Abstract
Background
In low-resource settings, many programs recommend that women who are infected with the human immunodeficiency virus (HIV) stop breast-feeding early. We conducted a randomized trial to evaluate whether abrupt weaning at 4 months as compared with the standard practice has a net benefit for HIV-free survival of children.
Methods
We enrolled 958 HIV-infected women and their infants in Lusaka, Zambia. All the women planned to breast-feed exclusively to 4 months; 481 were randomly assigned to a counseling program that encouraged abrupt weaning at 4 months, and 477 to a program that encouraged continued breast-feeding for as long as the women chose. The primary outcome was either HIV infection or death of the child by 24 months.
Results
In the intervention group, 69.0% of the mothers stopped breast-feeding at 5 months or earlier; 68.8% of these women reported the completion of weaning in less than 2 days. In the control group, the median duration of breast-feeding was 16 months. In the overall cohort, there was no significant difference between the groups in the rate of HIV-free survival among the children; 68.4% and 64.0% survived to 24 months without HIV infection in the intervention and control groups, respectively (P = 0.13). Among infants who were still being breast-fed and were not infected with HIV at 4 months, there was no significant difference between the groups in HIV-free survival at 24 months (83.9% and 80.7% in the intervention and control groups, respectively; P = 0.27). Children who were infected with HIV by 4 months had a higher mortality by 24 months if they had been assigned to the intervention group than if they had been assigned to the control group (73.6% vs. 54.8%, P = 0.007).
Conclusions
Early, abrupt cessation of breast-feeding by HIV-infected women in a low-resource setting, such as Lusaka, Zambia, does not improve the rate of HIV-free survival among children born to HIV-infected mothers and is harmful to HIV-infected infants. (ClinicalTrials.gov number, NCT00310726.)
Breast-feeding poses a dilemma for women who live in low-resource settings and who are infected with the human immunodeficiency virus (HIV) because the practice can transmit HIV but is the source of optimal nutrition and protection against other serious infectious diseases.1-4 Early cessation of breast-feeding has been recommended to balance these competing risks favorably — reducing postnatal transmission of HIV while preserving the nutritional and immunologic benefits of breast-feeding at the time when they are needed most.5-8 Postnatal transmission of HIV occurs throughout the duration of breast-feeding, but there are conflicting data on the question of whether the risks are evenly distributed between younger and older children.9-14 The benefits of breast-feeding for reducing the incidence of complications and death from non-HIV infectious disease, although known to extend into the second year, are greatest in the first few months of life.15-17
Exclusive breast-feeding confers lower risks of postnatal transmission of HIV than predominant or partial breast-feeding18-20 but is recommended only for 6 months, after which infants require other foods to complement breast milk.21 If early weaning is to be encouraged for HIV-infected women, the end of exclusive breast-feeding offers a logical end point. In low-resource settings, many programs that attempt to prevent mother-to-child transmission of HIV have recommended abrupt or rapid weaning to minimize the period of nonexclusive breast-feeding.
We conducted a randomized trial among HIV-infected women in Lusaka, Zambia, to evaluate whether exclusive breast-feeding to 4 months, followed by abrupt weaning, would reduce the postnatal transmission of HIV and mortality through the first 2 years of life. Four months was selected as the weaning time because this was the minimum duration of exclusive breast-feeding that was recommended at the time the study was designed22 and was considered to be a reasonable period for exclusive breast-feeding to be maintained. Equipoise existed to justify a randomized trial, since although the benefits of early abrupt weaning seemed plausible, there were no experimental, and only limited epidemiologic, data to justify it. The design of the study was constrained by ethical considerations regarding the random assignment of children to replacement feeding from birth, given the vulnerability to infectious diseases of non–breast-fed infants in Zambia, or to nonexclusive breast-feeding from birth, given the established benefits of exclusive breast-feeding for infant health15-17 and the increased risk of HIV transmission with mixed feeding.18-20
Methods
Study Design
The study was an unblinded, randomized trial of a behavioral intervention among HIV-infected women to encourage exclusive breast-feeding to 4 months, followed by the abrupt cessation of breast-feeding, as compared with the standard practice of continued breast-feeding for a longer period.23 The authors designed the study, supervised the clinical staff in the collection of the data, conducted the analyses, and wrote the manuscript. All of the authors vouch for the completeness and accuracy of the data and analyses.
Study Population
HIV-infected women were recruited from two antenatal clinics in Lusaka, Zambia, that offered voluntary HIV testing and counseling and single-dose nevirapine prophylaxis.24,25 Between May 2001 and September 2004, a total of 1435 HIV-seropositive women who were pregnant (less than 38 weeks' gestation) were recruited as potentially eligible trial participants. Women could volunteer if they intended to breast-feed for any length of time, accepted treatment with nevirapine, and agreed to be randomly assigned to the intervention or control group. Exclusion criteria were severe pregnancy complications (e.g., preeclampsia), previous cesarean delivery, and HIV-related conditions requiring hospitalization. Women were encouraged to involve their husband or partner or another family member before joining the study. All women provided written informed consent. The study was approved by human subjects committees at all the participating institutions.
Study Intervention
Participants were randomly assigned to one of two groups. The experimental intervention encouraged women to breast-feed exclusively to 4 months and then to stop breast-feeding abruptly, or as rapidly as possible. Beginning at the 2-month visit, women were counseled to prepare for abrupt cessation. Preparation included practice in cup-feeding of expressed milk, information about nutritional requirements during weaning, and correct preparation of formula and complementary foods, as well as counseling to anticipate possible weaning problems such as breast engorgement and the inability to comfort the child through breast-feeding. A 3-month supply of infant formula and fortified weaning cereal was provided. Extensive counseling was given to make replacement feeding as safe as possible. This included demonstrations and practical training sessions in preparing the products, education about the importance of using only boiled water for the preparation of formula and of not storing any remaining formula, correct feeding frequencies and dilutions, and household hygiene. Insulated thermoses for storing boiled water were provided. Cup-feeding was encouraged to minimize the hazards associated with the use of infant bottles. Around the time of weaning, there was weekly contact with the women, including at least two home visits. Women were encouraged to make the transition from formula to the weaning cereal (a product that was developed and tested in Zambia by the U.S. Department of Agriculture and was based on the local staple, maize meal, and fortified with milk powder, sugar, oil, and micronutrients) because the cooking process made the cereal a safer product. The infants were monitored over this period for any slowing in growth, and family planning was encouraged. Women in the control group were encouraged to breast-feed exclusively to 6 months, gradually introduce complementary foods (not provided), and continue to breast-feed for a duration of their own informed choice (standard practice).
Randomization
At 1 month post partum, participants who were still breast-feeding their infants (whose HIV status had not yet been determined) and were willing to continue with the study were randomly assigned to a study group with the use of a computer algorithm that was designed by the study statistician with a randomized permuted-block design within each site. Participants were informed of their assignment at the next (usually second-month) visit to ensure sufficient time for preparation.
Study Procedures
Blood was drawn at the time of enrollment, and two antenatal visits for counseling were scheduled before delivery. Sociodemographic and clinical data were collected at enrollment, and obstetric and neonatal data after delivery. Heel-stick blood samples were collected from the infants on filter paper on the day of birth, at 1 week, and at 1, 2, 3, 4, 4.5, 5, 6, 9, 12, 15, 18, 21, and 24 months of age. Clinic visits were scheduled at these time points, and information about infant-feeding practices was obtained by different members of the study staff from those performing the counseling. Breast-feeding duration was defined as the time from birth until the exact age that breast-feeding was first reported to have stopped. Children who died were assumed to have been breast-fed up to the date of death unless clinical records indicated that breast-feeding had been stopped before the illness that preceded the child's death. Home visits were scheduled at 4 days after birth and at time points that were interspersed between clinic visits so that contact occurred every 2 weeks for the first 5 months. The infants in the study, including those whose mothers died and those whose mothers did not adhere to the feeding protocol, were followed on this schedule of home and clinic visits through 24 months. Home-visit teams tracked the participants who did not return for appointments. Information about children's deaths was sought from hospital and clinic records and from interviews with caretakers and health care personnel. The circumstances of all deaths were reviewed to identify the causes of death.
Clinical Care and Treatment
Routine antenatal care included screening for syphilis with the use of rapid plasma reagin tests and the treatment of women who had a positive test and their partners with penicillin; prenatal multivitamins including iron; and malaria prophylaxis. Antiretroviral therapy became available in the public sector only after May 2004.26 Enrolled women who were eligible for treatment according to Zambian guidelines and who provided consent were started on first-line regimens. Co-trimoxazole was given to all infants (infected and uninfected) between 6 weeks and 12 months of age and, after November 2003, to women with CD4 cell counts of less than 200 per cubic millimeter.27 The children's growth was monitored monthly, and in both study groups, children with evidence of failure to thrive were provided with nutritional supplements.
Laboratory Studies
Maternal blood collected at enrollment was tested for CD4 and CD8 cell counts (FACSCount, BD Biosciences), hemoglobin (HemoCue system, HemoCue), and viral load (Amplicor HIV-1 Monitor Test, v1.5, Roche). Infant heel-stick samples were tested in batches for HIV-1 DNA by polymerase chain reaction (PCR).28 All positive results were confirmed in two or more samples, if available; if only one sample was available, it was retested for confirmation. To rule out false negative test results due to an inadequate sample, amplification of the beta-globin gene was performed. Infant diagnostic services were not available in Zambia during the time the study was conducted, so samples were tested in the United States. When the results became available, women were given the opportunity to learn their child's infection status and were recounseled about feeding choices.
Statistical analysis
The study was powered to detect a reduction of 50% or more in the combined outcome of HIV infection or death among the subjects who underwent randomization.23 All mother–child pairs randomly assigned to study groups (with the first-born infant selected in the case of multiple births) were included in the intention-to-treat analysis. Categorical characteristics were compared between groups with the use of chi-square tests, normally distributed continuous variables with the use of t-tests, and nonnormal continuous variables with the use of Wilcoxon tests. Child death, HIV-free survival, HIV transmission, and breast-feeding duration were treated as time-to-event variables and were analyzed with Kaplan–Meier methods and log-rank tests. The results of these analyses are expressed as Kaplan–Meier probabilities of the end points by a specified time per 100 study participants. For HIV transmission, the midpoint between the last negative and the first positive PCR test was imputed as the event time. For an analysis of death among uninfected children, data were censored at the time of the last negative test.
Results
Study Population
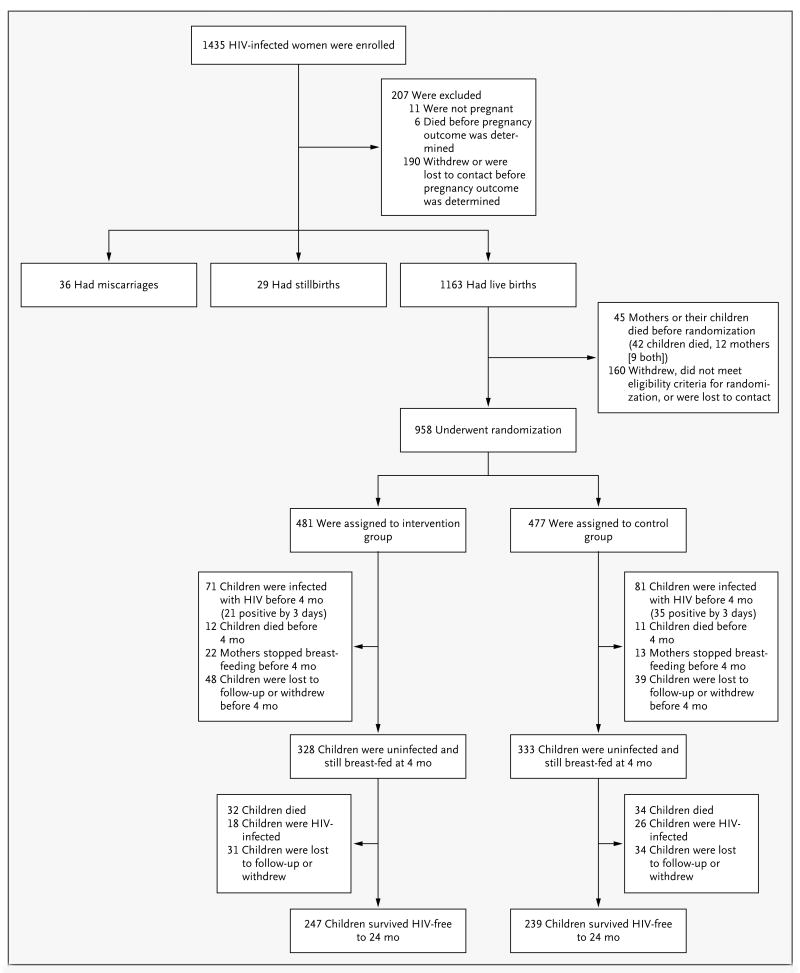
Of the 1435 HIV-infected pregnant women enrolled in the study, 958 women and their infants were randomly assigned to a study group (Fig. 1). Of those assigned to study groups, 84% in the intervention group and 86% in the control group were followed to 24 months or reached the study end points of HIV infection or death. The characteristics of the women who were assigned to the two groups were similar at baseline (Table 1). Child mortality rates were similar by 24 months in the two groups: 23.9% in the intervention group and 24.6% in the control group (P = 0.96) (Table 2 and Fig. 2A). Maternal mortality through 24 months was 9.0% in the intervention group and 8.7% in the control group (P = 0.85).
Figure 1. Study Enrollment, Randomization, and Outcomes.
Table 1. Baseline Characteristics of the 958 HIV-Infected Women and Their Infants According to Study Group.*.
| Characteristic | Intervention Group
(N = 481) |
Control Group
(N = 477) |
P Value |
|---|---|---|---|
| Maternal CD4 cell count | |||
| Median — cells/mm3 | 325 | 332 | 0.30 |
| <200 cells/mm3 — no. (%) | 120 (24.9) | 108 (22.6) | |
| 200–349 cells/mm3 — no. (%) | 150 (31.2) | 144 (30.2) | |
| 350–499 cells/mm3 — no. (%) | 115 (23.9) | 113 (23.7) | |
| ≥500 cells/mm3 — no. (%) | 96 (20.0) | 112 (23.5) | 0.56 |
| Maternal plasma viral load | |||
| Median — copies/ml | 41,374 | 36,054 | 0.88 |
| <1000 copies/ml — no./total no. (%) | 35/480 (7.3) | 29/476 (6.1) | |
| 1000–9999 copies/ml — no./total no. (%) | 90/480 (18.8) | 88/476 (18.5) | |
| 10,000–99,999 copies/ml — no./total no. (%) | 213/480 (44.4) | 210/476 (44.1) | |
| ≥100,000 copies/ml — no./total no. (%) | 142/480 (29.6) | 149/476 (31.3) | 0.86 |
| Hemoglobin — no./total no. (%) | 0.23 | ||
| <10 g/dl | 128/476 (26.9) | 143/470 (30.4) | |
| ≥10 g/dl | 348/476 (73.1) | 327/470 (69.6) | |
| Antiretroviral therapy — no. (%) | 0.76 | ||
| Eligible | 177 (36.8) | 171 (35.8) | |
| Not eligible | 304 (63.2) | 306 (64.2) | |
| Body-mass index 1 mo post partum — no./total no. (%)† | 0.76 | ||
| <18.5 | 68/476 (14.3) | 71/474 (15.0) | |
| ≥18.5 | 408/476 (85.7) | 403/474 (85.0) | |
| Mean age — yr | 26.1 | 26.2 | 0.73 |
| Parity — no. (%) | |||
| First child | 73 (15.2) | 68 (14.3) | |
| Second or third child | 222 (46.2) | 224 (47.0) | |
| ≥Fourth child | 186 (38.7) | 185 (38.8) | 0.92 |
| Death of one or more previous children — no./total no. (%)‡ | 180/401 (44.9) | 174/404 (43.1) | 0.60 |
| Median duration of breast-feeding for last child — mo§ | 18 | 18 | 0.65 |
| Positive rapid plasma reagin test — no./total no. (%) | 81/451 (18.0) | 76/450 (16.9) | 0.67 |
| HIV status disclosed to partner — no. (%) | 265 (55.1) | 281 (58.9) | 0.23 |
| Marital status — no. (%) | 0.85 | ||
| Married | 405 (84.2) | 407 (85.3) | |
| Single | 46 (9.6) | 44 (9.2) | |
| Widowed, divorced, or separated | 30 (6.2) | 26 (5.5) | |
| Education — no. (%) | 0.56 | ||
| No school | 28 (5.8) | 27 (5.7) | |
| Primary school (<8 yr) | 253 (52.6) | 237 (49.7) | |
| Some high school (≥8 yr) | 153 (31.8) | 172 (36.1) | |
| High school completed or more | 47 (9.8) | 41 (8.6) | |
| Domestic water source — no. (%) | 0.42 | ||
| Tap within dwelling | 32 (6.7) | 30 (6.3) | |
| Tap on property outside | 47 (9.8) | 51 (10.7) | |
| Community tap | 382 (79.4) | 385 (80.7) | |
| Other | 20 (4.2) | 11 (2.3) | |
| Electricity in the home — no. (%) | 0.81 | ||
| Yes | 190 (39.5) | 192 (40.3) | |
| No | 291 (60.5) | 285 (59.7) | |
| Cooking facilities — no./total no. (%) | 0.99 | ||
| Stove or hotplate | 167/481 (34.7) | 165/476 (34.7) | |
| Charcoal or wood | 314/481 (65.3) | 311/476 (65.3) | |
| No food at home on ≥1 day in previous mo — no. (%) | 113 (23.5) | 106 (22.2) | 0.64 |
| Full-time paid job — no. (%) | 40 (8.3) | 30 (6.3) | 0.23 |
| Treatment with nevirapine — no. (%) | |||
| Mother (single dose before delivery) | 464 (96.5) | 462 (96.9) | 0.74 |
| Infant | 446 (92.7) | 446 (93.5) | 0.64 |
| Place of birth — no. (%) | 0.66 | ||
| Home | 42 (8.7) | 49 (10.3) | |
| Clinic | 366 (76.1) | 360 (75.5) | |
| Hospital | 67 (13.9) | 65 (13.6) | |
| Other | 6 (1.2) | 3 (0.6) | |
| Infant sex — no./total no. (%) | 0.35 | ||
| Male | 255/481 (53.0) | 238/476 (50.0) | |
| Female | 226/481 (47.0) | 238/476 (50.0) | |
| Mode of delivery — no. (%) | 0.52 | ||
| Vaginal | 469 (97.5) | 468 (98.1) | |
| Cesarean | 12 (2.5) | 9 (1.9) | |
| Birth weight | |||
| Mean — g | 2992 | 3006 | 0.68 |
| <2500 g — no./total no. (%) | 51/471 (10.8) | 54/466 (11.6) | |
| ≥2500 g — no./total no. (%) | 420/471 (89.2) | 412/466 (88.4) | 0.71 |
| Multiple birth — no. (%) | 11 (2.3) | 7 (1.5) | 0.35 |
Maternal CD4 cell counts, viral load, hemoglobin, rapid plasma reagin status, and eligibility for antiretroviral therapy (i.e., a CD4 cell count of <200 per cubic millimeter or a CD4 cell count of <350 per cubic millimeter and World Health Organization stage III conditions) were determined on the basis of blood samples and clinical history obtained at enrollment during pregnancy; social and economic characteristics were also measured at this time.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Of 408 women in the intervention group and 409 women in the control group who had a previous live birth, data on the death of one or more previous children were available for 401 women and 404 women, respectively.
Of 408 women in the intervention group and 409 women in the control group who had a previous live birth, data on the median duration of breast-feeding for last child were available for 394 women and 389 women, respectively.
Table 2. Mortality Rates and Breast-Feeding Duration in the Overall Cohort, Rates of HIV Infection or Death among Children Uninfected and Still Breast-Fed at 4 Months, and Mortality among Children Infected by 4 Months in the Intervention and Control Groups.*.
| Subjects | Intervention Group | Control Group | P Value |
|---|---|---|---|
| Overall cohort | |||
| No. of mother–child pairs | 481 | 477 | |
| Children who died — no. (cumulative mortality rate) | 0.96 | ||
| <4 mo | 21 (0.046) | 22 (0.048) | |
| <12 mo | 79 (0.185) | 60 (0.139) | |
| <24 mo | 100 (0.239) | 102 (0.246) | |
| Mothers who stopped breast-feeding — no. (cumulative probability) | <0.0001 | ||
| <4 mo | 37 (0.084) | 18 (0.040) | |
| ≤4 mo | 270 (0.642) | 26 (0.060) | |
| ≤5 mo | 289 (0.690) | 32 (0.074) | |
| ≤12 mo | 309 (0.746) | 129 (0.343) | |
| ≤18 mo | 350 (0.872) | 261 (0.756) | |
| Children uninfected with HIV and still breast-feeding at 4 mo | |||
| No. of children | 328 | 333 | |
| Children who died or acquired HIV — no. (cumulative probability) | 0.27 | ||
| <12 mo | 31 (0.098) | 37 (0.117) | |
| <18 mo | 43 (0.137) | 58 (0.186) | |
| <24 mo | 50 (0.161) | 60 (0.193) | |
| Children infected with HIV by 4 mo | |||
| No. of children | 71 | 81 | |
| Children who died — no. (cumulative mortality rate) | 0.02 | ||
| <4 mo | 9 (0.132) | 11 (0.138) | |
| <12 mo | 40 (0.630) | 29 (0.395) | |
| <24 mo | 48 (0.771) | 43 (0.611) | |
All rates and probabilities were calculated with Kaplan-Meier methods. P values are based on the log-rank test.
Figure 2. Probability of Child Survival to 24 Months and Duration of Breast-Feeding, According to Study Group.
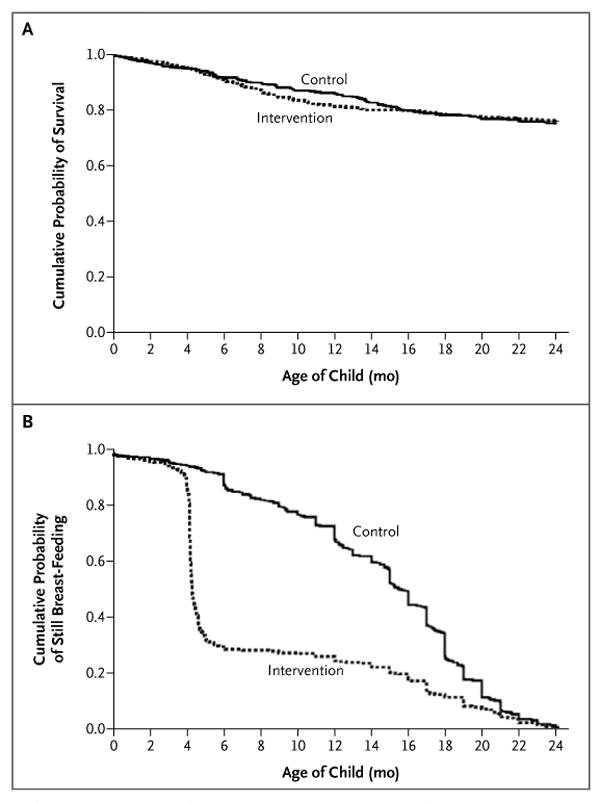
Panel A shows survival to 24 months among 481 children assigned to the intervention group, whose mothers were counseled to wean abruptly at 4 months, and among 477 children assigned to the control group, whose mothers were counseled to breast-feed longer than 4 months and to wean according to standard practice. Panel B shows the duration of breast-feeding according to the study-group assignment.
Adherence to the Intervention
In the intervention group, 69.0% of the women stopped breast-feeding by the end of 5 months. Of those who stopped, 68.8% reported stopping immediately or within 2 days, 25.1% within 2 to 7 days, and 6.1% in 7 days or more. In the control group, 7.4% of the women stopped breast-feeding by the end of 5 months, and 34.3% by the end of 12 months. The median duration of breast-feeding was 4 months (interquartile range, 4 to 14) in the intervention group and 16 months (interquartile range, 11 to 19) in the control group (P<0.001) (Fig. 2B).
HIV-free Survival at 24 Months
There was no significant difference in HIV-free survival between the two groups according to the intention-to-treat analysis: at 24 months of age, 68.4% of the children in the intervention group, as compared with 64.0% of those in the control group, were alive and not infected with HIV (P = 0.13). The rate of HIV transmission was slightly lower in the intervention group than in the control group (21.4% vs. 25.8%, P = 0.11), and the mortality rate for uninfected children was similar in the intervention and the control groups (13.6% and 14.4%, respectively; P = 0.81).
Since the feeding practices in the two groups were intentionally the same through 4 months, we restricted the analysis to children who survived without HIV infection (as determined by a negative PCR test at 4 months or later) and who were still being breast-fed at 4 months, in order to maximize the opportunity to detect differences between the groups. In this subgroup, prerandomization characteristics that were possibly related to HIV-free survival were balanced between the study groups (data not shown), but the women in this subgroup had less advanced HIV disease than the women in the overall study population, since women with more advanced HIV disease were more likely to have infants who were infected by 4 months and were therefore not included in this subgroup. The overall proportions of subjects who were excluded were similar in the two groups, but there were differences in the reasons for the exclusions (Fig. 1). The proportion of children who were excluded because of HIV transmission that occurred before 4 months of age (acquired intrauterine or intrapartum transmission or transmission through breast-feeding before the intervention could take effect) was similar in the intervention group (transmission rate, 15.6%; 71 children) and the control group (transmission rate, 17.6%; 81 children), but more children in the control group than in the intervention group were excluded because of HIV detected within the first 3 days of life (rate of acquired intrauterine transmission, 7.3% vs. 4.4%; P = 0.05). More children in the intervention group than in the control group were excluded because their mothers had stopped breast-feeding them before 4 months (8.4% vs. 4.0%, P = 0.01).
Among uninfected children who were still being breast-fed at 4 months, there was no significant difference between the groups in HIV-free survival at 24 months: 83.9% of the children in the intervention group survived to 24 months without HIV infection, as compared with 80.7% in the control group (P = 0.27) (Fig. 3A). Between 4 and 24 months, rates of postnatal HIV transmission were not significantly different (6.2% in the intervention group and 8.8% in the control group, P = 0.19), and mortality among uninfected children was similar in the two groups (0.7% in the intervention group and 11.7% in the control group, P = 0.71). The causes of death among uninfected children were predominantly diarrheal disease, which accounted for 59% of the deaths in the intervention group and 62% of those in the control group, and respiratory infections, which accounted for 44% and 50% of deaths in the two groups, respectively (more than one cause of death could be assigned). Nineteen percent of the children in the intervention group and 15% of those in the control group died from other causes (including malaria, malnutrition, measles, injury, and unknown causes). There was no significant difference in HIV-free survival between the groups when they were stratified according to the clinical site or evidence of an interaction between baseline characteristics and intervention group.
Figure 3. Cumulative Probability of HIV-free Survival among Uninfected Children and of Survival among Infected Children, According to Study Group.
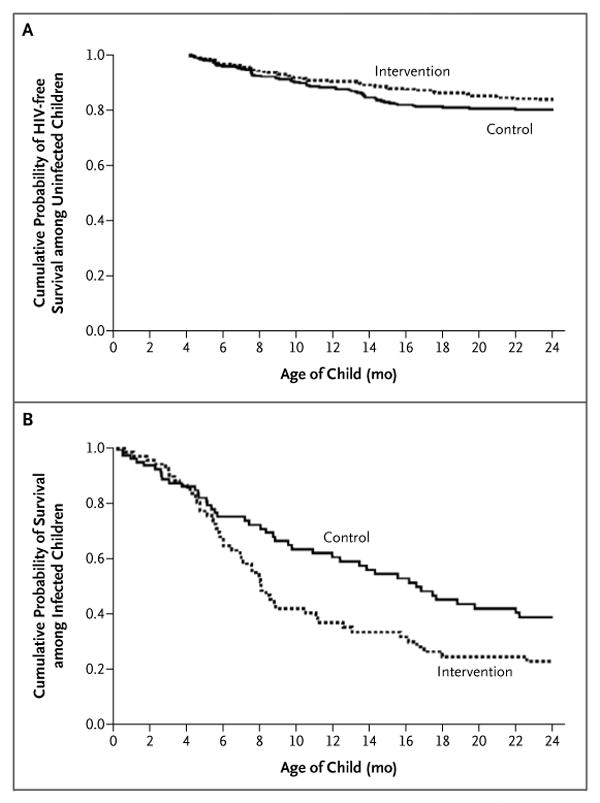
Panel A shows HIV-free survival from 4 to 24 months among children who were uninfected and still breast-feeding at 4 months of age (328 children assigned to the intervention group and 333 assigned to the control group). Panel B shows survival to 24 months among children who had HIV infection by 4 months of age (71 children assigned to the intervention group and 81 assigned to the control group).
Effects of Antiretroviral Therapy
The introduction of antiretroviral therapy toward the end of the study did not explain the absence of significant differences between the groups. Only 6 women started antiretroviral therapy before delivery (2 in the intervention group and 4 in the control group), and 107 women started treatment before 24 months (55 in the intervention group and 52 in the control group), a median of 16 months after delivery (interquartile range, 10 to 19) in the intervention group and 17 months (interquartile range, 11 to 21) in the control group. If maternal and infant follow-up time was censored once therapy was initiated, there was still no significant difference between the groups in the rate of HIV-free survival (84.5% in the intervention group and 80.9% in the control group, P = 0.20).
Mortality among HIV-infected Children
We examined the effects of the random assignment to a study group on 152 children with confirmed HIV infection before 4 months of age. The prognosis was poor in both groups, with cumulative mortality rates by 24 months of 77.1% in the intervention group (median survival, 8 months) and 61.1% in the control group (median survival, 17 months) (P = 0.02) (Table 2). There was no significant difference in mortality between the groups until 4 months of age, but among children who were alive at 4 months, mortality rates by 24 months were 73.6% in the intervention group and 54.8% in the control group (P = 0.007) (Fig. 3B).
Discussion
There was no significant benefit in HIV-free survival to 24 months among the infants of HIV-infected mothers who were encouraged to stop breast-feeding abruptly at 4 months as compared with the infants of mothers who were encouraged to wean their infants according to the standard practice and who continued breast-feeding for a median of 16 months. Early cessation of breast-feeding has substantial programmatic costs, including the provision of breast-milk substitutes, and carries risks that are difficult to quantify, including the disclosure of HIV status, stigmatization, increased fertility, and possible spillover effect in the uninfected population. The costs and risks of terminating breast-feeding early may be justifiable if a net benefit with respect to child health can be achieved, but radical changes in usual breast-feeding practices should not be encouraged in the absence of demonstrated benefits for HIV-free survival.
Early cessation of breast-feeding was not universally accepted in the study population. Despite consenting to this practice at enrollment, receiving intensive counseling, and being provided with formula and complementary foods, only approximately 70% of the women in the intervention group weaned their infants early. This finding is not surprising, since prolonged breast-feeding is the norm in Zambia. A limitation of our study is the incomplete compliance with early weaning, as well as the termination of breast-feeding earlier than expected in the control group (one third of the women stopping breast-feeding before 1 year). These observations highlight the difficulty of promoting the practice of early termination of breast-feeding and complicate the interpretation of the results. The failure of women in the intervention group to stop breast-feeding may have minimized the differences in HIV transmission between the two groups and may have made the benefit with respect to HIV prevention too small to offset the increased mortality due to early weaning.
The difference between the two groups in the rate of HIV transmission was less than expected,6-8 despite different feeding practices. We hypothesize that rapid weaning may be partially responsible. Abrupt weaning is associated with elevations in HIV levels in breast milk and with mastitis.29 Consequently, any exposure to breast milk during this period may be associated with an increased risk of infection. We underestimated the difficulty of completely eliminating all breast-milk exposures in this context even among women who were highly motivated to wean their infants. It is possible that more gradual weaning could reduce the risk of infection, but this possibility should be empirically evaluated. Estimates of reductions in transmission were based on extrapolation from observational studies (not from trials involving attempts to modify feeding practices) and may have overestimated the benefits of early weaning.30 If the weaning period itself is a time when the level of infectivity is elevated, then truncating breast-feeding early may have less effect than anticipated. In a post hoc calculation, our study had sufficient power to detect decreases in HIV transmission of 8% or more. A smaller benefit would not have been detectable, since we expected the benefit of early weaning to be greater than this.
Children who were already infected with HIV before weaning had significantly worse outcomes if they were assigned to the intervention group. We did not anticipate this finding and had initially expected that the progression of HIV disease would override any benefits of breast milk. Theoretically, since formula and weaning cereal were nutritionally replete and fortified with micronutrients, they may have conferred an advantage for HIV-infected children. Our observation of a clear benefit of breast-feeding for HIV-infected children highlights the importance of strengthening infant diagnostic services to triage HIV-infected children into HIV care and treatment31 and to provide encouragement for continued breast-feeding of infected children. Our data demonstrate the survival benefits, in this setting, of continued breast-feeding into the second year of life for HIV-infected children. Infant-feeding policies for HIV-infected women should take into consideration the special needs of HIV-infected children, since in most circumstances, their status will be unknown in early infancy.
Our results differ from those of a trial in Kenya32,33 but are consistent with those of a trial in Botswana, which showed that avoidance of all breast-feeding had no benefit with respect to HIV-free survival.34 Our data are consistent with recently updated recommendations from the World Health Organization that advise continued breast-feeding with complementary foods after 6 months “if replacement feeding is still not acceptable, feasible, affordable, sustainable and safe.”35 We intentionally studied women in a region where economic circumstances were insufficient to ensure safe replacement feeding, so that our results would be generalizable to the populations that are most affected by the epidemic of HIV and the acquired immunodeficiency syndrome in sub-Saharan Africa. Our results are applicable only to settings where the safety of replacement feeding cannot be ensured.
In the context of a clinical trial that included intensive counseling, provision of formula and complementary foods, use of cotrimoxazole, and modest bolstering of the health care infrastructure, abrupt cessation of breast-feeding at 4 months did not improve the outcomes for children born to HIV-infected mothers. These results suggest that early, abrupt cessation of breast-feeding for HIV-infected women in low-resource settings should be avoided.
Acknowledgments
Supported by grants from the National Institute of Child Health and Human Development (R01 HD 39611 and R01 HD 40777); the Centers for Disease Control and Prevention, through the President's Emergency Plan for AIDS Relief, for provision of antiretroviral treatment services; the Agency for International Development (GHS-A-00-00020-00); and the Stephen Lewis Foundation. Dr Aldrovandi is the recipient of a Scientist Award from the Elizabeth Glaser Pediatric AIDS Foundation.
We thank the Zambian families who participated in the research and all the study staff and volunteers; the members of the data safety and monitoring board (Drs. Elaine Abrams, Ted Colton, Wafaie Fawzi, and Saidi Kapiga); the Zambian Oversight Committee (Dr. Elwyn Chomba, chair); and Drs. Susan Allen, Chewe Luo, Lynne Mofenson, Ellen Piwoz, Kevin Ryan, Jon Simon, Zena Stein, Jeffrey Stringer, and Sten Vermund for assistance with aspects of the design and conduct of the study.
Footnotes
No potential conflict of interest relevant to this article was reported.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institutes of Health, the Centers for Disease Control and Prevention, or the Department of Health and Human Services.
References
- 1.Humphrey J, Iliff P. Is breast not best? Feeding babies born to HIV-positive mothers: bringing balance to a complex issue. Nutr Rev. 2001;59:119–27. doi: 10.1111/j.1753-4887.2001.tb06999.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilfert CM, Fowler MG. Balancing maternal and infant benefits and the consequences of breast-feeding in the developing world during the era of HIV infection. J Infect Dis. 2006;195:165–7. doi: 10.1086/510255. [DOI] [PubMed] [Google Scholar]
- 3.Kourtis AP, Butera S, Ibegbu C, Beled L, Duerr A. Breast milk and HIV-1: vector of transmission or vehicle of protection? Lancet Infect Dis. 2003;3:786–93. doi: 10.1016/s1473-3099(03)00832-6. [DOI] [PubMed] [Google Scholar]
- 4.Bulterys M, Fowler MG, Van Rompay KK, Kourtis AP. Prevention of mother-to-child transmission of HIV-1 through breast-feeding: past, present, and future. J Infect Dis. 2004;189:2149–53. doi: 10.1086/420835. [DOI] [PubMed] [Google Scholar]
- 5.Ekpini ER, Wiktor SZ, Satten GA, et al. Late postnatal mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire. Lancet. 1997;349:1054–9. doi: 10.1016/s0140-6736(96)06444-6. [DOI] [PubMed] [Google Scholar]
- 6.Nagelkerke NJ, Moses S, Embree JE, Jenniskens F, Plummer FA. The duration of breastfeeding by HIV-1-infected mothers in developing countries: balancing benefits and risks. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:176–81. [PubMed] [Google Scholar]
- 7.Kuhn L, Stein Z. Infant survival, HIV infection, and feeding alternatives in less-developed countries. Am J Public Health. 1997;87:926–31. doi: 10.2105/ajph.87.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piwoz EG, Ross JS. Use of population-specific infant mortality rates to inform policy decisions regarding HIV and infant feeding. J Nutr. 2005;135:1113–9. doi: 10.1093/jn/135.5.1113. [DOI] [PubMed] [Google Scholar]
- 9.Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission through breastfeeding: a study in Malawi. JAMA. 1999;282:744–9. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 10.Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 11.Fawzi W, Msamanga G, Spiegelman D, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–8. doi: 10.1097/00126334-200211010-00010. [DOI] [PubMed] [Google Scholar]
- 12.Van de Perre P, Simonon A, Msellati P, et al. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant: a prospective cohort study in Kigali, Rwanda. N Engl J Med. 1991;325:593–8. doi: 10.1056/NEJM199108293250901. [DOI] [PubMed] [Google Scholar]
- 13.Bertolli J, St Louis ME, Simonds RJ, et al. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J Infect Dis. 1996;174:722–6. doi: 10.1093/infdis/174.4.722. [DOI] [PubMed] [Google Scholar]
- 14.Embree JE, Njenga S, Datta P, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–41. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 15.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breast-feeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–5. Erratum, Lancet 2000;355: 1104. [PubMed] [Google Scholar]
- 16.Cunningham AS, Jelliffe DB, Jelliffe EF. Breast-feeding and health in the 1980s: a global epidemiologic review. J Pediatr. 1991;118:659–66. doi: 10.1016/s0022-3476(05)80023-x. [DOI] [PubMed] [Google Scholar]
- 17.Jelliffe DB, Jelliffe EF. Human milk in the modern world. New York: Oxford University Press; 1978. [Google Scholar]
- 18.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–87. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 19.Iliff PJ, Piwoz EG, Tavengwa NV, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 20.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breast-feeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–16. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 21.Fewtrell MS, Morgan JB, Duggan C, et al. Optimal duration of exclusive breast-feeding: what is the evidence to support current recommendations? Am J Clin Nutr. 2007;85(Suppl):635S–638S. doi: 10.1093/ajcn/85.2.635S. [DOI] [PubMed] [Google Scholar]
- 22.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 23.Thea DM, Vwalika C, Kasonde P, et al. Issues in the design of a clinical trial with a behavioral intervention — the Zambian Exclusive Breast-feeding Study. Control Clin Trials. 2004;25:353–65. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Stringer EM, Sinkala M, Stringer JS, et al. Prevention of mother-to-child transmission of HIV in Africa: successes and challenges in scaling-up a nevirapine-based program in Lusaka, Zambia. AIDS. 2003;17:1377–82. doi: 10.1097/01.aids.0000060395.18106.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stringer JS, Sinkala M, Maclean CC, et al. Effectiveness of a city-wide program to prevent mother-to-child HIV transmission in Lusaka, Zambia. AIDS. 2005;19:1309–15. doi: 10.1097/01.aids.0000180102.88511.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 27.Walter J, Mwiya M, Scott N, et al. Reduction in preterm delivery and neonatal mortality after the introduction of antenatal cotrimoxazole prophylaxis among HIV-infected women with low CD4 cell counts. J Infect Dis. 2006;194:1510–8. doi: 10.1086/508996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh MK, Kuhn L, West J, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol. 2003;41:2465–70. doi: 10.1128/JCM.41.6.2465-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thea DM, Aldrovandi G, Kankasa C, et al. Post-weaning breast milk HIV-1 viral load, blood prolactin levels and breast milk volume. AIDS. 2006;20:1539–47. doi: 10.1097/01.aids.0000237370.49241.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn DT, Tess BH, Rodrigues LC, Ades AE. Mother-to-child transmission of HIV: implications of variation in maternal infectivity. AIDS. 1998;12:2211–6. doi: 10.1097/00002030-199816000-00017. [DOI] [PubMed] [Google Scholar]
- 31.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 32.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 33.Mbori-Ngacha D, Nduati R, John G, et al. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: a randomized clinical trial. JAMA. 2001;286:2413–20. doi: 10.1001/jama.286.19.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 35.HIV and infant feeding: new evidence and programmatic experience — report of a technical consultation held on behalf of the Inter-agency Task Team (IATT) on Prevention of HIV Infections in Pregnant Women, Mothers and their Infants; Geneva, Switzerland. 25–27 October 2006; Geneva: World Health Organization; 2007. [May 27, 2008]. http://whqlibdoc.who.int/publications/2007/9789241595971_eng.pdf. [Google Scholar]