Abstract
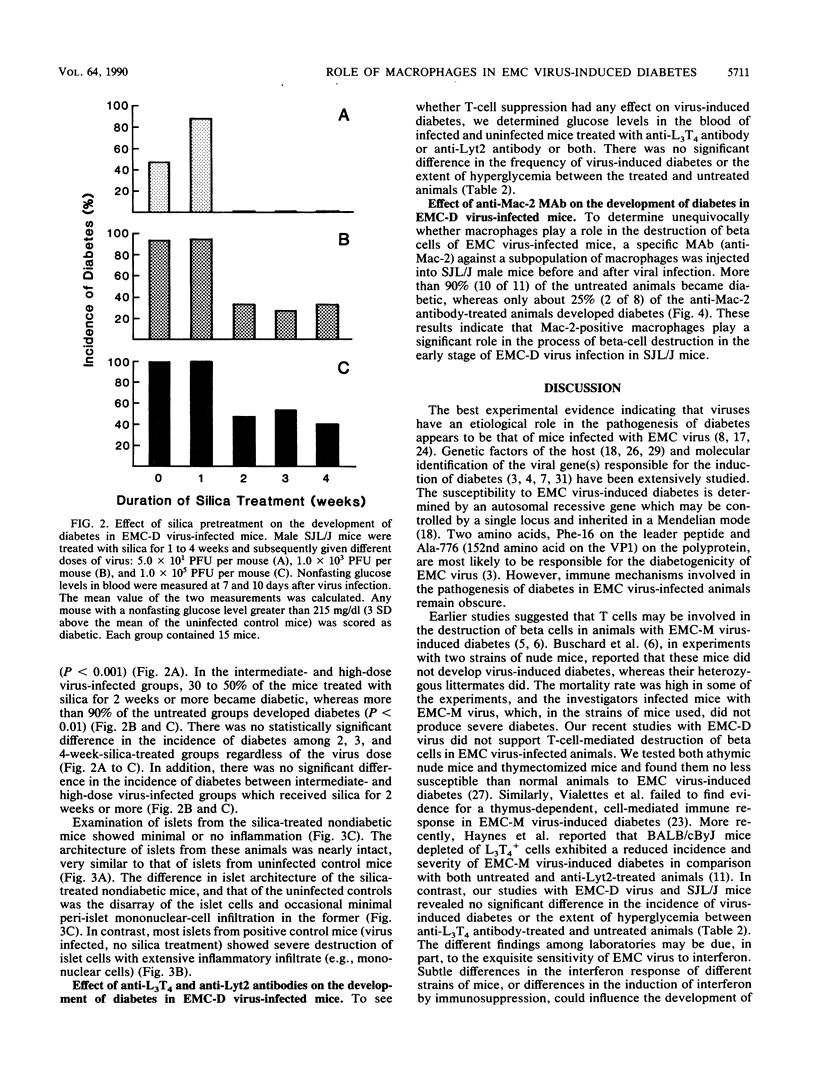
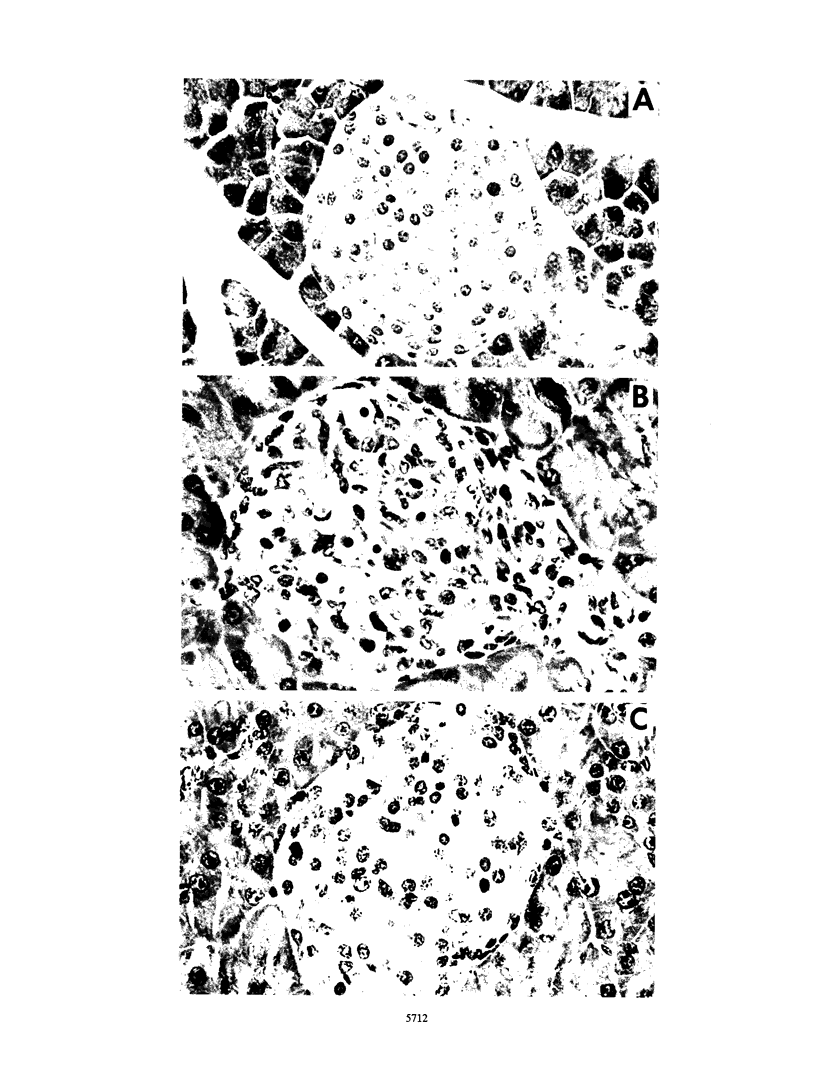
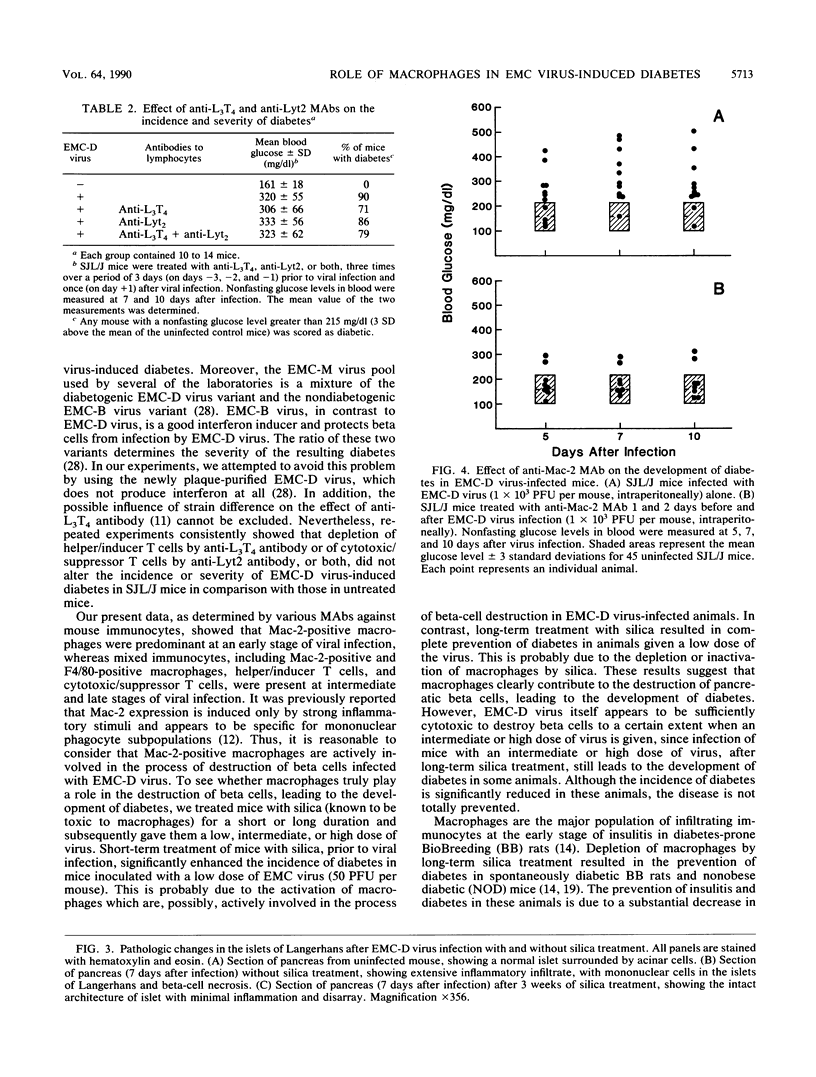
Pancreatic islets from SJL/J mice infected with the D variant of encephalomyocarditis virus (EMC-D virus) showed lymphocytic infiltration with moderate to severe destruction of beta cells. Immunohistochemical staining of the islet sections with several monoclonal antibodies, anti-Mac-1, anti-Mac-2, and F4/80 for macrophages, anti-L3T4 for helper/inducer T cells, and anti-Lyt2 for cytotoxic/suppressor T cells revealed that the major population of infiltrating cells at the early stage of viral infection was Mac-2-positive macrophages. In contrast, macrophages detected by anti-Mac-1 and F4/80 monoclonal antibodies were not found at the early stage of viral infection but were found at intermediate and late stages of viral infection. Helper/inducer T cells and cytotoxic/suppressor T cells also infiltrated the islets at intermediate and late stages of viral infection. Short-term treatment of mice with silica prior to viral infection resulted in an enhancement of beta-cell destruction, leading to the development of diabetes. In contrast, long-term treatment of mice with silica resulted in complete prevention of diabetes caused by a low dose of viral infection and a significant decrease in the incidence of diabetes caused by an intermediate or high dose of viral infection. Furthermore, depletion of macrophages by a specific monoclonal antibody (anti-Mac-2) resulted in a much greater decrease in the incidence of diabetes caused by an intermediate dose of viral infection. However, suppression of helper/inducer T cells and cytotoxic/suppressor T cells, by anti-L3T4 and anti-Lyt2 antibodies, respectively, did not alter the incidence of diabetes. On the basis of these data, it is concluded that macrophages, particularly Mac-2-positive macrophages, play a crucial role in the process of pancreatic beta-cell destruction at the early stage of encephalomyocarditis D virus infection in SJL/J mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Yoon J. W. Studies on autoimmunity for initiation of beta-cell destruction. V. Decrease of macrophage-dependent T lymphocytes and natural killer cytotoxicity in silica-treated BB rats. Diabetes. 1990 May;39(5):590–596. doi: 10.2337/diab.39.5.590. [DOI] [PubMed] [Google Scholar]
- Appels B., Burkart V., Kantwerk-Funke G., Funda J., Kolb-Bachofen V., Kolb H. Spontaneous cytotoxicity of macrophages against pancreatic islet cells. J Immunol. 1989 Jun 1;142(11):3803–3808. [PubMed] [Google Scholar]
- Bae Y. S., Eun H. M., Pon R. T., Giron D., Yoon J. W. Two amino acids, Phe 16 and Ala 776, on the polyprotein are most likely to be responsible for the diabetogenicity of encephalomyocarditis virus. J Gen Virol. 1990 Mar;71(Pt 3):639–645. doi: 10.1099/0022-1317-71-3-639. [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Eun H. M., Yoon J. W. Genomic differences between the diabetogenic and nondiabetogenic variants of encephalomyocarditis virus. Virology. 1989 May;170(1):282–287. doi: 10.1016/0042-6822(89)90379-6. [DOI] [PubMed] [Google Scholar]
- Buschard K., Hastrup N., Rygaard J. Virus-induced diabetes mellitus in mice and the thymus-dependent immune system. Diabetologia. 1983 Jan;24(1):42–46. doi: 10.1007/BF00275946. [DOI] [PubMed] [Google Scholar]
- Buschard K., Rygaard J., Lung E. The inability of a diabetogenic virus to induce diabetes mellitus in athymic (nude) mice. Acta Pathol Microbiol Scand C. 1976 Aug;84(4):299–303. doi: 10.1111/j.1699-0463.1976.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. H., Naviaux R. K., vanden Brink K. M., Jordan G. W. Comparison of the nucleotide sequences of diabetogenic and nondiabetogenic encephalomyocarditis virus. Virology. 1988 Oct;166(2):603–607. doi: 10.1016/0042-6822(88)90534-x. [DOI] [PubMed] [Google Scholar]
- Craighead J. E. The role of viruses in the pathogenesis of pancreatic disease and diabetes mellitus. Prog Med Virol. 1975;19:161–214. [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes M. K., Huber S. A., Craighead J. E. Helper-inducer T-lymphocytes mediate diabetes in EMC-infected BALB/c ByJ mice. Diabetes. 1987 Jul;36(7):877–881. doi: 10.2337/diab.36.7.877. [DOI] [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Koike T., Itoh Y., Ishii T., Ito I., Takabayashi K., Maruyama N., Tomioka H., Yoshida S. Preventive effect of monoclonal anti-L3T4 antibody on development of diabetes in NOD mice. Diabetes. 1987 Apr;36(4):539–541. doi: 10.2337/diab.36.4.539. [DOI] [PubMed] [Google Scholar]
- Lee K. U., Amano K., Yoon J. W. Evidence for initial involvement of macrophage in development of insulitis in NOD mice. Diabetes. 1988 Jul;37(7):989–991. doi: 10.2337/diab.37.7.989. [DOI] [PubMed] [Google Scholar]
- Like A. A., Biron C. A., Weringer E. J., Byman K., Sroczynski E., Guberski D. L. Prevention of diabetes in BioBreeding/Worcester rats with monoclonal antibodies that recognize T lymphocytes or natural killer cells. J Exp Med. 1986 Oct 1;164(4):1145–1159. doi: 10.1084/jem.164.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like A. A., Rossini A. A., Guberski D. L., Appel M. C., Williams R. M. Spontaneous diabetes mellitus: reversal and prevention in the BB/W rat with antiserum to rat lymphocytes. Science. 1979 Dec 21;206(4425):1421–1423. doi: 10.1126/science.388619. [DOI] [PubMed] [Google Scholar]
- Onodera T., Yoon J. W., Brown K. S., Notkina A. L. Evidence for a single locus controlling susceptibility to virus-induced diabetes mellitus. Nature. 1978 Aug 17;274(5672):693–696. doi: 10.1038/274693a0. [DOI] [PubMed] [Google Scholar]
- Oschilewski U., Kiesel U., Kolb H. Administration of silica prevents diabetes in BB-rats. Diabetes. 1985 Feb;34(2):197–199. doi: 10.2337/diab.34.2.197. [DOI] [PubMed] [Google Scholar]
- Pukel C., Baquerizo H., Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988 Jan;37(1):133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- Ross M. E., Onodera T., Brown K. S., Notkins A. L. Virus-induced diabetes mellitus. IV. Genetic and environmental factors influencing the development of diabetes after infection with the M variant of encephalomyocarditis virus. Diabetes. 1976 Mar;25(3):190–197. doi: 10.2337/diab.25.3.190. [DOI] [PubMed] [Google Scholar]
- Vialettes B., Baume D., Charpin C., De Maeyer-Guignard J., Vague P. Assessment of viral and immune factors in EMC virus-induced diabetes: effects of cyclosporin A and interferon. J Clin Lab Immunol. 1983 Jan;10(1):35–40. [PubMed] [Google Scholar]
- Yoon J. W., Austin M., Onodera T., Notkins A. L. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979 May 24;300(21):1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., Lesniak M. A., Fussganger R., Notkins A. L. Genetic differences in susceptibility of pancreatic beta cells to virus-induced diabetes mellitus. Nature. 1976 Nov 11;264(5582):178–180. doi: 10.1038/264178a0. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., McClintock P. R., Bachurski C. J., Longstreth J. D., Notkins A. L. Virus-induced diabetes mellitus. No evidence for immune mechanisms in the destruction of beta-cells by the D-variant of encephalomyocarditis virus. Diabetes. 1985 Sep;34(9):922–925. doi: 10.2337/diab.34.9.922. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., McClintock P. R., Onodera T., Notkins A. L. Virus-induced diabetes mellitus. XVIII. Inhibition by a nondiabetogenic variant of encephalomyocarditis virus. J Exp Med. 1980 Oct 1;152(4):878–892. doi: 10.1084/jem.152.4.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W., Notkins A. L. Virus-induced diabetes mellitus. VI. Genetically determined host differences in the replicating of encephalomyocarditis virus in pancreatic beta cells. J Exp Med. 1976 May 1;143(5):1170–1185. doi: 10.1084/jem.143.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J. W. Pathogenic mechanisms of virus-induced type 1 diabetes. Microb Pathog. 1988 Aug;5(2):77–86. doi: 10.1016/0882-4010(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Yoon J. W., Wong A. K., Bae Y. S., Eun H. M. An apparent deletion of an oligonucleotide detected by RNA fingerprint in the nondiabetogenic B variant of encephalomyocarditis virus is caused by a point mutation. J Virol. 1988 Feb;62(2):637–640. doi: 10.1128/jvi.62.2.637-640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Onodera T., Notkins A. L. Virus-induced diabetes mellitus: VIII. Passage of encephalomyocarditis virus and severity of diabetes in susceptible and resistant strains of mice. J Gen Virol. 1977 Nov;37(2):225–232. doi: 10.1099/0022-1317-37-2-225. [DOI] [PubMed] [Google Scholar]