Abstract
Transformation of primary B lymphocytes by Epstein-Barr virus requires the establishment of a strictly latent infection, the expression of several latent viral proteins, and sustained telomerase activity. Our previous findings indicated that induction of hTERT, the rate-limiting catalytic unit of the telomerase complex, was associated with the expression of the viral latent membrane protein 1 (LMP1). In the present study, we demonstrate that ectopic expression of LMP1 in BJAB and Ramos B cells resulted in an increase of hTERT transcripts, thus suggesting that LMP1 acts at the transcriptional level. This was confirmed by transient expression of a luciferase reporter plasmid containing the hTERT promoter cotransfected with an LMP1-expressing vector or transfected into B cells in which LMP1 expression was inducible. Consistently, silencing of LMP1 by small interfering RNA resulted in a reduction of hTERT transcripts. We also provide evidence indicating that LMP1-induced hTERT activation is independently mediated by NF-κB and by mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 pathways, whereas CD40, Akt, and mTOR signaling has no involvement. Moreover, our results do not support a role for c-Myc in mediating these effects on hTERT, since ectopic expression of LMP1 did not upregulate c-Myc and silencing of this oncogene or E box mutagenesis failed to inhibit LMP1-induced hTERT activation. These findings indicate that LMP1 simultaneously modulates multiple signal transduction pathways in B cells to transactivate the hTERT promoter and enhance telomerase activity, thus confirming the pleiotropic nature of this viral oncoprotein.
Epstein-Barr virus (EBV) is a ubiquitous human gammaherpesvirus that establishes a life-long asymptomatic infection in immunocompetent hosts by colonizing memory B lymphocytes. EBV has a potent transforming ability, being able to efficiently induce blast transformation and uncontrolled proliferation of infected B lymphocytes in vitro. Available evidence, particularly the presence of EBV genomes and the constant expression of viral proteins, strongly supports a relevant role for EBV in the pathogenesis of a wide spectrum of human malignancies, most of which are derived from B lymphocytes (12, 44). Latently EBV-infected B cells may express a defined set of latency genes that include those encoding six nuclear antigens (EBNAs) and three latent membrane proteins (latent membrane protein 1 [LMP1], LMP2A, and LMP2B). Among the EBV latency gene products, LMP1 is considered the strongest oncoprotein, being essential for immortalization of B cells. The N terminus and the six transmembrane domains of the protein form aggregates in the cytoplasmic membrane, allowing LMP1 to act like a constitutively activated receptor (5, 15). Indeed, LMP1 shares functional properties with members of the tumor necrosis factor (TNF) receptor superfamily, particularly CD40, and induces the expression of NF-κB through activation of the TNF receptor-associated factor signaling pathway (31, 40). Consistent with this, it has been shown that LMP1 can partly restore the wild-type phenotype to mice deficient in CD40 (57). However, unlike the TNF receptor, LMP1 engages at least part of the CD40 pathway in a ligand-independent manner. In addition, LMP1 also activates other molecules affecting diverse signaling cascades, including c-Jun NH2-terminal kinase (62), p38 mitogen-activated protein kinase (MAPK) (14), extracellular signal-regulated kinases (ERKs) (36, 47), Janus kinase (19), and phosphatidylinositol 3-kinase (9), thus behaving as a “pleiotropic” viral oncogene. The hijacking of multiple cellular signaling pathways by LMP1 likely contributes to the pathogenesis of most EBV-associated disorders through the simultaneous or sequential triggering of signals involved in the promotion of cell activation, growth, and survival.
Expression of latent EBV proteins does not suffice to fully immortalize EBV-infected B cells. In fact, only EBV-carrying B cells with sustained telomerase activity are truly immortalized, whereas telomerase-negative cells, although exhibiting a prolonged life span, eventually undergo cellular senescence and terminate their life span by shortening of telomeres (54). Telomerase, a ribonucleoprotein complex containing an internal RNA template (hTR) and a catalytic protein with a telomere-specific reverse transcriptase activity (hTERT), extends telomeres at the end of eukaryotic chromosomes, thus preventing cell senescence and death. While hTR is constitutively present in normal and tumor cells, hTERT is the rate-limiting component of the telomerase complex, and its expression correlates with telomerase activity (41). hTERT activity is repressed in somatic tissues, but both hTERT expression and telomerase activity are elevated in most human tumors (28, 35). Ectopic expression of hTERT in telomerase-negative human cells is associated with the extension of cellular life span, whereas inhibition of hTERT limits the growth of cancer cells (22). Furthermore, several pieces of data suggest that besides the maintenance of telomere length, hTERT is also involved in other cellular functions, including promotion of cell growth (6, 49) and survival (10, 39, 45), thus contributing to tumorigenesis by mechanisms independent of its ability to prevent telomere erosion (53).
We recently demonstrated that in early-passage EBV-infected B lymphocytes, activation of hTERT depends on the balance between latent and lytic EBV gene expression, with latent genes being positively associated with telomerase activation (55). We also showed that ectopic hTERT expression inhibits EBV replication and promotes the growth of primary B lymphocytes, suggesting that hTERT contributes to EBV-driven B-cell transformation by multiple pathways (55). Nevertheless, the mechanisms responsible for EBV-induced hTERT activation in B cells are still poorly elucidated. In the present study, we provide evidence indicating that, in B lymphocytes, LMP1 directly promotes the activation of telomerase by acting at the transcriptional level on the hTERT promoter. These effects are mediated by engagement of the NF-κB, MAPK, and ERK1/2 pathways.
MATERIALS AND METHODS
Cell lines.
BJAB and Ramos are EBV-negative B-lymphoma cell lines, with c-Myc in germ line configuration (BJAB) or translocated (Ramos) (64). The BJABtet-LMP1 and Ramos mT-LMP1 cell lines, which express LMP1 under the control of an inducible promoter, and their LMP1-negative counterparts (BJABtet and Ramos mT) were used. BJABtet and BJABtet-LMP1 were maintained in standard medium supplemented with 1 μg/ml puromycin and 1 mg/ml Geneticin (G418; Gibco). For LMP1 induction, 1 or 10 ng/ml tetracycline (TC) was added to cell cultures, and cells were harvested after 48 h. Ramos mT and Ramos mT-LMP1 cells were cultured in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum (FCS), 450 μg/ml hygromycin, and 0.5 mmol/liter ethylenediaminetetracetic acid. For LMP1 induction, cells were washed with phosphate-buffered saline, 1 μmol/liter cadmium chloride was added, and cells were harvested after 24 h (20). BJAB and BJAB/LMP1 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2% glutamine, and 750 μg/ml of Geneticin. DG75 and DG75 tTA-LMP1 cells were maintained in RPMI 1640 medium supplemented with 10% FCS, 2% glutamine, 500 μg/ml hygromycin, and 1 mg/ml Geneticin. The addition of TC (1 μg/ml) represses LMP1 transcription (16). HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FCS. 4134 LCL cells were obtained by infecting peripheral blood from a healthy donor with the B95.8 EBV strain. These cells were used at passage 103 and were positive for hTERT and LMP1 expression, as previously reported (55). Cells were maintained in RPMI 1640 medium supplemented with 10% FCS.
Northern blotting, reverse transcriptase PCR, and real-time PCR.
Total cellular RNA was extracted from 1 × 106 to 5 × 106 cells by use of TRIzol reagent (Invitrogen). Contaminating genomic DNA was removed using a DNase I Amp-grade kit (Invitrogen). The integrity of RNA was evaluated by visualizing the 18S and 28S RNAs through agarose gel electrophoresis, and the relative amount was quantified by spectrophotometry. Northern blotting analysis of c-Myc mRNA expression was carried out as previously described (7). For the reverse transcriptase PCR and real-time PCR experiments, 1 μg RNA was retrotranscribed into cDNA by using the SuperScript III RNase reverse transcriptase assay (Invitrogen) according to the manufacturer's instructions. Either all hTERT transcripts (hTERT-AT) or the full-length hTERT transcript (hTERT-FL), which encodes the functional protein (58), were quantified by real-time PCR, using the AT1/AT2 and FL1/FL2 primer pairs, respectively, as previously described (55). The LMP1 gene transcript was detected using the LMP1-5′ and LMP1-3′ primer pair (42, 46). The transcript of the housekeeping gene GAPDH was detected by using the forward primer 5′-TCGGAGTCAACGGATTTGGT-3′ and the reverse primer 5′-TGATGACAAGCTTCCCGTTG-3′. The c-Myc transcript was detected using the primer pair 5′-CGAGACCTTCATCAAAAACATCAT-3′ (forward) and 5′-GGAGGCCAGCTTCTCTGAGG-3 (reverse). A PCR was performed in a 50-μl final mixture containing 10 μl of cDNA template and 700 nM of each primer. Amplifications were carried out for 35 cycles of 30 s at 94°C, 30 s at 55°C, and 30 s at 72°C. Twenty microliters of the PCR mixture was electrophoresed in a 2% agarose gel.
Telomerase activity detection assay.
Telomerase activity was evaluated by using a PCR-based telomeric repeat amplification protocol (TRAP) as previously reported (4). The TRAP assay was performed using 1 μg of total cell lysate, unless otherwise indicated. The amount of telomerase activity in each sample was expressed as the ratio between the counts per min (cpm) relative to the telomerase ladder (cpm TL) and the cpm of the internal telomerase assay standard control (cpm ITAS). cpm TL/cpm ITAS ratios provided a more accurate quantification of telomerase levels and a wider range of linear relationship than those obtained by comparing sample values against an external standard control (4, 56).
Plasmids.
The phTERTpromoterLuc plasmid contains a PCR-generated 800-bp fragment upstream of the hTERT translational start site (65). The phTERTpromoterLucDM plasmid contains the two c-Myc-binding E box sites mutated from CACGTC to CACCTG (65). The phTERTpromoterLucNF2 plasmid contains mutations in the NF-κB binding site mapped from positions −664 to −654 (67). Mutations were introduced into the phTERT 800-bp promoter by a PCR-based site-directed mutagenesis method (QuikChange X site-directed mutagenesis kit; Stratagene), and the site was mutated from GGGAGGTCCC to TTATGGGAAA. Products of in vitro mutagenesis were verified by DNA sequencing using an ABI Prism 3130xl genetic analyzer (PE Applied Biosystems) and a BigDye Terminator v1.1 cycle sequencing ready reaction kit (PE Applied Biosystems) according to the manufacturer's instructions.
Luciferase reporter assay.
HeLa cells (1.5 × 105) were transiently transfected with 0.3 μg phTERTpromoterLuc (65), 0.5 μg pcDNA3LMP1, or empty pcDNA3 and 0.5 μg cytomegalovirus β-galactosidase (CMV β-Gal) by use of Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. HeLa cells were transiently transfected with the following vectors: 0.3 μg phTERTpromoter, i.e., the wild type (Luc) or one containing mutations in the c-Myc (DM) or NF-κB (NF2) binding sites, 0.5 μg pMT2T c-myc 2.3wt or empty pMT2T (65), and 0.5 μg CMV β-Gal. The amount of DNA in each transfection mix was kept constant by the addition of empty pBluescript vector. DG75 tTA-LMP1 and BJABtet-LMP1 cells and their counterpart DG75tTA and BJABtet cells were seeded in duplicate at 1 × 106 cells/well into 12-well plates. After 24 h, cells were transfected with 4 μg phTERTpromoter and 1 μg pCMVβ-gal by use of a Polyplus transfection kit (jectPEI; Celbio). After 72 h, the luciferase activity was estimated with a dual-luciferase assay system (Promega); values were normalized for transfection efficiency by expressing them for the same amount of β-Gal counts.
RNA interference.
Cells in the logarithmic growth phase (4.5 × 105 cells/well) were lipofected with small interfering RNA (siRNA) for LMP1 or c-Myc (Dharmacon, Lafayette, CO) or with control siRNA (siCONTROL nontargeting siRNA#2; Dharmacon) at different final concentrations, using Dharmafect 2 transfection reagent (Dharmacon) according to the manufacturer's instructions.
Antibodies and reagents.
Phospho-ERK1/2 (T202/Y204), ERK1/2, phospho-Akt (S473), Akt, phospho-IκBα (S32), IκBα, phospho-NF-κB p65 (S536), NF-κB2 p100/p52, and NF-κB p105/p50 antibodies were purchased from Cell Signaling Technology; NF-κB p65 (F-6) and β-tubulin (H435) antibodies were purchased from Santa Cruz Biotechnology; and phycoerythrin (PE)-CD54 and PE-Fas antibodies were purchased from BD Pharmingen (San Diego, CA). The SH5 Akt inhibitor was purchased from Alexis Biochemicals; UO126, BAY-11-7082, and 6-amino-4-(4-phenoxyphenylethylamino) quinazoline were purchased from Calbiochem; and rapamycin was purchased from Sigma.
Analysis of CD40 activation.
As indicated, the cells were exposed to recombinant human soluble CD40 ligand (CD40L; Alexis, Rome, Italy) and interleukin-4 (IL-4; R&D Systems, Minneapolis, MN). Activation of CD40 signaling was verified by upregulation of membrane intercellular adhesion molecule 1 (ICAM-1)/CD54 and Fas/CD95 (23) by immunofluorescence staining and flow cytometric analysis (FC500; Beckman Coulter, Milan, Italy).
Immunoblotting.
Whole-cell lysates were prepared in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2 mM EGTA, 2 mM EDTA, 2 mM Na3VO4, 25 mM β-glycerophosphate, 25 mM NaF, 1 μM okadaic acid, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 0.2% Triton X-100, and 0.3% NP-40) and lysed for 1 h on ice. Total protein extracts were obtained by centrifugation at 16,000 × g for 20 min at 4°C, and protein concentrations were determined by the Bio-Rad Bradford protein assay. Proteins were fractionated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. Immunoblotting was performed using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech).
No-shift NF-κB p65 transcription factor DNA-binding activity assay.
For nuclear protein extracts, cells were lysed with buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 0.5 mM PMSF) on ice for 30 min, 0.5% NP-40 was added, and the samples were vortexed for 1 min and centrifuged at 4,000 × g for 20 s at 4°C. The supernatants (cytoplasmic fraction) were recovered, and the pellets (nuclear fraction) were resuspended in ice-cold extraction buffer C (20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25% [vol/vol] glycerol, 5 μg/ml leupeptin, 1 μg/ml aprotinin, 0.5 mM PMSF) and incubated on ice for 1 h. Nuclear extracts were recovered after centrifugation at 16,000 × g for 20 min at 4°C. Protein concentrations were determined by the Bio-Rad Bradford protein assay. Ten micrograms of nuclear extract was used to analyze NF-κB p65 DNA-binding activity, using an EZ-Detect chemiluminescent transcription factor assay kit (Pierce Biotechnology).
RESULTS
LMP1 upregulates hTERT expression in B-lymphoma cell lines.
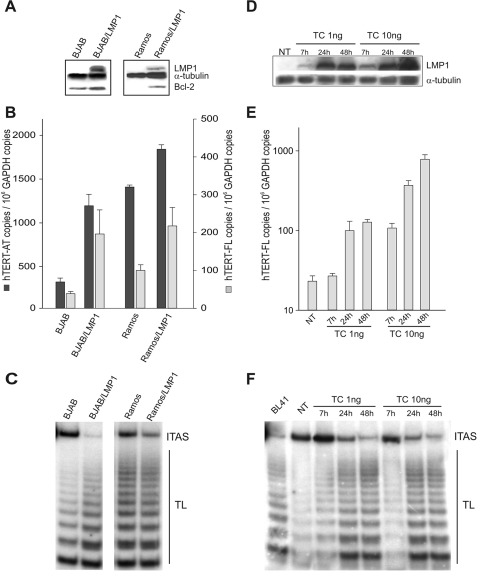
As a first step, hTERT transcript levels and telomerase activity were analyzed in transformed B cells in which ectopic LMP1 expression was constitutive (BJAB/LMP1 cells) or inducible (Ramos cells) (Fig. 1A). BJAB cells contain a germ line nontranslocated c-Myc proto-oncogene, as usually occurs in lymphoblastoid cell lines (LCLs), and basally showed low levels of all hTERT transcripts (450 ± 40 hTERT-AT copies/106 glyceraldehyde-3-phosphate dehydrogenase [GAPDH] copies) and the full-length hTERT transcript encoding functional protein (40 ± 12 hTERT-FL copies/106 GAPDH copies) (Fig. 1B). Ramos cells carry a translocated c-Myc gene and showed >10-fold higher baseline levels of hTERT mRNA, consistent with the capacity of c-Myc to activate the hTERT promoter (Fig. 1B) (65). In both cellular systems, ectopic LMP1 expression was associated with induction/upregulation of the Bcl-2 protein (Fig. 1A). Constitutive LMP1 expression in BJAB cells resulted in a four- to fivefold increase in the levels of both hTERT-AT and hTERT-FL transcripts (Fig. 1B), as well as a marked enhancement of telomerase activity (Fig. 1C). A less striking increase in hTERT transcript levels and telomerase activity was also observed in Ramos cells expressing LMP1 under the control of an inducible promoter (Fig. 1B and C). The effects of LMP1 on hTERT activation were investigated in more detail by using an established, tightly regulatable TC-based system to express LMP1 in BJAB cells (BJABtet-LMP1 cells). As shown in Fig. 1D, treatment with 1 or 10 ng of TC induced a dose-dependent upregulation of LMP1, starting from 7 h and progressively increasing up to 48 h of treatment. Notably, the extent of LMP1 induction in BJAB cells was strictly associated with a dose-dependent increase of hTERT-FL transcripts (Fig. 1E) and telomerase activity (Fig. 1F).
FIG. 1.
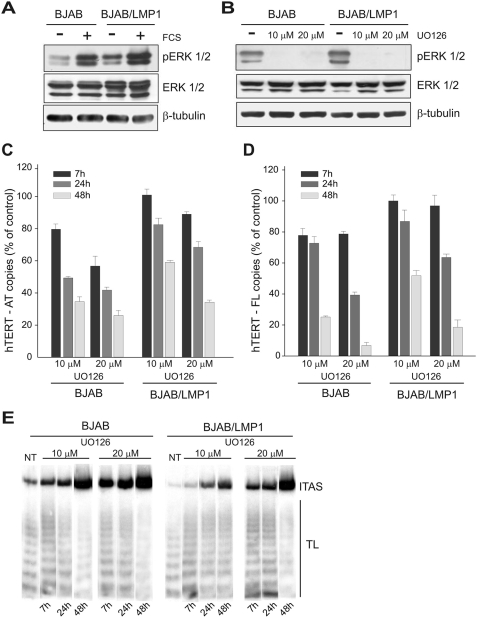
LMP1 expression upregulates hTERT in B-lymphoma cell lines. (A) BJAB and Ramos cells and their counterparts expressing LMP1 (BJAB/LMP1 and Ramos/LMP1) were analyzed for LMP1, Bcl-2, and α-tubulin expression by Western blotting. (B) hTERT-AT and hTERT-FL transcripts were quantified by real-time PCR. Means and standard deviations (SD) (error bars) for three replicates are shown. (C) Telomerase activity was tested by TRAP assay. The panel is representative of three separate experiments. TL, telomerase ladder; ITAS, internal telomerase assay standard. (D) BJABtet-LMP1 cells expressing LMP1 under the control of an inducible promoter were treated with 1 or 10 ng TC and analyzed before (NT) and at 7, 24, and 48 h of treatment for LMP1 expression by Western blotting. α-Tubulin expression was used for sample comparison. (E) hTERT-FL transcripts were measured by real-time PCR. Values are the means and SD (error bars) for three replicates. (F) Telomerase activity was determined by TRAP assay. TL, telomerase ladder; ITAS, internal telomerase assay standard.
siRNA knockdown of LMP1 decreases hTERT transcription.
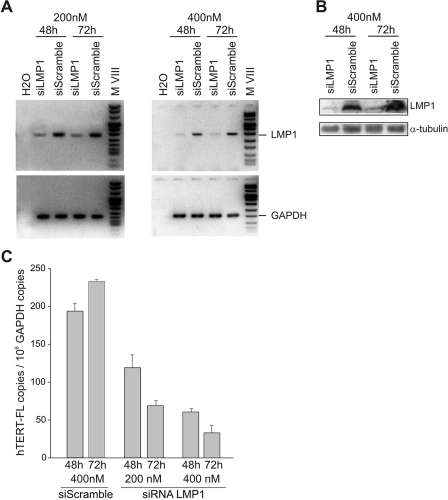
Silencing of LMP1 in the 4134 LCL cells by using a specific siRNA efficiently induced a downregulation of LMP1 mRNA and protein (Fig. 2A and B). A nonsilencing, scrambled siRNA used at the same concentrations (200 and 400 nM) served as a negative control (Fig. 2A and B). Knocking down LMP1 resulted in a dose-dependent decrease in the level of hTERT transcripts (Fig. 2C). After 72 h of exposure to 400 nM anti-LMP1 siRNA, the levels of hTERT-FL mRNA were reduced >80% compared to those in control cells (Fig. 2C). Similar inhibition of hTERT mRNA was obtained when BJAB/LMP1 cells were exposed to 400 nM anti-LMP1 siRNA (not shown).
FIG. 2.
siRNA knockdown of LMP1 decreases hTERT transcription. 4134 LCL cells were lipofected with different concentrations (200 nM and 400 nM) of anti-LMP1 siRNA (siLMP1) or control siRNA (siScramble) and analyzed after 48 and 72 h. (A) LMP1 (upper panels) and GAPDH (lower panels) mRNAs were analyzed by reverse transcription-PCR. (B) Expression of LMP1 protein was assessed by Western blotting. α-Tubulin expression was used for sample comparison. (C) hTERT-FL mRNA levels were quantified by real-time PCR with 4134 cells treated with siRNA LMP1 or control siRNA (siScramble). Means and SD (error bars) for three replicates are shown.
LMP1 transactivates the hTERT promoter in both epithelial and B cells.
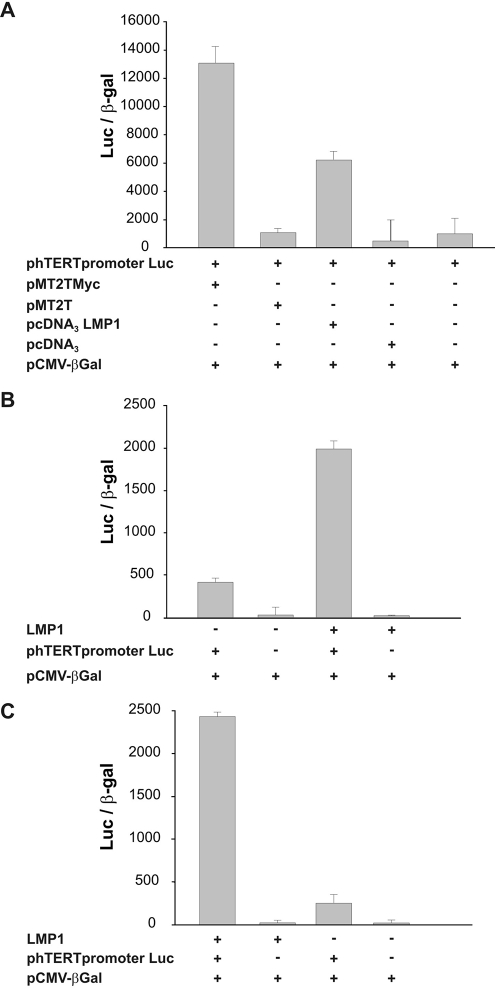
The findings that LMP1 induced hTERT mRNA and that LMP1 silencing resulted in hTERT mRNA downregulation prompted us to assess whether the hTERT promoter is regulated by LMP1. HeLa cells were cotransfected with phTERTpromoterLuc, expressing luciferase under the control of the hTERT promoter, and pcDNA3LMP1, which expresses the LMP1 protein, or the empty pcDNA3 vector as a control. As shown in Fig. 3A, the hTERT promoter was induced by ectopic LMP1 expression. We also observed that LMP1 induced hTERT promoter activity to a slightly lower level than that induced by ectopic expression of c-Myc, a known transcriptional regulator of hTERT (Fig. 3A). To verify the ability of LMP1 to induce the hTERT promoter in the B-cell background, the phTERTpromoterLuc plasmid was transfected into BJABtet-LMP1 and DG75 cells expressing LMP1 under the control of a TC-dependent promoter. In both cellular systems, LMP1 induction was associated with a strong activation of the hTERT promoter (Fig. 3B and C).
FIG. 3.
Transcriptional activation of hTERT promoter by LMP1. (A) HeLa cells were cotransfected with vectors allowing the expression of c-Myc (pMT2TMyc) or LMP1 (pcDNA3LMP1) or with control vectors (pMT2T and pcDNA3) and with a plasmid expressing luciferase under the control of the hTERT promoter (phTERTpromoterLuc). (B) BJABtet-LMP1 cells, expressing LMP1 under the control of a TC-inducible promoter, were cultured without (LMP1−) or with (LMP1+) 10 ng TC for 48 h and then transfected with phTERTpromoterLuc. (C) DG75 tTA cells expressing LMP1 after TC removal were cultured with 1 μg TC (LMP1−) or without TC (LMP1+) for 48 h and then transfected with phTERTpromoterLuc. In each experiment, the total amount of transfected DNA was kept constant by adding the pBluescript vector. A plasmid expressing the bacterial β-Gal gene was also cotransfected in each experiment as an internal control for transfection efficiency. Values were normalized for transfection efficiency by expressing them for the same amount of β-Gal counts.
CD40 signaling is not involved in mediating the transcriptional activation of hTERT and the increase of telomerase activity induced by LMP1 in B cells.
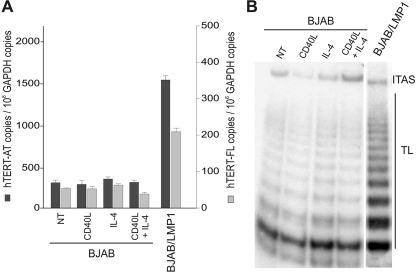
Considering that LMP1 engages at least part of the CD40 signaling pathway (31, 40, 57) and that CD40 activation may contribute to the induction of telomerase activity in B lymphocytes (25, 26, 63), we investigated whether the CD40 pathway could mediate LMP1-dependent induction of hTERT mRNA and telomerase activity in B cells. Treatment of BJAB cells (24 h) with recombinant human soluble CD40L (0.5 μg/ml), IL-4 (0.1 μg/ml), or their combination did not increase the levels of hTERT-AT and hTERT-FL transcripts (Fig. 4A), nor did it enhance telomerase activity (Fig. 4B). Activation of CD40 signaling in these cells was verified by upregulation of cell surface expression of ICAM-1/CD54 and Fas/CD95 (23; data not shown). These findings rule out the possibility that the effects induced by LMP1 on hTERT expression and telomerase activity are due to the ability of this viral protein to hijack CD40-dependent signaling.
FIG. 4.
CD40L and IL-4 do not upregulate hTERT expression in B-cell lymphoma. BJAB cells were cultured in the presence of solvent alone (NT) or treated with recombinant human soluble CD40L (0.5 μg/ml), IL-4 (0.1 μg/ml), or their combination for 24 h. Activation of CD40 signaling was verified by upregulation of membrane ICAM-1/CD54 and Fas/CD95 (23), detected by immunofluorescence and flow cytometry analysis (not shown). (A) Levels of hTERT-AT and hTERT-FL transcripts were quantified by real-time PCR. Values are means and SD (error bars) for three replicates. (B) Telomerase activity was tested by TRAP assay. TL, telomerase ladder; ITAS, internal telomerase assay standard.
The p42/p44 MAPK pathway contributes to LMP1-dependent induction of hTERT mRNA and telomerase activity.
The LMP1 protein engages several signaling pathways that are involved in the induction of hTERT mRNA and/or telomerase activity. In particular, LMP1 is a potent stimulator of the activity of ERKs (36, 47), and it has been shown that MAPK and ERK1/2 are involved in the induction of hTERT expression and/or telomerase activity mediated by stress or growth stimuli (18, 38). Analysis of ERK1/2 activation in BJAB cells showed that LMP1 markedly enhanced the phosphorylation of ERK1/2 both in the presence and in the absence of serum, indicating that LMP1 activates the ERK1/2 pathway (Fig. 5A). Similar findings were observed in DG75 tTA-LMP1 cells (not shown). Pharmacologic inhibition of ERK1/2 by UO126 (Fig. 5B) resulted in dose- and time-dependent decreases in the levels of hTERT-AT and hTERT-FL transcripts and in telomerase activity in both BJAB and BJAB/LMP1 cells (Fig. 5C, D, and E). Notably, the inhibition was lower in BJAB/LMP1 cells than in parental BJAB cells, as a possible consequence of the higher levels of ERK1/2 activation and hTERT expression in LMP1 transfectants. These findings indicate that ERK1/2 is involved in the LMP1-dependent induction of hTERT mRNA and telomerase activity in B cells.
FIG. 5.
LMP1 engages the ERK1/2 pathway to activate hTERT. BJAB and BJAB/LMP1 cells were cultured in the presence or absence of 10% FCS (A) or treated with UO126 (10 μM or 20 μM) (B) for 24 h and analyzed by Western blotting using specific antibodies to phospho-ERK1/2 (T202/Y204), ERK1/2, and β-tubulin. (C and D) BJAB and BJAB/LMP1 cells were treated with solvent alone (dimethyl sulfoxide) or with UO126 (10 μM or 20 μM) and analyzed for hTERT transcription and telomerase activity after 7, 24, and 48 h of treatment. Levels of hTERT-AT (C) and hTER-FL (D) transcripts were determined by real-time PCR and expressed as percentages of hTERT levels quantified in the corresponding untreated cells. Means and SD (error bars) for three replicates are shown. (E) Telomerase activity was analyzed by TRAP assay. The panel is representative of three separate experiments. TL, telomerase ladder; ITAS, internal telomerase assay standard.
NF-κB but not Akt or mTOR signaling mediates LMP1-dependent induction of hTERT mRNA and telomerase activity.
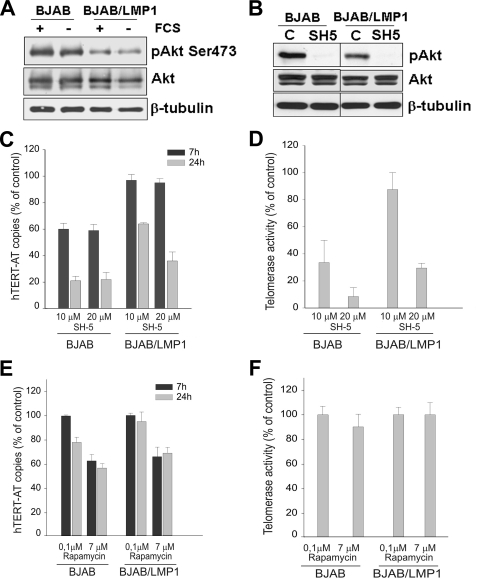
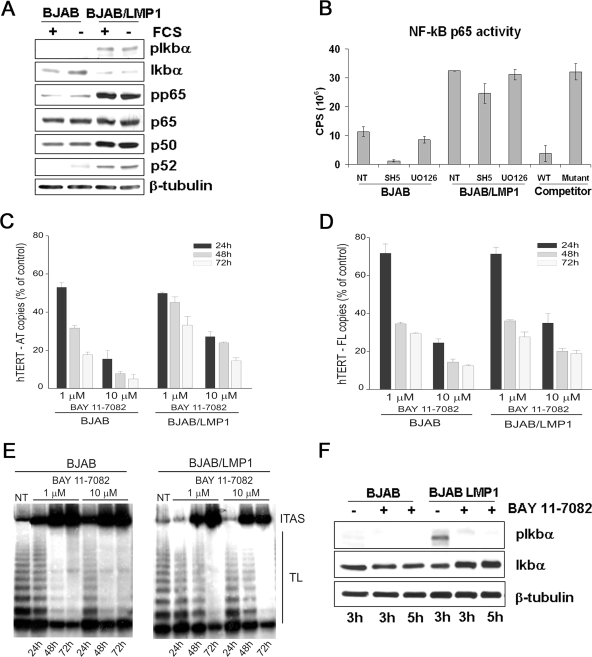
Previous studies have shown that LMP1 activates the phosphatidylinositol 3-kinase/Akt pathway (9), a cascade that regulates telomerase activity by both transcriptional and posttranscriptional mechanisms (29, 30, 68). Ectopic expression of LMP1 in BJAB cells (Fig. 6A) or in DG75 cells (not shown) did not increase the level of the phosphorylated, active form of Akt, which was instead lower than that in parental cells. Nevertheless, pharmacologic inhibition of Akt (SH5) (Fig. 6B) induced more pronounced decreases of hTERT mRNA and telomerase activity in parental BJAB cells than in LMP1 transfectants, despite the lower level of constitutive Akt activation in the latter cells (Fig. 6C and D). Pharmacologic inhibition of the mTOR kinase, a downstream target of Akt, induced only at high doses a slight decrease in the level of hTERT mRNA in both BJAB and BJAB/LMP1 cells, with no effect on telomerase activity (Fig. 6E and F). Considering that NF-κB is a downstream target of Akt (37, 43, 48, 52) and that activation of this transcription factor is crucial for LMP1-driven B-cell transformation (27, 40), we then investigated the role of NF-κB in LMP1-induced upregulation of hTERT mRNA and telomerase activity. NF-κB activation by LMP1 was demonstrated by the upregulation of the p50 and p52 subunits and the higher levels of pp65(Ser536) and pIκBα (Ser32) in BJAB/LMP1 cells than in parental BJAB cells (Fig. 7A), consistent with previous findings (2, 24). LMP1-induced NF-κB activation was also confirmed using a no-shift p65 transcription factor assay (Fig. 7B). Pharmacologic inhibition of NF-κB by BAY-11-7082 resulted in dose- and time-dependent decreases in the levels of the hTERT-AT and hTERT-FL transcripts and in telomerase activity in both BJAB and BJAB/LMP1 cells (Fig. 7C, D, and E). Similar dose- and time-dependent decreases of hTERT mRNAs and telomerase activity were also observed when NF-κB was inhibited by 6-amino-4-(4-phenoxyphenylethylamino) quinazoline (not shown). Notably, the inhibition was lower in BJAB/LMP1 cells than in BJAB cells, a possible consequence of the higher levels of hTERT expression and NF-κB activation in LMP1 transfectants. NF-κB inhibition by BAY-11-7082 was confirmed by pIκBα downregulation and increased levels of the IκBα protein, as a likely consequence of its reduced degradation (Fig. 7F). These findings are consistent with a direct involvement of NF-κB in LMP1-mediated hTERT transcriptional activation. Experiments carried out with the SH5 Akt inhibitor confirmed that NF-κB is a downstream target of Akt in BJAB cells. In particular, this inhibitor decreased the phosphorylation of p65 at Ser536, downregulated the p50 and p65 proteins (not shown), and inhibited NF-κB activity, with a stronger effect in BJAB than in BJAB/LMP1 cells, where LMP1 enhances NF-κB activation (Fig. 7B). These results suggest that the downregulation of hTERT mRNA and telomerase activity observed after Akt inhibition is probably dependent on downstream effects mediated by the NF-κB transcription factor. Conversely, UO126 treatment had no effect on NF-κB activity, ruling out a possible cross talk between ERK1/2 and NF-κB in these cells (Fig. 7B).
FIG. 6.
Analysis of the role of Akt- and mTOR-dependent pathways. BJAB and BJAB/LMP1 cells were cultured in the absence or presence of 10% FCS (A) or treated with SH5, an inhibitor of Akt, at 20 μM (B) for 24 h. Whole-cell lysates were analyzed by Western blotting using anti-phospho-Akt (Ser473), anti-Akt, and anti-β-tubulin. BJAB and BJAB/LMP1 cells were cultured with solvent alone or with SH5 at 10 μM or 20 μM (C and D) or rapamycin, an inhibitor of mTOR, at 0.1 μM and 7 μM (E and F) and analyzed at 7 and 24 h of treatment. Levels of hTERT-AT transcripts (C and E) were quantified by real-time PCR and expressed as percentages of the hTERT-AT levels quantified in the corresponding untreated cells. (D and F) Telomerase activity was tested by TRAP assay, and levels were reported as percentages of telomerase activity quantified in the corresponding untreated cells. Values are the means and SD (error bars) for three separate experiments.
FIG. 7.
LMP1 engages the NF-κB pathway to activate hTERT. (A) BJAB and BJAB/LMP1 cells were cultured in the presence or absence of 10% FCS for 24 h, and whole-cell lysates were analyzed by immunoblotting for the indicated proteins. (B) BJAB and BJAB/LMP1 cells were treated with solvent alone (NT), 20 μM SH5, or 10 μM UO126 for 24 h. Ten micrograms of nuclear protein extract was analyzed for NF-κB p65 DNA-binding activity, using an EZ-Detect chemiluminescent transcription factor assay kit from Pierce Biotechnology. Wild-type and mutant NF-κB competitor duplexes were used as signal specificity controls. Histograms are representative of three separate experiments with virtually identical results. (C and D) BJAB and BJAB/LMP1 cells were treated with BAY-11-7082 at 1 μM or 10 μM and analyzed after 24, 48, and 72 h of treatment. Levels of hTERT-AT (C) and hTERT-FL (D) transcripts were quantified by real-time PCR and expressed as percentages of the hTERT levels quantified in the corresponding untreated cells. Values are the means and SD (error bars) for three separate experiments. (E) Telomerase activity was analyzed by TRAP assay. The panel is representative of three separate experiments. TL, telomerase ladder; ITAS, internal telomerase assay standard. (F) BJAB and BJAB/LMP1 cells were treated with 3 μM BAY-11-7082 for 3 and 5 h and analyzed by Western blotting for phospho-IκBα (Ser32), IκBα, and β-tubulin expression.
LMP1 does not engage c-Myc to activate hTERT.
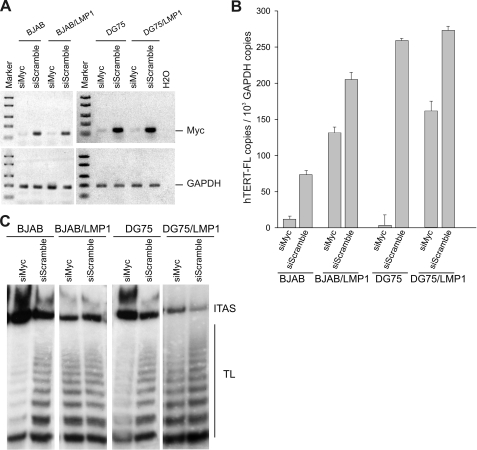
The recent demonstration that LMP1 may upregulate hTERT expression through c-Myc activation in epithelial cells (66) prompted us to investigate the possible involvement of c-Myc in B lymphocytes. Northern blot analysis showed that ectopic expression of LMP1 in BJAB cells did not increase the level of c-Myc mRNA (not shown). We also analyzed the effects of c-Myc silencing by using a specific siRNA (Fig. 8A). A scramble siRNA was used as a negative control, whereas DG75 cells carrying a translocated c-Myc were used as a positive control (Fig. 8A). Silencing of c-Myc resulted in a strong inhibition of hTERT transcripts and telomerase activity in both DG75 and BJAB cells, while only a slight reduction of hTERT mRNA was observed in BJAB/LMP1 and DG75/LMP1 cells (Fig. 8B and C). These findings suggest that LMP1 acts as an hTERT transcriptional activator independently of c-Myc expression/activation. To verify the ability of LMP1 to induce the hTERT promoter independently of c-Myc and dependent on the NF-κB pathway, BJABtet-LMP1 cells were transfected with hTERTpromoterLucDM, containing mutations in the E box, or with a plasmid containing mutations in the NF-κB binding site. As shown in Fig. 9, disruption of the E box did not prevent LMP1-induced hTERT activation, thus confirming that LMP1 activates hTERT independently of c-Myc. In contrast, mutagenesis in the NF-κB binding site impaired LMP1-induced activation of the hTERT promoter (Fig. 9).
FIG. 8.
LMP1 does not engage c-Myc to activate hTERT. BJAB, BJAB/LMP1, DG75, and DG75/LMP1 cells were lipofected with 400 nM anti-c-Myc siRNA (siMyc) or with control siRNA (siScramble) and were analyzed after 24 h. (A) c-Myc (top) and GAPDH (bottom) mRNAs were analyzed by reverse transcription-PCR. (B) hTERT-FL mRNA levels were quantified by real-time PCR. Means and SD (error bars) for three replicates are shown. (C) Telomerase activity was analyzed by TRAP assay. TL, telomerase ladder; ITAS, internal telomerase assay standard.
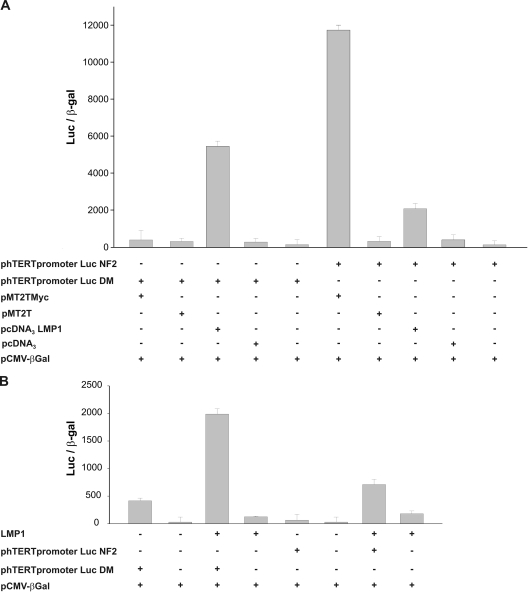
FIG. 9.
Transcriptional activation of the hTERT promoter by LMP1. (A) HeLa cells were cotransfected with vectors allowing the expression of c-Myc (pMT2TMyc) or LMP1 (pcDNA3LMP1) or with control vectors (pMT2T and pcDNA3) and with a plasmid expressing luciferase under the control of the hTERT promoter containing mutations in the c-Myc binding sites (phTERTpromoterLucDM) or NF-κB binding site (phTERTpromoterLucNF2). (B) BJABtet-LMP1 cells, expressing LMP1 under the control of a TC-inducible promoter, were cultured without (LMP1−) or with (LMP1+) 10 ng TC for 48 h and then transfected with the phTERTpromoterLucDM or phTERTpromoterLucNF2 plasmid. The total amount of transfected DNA was kept constant in each experiment by adding the pBluescript vector. A plasmid expressing the bacterial β-Gal gene was also cotransfected in each experiment as an internal control for transfection efficiency. Values were normalized for transfection efficiency by expressing them for the same amount of β-Gal counts.
DISCUSSION
Telomerase activation is a critical step of EBV-driven cell immortalization. We recently demonstrated that activation of hTERT in early-passage EBV-infected B lymphocytes is associated with a progressive increase in LMP1 expression and inhibition of viral lytic replication (55). The observation that LMP1 may activate telomerase activity in nasopharyngeal carcinoma cells (11, 66) prompted us to investigate the effects of LMP1 on hTERT expression and telomerase activation in the B-cell system. Here we provide evidence that ectopic expression of LMP1 in different B-cell backgrounds induces a dose-dependent increase of hTERT mRNA and telomerase activity. Consistently, inhibition of LMP1 expression by siRNA reduced the levels of hTERT transcripts, again in a dose-dependent fashion. Moreover, cotransfection experiments demonstrated that LMP1 transactivates the hTERT promoter in both carcinoma (HeLa) cells and transformed B lymphocytes (BJAB and DG75 cells). Overall, these findings indicate that LMP1 directly promotes the activation of hTERT in B cells by acting at the transcriptional level. In this respect, LMP1 mimics the effects of other proteins carried by oncogenic viruses that activate hTERT transcriptionally, such as Tax of human T-cell lymphotropic virus type 1 (51), E6 of human papillomavirus (33, 60), latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (61), and the preS2 gene product of hepatitis B virus (34). Moreover, this additional property of LMP1 reinforces the notion that this pleiotropic viral oncogene is one of the major contributors to EBV-driven B-cell transformation. It should be considered, however, that LMP1 expression alone may not be sufficient to activate telomerase in primary B cells, since the B-cell lines we used already have baseline levels of hTERT expression and telomerase activity.
LMP1 hijacks cellular signaling pathways that are critical for B-cell growth and survival, including some cascades that are also known to regulate hTERT expression and telomerase activity. In particular, LMP1 engages at least part of the CD40 signaling pathway (31, 40, 57), which may contribute to the induction of telomerase activity in B lymphocytes (25, 26, 63). Nevertheless, CD40 triggering in BJAB cells, even in combination with IL-4 costimulation, failed to reproduce LMP1-induced upregulation of hTERT expression and telomerase activity, ruling out a possible involvement of CD40 signaling in LMP1-mediated hTERT activation in this cellular system. Among the other pathways that may regulate telomerase activity, we showed that the ERK1/2 and NF-κB pathways are strongly activated in BJAB cells following ectopic expression of LMP1. To our knowledge, this is the first demonstration that LMP1 activates ERK1/2 in B lymphocytes, since available data refer to experiments carried out with epithelial cells of various origins or with fibroblasts (17, 36, 47). Notably, pharmacologic inhibition of the ERK1/2 and NF-κB pathways markedly decreased hTERT mRNA expression and telomerase activity in parental BJAB cells and, at lower levels, in the LMP1 transfectants. These are specific, not toxic, effects, since other inhibitors (i.e., rapamycin) failed to block the activation. These findings support a role of ERK1/2 and NF-κB in mediating LMP1-dependent hTERT transactivation. Pathways involving ERK1/2 activation are known to regulate telomerase activity in response to exogenous growth stimuli, even independent of proliferation (21, 38). Our results suggest that LMP1 expression in B lymphocytes may mimic the effects of growth factors by directly activating telomerase via ERK1/2, thus contributing to cell immortalization. In epithelial cells, the Ets transcription factor family, downstream of the MAPK and ERK1/2 signaling pathways, was shown to regulate telomerase activity at the transcriptional level, both directly and indirectly through the proto-oncogene c-Myc (13). Further studies are needed to assess whether Ets or other transcription factors are involved in ERK1/2-dependent activation of telomerase in B cells induced by LMP1.
The observation that NF-κB is involved in mediating LMP1-dependent hTERT transactivation in B lymphocytes is consistent with findings obtained with other cellular systems supporting a role for this transcription factor in regulating telomerase (1, 50, 51). LMP1 was shown to induce telomerase activity in nasopharyngeal carcinoma cells through NF-κB activation (11), an effect that was c-Myc dependent, since mutagenesis of c-Myc-responsive E box elements in the hTERT promoter inhibited hTERT transactivation induced by LMP1 (66). Our results, however, do not support a role for c-Myc in mediating the hTERT expression and telomerase activation induced by LMP1 in B lymphocytes. In these cells, in fact, ectopic expression of LMP1 did not upregulate c-Myc expression, and silencing of this oncogene failed to inhibit LMP1-induced telomerase activation. Furthermore, mutagenesis in the NF-κB binding site, but not in the c-Myc binding sites, inhibited LMP1-induced activation of the hTERT promoter.
Although NF-κB may be positively regulated by ERKs (8, 59), LMP1-induced activation of telomerase via NF-κB in B cells is independent of ERK1/2, as shown by the finding that inhibition of the MAPK/ERK pathway did not affect NF-κB activation in LMP1-expressing cells. We also investigated the possible involvement of Akt- and mTOR-dependent signaling, two pathways that may regulate telomerase (3, 29, 30, 32, 68, 69). Although we confirmed a role for Akt in regulating telomerase in B lymphocytes, LMP1 failed to activate Akt in BJAB cells, as shown by the downregulation of the phosphorylated form of Akt in LMP1-expressing cells. The slight resistance of LMP1 transfectants to Akt-dependent inhibition of hTERT expression and telomerase activity is probably due to the greater activation of NF-κB downstream of Akt. Taken together, these findings do not support a role for Akt in mediating LMP1-dependent induction of hTERT expression. Available evidence indicates that the mTOR kinase, which is downstream of Akt, may regulate hTERT expression at the transcriptional level in gynecologic tumors (68). However, mTOR inhibition by rapamycin did not affect hTERT mRNA levels or telomerase activity in B cells, ruling out a possible involvement of this kinase in LMP1-mediated hTERT activation.
Overall, the results of the present study demonstrate that the hTERT activation induced by LMP1 in B lymphocytes occurs at the transcriptional level through NF-κB- and ERK-dependent pathways. These findings also confirm the pleiotropic nature of this viral oncoprotein, which simultaneously modulates multiple signal transduction pathways to activate the hTERT promoter and enhance telomerase activity. On these grounds and considering the pivotal role of telomerase in the establishment of EBV latency (55) and B-cell transformation, therapeutic approaches targeting LMP1 may prove effective in the prevention and treatment of EBV-associated B-cell lymphoproliferative disorders.
Acknowledgments
We thank Riccardo Dalla Favera (Columbia University, NY) for providing the BJABtet, BJABtet-LMP1, Ramos mT, and Ramos mT-LMP1 cell lines and the pMT2T c-myc, pMT2T, phTERTpromoterLuc, and phTERTpromoterLuc DM plasmids; Ethel Cesarman (Weill Cornell Medical College, NY) for providing the pcDNA3LMP1 and pcDNA3 plasmids; Pierantonio Gallo for artwork; and Lisa Smith for editorial assistance. We thank Donna D'Agostino (Department of Oncology and Surgical Sciences, University of Padova) for help in constructing the phTERTpromoterLucNF2 plasmid.
This work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC) and Programmi di Ricerca di Rilevante Interesse Nazionale (PRIN) 2005-2006. Jessica Dal Col is a fellowship recipient of AIRC.
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Akiyama, M., T. Hideshima, T. Hayashi, Y. T. Tai, C. S. Mitsiades, N. Mitsiades, D. Chauhan, P. Richardson, N. C. Munshi, and K. C. Anderson. 2003. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res. 6318-21. [PubMed] [Google Scholar]
- 2.Atkinson, P. G., H. J. Coope, M. Rowe, and S. C. Ley. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 27851134-51142. [DOI] [PubMed] [Google Scholar]
- 3.Bae-Jump, V. L., C. Zhou, P. A. Gehrig, Y. E. Whang, and J. F. Boggess. 2006. Rapamycin inhibits hTERT telomerase mRNA expression, independent of cell cycle arrest. Gynecol. Oncol. 100487-494. [DOI] [PubMed] [Google Scholar]
- 4.Ballon, G., L. Ometto, E. Righetti, A. M. Cattelan, S. Masiero, M. Zanchetta, L. Chieco-Bianchi, and A. De Rossi. 2001. Human immunodeficiency virus type 1 modulates telomerase activity in peripheral blood lymphocytes. J. Infect. Dis. 183417-424. [DOI] [PubMed] [Google Scholar]
- 5.Cahir McFarland, E. D., K. M. Izumi, and G. Mosialos. 1999. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-kappaB. Oncogene 186959-6964. [DOI] [PubMed] [Google Scholar]
- 6.Cao, Y., H. Li, S. Deb, and J. P. Liu. 2002. TERT regulates cell survival independent of telomerase enzymatic activity. Oncogene 213130-3138. [DOI] [PubMed] [Google Scholar]
- 7.Cariati, R., P. Zancai, M. Quaia, G. Cutrona, F. Giannini, S. Rizzo, M. Boiocchi, and R. Dolcetti. 2000. Retinoic acid induces persistent, RARalpha-mediated anti-proliferative responses in Epstein-Barr virus-immortalized B lymphoblasts carrying an activated c-Myc oncogene but not in Burkitt's lymphoma cell lines. Int. J. Cancer 86375-384. [DOI] [PubMed] [Google Scholar]
- 8.Craig, R., A. Larkin, A. M. Mingo, D. J. Thuerauf, C. Andrews, P. M. McDonough, and C. C. Glembotski. 2000. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 27523814-23824. [DOI] [PubMed] [Google Scholar]
- 9.Dawson, C. W., G. Tramountanis, A. G. Eliopoulos, and L. S. Young. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 2783694-3704. [DOI] [PubMed] [Google Scholar]
- 10.Del Bufalo, D., A. Rizzo, D. Trisciuoglio, G. Cardinali, M. R. Torrisi, U. Zangemeister-Wittke, G. Zupi, and A. Biroccio. 2005. Involvement of hTERT in apoptosis induced by interference with Bcl-2 expression and function. Cell Death Differ. 121429-1438. [DOI] [PubMed] [Google Scholar]
- 11.Ding, L., L. L. Li, J. Yang, Y. G. Tao, M. Ye, Y. Shi, M. Tang, W. Yi, X. L. Li, J. P. Gong, and Y. Cao. 2005. Epstein-Barr virus encoded latent membrane protein 1 modulates nuclear translocation of telomerase reverse transcriptase protein by activating nuclear factor-kappaB p65 in human nasopharyngeal carcinoma cells. Int. J. Biochem. Cell. Biol. 371881-1889. [DOI] [PubMed] [Google Scholar]
- 12.Dolcetti, R., and M. G. Masucci. 2003. Epstein-Barr virus: induction and control of cell transformation. J. Cell. Physiol. 196207-218. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer, J., H. Li, D. Xu, and J. P. Liu. 2007. Transcriptional regulation of telomerase activity: roles of the Ets transcription factor family. Ann. N. Y. Acad. Sci. 111436-47. [DOI] [PubMed] [Google Scholar]
- 14.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 27416085-16096. [DOI] [PubMed] [Google Scholar]
- 15.Eliopoulos, A. G., and L. S. Young. 2001. LMP1 structure and signal transduction. Semin. Cancer Biol. 11435-444. [DOI] [PubMed] [Google Scholar]
- 16.Floettmann, J. E., K. Ward, A. B. Rickinson, and M. Rowe. 1996. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology 22329-40. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda, M., W. Kurosaki, K. Yanagihara, H. Kuratsune, and T. Sairenji. 2002. A mechanism in Epstein-Barr virus oncogenesis: inhibition of transforming growth factor-beta 1-mediated induction of MAPK/p21 by LMP1. Virology 302310-320. [DOI] [PubMed] [Google Scholar]
- 18.Ge, Z., C. Liu, M. Björkholm, A. Gruber, and D. Xu. 2006. Mitogen-activated protein kinase cascade-mediated histone H3 phosphorylation is critical for telomerase reverse transcriptase expression/telomerase activation induced by proliferation. Mol. Cell. Biol. 26230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gires, O., F. Kohlhuber, E. Kilger, M. Baumann, A. Kieser, C. Kaiser, R. Zeidler, B. Scheffer, M. Ueffing, and W. Hammerschmidt. 1999. Latent membrane protein 1 of Epstein-Barr virus interacts with JAK3 and activates STAT proteins. EMBO J. 183064-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gloghini, A., G. Gaidano, L. M. Larocca, F. Pierconti, A. Cingolani, L. Dal Maso, D. Capello, S. Franceschi, U. Tirelli, M. Libra, H. Niu, R. Dalla-Favera, and A. Carbone. 2002. Expression of cyclin-dependent kinase inhibitor p27(Kip1) in AIDS-related diffuse large-cell lymphomas is associated with Epstein-Barr virus-encoded latent membrane protein 1. Am. J. Pathol. 161163-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goueli, B. S., and R. Janknecht. 2004. Upregulation of the catalytic telomerase subunit by the transcription factor ER81 and oncogenic HER2/Neu, Ras, or Raf. Mol. Cell. Biol. 2425-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahn, W. C., S. A. Stewart, M. W. Brooks, S. G. York, E. Eaton, A. Kurachi, R. L. Beijersbergen, J. H. Knoll, M. Meyerson, and R. A. Weinberg. 1999. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 51164-1170. [DOI] [PubMed] [Google Scholar]
- 23.Henriquez, N. V., E. Floettmann, M. Salmon, M. Rowe, and A. B. Rickinson. 1999. Differential responses to CD40 ligation among Burkitt lymphoma lines that are uniformly responsive to Epstein-Barr virus latent membrane protein 1. J. Immunol. 1623298-3307. [PubMed] [Google Scholar]
- 24.Herrero, J. A., P. Mathew, and C. V. Paya. 1995. LMP-1 activates NF-kappa B by targeting the inhibitory molecule I kappa B alpha. J. Virol. 692168-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu, B. T., and R. A. Insel. 1999. Up-regulation of telomerase in human B lymphocytes occurs independently of cellular proliferation and with expression of the telomerase catalytic subunit. Eur. J. Immunol. 293745-3753. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi, H., and N. Sakaguchi. 1997. Telomerase activity is induced in human peripheral B lymphocytes by the stimulation to antigen receptor. Blood 891299-1307. [PubMed] [Google Scholar]
- 27.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc. Natl. Acad. Sci. USA 9412592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janknecht, R. 2004. On the road to immortality: hTERT upregulation in cancer cells. FEBS Lett. 5649-13. [DOI] [PubMed] [Google Scholar]
- 29.Kang, S. S., T. Kwon, D. Y. Kwon, and S. I. Do. 1999. Akt protein kinase enhances human telomerase activity through phosphorylation of telomerase reverse transcriptase subunit. J. Biol. Chem. 27413085-13090. [DOI] [PubMed] [Google Scholar]
- 30.Kawauchi, K., K. Ihjima, and O. Yamada. 2005. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3′-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J. Immunol. 1745261-5269. [DOI] [PubMed] [Google Scholar]
- 31.Kieser, A., C. Kaiser, and W. Hammerschmidt. 1999. LMP1 signal transduction differs substantially from TNF receptor 1 signaling in the molecular functions of TRADD and TRAF2. EMBO J. 182511-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura, A., M. Ohmichi, J. Kawagoe, S. Kyo, S. Mabuchi, T. Takahashi, C. Ohshima, E. Arimoto-Ishida, Y. Nishio, M. Inoue, H. Kurachi, K. Tasaka, and Y. Murata. 2004. Induction of hTERT expression and phosphorylation by estrogen via Akt cascade in human ovarian cancer cell lines. Oncogene 234505-4515. [DOI] [PubMed] [Google Scholar]
- 33.Klingelhutz, A. J., S. A. Foster, and J. K. McDougall. 1996. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 38079-82. [DOI] [PubMed] [Google Scholar]
- 34.Liu, H., F. Luan, Y. Ju, H. Shen, L. Gao, X. Wang, S. Liu, L. Zhang, W. Sun, and C. Ma. 2007. In vitro transfection of the hepatitis B virus PreS2 gene into the human hepatocarcinoma cell line HepG2 induces upregulation of human telomerase reverse transcriptase. Biochem. Biophys. Res. Commun. 355379-384. [DOI] [PubMed] [Google Scholar]
- 35.Liu, L., S. Lai, L. G. Andrews, and T. O. Tollefsbol. 2004. Genetic and epigenetic modulation of telomerase activity in development and disease. Gene 3401-10. [DOI] [PubMed] [Google Scholar]
- 36.Liu, L. T., J. P. Peng, H. C. Chang, and W. C. Hung. 2003. RECK is a target of Epstein-Barr virus latent membrane protein 1. Oncogene 228263-8270. [DOI] [PubMed] [Google Scholar]
- 37.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappaB. Mol. Cell. Biol. 201626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maida, Y., S. Kyo, T. Kanaya, Z. Wang, N. Yatabe, M. Tanaka, M. Nakamura, M. Ohmichi, N. Gotoh, S. Murakami, and M. Inoue. 2002. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene 214071-4079. [DOI] [PubMed] [Google Scholar]
- 39.Massard, C., Y. Zermati, A. L. Pauleau, N. Larochette, D. Métivier, L. Sabatier, G. Kroemer, and J. C. Soria. 2006. hTERT: a novel endogenous inhibitor of the mitochondrial cell death pathway. Oncogene 254505-4514. [DOI] [PubMed] [Google Scholar]
- 40.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80389-399. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura, T. M., G. B. Morin, K. B. Chapman, S. L. Weinrich, W. H. Andrews, J. Lingner, C. B. Harley, and T. R. Cech. 1997. Telomerase catalytic subunit homologs from fission yeast and human. Science 277955-959. [DOI] [PubMed] [Google Scholar]
- 42.Ometto, L., C. Menin, S. Masiero, L. Bonaldi, A. Del Mistro, A. M. Cattelan, E. D'Andrea, A. De Rossi, and L. Chieco-Bianchi. 1997. Molecular profile of Epstein-Barr virus in human immunodeficiency virus type 1-related lymphadenopathies and lymphomas. Blood 90313-322. [PubMed] [Google Scholar]
- 43.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 40182-85. [DOI] [PubMed] [Google Scholar]
- 44.Pattle, S. B., and P. J. Farrell. 2006. The role of Epstein-Barr virus in cancer. Expert Opin. Biol. Ther. 61193-1205. [DOI] [PubMed] [Google Scholar]
- 45.Rahman, R., L. Latonen, and K. G. Wiman. 2005. hTERT antagonizes p53-induced apoptosis independently of telomerase activity. Oncogene 241320-1327. [DOI] [PubMed] [Google Scholar]
- 46.Righetti, E., G. Ballon, L. Ometto, A. M. Cattelan, C. Menin, M. Zanchetta, L. Chieco-Bianchi, and A. De Rossi. 2002. Dynamics of Epstein-Barr virus in HIV-1-infected subjects on highly active antiretroviral therapy. AIDS 1663-73. [DOI] [PubMed] [Google Scholar]
- 47.Roberts, M. L., and N. R. Cooper. 1998. Activation of a ras-MAPK-dependent pathway by Epstein-Barr virus latent membrane protein 1 is essential for cellular transformation. Virology 24093-99. [DOI] [PubMed] [Google Scholar]
- 48.Romashkova, J. A., and S. S. Makarov. 1999. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 40186-90. [DOI] [PubMed] [Google Scholar]
- 49.Rufer, N., M. Migliaccio, J. Antonchuk, R. K. Humphries, E. Roosnek, and P. M. Lansdorp. 2001. Transfer of the human telomerase reverse transcriptase (TERT) gene into T lymphocytes results in extension of replicative potential. Blood 98597-603. [DOI] [PubMed] [Google Scholar]
- 50.Sheng, W. Y., Y. R. Chen, and T. C. Wang. 2006. A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett. 5806819-6824. [DOI] [PubMed] [Google Scholar]
- 51.Sinha-Datta, U., I. Horikawa, E. Michishita, A. Datta, J. C. Sigler-Nicot, M. Brown, M. Kazanji, J. C. Barrett, and C. Nicot. 2004. Transcriptional activation of hTERT through the NF-kappaB pathway in HTLV-I-transformed cells. Blood 1042523-2531. [DOI] [PubMed] [Google Scholar]
- 52.Sizemore, N., S. Leung, and G. R. Stark. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell. Biol. 194798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stewart, S. A., W. C. Hahn, B. F. O'Connor, E. N. Banner, A. S. Lundberg, P. Modha, H. Mizuno, M. W. Brooks, M. Fleming, D. B. Zimonjic, N. C. Popescu, and R. A. Weinberg. 2002. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc. Natl. Acad. Sci. USA 9912606-12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugimoto, M., H. Tahara, T. Ide, and Y. Furuichi. 2004. Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res. 643361-3364. [DOI] [PubMed] [Google Scholar]
- 55.Terrin, L., R. Dolcetti, I. Corradini, S. Indraccolo, J. Dal Col, R. Bertorelle, L. Bonaldi, G. Esposito, and A. De Rossi. 2007. hTERT inhibits the Epstein-Barr virus lytic cycle and promotes the proliferation of primary B lymphocytes: implications for EBV-driven lymphomagenesis. Int. J. Cancer 121576-587. [DOI] [PubMed] [Google Scholar]
- 56.Trentin, L., G. Ballon, L. Ometto, A. Perin, U. Basso, L. Chieco-Bianchi, G. Semenzato, and A. De Rossi. 1999. Telomerase activity in chronic lymphoproliferative disorders of B-cell lineage. Br. J. Haematol. 106662-668. [DOI] [PubMed] [Google Scholar]
- 57.Uchida, J., T. Yasui, Y. Takaoka-Shichijo, M. Muraoka, W. Kulwichit, N. Raab-Traub, and H. Kikutani. 1999. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 286300-303. [DOI] [PubMed] [Google Scholar]
- 58.Ulaner, G. A., J.-F. Hu, T. H. Vu, L. C. Giudice, and R. Hoffman. 2001. Tissue-specific alternate splicing of human telomerase reverse transcriptase (hTERT) influences telomere lengths during human development. Int. J. Cancer 91644-649. [PubMed] [Google Scholar]
- 59.Vanden Berghe, W., S. Plaisance, E. Boone, K. De Bosscher, M. L. Schmitz, W. Fiers, and G. Haegeman. 1998. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J. Biol. Chem. 2733285-3290. [DOI] [PubMed] [Google Scholar]
- 60.Veldman, T., I. Horikawa, J. C. Barrett, and R. Schlegel. 2001. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J. Virol. 754467-4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verma, S. C., S. Borah, and E. S. Robertson. 2004. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 7810348-10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wan, J., L. Sun, J. W. Mendoza, Y. L. Chui, D. P. Huang, Z. J. Chen, N. Suzuki, S. Suzuki, W. C. Yeh, S. Akira, K. Matsumoto, Z. G. Liu, and Z. Wu. 2004. Elucidation of the c-Jun N-terminal kinase pathway mediated by Epstein-Barr virus-encoded latent membrane protein 1. Mol. Cell. Biol. 24192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weng, N. P., L. Granger, and R. J. Hodes. 1997. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci. USA 9410827-10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wennborg, A., P. Aman, D. Saranath, W. Pear, J. Sumegi, and G. Klein. 1987. Conversion of the lymphoma line “BJAB” by Epstein-Barr virus into phenotypically altered sublines is accompanied by increased c-myc mRNA levels. Int. J. Cancer 40202-206. [DOI] [PubMed] [Google Scholar]
- 65.Wu, K. J., C. Grandori, M. Amacker, N. Simon-Vermot, A. Polack, J. Lingner, and R. Dalla-Favera. 1999. Direct activation of TERT transcription by c-Myc. Nat. Genet. 21220-224. [DOI] [PubMed] [Google Scholar]
- 66.Yang, J., X. Deng, L. Deng, H. Gu, W. Fan, and Y. Cao. 2004. Telomerase activation by Epstein-Barr virus latent membrane protein 1 is associated with c-Myc expression in human nasopharyngeal epithelial cells. J. Exp. Clin. Cancer Res. 23495-506. [PubMed] [Google Scholar]
- 67.Ying, L., A. Hubbard, and C. Giardina. 2000. NF-κB regulates transcription of the mouse telomerase catalytic subunit. J. Biol. Chem. 27536671-36675. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, C., V. L. Bae-Jump, Y. E. Whang, P. A. Gehrig, and J. F. Boggess. 2006. The PTEN tumor suppressor inhibits telomerase activity in endometrial cancer cells by decreasing hTERT mRNA levels. Gynecol. Oncol. 101305-310. [DOI] [PubMed] [Google Scholar]
- 69.Zhou, C., P. A. Gehrig, Y. E. Whang, and J. F. Boggess. 2003. Rapamycin inhibits telomerase activity by decreasing the hTERT mRNA level in endometrial cancer cells. Mol. Cancer Ther. 2789-795. [PubMed] [Google Scholar]