Abstract
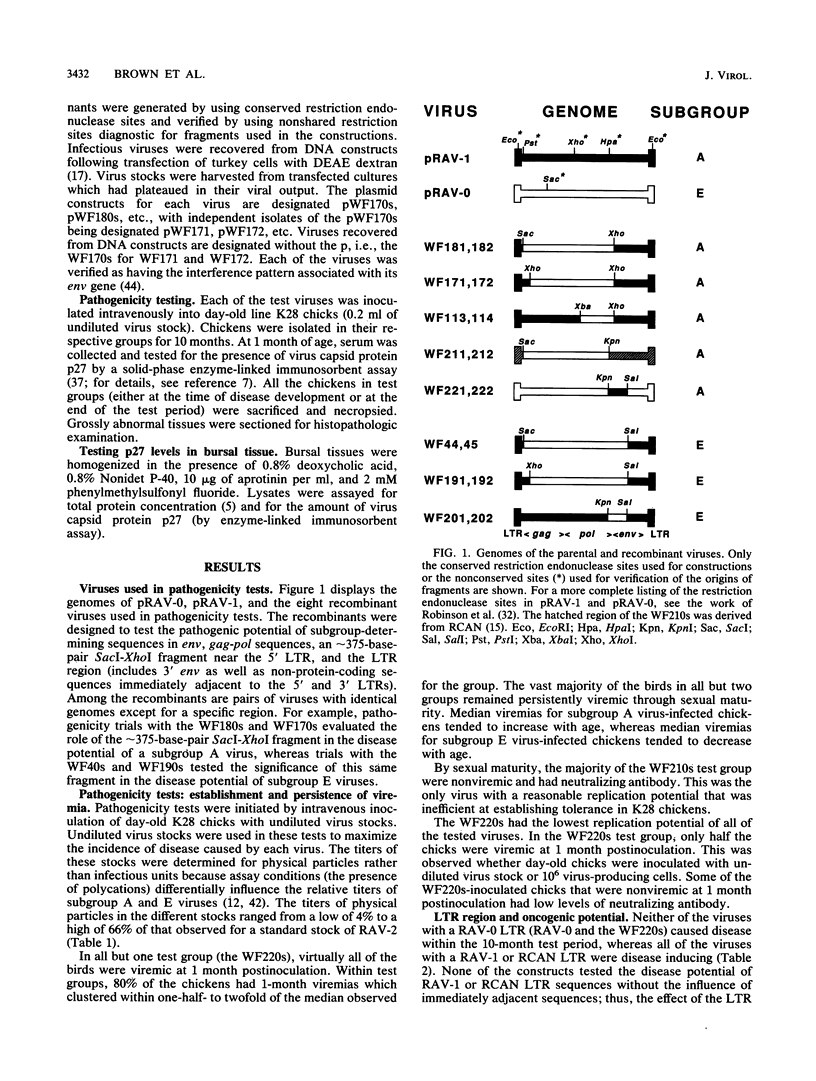
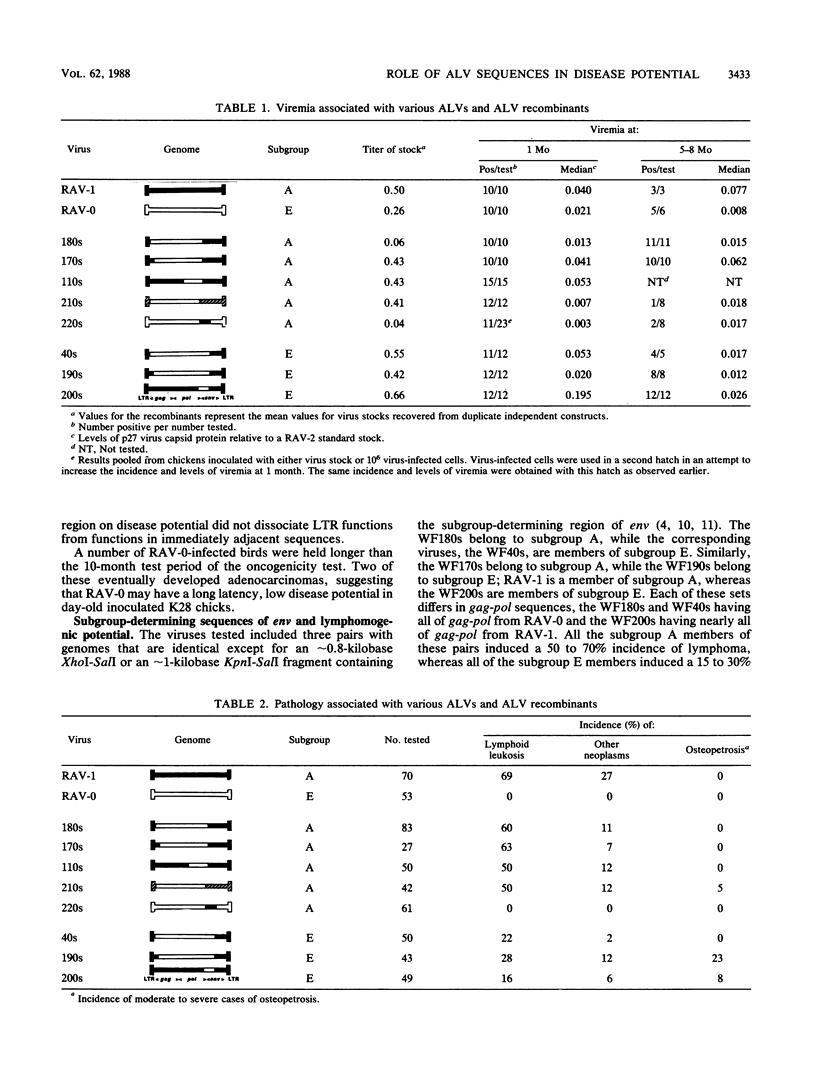
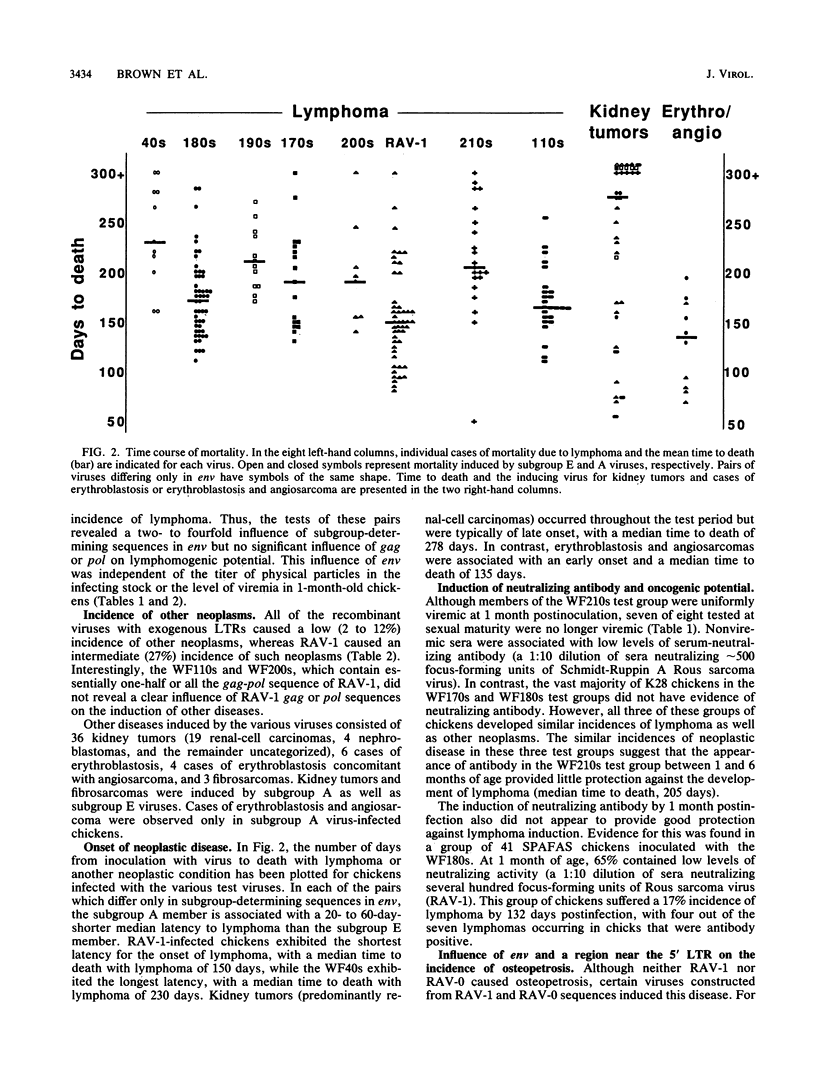
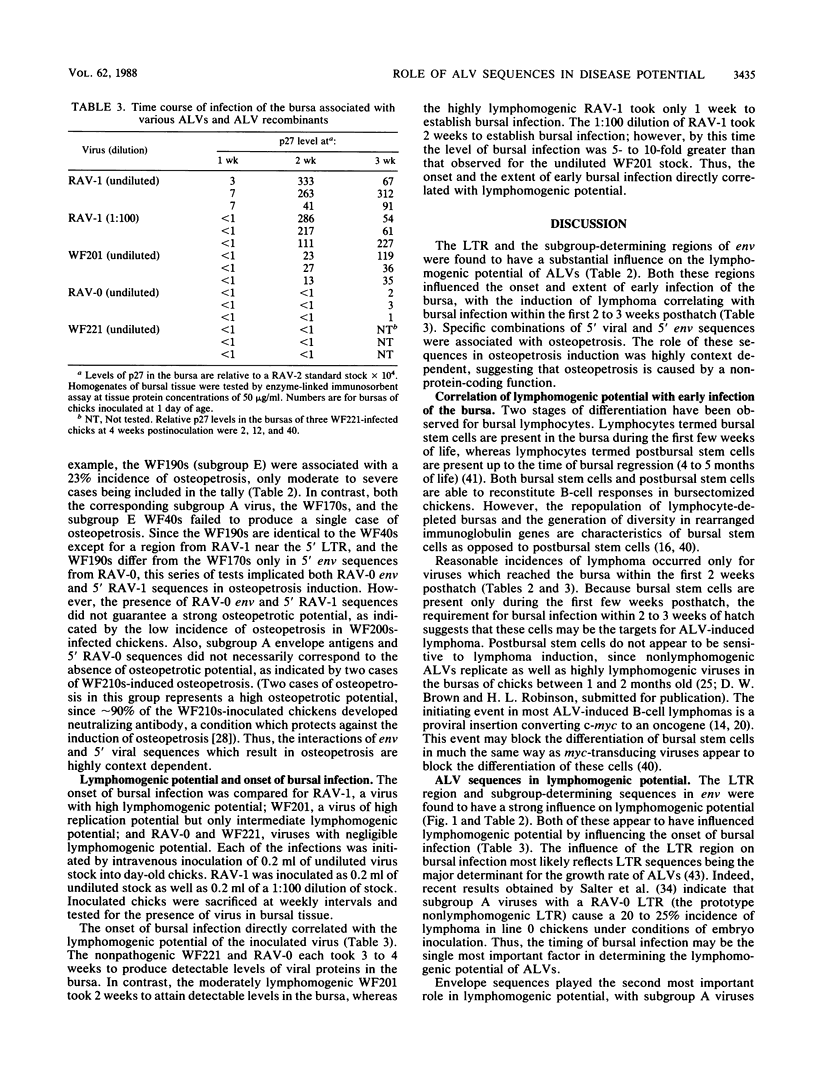
A series of recombinants between Rous-associated virus type 0 (RAV-0), RAV-1, and a replication-competent avian leukosis virus vector (RCAN) have been tested for disease potential in day-old inoculated K28 chicks. RAV-0 is a benign virus, whereas RAV-1 and RCAN induce lymphoma and a low incidence of a variety of other neoplasms. The results of the oncogenicity tests indicate that (i) the long terminal repeat regions of RAV-1 and RCAN play a major role in disease potential, (ii) subgroup A envelope glycoproteins are associated with a two- to fourfold higher incidence of lymphoma than subgroup E glycoproteins, and (iii) certain combinations of 5' viral and env sequences cause osteopetrosis in a highly context-dependent manner. Long terminal repeat and env sequences appeared to influence lymphomogenic potential by determining the extent of bursal infection within the first 2 to 3 weeks of life. This would suggest that bursal but not postbursal stem cells are targets for avian leukosis virus-induced lymphomogenesis. The induction of neutralizing antibody had no obvious influence on the incidence of lymphoma.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Crittenden L. B., Buss E. G. Ev 2, a genetic locus containing structural genes for endogenous virus, codes for Rous-associated virus type 0 produced by line 72 chickens. J Virol. 1980 Jan;33(1):250–255. doi: 10.1128/jvi.33.1.250-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L. Gs, an allele of chickens for endogenous avian leukosis viral antigens, segregates with ev 3, a genetic locus that contains structural genes for virus. J Virol. 1979 Aug;31(2):420–425. doi: 10.1128/jvi.31.2.420-425.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon L. D., Witter R. L., Crittenden L. B., Fadly A., Motta J. B-haplotype influence on Marek's disease, Rous sarcoma, and lymphoid leukosis virus-induced tumors in chickens. Poult Sci. 1981 Jun;60(6):1132–1139. doi: 10.3382/ps.0601132. [DOI] [PubMed] [Google Scholar]
- Bova C. A., Manfredi J. P., Swanstrom R. env genes of avian retroviruses: nucleotide sequence and molecular recombinants define host range determinants. Virology. 1986 Jul 30;152(2):343–354. doi: 10.1016/0042-6822(86)90137-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Briles W. E., Bumstead N., Ewert D. L., Gilmour D. G., Gogusev J., Hála K., Koch C., Longenecker B. M., Nordskog A. W., Pink J. R. Nomenclature for chicken major histocompatibility (B) complex. Immunogenetics. 1982;15(5):441–447. doi: 10.1007/BF00345903. [DOI] [PubMed] [Google Scholar]
- Brown D. W., Robinson H. L. Role of RAV-0 genes in the permissive replication of subgroup E avian leukosis viruses on line 15Bev1 CEF. Virology. 1988 Jan;162(1):239–242. doi: 10.1016/0042-6822(88)90414-x. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971 Feb 26;171(3973):813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Stoye J. P., Coffin J. M. Molecular basis of host range variation in avian retroviruses. J Virol. 1985 Jan;53(1):32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc-Nguyen H. Enhancing effect of diethylaminoethyl-dextran on the focus-forming titer of a murine sarcoma virus (Harvey strain). J Virol. 1968 Jun;2(6):643–644. doi: 10.1128/jvi.2.6.643-644.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Granfors K., Toivanen P. Defect in the generation of light-chain diversity in bursectomized chickens. Nature. 1984 Sep 6;311(5981):69–71. doi: 10.1038/311069a0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta J. V., Crittenden L. B., Purchase H. G., Stone H. A., Witter R. L. Low oncogenic potential of avian endogenous RNA tumor virus infection or expression. J Natl Cancer Inst. 1975 Sep;55(3):685–689. doi: 10.1093/jnci/55.3.685. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Gilmour D. G., Romero C. H., Okazaki W. Post-infection genetic resistance to avian lymphoid leukosis resides in B target cell. Nature. 1977 Nov 3;270(5632):61–62. doi: 10.1038/270061a0. [DOI] [PubMed] [Google Scholar]
- Purchase H. G., Okazaki W., Vogt P. K., Hanafusa H., Burmester B. R., Crittenden L. B. Oncogenicity of avian leukosis viruses of different subgroups and of mutants of sarcoma viruses. Infect Immun. 1977 Feb;15(2):423–428. doi: 10.1128/iai.15.2.423-428.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN H., VOGT P. K. An avian leukosis virus associated with stocks of Rous sarcoma virus. Virology. 1962 May;17:184–194. doi: 10.1016/0042-6822(62)90096-x. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Blais B. M., Tsichlis P. N., Coffin J. M. At least two regions of the viral genome determine the oncogenic potential of avian leukosis viruses. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1225–1229. doi: 10.1073/pnas.79.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978;83:1–36. doi: 10.1007/978-3-642-67087-9_1. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Jensen L., Coffin J. M. Sequences outside of the long terminal repeat determine the lymphomogenic potential of Rous-associated virus type 1. J Virol. 1985 Sep;55(3):752–759. doi: 10.1128/jvi.55.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Lamoreux W. F. Expression of endogenous ALV antigens and susceptibility to subgroup E ALV in three strains of chickens (endogenous avian C-type virus). Virology. 1976 Jan;69(1):50–62. doi: 10.1016/0042-6822(76)90193-8. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Miles B. D. Avian leukosis virus-induced osteopetrosis is associated with the persistent synthesis of viral DNA. Virology. 1985 Feb;141(1):130–143. doi: 10.1016/0042-6822(85)90189-8. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Miles B. D., Catalano D. E., Briles W. E., Crittenden L. B. Susceptibility to erbB-induced erythroblastosis is a dominant trait of 151 chickens. J Virol. 1985 Sep;55(3):617–622. doi: 10.1128/jvi.55.3.617-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Pearson M. N., DeSimone D. W., Tsichlis P. N., Coffin J. M. Subgroup-E avian-leukosis-virus-associated disease in chickens. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1133–1141. doi: 10.1101/sqb.1980.044.01.122. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Reinsch S. S., Shank P. R. Sequences near the 5' long terminal repeat of avian leukosis viruses determine the ability to induce osteopetrosis. J Virol. 1986 Jul;59(1):45–49. doi: 10.1128/jvi.59.1.45-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Smith E. J., Hughes S. H., Wright S. E., Fadly A. M., Witter R. L., Crittenden L. B. Gene insertion into the chicken germ line by retroviruses. Poult Sci. 1986 Aug;65(8):1445–1458. doi: 10.3382/ps.0651445. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Schatz P. J., Jensen L. M., Tsichlis P. N., Coffin J. M., Robinson H. L. Sequences in the gag-pol-5'env region of avian leukosis viruses confer the ability to induce osteopetrosis. Virology. 1985 Aug;145(1):94–104. doi: 10.1016/0042-6822(85)90204-1. [DOI] [PubMed] [Google Scholar]
- Smith E. J., Fadly A., Okazaki W. An enzyme-linked immunosorbent assay for detecting avian leukosis-sarcoma viruses. Avian Dis. 1979 Jul-Sep;23(3):698–707. [PubMed] [Google Scholar]
- Smith R. E. Avian osteopetrosis. Curr Top Microbiol Immunol. 1982;101:75–94. doi: 10.1007/978-3-642-68654-2_4. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Moscovici C. The oncogenic effects of nontransforming viruses from avian myeloblastosis virus. Cancer Res. 1969 Jul;29(7):1356–1366. [PubMed] [Google Scholar]
- Thompson C. B., Humphries E. H., Carlson L. M., Chen C. L., Neiman P. E. The effect of alterations in myc gene expression on B cell development in the bursa of Fabricius. Cell. 1987 Nov 6;51(3):371–381. doi: 10.1016/0092-8674(87)90633-7. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A. Bursal and postbursal stem cells in chicken. Functional characteristics. Eur J Immunol. 1973 Sep;3(9):585–595. doi: 10.1002/eji.1830030912. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]


