Abstract
A critical goal of vaccine development for a wide variety of pathogens is the induction of potent and durable mucosal immunity. However, it has been assumed that this goal would be difficult to achieve by systemic vaccination due to the anatomic and functional distinctness of the systemic and mucosal immune systems and the resultant compartmentalization of immune responses. Here we show that antigen-specific CD8+ T-lymphocytes traffic efficiently to mucosal surfaces following systemic vaccination. Intramuscular immunization with recombinant adenovirus (rAd) vector-based vaccines expressing SIV Gag resulted in potent, durable and functional CD8+ T lymphocyte responses at multiple mucosal effector sites in both mice and rhesus monkeys. In adoptive transfer studies in mice, vaccine-elicited CD8+ T-lymphocytes exhibited phenotypic plasticity, upregulated mucosal homing integrins and chemokine receptors, and trafficked rapidly to mucosal surfaces. Moreover, the migration of systemic CD8+ T-lymphocytes to mucosal compartments accounted for the vast majority of antigen-specific mucosal CD8+ T-lymphocytes induced by systemic vaccination. Thus, intramuscular vaccination can overcome immune compartmentalization and generate robust mucosal CD8+ T-lymphocyte memory. These data demonstrate that the systemic and mucosal immune systems are highly coordinated following vaccination.
Keywords: AIDS, Vaccination, Mucosa
Introduction
The ability to generate potent mucosal immune responses is an important goal of vaccine development. Most vector-based vaccine candidates utilize exclusively systemic immunization strategies as a result of the logistic and potential safety concerns associated with the delivery of recombinant vaccine vectors by mucosal routes. However, it has been assumed that potent mucosal immunity would be difficult to generate by systemic vaccination as a result of the anatomic and functional distinctness of the systemic and mucosal immune systems and the resultant compartmentalization of immune responses (1, 2).
Anatomic compartmentalization of cellular immune memory has been shown to be influenced by the initial site of antigen exposure in both humans and animal models of localized infections or malignancies (3-5). Similar anatomically biased primary and recall responses have been observed in certain models of vaccination (6-10). In addition, dendritic cells isolated from specific anatomic sites have been reported to influence patterns of chemokine receptor and integrin expression and tissue homing specificities of primed CD8+ T-lymphocytes (11-13). In contrast, global CD8+ T-lymphocyte responses bridging systemic and mucosal compartments have been observed in models of localized infection with rotavirus, Sendai virus and Listeria (14, 15). Moreover, the homing specificities initially imprinted on CD8+ T-lymphocytes have been shown to be modulated upon subsequent trafficking to different microenvironments (16, 17). It is not known, however, whether vaccine-elicited CD8+ T-lymphocytes can overcome immune compartmentalization to establish broadly distributed cellular immune memory.
Mucosal immunity will likely prove particularly important for an HIV-1 vaccine, not only because the genital and rectal mucosa represent the primary portals of virus entry but also because the gastrointestinal mucosa is the predominant site of destruction of memory CD4+ T-lymphocytes during acute infection (18-21). Moreover, vaccine efficacy has been correlated with preservation of mucosal memory CD4+ T-lymphocytes after SIV challenge in rhesus monkeys (22). Studies in rhesus monkeys (23-25) and humans (26-29) have suggested that CD8+ T-lymphocyte responses likely contribute to control of SIV and HIV-1 replication, and thus vaccines that induce potent, durable and protective mucosal cellular immunity would likely be beneficial. However, it is also possible that vaccine-elicited mucosal CD4+ T-lymphocytes could theoretically result in increased targets of HIV-1 infection, demonstrating the importance of improving our understanding of cellular mucosal immune responses following vaccination. Although the majority of HIV-1 vaccine candidates are administered by the intramuscular route, the ability of intramuscular immunization to influence patterns of CD8+ and CD4+ T-lymphocyte trafficking and to generate potent and durable mucosal cellular immune memory has not previously been elucidated in detail.
In this study, we evaluated the magnitude, kinetics, phenotype and durability of mucosal cellular immune responses in mice and rhesus monkeys after systemic immunization with various novel replication-incompetent recombinant adenovirus (rAd) vectors, administered either alone or in heterologous prime-boost regimens. We found that systemic immunization induced remarkably potent and durable CD8+ T-lymphocyte responses at multiple mucosal surfaces. We also assessed the mechanism of Tlymphocyte trafficking following systemic immunization. Our data show that vaccine-activated CD8+ T-lymphocytes, but not quiescent CD8+ T-lymphocytes, upregulated mucosal homing markers and migrated rapidly from systemic to mucosal immune compartments to generate potent and durable mucosal immune responses. Moreover, the migration of systemic CD8+ T-lymphocytes to mucosal compartments accounted for the vast majority of mucosal CD8+ T-lymphocytes induced by intramuscular vaccination. These results have important implications for vaccination strategies aimed at inducing mucosal cellular immunity.
Materials and Methods
Animals, vectors and immunizations
The construction of rAd5, rAd26 and rAd5HVR48 vectors expressing SIVmac239 Gag has been described previously (30-33). C57BL/6 and B6.SJL-Ptprca Pepcb/BoyJ mice were obtained from Jackson Laboratories. Six- to eight-week old mice were injected intramuscularly with 109 VP of various replication-incompetent rAd vectors expressing SIV Gag in 100 μl sterile PBS divided equally between both quadriceps muscles. For prime-boost regimens, mice were immunized as above with injections spaced 6-8 weeks apart.
Adult, outbred rhesus monkeys (Macaca mulatta) were housed at the New England Primate Research Center, Southborough, MA. Presence of the Mamu-A*01 allele was determined by PCR and sequencing (34). Monkeys were injected intramuscularly with 1011 VP rAd5HVR48 expressing SIV Gag in 1 ml sterile PBS divided equally between both quadriceps muscles. Monkeys were anesthetized prior to endoscopic acquisition of biopsy specimens. All animals used in this study were maintained in accordance with institutional guidelines, and studies were reviewed and approved by the relevant institutional animal care and use committees.
Mucosal lymphocyte isolation
Murine small and large bowel, vaginal tract and respiratory tract were dissected free of associated connective tissue and cut into small pieces using straight scissors. Bowel specimens were washed extensively with HBSS and incubated with HBSS supplemented with 0.1 mm EDTA and 10% FBS at 37°C for 30 minutes with vigorous shaking. The specimens were then vortexed and washed again with fresh media. Pooled supernatants contained the IEL population. Cells were resuspended in 40% Percoll (Sigma Chemical) and layered over 67% Percoll; samples were centrifuged at 1000g for 25 minutes. The interface between the two Percoll layers contained the lymphocyte population. After IEL removal, bowel specimens were washed three times with RPMI 1640 supplemented with 5% FBS to remove all traces of EDTA. Mucosal tissues were then digested by two serial 30 minute incubations at 37°C in RPMI 1640 containing 5%FBS supplemented with type IV collagenase (Sigma Chemical) at 300 U/ml with vigorous shaking to isolate small and large bowel LPL as well as vaginal tract and respiratory tract lymphocytes. Pooled supernatants from serial incubations were purified with a Percoll gradient as above to isolate the lymphocyte population. Lymphocytes were isolated from monkey mucosal biopsies by incubating samples in RPMI 1640 supplemented with 10% FBS, type IV collagenase at 300 U/ml and DNaseI (Sigma Chemical) at 30 U/ml at 37°C for 45 minutes with vigorous shaking. Cells were washed once with RPMI 1640 containing DNaseI at 30 U/ml, and lymphocytes were purified with a Percoll gradient as above.
Tetramer binding assays
For murine tetramer binding assays, H-2Db tetramers labeled with phycoerytherin and folded around the immunodominant SIV Gag epitope AL11 (AAVKNWMTQTL) were used to stain peripheral blood and tissue lymphocytes as described (30). Samples were analyzed using an LSRII flow cytometer (Becton Dickinson) and FloJo software (Tree Star). CD8+ T lymphocytes from naïve mice were utilized as negative controls and exhibited <0.1% tetramer staining at all anatomic sites. Monoclonal antibodies used in multiparameter flow cytometry were purchased from BD Biosciences (CD44-FITC (IM7), TCRγδ-FITC (GL3), β7 integrin-FITC (M293), CD4-PE or Pacific Blue (L3T4), CD8α-PerCP-Cy5.5 (53-6.7) and CD3-APC (145-2C111)) and eBioscience (CD127-PECy7 (A7R34), CD62L-APC-AlexaFluor750 (Mel-14), CD45.1-PE-Cy7 (A20), CD45.2 APC-AlexaFluor750 (104), CD3-AlexaFluor 700 (17A2), CD103-FITC (2E7) and CCR9-FITC (eBioCW-1.2)). LIVE/DEAD Fixable Violet was used for vital dye exclusion in flow cytometric assays according to the manufacturer's instructions (Invitrogen). For rhesus monkey tetramer binding assays, Mamu-A*01 tetramers labeled with phycoerythrin and folded around the immunodominant SIV Gag epitope CM9 (CTPYDINQM; also known as p11c) (35) were used in conjunction with monoclonal antibodies against CD3-Alexa700 (SP34), CD8-APC-Cy7 (SK1), CD28-PerCP-Cy5.5 (L293) and CD95-PE (DX2) (BD Biosciences) to stain CD8+ T lymphocytes from peripheral blood and extracted from tissue biopsy specimens. Samples were analyzed using an LSRII flow cytometer and FloJo software as described above.
Intracellular cytokine staining assays
Murine intracellular cytokine staining (ICS) assays were performed as previously described (36). Briefly, lymphocytes isolated from various anatomic sites were stimulated at 37°C in 200 μl media containing 4 μg/ml AL11 peptide or pooled overlapping SIV Gag peptides. After 2 hours, 50 μl media containing 100 μg/ml GolgiStop (BD Biosciences) was added, and the cells were cultured for an additional 4 hours at 37°C. Cells were stained with fluorescently-conjugated anti-CD3α, CD4, CD8, CD44, CD62L and CD127 monoclonal antibodies as above and then fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences). Permeablized cells were incubated with PE-conjugated anti-interleukin-2 (JES6-5H4; BD Biosciences) and APCconjugated anti-IFN-γ (XMG1.2; BD Biosciences) antibodies and were washed and resuspended in PBS containing 1.5% formaldehyde. Samples were analyzed using an LSRII flow cytometer and FloJo software.
Adoptive transfer studies
Spleens from donor animals were isolated and perfused gently with cold HBSS containing 4% FBS using a 1 cc insulin syringe to release splenocytes. CD4+ or CD8+ T-lymphocytes were purified from splenocytes by negative selection using immunomagnetic beads (CD4+ and CD8+ T-cell isolation kits; Miltenyi Biotec), according to the manufacturer's instructions. CD4+ and CD8+ T-lymphocytes were >90% pure by flow cytometric analysis. Purified cells were washed twice with PBS and resuspended at 2.5-5.0 × 106 cells/10 μl in cold sterile PBS. Cells were transferred to recipient mice by tail vein injection.
Statistical analyses
Statistical analyses were performed with GraphPad Prism version 4.01 (GraphPad Software, Inc., 2004). Immune responses among groups of mice are presented as means with standard errors. Comparisons of mean immune responses were performed using two-sided t-tests. In all cases, p-values of less than 0.05 were considered significant.
Results
Intramuscular rAd immunization induces potent and durable mucosal CD8+ T-lymphocyte responses
We first studied the ability of intramuscularly administered rAd serotype 5 (rAd5) vectors to elicit mucosal cellular immune responses. C57BL/6 mice were immunized intramuscularly with 109 viral particles (VP) of rAd5 expressing SIV Gag (rAd5-Gag), and Gag-specific CD8+ T-lymphocyte responses specific for the dominant Db-restricted epitope AL11 (AAVKNWMTQTL) (30) were assessed by Db/AL11 tetramer binding assays over 24 weeks. We evaluated the magnitude and kinetics of AL11-specific CD8+ T-lymphocyte responses in multiple systemic and mucosal compartments, including peripheral blood, spleen, inguinal and mesenteric lymph nodes, Peyer's patches, vaginal mucosa, and the intraepithelial and lamina propria lymphocyte populations (IEL and LPL) of both the small and large intestines. To determine the effector or memory phenotype of AL11-specific CD8+ T-lymphocytes, multiparameter flow cytometry was utilized to assess CD44, CD62L and CD127 expression (37-39). Lymphocytes from systemic and mucosal compartments exhibited comparable viability as determined by vital dye exclusion and their ability to produce interferon-γ (IFN-γ) and interleukin-2 (IL-2) following stimulation with phytohemagglutinin and ionomycin (data not shown). Lymphocytes isolated from mucosal effector surfaces were also free of contamination from blood or inductive lymphoid tissue, as demonstrated by minimal numbers of naïve (CD44-CD62L+) and central memory (CD44+CD62L+) T-lymphocytes in the cell populations isolated from these compartments (data not shown).
After a single intramuscular immunization with 109 VP rAd5-Gag, high frequency AL11-specific CD8+ T-lymphocyte responses were observed in multiple systemic and mucosal compartments, as shown for a representative experiment (Fig. 1A) or in summary for 4-6 mice per time point (Fig. 1B). The kinetics of the responses were similar at all anatomic sites evaluated. At week 2 following vaccination, mean peak AL11-specific CD8+ T-lymphocyte responses in blood were 6.4% of total CD8+ T-lymphocytes, while mean peak responses in spleen were 4.0%. Mean peak responses in both systemic and mucosal lymphoid inductive sites were several-fold lower (inguinal lymph nodes 0.6%, mesenteric lymph nodes 0.7% and Peyer's patches 1.3%). Surprisingly, mean peak responses in small and large bowel lamina propria (6.1% and 4.0%, respectively) were comparable in magnitude to those seen in blood and spleen, although mean peak responses in the small and large bowel IEL compartment were several-fold lower (1.6% in each compartment). In the vaginal tract, mean peak responses were, remarkably, 33% of CD8+ T-lymphocytes. In all anatomic compartments, AL11-specific CD8+ T-lymphocyte responses exhibited considerable durability up to 24 weeks following a single immunization. A similar anatomic distribution of CD8+ T lymphocyte responses was observed after subcutaneous immunization (data not shown).
FIGURE 1.
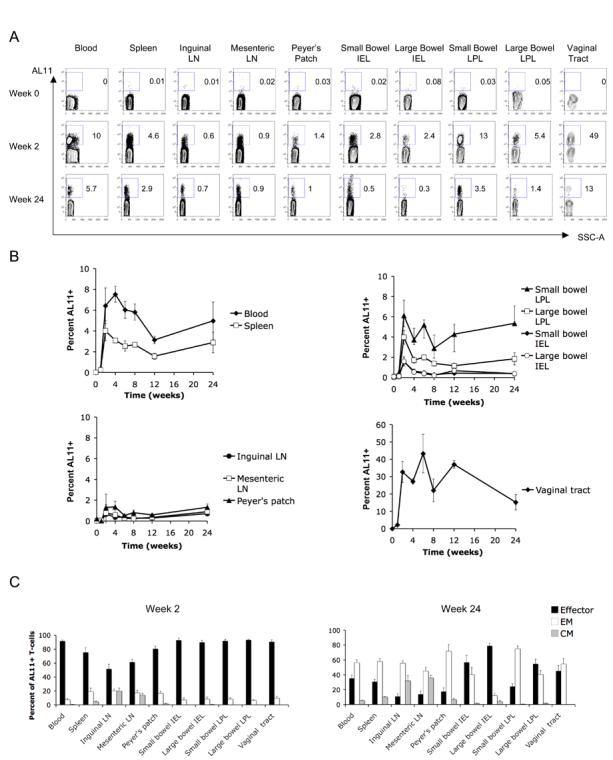
Intramuscular rAd immunization induces durable high frequency CD8+ T-lymphocyte memory in multiple mucosal compartments. C57BL/6 mice were immunized intramuscularly with 109 VP rAd5-Gag, and AL11-specific CD8+ T-lymphocyte responses were followed over a 24-week time course in multiple anatomic compartments using Db/AL11 tetramer binding assays, as shown for a representative experiment (A) and in summary for 4-6 mice/time point in (B). In (C), the memory phenotype (effector, black bars; effector memory, white bars; and central memory, gray bars) of the AL11-specific CD8+ T-lymphyocyte population was determined at weeks 2 and 24 post-immunization based on expression of CD62L and CD127. Error bars are +/− S.E.
At week 2, AL11-specific CD8+ T-lymphocytes from all anatomic sites exhibited predominantly an effector (CD62L− CD127 low) phenotype (Fig. 1C). However, by week 24, AL11-specific CD8+ T-lymphocyte responses transitioned to a memory phenotype, with effector memory lymphocytes (CD62L− CD127 high) present at all anatomic sites and central memory lymphocytes (CD62L+ CD127 high) accumulating preferentially at both mucosal and systemic lymphoid inductive sites (Fig. 1C). These data demonstrate the phenotypic changes of vaccine-elicited CD8+ T-lymphocytes over time in the development of systemic and mucosal CD8+ T-lymphocyte memory at both inductive and effector sites.
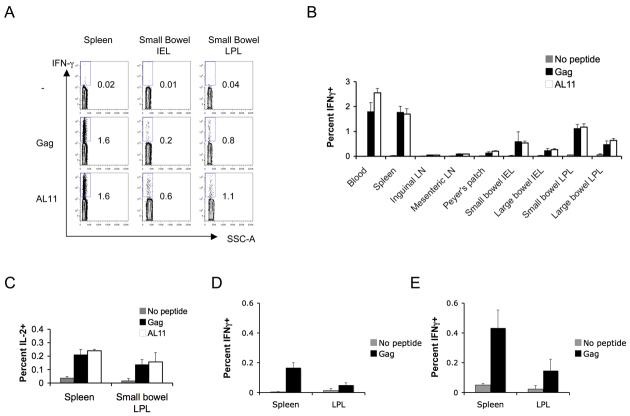
To assess the functionality of vaccine-elicited mucosal CD8+ T-lymphocyte responses, we performed intracellular cytokine staining (ICS) assays to evaluate antigen-specific IFN-γ and IL-2 production at week 2 following intramuscular vaccination with 109 VP rAd5-Gag. High-frequency IFN-γ+ CD8+ T-lymphocyte responses were observed in multiple systemic and mucosal compartments following stimulation with either pooled SIV Gag peptides or the immunodominant AL11 peptide, and the anatomic distribution of these responses was concordant with the tetramer binding assays, as shown in representative mice (Fig. 2A) and in summary for 6 mice per group (Fig. 2B). IL-2+ CD8+ T-lymphocyte responses were of lower magnitude as compared with IFN-γ responses, consistent with our ongoing studies of rAd5 vectors in rhesus monkeys (40), but the magnitude of IL-2 responses nevertheless remained comparable between spleen and small bowel lamina propria (Fig. 2C). The majority of IL-2-secreting cells also produced IFN-γ (data not shown). In contrast, little to no IL-4 and IL-10 was secreted by these cell populations (data not shown). Thus, intramuscular immunization with rAd5-Gag induced the accumulation of potent, durable and functional antigen-specific memory CD8+ T-lymphocytes in multiple mucosal compartments. The functionality of the mucosal AL11-specific CD8+ T-lymphocytes elicited by this regimen was further confirmed by their capacity to expand and to control an intranasal rVaccinia-Gag challenge (data not shown).
FIGURE 2.
Intramuscular rAd immunization induces high frequency functional mucosal CD8+ T-lymphocyte responses but low frequency mucosal CD4+ T-lymphocyte responses. C57BL/6 mice were immunized intramuscularly with 109 VP rAd5-Gag, and CD8+ T-lymphocyte responses to pooled Gag peptides or to the AL11 epitope peptide were monitored at week 2 post-immunization by intracellular cytokine staining for IFN-γ, as shown for a representative experiment in (A) and in summary for 6 mice/group in (B), or for IL-2 (C). C57BL/6 mice (n=6/group) were also immunized intramuscularly with 109 VP of rAd5-Gag (D) or primed with 109 VP rAd5-Gag and boosted with 109 VP rAd5HVR48-Gag (E), and CD4+ T-cell responses were assessed by intracellular cytokine staining for IFN-γ after stimulation with pooled Gag peptides at week 2 post-immunization. Error bars are +/− S.E.
Although mucosal antigen-specific CD8+ T-lymphocytes may be desirable for a candidate HIV-1 vaccine, it is possible that vaccine-elicited mucosal CD4+ T-lymphocytes may serve as additional targets of HIV-1 infection and prove detrimental. We therefore evaluated the mucosal CD4+ T-lymphocyte responses elicited by intramuscular rAd5-Gag immunization. At week 2 following a single immunization with 109 VP rAd5-Gag, low-frequency (0.18%) IFN-γ-secreting CD4+ T-lymphocytes could be detected in spleen following stimulation with pooled SIV Gag peptides (Fig. 2D). However, IFN-γ secreting CD4+ T-lymphocytes in the small bowel mucosa were not significantly above background despite clearly detectable CD8+ T-lymphocyte responses in this anatomic compartment (Fig. 2A-B). Moreover, IL-2 and IL-4 secreting CD4+ T lymphocytes were not detected in both systemic and mucosal compartments (data not shown). To increase our capacity to detect mucosal CD4+ T-lymphocyte responses, C57BL/6 mice were primed intramuscularly with 109 VP rAd5-Gag and boosted with 109 VP of the heterologous hexon-chimeric vector rAd5HVR48-Gag (31). At week 2 following the boost immunization, increased Gag-specific CD4+ T-lymphocyte responses were observed in splenocytes, but only low responses were observed in the small bowel mucosa (Fig. 2E). Thus, while high frequency IFN-γ and low frequency IL-2 secreting mucosal Gag-specific CD8+ T-lymphocytes were induced following intramuscular rAd5 immunization, Gag-specific CD4+ T-lymphocyte responses were over 10-fold lower in magnitude and only marginally detectable in this model.
We also assessed Gag-specific antibodies in serum, rectal washes and vaginal washes by ELISA in similarly vaccinated mice. Gag-specific IgG was detected in serum following immunization with 1010 VP rAd5HVR48-Gag, but no Gag-specific IgG or IgA was detected in rectal or vaginal washes (data not shown).
Systemic CD8+ T-lymphocytes rapidly traffic to mucosal surfaces after intramuscular rAd vaccination
The comparable magnitude and kinetics of CD8+ T-lymphocyte responses in systemic and mucosal compartments following intramuscular rAd vaccination suggested a coordinated cellular immune response that bridged anatomic sites, contrasting with the anatomically skewed cellular immune responses reported in prior studies of several different vaccine modalities (6-10). One possibility was that the rAd vectors directly distributed to mucosal sites and primed local responses simultaneously in multiple anatomic compartments. However, GLP-grade rAd biodistribution studies in rabbits supporting regulatory submissions to the FDA showed no evidence of direct vector trafficking to mucosal lymphoid inductive sites following intramuscular immunization utilizing an ultrasensitive and validated quantitative PCR-based assay (D.H.B., unpublished data). These data strongly suggest that T-lymphocyte priming was restricted to systemic inductive sites. We therefore hypothesized that systemic CD8+ T-lymphocytes may have acquired the capacity to migrate to mucosal surfaces and to persist at those sites following intramuscular rAd vaccination.
To explore this possibility, we performed adoptive transfer studies to evaluate the trafficking of systemic CD8+ T-lymphocytes activated by rAd immunization. C57BL/6 mice were primed intramuscularly with 109 VP of the rare serotype vector rAd26-Gag (32), and boosted 6 weeks later with 109 VP rAd5HVR48-Gag to generate high frequencies of AL11-specific CD8+ T-lymphocytes (10% of CD8+ T-lymphocytes in spleen). On day 10 after the boost immunization, systemic CD8+ T-lymphocytes were purified from splenocytes by negative selection using immunomagnetic beads. CD8+ T-lymphocytes were then transferred intravenously to naïve recipient mice, and the anatomic distribution and phenotype of the transferred AL11-specific CD8+ T-lymphocytes were determined 14 days later. AL11-specific CD8+ T-lymphocytes from spleen rapidly migrated from the blood to all anatomic sites examined and established a tissue distribution pattern that recapitulated that seen after direct immunization, as shown for a representative experiment (Fig. 3A) and in summary for 5 mice (Fig. 3B). Moreover, the anatomic distribution of effector and memory phenotypes of the transferred AL11-specific CD8+ T-lymphocytes (Fig. 3C) proved comparable with that seen after active immunization (Fig. 1C), with central memory cells accumulating at systemic and mucosal inductive sites but largely excluded from mucosal effector surfaces. Importantly, transferred AL11-specific CD8+ T-lymphocytes that trafficked to the gastrointestinal tract also markedly increased expression of integrins and chemokine receptors critical for intestinal homing. β7 integrin, CCR9 and CD103 (integrin αIEL) (1, 41) were dramatically upregulated on AL11-specific CD8+ T-lymphocytes migrating to gastrointestinal LPL and IEL compartments despite being expressed at very low levels on donor lymphocytes prior to adoptive transfer (Fig. 3D). These findings suggest that vaccine-activated, systemic CD8+ T-lymphocytes exhibited substantial phenotypic plasticity as well as the capacity to traffic widely to mucosal tissues.
FIGURE 3.
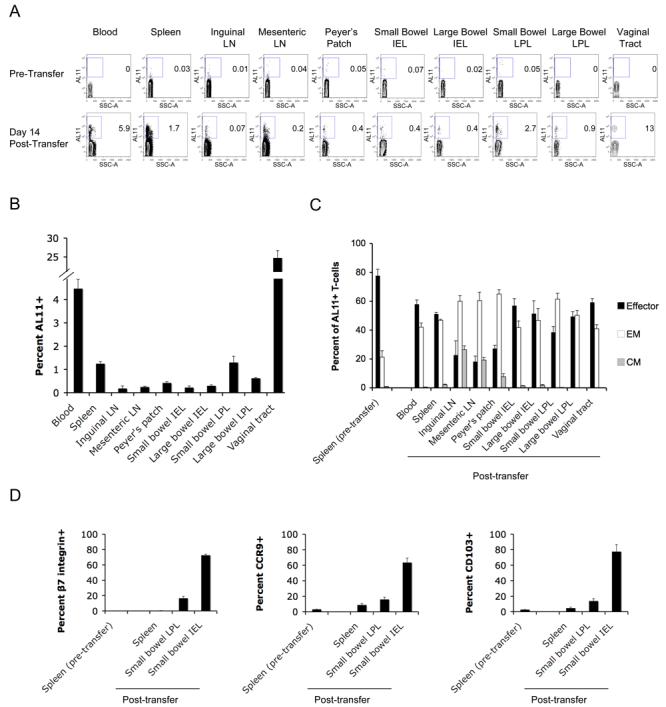
Systemic CD8+ T-lymphocytes upregulate mucosal homing markers and migrate to multiple mucosal compartments after adoptive transfer. Donor C57BL/6 mice were primed intramuscularly at week 0 with 109 VP rAd26-Gag and boosted at week 6 with 109 VP rAd5HVR48-Gag. On day 10 following the boost immunization, CD8+ T-lymphocytes were purified from splenocytes by negative immunomagnetic selection, and 2×107 purified lymphocytes were injected intravenously into naïve recipient mice (n=5/experiment). The tissue distribution of transferred AL11-specific CD8+ T-lymphocytes was determined at week 2 after adoptive transfer, as shown for a representative experiment (A) and in summary for 5 mice/group in (B). The effector and memory phenotypes (C) and the pattern of mucosal homing marker expression (D) were determined for AL11-specific CD8+ T-lymphocytes both prior to transfer and at week 2 post-transfer.
Given these observations, we hypothesized that intramuscular vaccination altered the migration patterns of antigen-specific CD8+ T-lymphocytes and conferred mucosal homing capacity to these cells, which typically have more restricted trafficking patterns. To compare directly the tissue migration patterns of quiescent and vaccine-activated systemic CD8+ T-lymphocytes, we performed adoptive transfer experiments utilizing congenic mice. Systemic CD8+ T-lymphocytes were purified from splenocytes of naïve CD45.1+ mice (B6.SJL) and transferred intravenously to naïve CD45.2-congenic recipients (C57BL/6). As expected, transferred naïve CD8+ T-lymphocytes migrated rapidly to the spleen and lymph nodes in recipient mice (Fig. 4A). However, in the absence of immunization, trafficking of adoptively transferred CD8+ T-lymphocytes to mucosal effector sites was highly restricted at day 14 post-transfer, as demonstrated by the low proportion of CD45.1+ to CD45.2+ CD8+ T-lymphocytes in the gastrointestinal IEL and LPL compartments (Fig. 4A). In contrast, intramuscular immunization of the recipient mice with 109 VP rAd5-Gag on day 2 post-transfer generated AL11-specific CD45.1+ CD8+ T-lymphocytes that efficiently migrated to gastrointestinal mucosa, as shown by the comparable proportions of these cells relative to AL11-specific CD45.2+ CD8+ T-lymphocytes across both systemic and mucosal compartments on day 14 (Fig. 4B). These findings demonstrate that vaccine-activated, but not quiescent, peripheral CD8+ T-lymphocytes have the capacity to migrate rapidly and extensively to multiple mucosal tissues. Moreover, the congenic adoptive transfer system allowed for a comparison of the relative magnitudes of mucosal immune responses generated by adoptively transferred and native CD8+ T-lymphocytes within the same mouse. The comparable responses in multiple anatomic compartments (Fig. 4B) suggest that trafficking of systemic lymphocytes to mucosal sites accounted for the vast majority of antigen-specific mucosal CD8+ T-lymphocytes. Thus, trafficking of systemic lymphocytes, rather than direct local priming at mucosal sites, appears to be the predominant mechanism for generating widespread mucosal immunity following systemic vaccination with rAd vectors.
FIGURE 4.
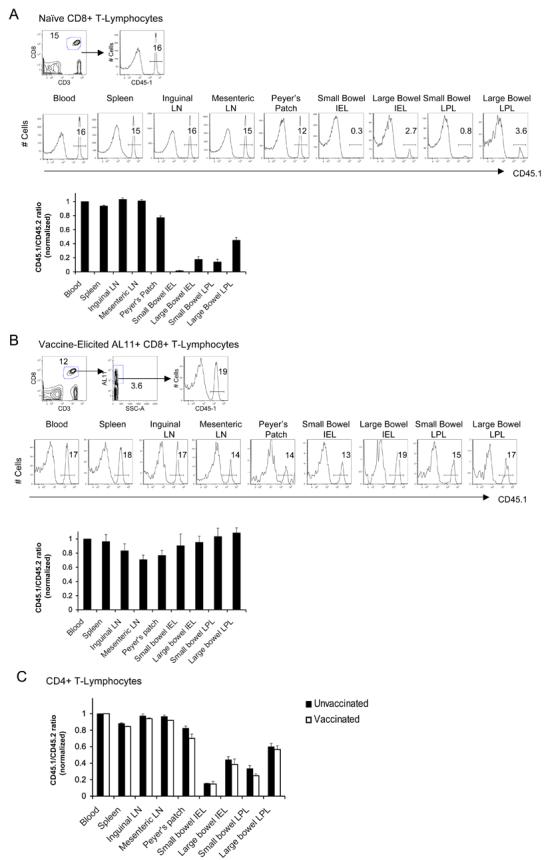
Vaccination facilitates trafficking of systemic CD8+ T-lymphocytes to mucosal surfaces. 107 purified systemic CD8+ T-lymphocytes were isolated from splenocytes of naïve CD45.1+ donors and adoptively transferred into naïve congenic CD45.2+ recipients. The ratio of transferred/native CD8+ T-lymphocytes was determined on day 12 post-transfer for the total CD8+ T-lymphocyte population at each anatomic site in the absence of immunization (A) or for the responding AL11-specific CD8+ T-lymphocyte population at each anatomic site on day 14 post-immunization with rAd5-Gag (B). In each case, the top panel demonstrates the gating strategy used for analysis, the middle panel shows a representative experiment, and the bottom panel shows the ratio of transferred/native CD8+ T-lymphocytes at each anatomic site averaged for 4 mice/group. (C) 107 purified systemic CD4+ T-lymphocytes were isolated from splenocytes of naïve CD45.1+ donors and adoptively transferred into naïve congenic CD45.2+ recipients. The ratio of transferred/native CD4+ T-lymphocytes was determined on day 14 post-transfer at each anatomic site in the absence of immunization (black bars) or following intramuscular immunization with rAd5-Gag on day 2 (white bars). Error bars are +/− S.E.
We also assessed the capacity of CD4+ T-lymphocytes to traffic to mucosal sites utilizing the same adoptive transfer and immunization protocol. Naïve CD4+ T-lymphocytes exhibited limited capacity to migrate to mucosal sites (Fig. 4C, black bars), comparable with the restricted trafficking observed with naïve CD8+ T-lymphocytes (Fig. 4A). Vaccination did not detectably increase the capacity of total CD4+ T-lymphocytes to migrate to mucosal surfaces (Fig. 4C, white bars). However, we were unable to study the trafficking of antigen-specific CD4+ T-lymphocytes to mucosal surfaces as a result of the low magnitude of Gag-specific CD4+ T-lymphocyte responses in this experimental model (Fig. 2D-E).
Heterologous prime-boost regimens with rare serotype and hexon-chimeric rAd vectors elicit potent anamnestic mucosal cellular immune responses
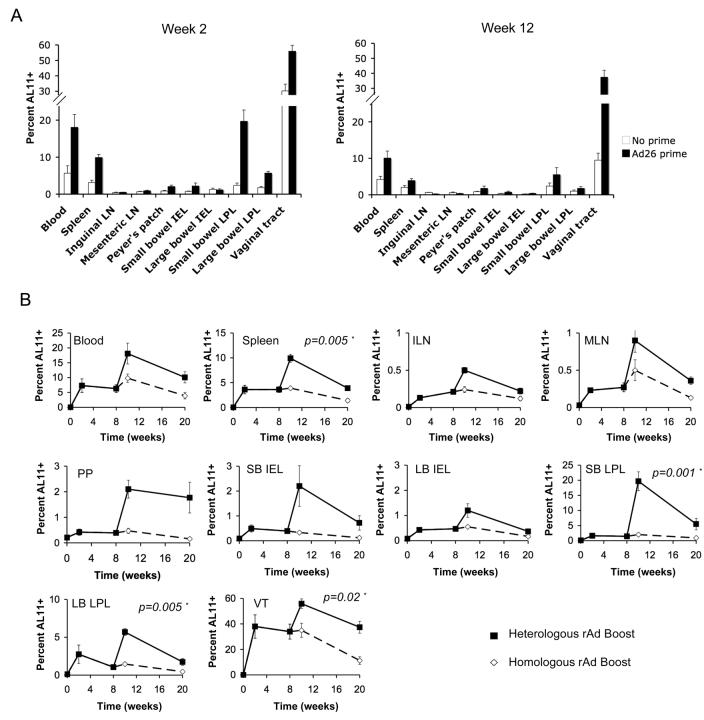
The capacity of antigen-specific CD8+ T-lymphocytes to traffic from systemic to mucosal compartments after a single immunization raised the possibility that mucosal cellular immune responses may increase further following heterologous boost immunizations. However, the anatomic distribution of recall responses has previously been reported to be biased by the site of initial antigen exposure (1, 2). Therefore, we investigated whether repeated intramuscular administration of rAd vectors in heterologous prime-boost regimens would augment mucosal responses or, alternatively, would bias recall responses away from mucosal surfaces. The utility of homologous rAd prime-boost regimens is limited by the inability of homologous vector readministration to boost responses efficiently, as a result of the generation of potent vector-specific neutralizing antibodies by the priming immunization (30, 42, 43). Therefore, we utilized serologically distinct rare serotype and hexon-chimeric rAd vectors for these studies.
Naïve C57BL/6 mice or mice previously primed with 109 VP rAd26-Gag were boosted intramuscularly with 109 VP rAd5HVR48-Gag, and CD8+ T-lymphocyte responses were examined in multiple anatomic compartments. As compared with naïve mice, mice previously primed with rAd26-Gag exhibited substantially higher peak frequencies of AL11-specific CD8+ T-lymphocytes following rAd5HVR48-Gag immunization in both systemic and mucosal compartments (Fig. 5A), and the magnitude of the boost effect was comparable at systemic and mucosal sites. After boosting, frequencies of AL11-specific CD8+ T-lymphocytes approached 20% in the small bowel lamina propria and exceeded 60% in the vaginal tract, and these responses persisted for over 12 weeks. Thus, rather than directing CD8+ T-lymphocyte responses away from mucosal surfaces, boosting with a heterologous vector resulted in potent and persistent secondary recall responses in multiple mucosal compartments.
FIGURE 5.
Heterologous rAd prime-boost regimens are significantly superior to homologous regimens in inducing mucosal CD8+ T-lymphocyte recall responses. (A) To compare primary and recall responses, AL11-specific CD8+ T-lymphocyte responses at week 2 and week 12 following immunization with 109 VP rAd5HVR48-Gag were evaluated in previously naïve C57BL/6 mice (white bars) or in mice primed 6 weeks earlier with 109 VP rAd26-Gag (black bars) (n=4/group at each time point). (B) C57BL/6 mice (n=4/group at each time point) were primed intramuscularly at week 0 with 109 VP rAd26-Gag and boosted at week 8 with either 109 VP rAd26-Gag (homologous vector; dashed lines) or 109 VP rAd5HVR48-Gag (heterologous vector; solid lines). AL11-specific CD8+ T-lymphocyte responses were assessed at multiple time points following the priming and boosting immunizations. Means +/− S.E. for each group are shown. Asterisks denote two-sided t-tests. ILN, inguinal lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer's patches; SB, small bowel; LB, large bowel; IEL, intraepithelial lymphocytes; LPL, lamina propria lymphocytes; VT, vaginal tract.
We next directly compared the magnitude and kinetics of mucosal CD8+ T-lymphocyte responses elicited by systemic heterologous versus homologous rAd prime-boost regimens. C57BL/6 mice were primed intramuscularly at week 0 with rAd26-Gag and were boosted intramuscularly at week 8 with the homologous rAd26-Gag vector or the heterologous rAd5HVR48-Gag vector. Systemic and mucosal AL11-specific CD8+ T-lymphocyte responses were assessed for 12 weeks following boost immunization. Homologous boosting with rAd26-Gag resulted in little to no increase in CD8+ T-lymphocyte responses as expected (Fig. 5B). In contrast, heterologous boosting with rAd5HVR48-Gag generated significantly enhanced peak and memory CD8+ T-lymphocyte responses in both systemic and mucosal compartments (p=0.005 for spleen, p=0.001 for small bowel LPL, p=0.005 for large bowel LPL, and p=0.02 for vaginal tract lymphocytes comparing heterologous versus homologous responses at week 10 using two-tailed t-tests). These data demonstrate that heterologous rAd prime-boost regimens were significantly superior to homologous rAd regimens for generating potent and durable cellular immune memory in the gastrointestinal and vaginal tracts.
Intramuscular rAd immunization induces high frequency, durable mucosal CD8+ T-lymphocyte memory in rhesus monkeys
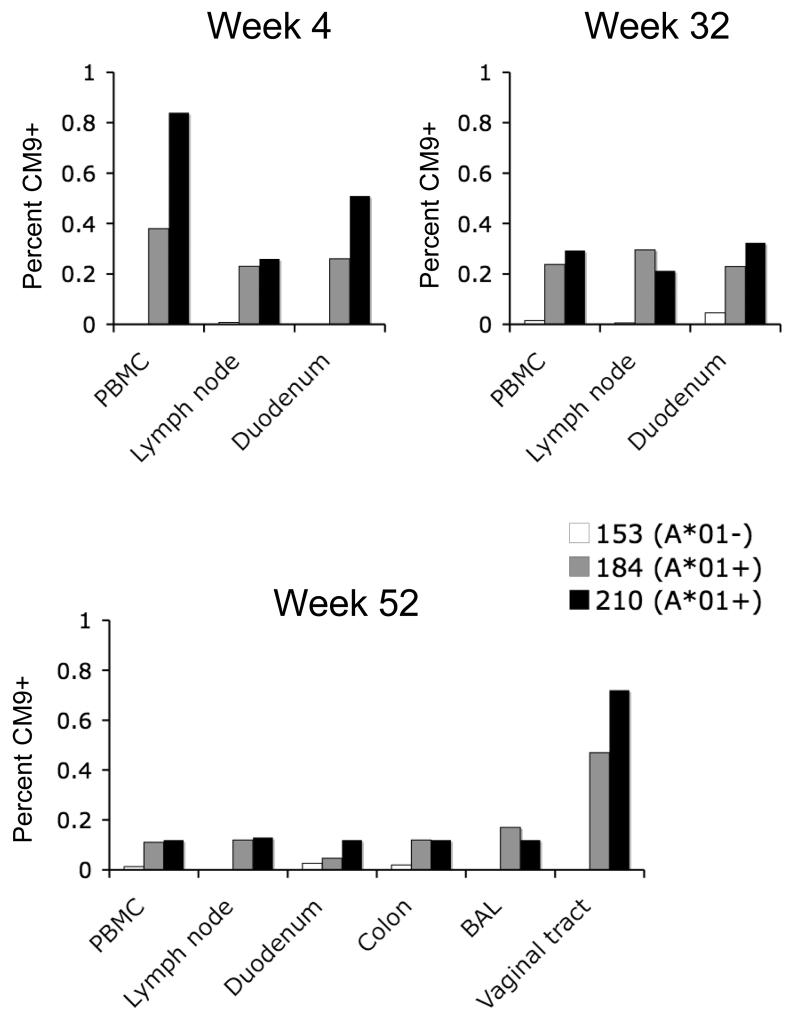
We next investigated whether our findings of robust mucosal cellular immunity in intramuscularly rAd vaccinated mice would translate into nonhuman primates. We immunized three rhesus monkeys (two that expressed the MHC class I allele MamuA*01 and one that did not express this allele) intramuscularly with 1011 VP of rAd5HVR48-Gag. Mamu-A*01-restricted CD8+ T-lymphocyte responses to the highly immunodominant Gag epitope CM9 (CTPYDINQM) (44) were assessed by multiparameter tetramer binding assays for up to 1 year following immunization in multiple systemic and mucosal compartments. CD8+ T-lymphocyte responses were observed in duodenal mucosa as well as in blood and lymph nodes in the Mamu-A*01-positive animals at weeks 4 and 32 after vaccination, and the magnitude of mucosal responses proved comparable with the magnitude of systemic responses (Fig. 6). Moreover, CM9-specific memory CD8+ T-lymphocytes persisted for at least 52 weeks following vaccination. At this late time point, CM9-specific CD8+ T-lymphocytes were still detected in duodenal mucosa, colorectal mucosa, bronchoalveolar lavage and vaginal mucosa. Importantly, the magnitude of these long-term mucosal responses proved comparable with those found in blood and lymph nodes, except for responses in vaginal mucosa that were approximately 5-fold higher in magnitude than responses in blood, consistent with our mouse studies (Figs. 1, 2). At all anatomic sites, CM9-specific CD8+ T-lymphocytes were predominantly of a CD28+CD95+ memory phenotype (45). These data demonstrate that a single intramuscular rAd vaccination generated potent and durable CD8+ T-lymphocyte memory that persisted for over one year in multiple mucosal tissues in nonhuman primates.
FIGURE 6.
Mucosal cellular immune memory in rhesus monkeys after intramuscular rAd vaccination. Two rhesus monkeys expressing the MHC class I allele Mamu-A*01 (animals 184-03, gray bars, and 210-03, black bars) and one monkey negative for this allele (153-03, white bars) were vaccinated intramuscularly with a single injection of 1011 VP rAd5HVR48-Gag. Gag CM9-specific CD8+ T-lymphocyte responses were evaluated in systemic and mucosal compartments at weeks 4, 32, and 52 following vaccination. The memory phenotype of the responding lymphocytes was determined by CD28 and CD95 expression.
Discussion
The induction of potent and durable mucosal immunity is a critical objective of vaccine development for mucosal pathogens. However, circulating systemic T-lymphocytes often exhibit limited capacity to traffic to mucosal surfaces (1, 46), and the resultant anatomic compartmentalization of responses has been considered a major barrier to generating effective mucosal cellular immunity by intramuscular vaccination. In this study, we demonstrated that intramuscular immunization with rAd vectors, either individually or in heterologous prime boost regimens, elicited potent, durable and functional CD8+ T-lymphocyte memory at multiple mucosal sites in both mice and rhesus monkeys. We also observed that intramuscular vaccination activated systemic CD8+ T-lymphocytes to upregulate mucosal homing integrins and chemokine receptors, to traffic rapidly to mucosal surfaces, and to adopt memory phenotypes characteristic of resident mucosal T-lymphocytes. These data support a model in which systemic CD8+ T-lymphocytes overcome immune compartmentalization following intramuscular immunization and result in potent cellular immune responses at multiple anatomic sites.
Intramuscular rAd vaccination resulted in substantial alterations to the patterns of CD8+ T-lymphocyte trafficking. While naïve systemic CD8+ T-lymphocytes were highly restricted in their ability to migrate to mucosal surfaces (Fig. 4A), vaccine-activated systemic CD8+ T-lymphocytes trafficked readily to these anatomic compartments (Fig. 3A-B, Fig. 4B), where they established persistent populations of memory CD8+ T-lymphocytes. This trafficking was accompanied by substantial upregulation of β7 integrin, CD103 (αE integrin) and CCR9, which are critical for Tlymphocyte homing to intestinal mucosa (Fig. 3D), as well as the acquisition of an effector/effector memory phenotype (CD44−, CD62L−) typical of mucosal lymphocytes (Fig. 3C). Moreover, in congenic adoptive transfer studies, comparable tetramer responses in mucosal compartments were generated by intravenously transferred CD8+ T-lymphocytes as compared with native CD8+ T-lymphocytes (Fig. 4B), suggesting that the migration of systemic lymphocytes to mucosal sites after vaccination likely accounted for the vast proportion of responses observed at mucosal surfaces. Thus, intramuscular rAd vaccination confers remarkable migratory and phenotypic plasticity on systemic CD8+ T-lymphocytes. We hypothesize that the mechanism underlying this observation involves cellular activation and upregulation of mucosal homing markers following vaccination, although it has also been reported that specific imprinting may occur following migration of CD8+ T-lymphocytes to tissue-specific lymphoid inductive sites (16). Our data extend previous observations that highlight the plasticity of memory CD8+ T-lymphocyte phenotypes in systemic and mucosal microenvironments (17, 47) and demonstrate for the first time the functional relevance of this phenomenon for vaccine-elicited mucosal immunity.
Several preclinical and clinical studies of localized infection and vaccination utilizing different vaccine modalities have shown anatomic skewing of primary and recall responses to the initial site of antigen exposure (3-10). For example, Gallichan et al. observed durable mucosal CD8+ T-lymphocyte responses in mice challenged intranasally with HSV following intranasal but not systemic immunization with an rAd vector expressing HSV glycoprotein B (6). Similarly, Belyakov et al. observed that intrarectal immunization of mice and macaques with a peptide vaccine generated CD8+ T-lymphocyte memory in intestinal mucosa but not in systemic compartments, while systemic immunization induced CD8+ T-lymphocyte memory systemically but not in the mucosa (7, 8). In contrast with these studies, we observed that systemic vaccination with rAd vectors elicited rapid, potent and durable mucosal cellular immune responses in both mice and rhesus monkeys with magnitudes that were comparable with systemic cellular immune responses. It is possible that intrinsic biologic differences among the vectors as well as differences in the immunologic assays may be responsible for these divergent observations. Importantly, the degree to which other vaccine modalities may similarly generate robust and widespread mucosal cellular immune responses remains to be determined, although the anatomically widespread cellular immune responses reported in murine models of localized rotavirus, Sendai virus and Listeria infection (14, 15) suggest the potential generalizability of our findings.
After mucosal transmission, HIV-1 replicates locally within the genital or rectal mucosa and associated lymphoid tissues before disseminating (48-50). Potent mucosal immune responses at the portal of virus entry might therefore be able to alter dynamics between the virus and the host. Moreover, peak viral replication in acute HIV-1 infection is accompanied by a massive, irreversible destruction of memory CD4+ T-lymphocytes, particularly in gastrointestinal mucosal tissues (18-21). HIV-1 vaccination strategies that drive potent mucosal cellular immune responses may therefore prove critical for CD4+ Tlymphocyte preservation and control of viral replication. In the present study, we demonstrated that novel heterologous rAd prime-boost regimens generated potent secondary mucosal cellular immune responses that were significantly superior to those induced by homologous rAd regimens (Fig. 5), which are limited by anti-vector neutralizing antibodies as a result of the priming immunization (30, 42, 43).
It is also possible that the potential utility of vaccine-elicited mucosal CD8+ T-lymphocytes could be counterbalanced by the induction of activated CD4+ T-lymphocytes that could theoretically increase the number of target cells available for HIV-1 infection in the mucosa. Whether or not this mechanism might account for the observed increase in HIV-1 acquisition in rAd5 vaccinees with pre-existing Ad5-specific neutralizing antibodies in the STEP study (51) is unclear but is an area of active investigation. In the present studies, we observed only minimal antigen-specific mucosal CD4+ T-lymphocyte responses by intracellular cytokine staining assays (Fig. 2D), despite high frequency mucosal CD8+ T-lymphocyte responses (Fig. 2A-C). As a result, we were unable to perform a detailed analysis of the trafficking or the phenotypes of antigen-specific CD4+ T-lymphocyte responses in this experimental system. Further studies will therefore be required to evaluate antigen-specific and vector-specific CD8+ and CD4+ T-lymphocyte responses induced by intramuscular rAd vaccination in mucosal tissues in nonhuman primates.
Our data demonstrate that systemic immunization with rAd vectors can overcome immune compartmentalization and generate potent and durable mucosal cellular immunity. Moreover, the capacity of heterologous rAd prime-boost regimens to induce high frequency recall responses in mucosal compartments suggests the functional quality of these mucosal immune responses. These findings suggest that mucosal immunization strategies may not be required for inducing mucosal immunity as a result of the plasticity of vaccine-activated systemic T-lymphocytes. Further delineation of the detailed molecular events associated with lymphocyte activation and mucosal trafficking following systemic immunization will facilitate the future development of vaccines against mucosal pathogens.
Acknowledgements
We thank N. Simmons, D. Miller, M. Denholtz, B. Ewald, D. Lynch, P. Abbink, M. Lifton, K. Furr, K. Carlson, F. Stephens, A. Hovav, M. Pau, R. Vogels, A. Lemckert, and R. Veazey for generous advice, assistance and reagents.
Footnotes
We acknowledge grant support from the NIH (U19 AI066305, R01 AI066924) and the Bill & Melinda Gates Foundation (#38614) to D.H.B. and an NIH training grant (T32 AI07387) to D.R.K.
References
- 1.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat. Rev. Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 3.Offit PA, Cunningham SL, Dudzik KI. Memory and distribution of virus-specific cytotoxic T lymphocytes (CTLs) and CTL precursors after rotavirus infection. J. Virol. 1991;65:1318–1324. doi: 10.1128/jvi.65.3.1318-1324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J. Clin. Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belyakov IM, Derby MA, Ahlers JD, Kelsall BL, Earl P, Moss B, Strober W, Berzofsky JA. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. U.S A. 1998;95:1709–1714. doi: 10.1073/pnas.95.4.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belyakov IM, Hel Z, Kelsall B, Kuznetsov VA, Ahlers JD, Nacsa J, Watkins DI, Allen TM, Sette A, Altman J, Woodward R, Markham PD, Clements JD, Franchini G, Strober W, Berzofsky JA. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 9.Kantele A, Zivny J, Hakkinen M, Elson CO, Mestecky J. Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 1999;162:5173–5177. [PubMed] [Google Scholar]
- 10.Santosuosso M, Zhang X, McCormick S, Wang J, Hitt M, Xing Z. Mechanisms of mucosal and parenteral tuberculosis vaccinations: adenoviral-based mucosal immunization preferentially elicits sustained accumulation of immune protective CD4 and CD8 T cells within the airway lumen. J. Immunol. 2005;174:7986–7994. doi: 10.4049/jimmunol.174.12.7986. [DOI] [PubMed] [Google Scholar]
- 11.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 12.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 15.Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Fuhlbrigge RC, Karibian K, Tian T, Kupper TS. Dynamic programming of CD8+ T cell trafficking after live viral immunization. Immunity. 2006;25:511–520. doi: 10.1016/j.immuni.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 18.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 19.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 21.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 22.Mattapallil JJ, Douek DC, Buckler-White A, Montefiori D, Letvin NL, Nabel GJ, Roederer M. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2006;203:1533–1541. doi: 10.1084/jem.20060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 24.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, Yang ZY, Chakrabarti B, Rao SS, Schmitz JE, Montefiori DC, Barker BR, Bookstein FL, Nabel GJ. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312:1530–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, Kaslow R, Buchbinder S, Hoots K, O'Brien SJ. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 27.Barouch DH, Kunstman J, Kuroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gorgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- 28.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 29.Goulder PJ, Watkins DI. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 2004;4:630–640. doi: 10.1038/nri1417. [DOI] [PubMed] [Google Scholar]
- 30.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, Truitt DM, Sumida SM, Kishko MG, Arthur JC, Korioth-Schmitz B, Newberg MH, Gorgone DA, Lifton MA, Panicali DL, Nabel GJ, Letvin NL, Goudsmit J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004;172:6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 31.Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, Liu J, Thorner AR, Swanson PE, Gorgone DA, Lifton MA, Lemckert AA, Holterman L, Chen B, Dilraj A, Carville A, Mansfield KG, Goudsmit J, Barouch DH. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 32.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O'Brien KL, Carville A, Mansfield KG, Goudsmit J, Havenga MJ, Barouch DH. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Bethune MP, Kostense S, Penders G, Helmus N, Koudstaal W, Cecchini M, Wetterwald A, Sprangers M, Lemckert A, Ophorst O, Koel B, van Meerendonk M, Quax P, Panitti L, Grimbergen J, Bout A, Goudsmit J, Havenga M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003;77:8263–8271. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroda MJ, Schmitz JE, Charini WA, Nickerson CE, Lord CI, Forman MA, Letvin NL. Comparative analysis of cytotoxic T lymphocytes in lymph nodes and peripheral blood of simian immunodeficiency virus-infected rhesus monkeys. J. Virol. 1999;73:1573–1579. doi: 10.1128/jvi.73.2.1573-1579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen TM, Sidney J, del Guercio MF, Glickman RL, Lensmeyer GL, Wiebe DA, DeMars R, Pauza CD, Johnson RP, Sette A, Watkins DI. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 36.Liu J, Ewald BA, Lynch DM, Nanda A, Sumida SM, Barouch DH. Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J. Virol. 2006;80:11991–11997. doi: 10.1128/JVI.01348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Budd RC, Cerottini JC, Horvath C, Bron C, Pedrazzini T, Howe RC, MacDonald HR. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 1987;138:3120–3129. [PubMed] [Google Scholar]
- 38.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 39.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. U S A. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, Carville A, Mansfield KG, Havenga MJ, Goudsmit J, Barouch DH. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 2008;82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefrancois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu. Rev. Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]
- 42.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 43.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, Tang A, Chen M, Huang L, Harris V, Freed DC, Wilson KA, Dubey S, Zhu DM, Nawrocki D, Mach H, Troutman R, Isopi L, Williams D, Hurni W, Xu Z, Smith JG, Wang S, Liu X, Guan L, Long R, Trigona W, Heidecker GJ, Perry HC, Persaud N, Toner TJ, Su Q, Liang X, Youil R, Chastain M, Bett AJ, Volkin DB, Emini EA, Shiver JW. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 2003;77:6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuroda MJ, Schmitz JE, Barouch DH, Craiu A, Allen TM, Sette A, Watkins DI, Forman MA, Letvin NL. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 1998;187:1373–1381. doi: 10.1084/jem.187.9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, Axthelm MK, Picker LJ. Development and homeostasis of T cell memory in rhesus macaque. J. Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 46.Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 47.Marzo AL, Yagita H, Lefrancois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joag SV, Adany I, Li Z, Foresman L, Pinson DM, Wang C, Stephens EB, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J. Virol. 1997;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, La Franco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J. Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinbrook R. One step forward, two steps back--will there ever be an AIDS vaccine? N. Engl. J. Med. 2007;357:2653–2655. doi: 10.1056/NEJMp0708117. [DOI] [PubMed] [Google Scholar]