jim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adler SP, Chandrika T, Lawrence L, Baggett J. Cytomegalovirus infections in neonates acquired by blood transfusions. Pediatr Infect Dis. 1983;2(2):114–118. doi: 10.1097/00006454-198303000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Yeager AS, Grumet FC, Hafleigh EB, Arvin AM, Bradley JS, Prober CG. Prevention of transfusion-acquired cytomegalovirus infections in newborn infants. J Pediatr. 1981;98(2):281–287. doi: 10.1016/s0022-3476(81)80662-2. [DOI] [PubMed] [Google Scholar]
- 25.Nigro G, Adler SP, La Torre R, Best AM. Passive Immunization during Pregnancy for Congenital Cytomegalovirus Infection. N Engl J Med. 2005;353(13):1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen L, Matkin C, Spaete R, Pachl C, Merigan TC. Antibody response to human cytomegalovirus glycoproteins gB and gH after natural infection in humans. J Infect Dis. 1991;164(5):835–842. doi: 10.1093/infdis/164.5.835. [DOI] [PubMed] [Google Scholar]
- 27.Keay S, Baldwin B. Anti-idiotype antibodies that mimic gp86 of human cytomegalovirus inhibit viral fusion but not attachment. J Virol. 1991;65(9):5124–5128. doi: 10.1128/jvi.65.9.5124-5128.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryckman BJ, Rainish BL, Chase MC, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2007 doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Yu QC, Schroer J, Murphy E, Shenk T. Human cytomegalovirus uses two distinct pathways to enter retinal pigmented epithelial cells. Proc Natl Acad Sci U S A. 2007;104(50):20037–20042. doi: 10.1073/pnas.0709704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerna G, Percivalle E, Lilleri D, et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J Gen Virol. 2005;86(Pt 2):275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 31.Sinzger C. Entry route of HCMV into endothelial cells. J Clin Virol. 2008;41(3):174–179. doi: 10.1016/j.jcv.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Plotkin SA, Smiley ML, Friedman HM, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet. 1984;1(8376):528–530. doi: 10.1016/s0140-6736(84)90930-9. [DOI] [PubMed] [Google Scholar]
- 33.Adler SP, Starr SE, Plotkin SA, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age [published erratum appears in J Infect Dis 1995 Apr;171(4):1080] J Infect Dis. 1995;171(1):26–32. doi: 10.1093/infdis/171.1.26. [DOI] [PubMed] [Google Scholar]
- 34.Pass R, Zhang C, Simpson T, et al. Infectious Diseases Society of America. San Diego California: 2007. Cytomegalovirus (CMV) Envelope Glycoprotein B (gB) Vaccine in Young Women. [Google Scholar]