Abstract
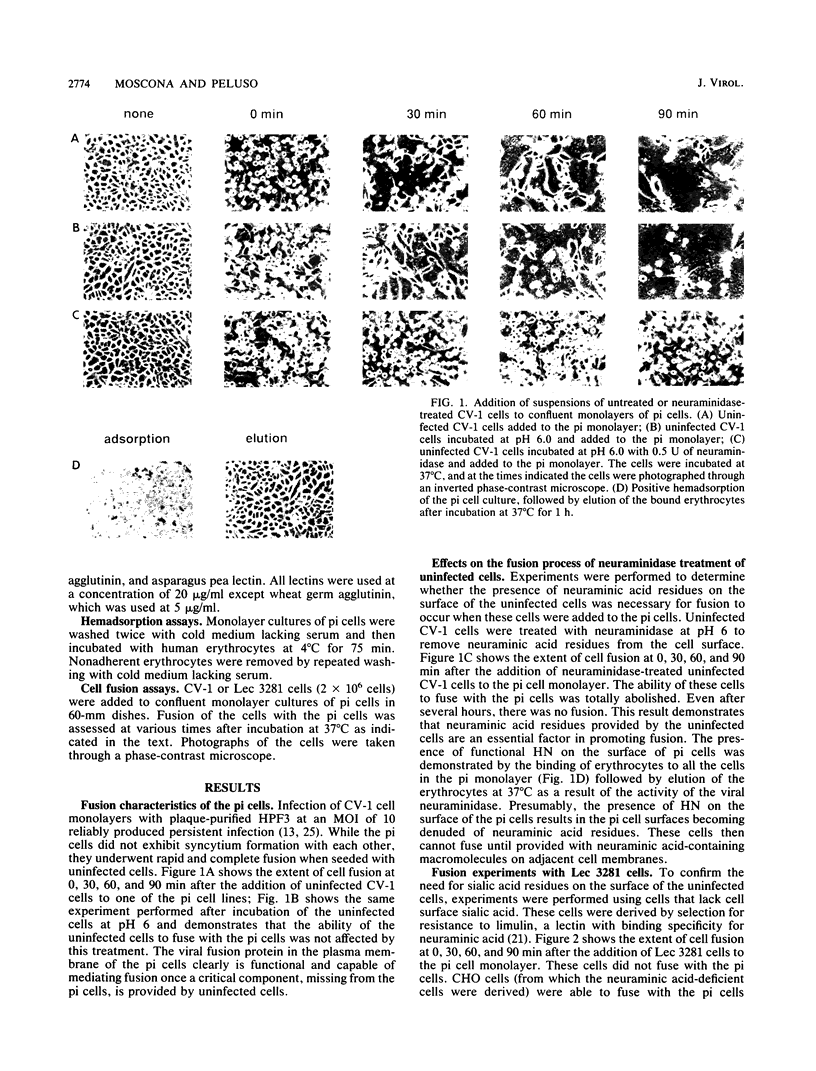
Cells persistently infected with human parainfluenza virus type 3 (HPF3) exhibit a novel phenotype. They are completely resistant to fusion with each other but readily fuse with uninfected cells. We demonstrate that the inability of these cells to fuse with each other is due to a lack of cell surface neuraminic acid. Neuraminic acid is the receptor for the HPF3 hemagglutinin-neuraminidase (HN) glycoprotein, the molecule responsible for binding of the virus to cell surfaces. Uninfected CV-1 cells were treated with neuraminidase and then tested for their ability to fuse with the persistently infected (pi) cells. Neuraminidase treatment totally abolished cell fusion. To extend this result, we used a cell line deficient in sialic acid and demonstrated that these cells, like the neuraminidase-treated CV-1 cells, were unable to fuse with pi cells. We then tested whether mimicking the agglutinating function of the HN molecule with lectins would result in cell fusion. We added a panel of five lectins to the neuraminic acid-deficient cells and showed that binding of these cells to the pi cells did not result in fusion; the lectins could not substitute for interaction of neuraminic acid with the HN molecule in promoting membrane fusion. These results provide compelling evidence that the HN molecule of HPF3 and its interaction with neuraminic acid participate in membrane fusion and that cell fusion is mediated by an interaction more complex than mere juxtaposition of the cell membranes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Yanai P., Loyter A. The use of circular dichroism to study conformational changes induced in Sendai virus envelope glycoproteins. A correlation with the viral fusogenic activity. J Biol Chem. 1986 Feb 15;261(5):2235–2239. [PubMed] [Google Scholar]
- Gibson S., Bundo-Morita K., Portner A., Lenard J. Fusion of a Sendai mutant deficient in HN protein (ts271) with cardiolipin liposomes. Virology. 1988 Mar;163(1):226–229. doi: 10.1016/0042-6822(88)90254-1. [DOI] [PubMed] [Google Scholar]
- Gitman A. G., Loyter A. Construction of fusogenic vesicles bearing specific antibodies. Targeting of reconstituted Sendai virus envelopes towards neuraminidase-treated human erythrocytes. J Biol Chem. 1984 Aug 10;259(15):9813–9820. [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Portner A., Schwartz A. L. An alternative route of infection for viruses: entry by means of the asialoglycoprotein receptor of a Sendai virus mutant lacking its attachment protein. Proc Natl Acad Sci U S A. 1985 Feb;82(4):978–982. doi: 10.1073/pnas.82.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Scheid A., Choppin P. W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J Exp Med. 1980 Feb 1;151(2):275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Biochemical features of mumps virus neuraminidases and their relationship with pathogenicity. Virology. 1981 Oct 15;114(1):218–227. doi: 10.1016/0042-6822(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Merz D. C., Wolinsky J. S. Conversion of nonfusing mumps virus infections to fusing infections by selective proteolysis of the HN glycoprotein. Virology. 1983 Dec;131(2):328–340. doi: 10.1016/0042-6822(83)90501-9. [DOI] [PubMed] [Google Scholar]
- Miura N., Uchida T., Okada Y. HVJ (Sendai virus)-induced envelope fusion and cell fusion are blocked by monoclonal anti-HN protein antibody that does not inhibit hemagglutination activity of HVJ. Exp Cell Res. 1982 Oct;141(2):409–420. doi: 10.1016/0014-4827(82)90229-4. [DOI] [PubMed] [Google Scholar]
- Morrison T. G. Structure, function, and intracellular processing of paramyxovirus membrane proteins. Virus Res. 1988 May;10(2-3):113–135. doi: 10.1016/0168-1702(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Moscona A., Galinski M. S. Characterization of human parainfluenza virus type 3 persistent infection in cell culture. J Virol. 1990 Jul;64(7):3212–3218. doi: 10.1128/jvi.64.7.3212-3218.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Biological activities of glycoproteins of HVJ (Sendai virus) studied by reconstitution of hybrid envelope and by concanavalin A-mediated binding: a new function of HANA protein and structural requirement of F protein in hemolysis. Virology. 1979 Nov;99(1):197–202. doi: 10.1016/0042-6822(79)90055-2. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Hiebert S. W., Lamb R. A. Expression at the cell surface of biologically active fusion and hemagglutinin/neuraminidase proteins of the paramyxovirus simian virus 5 from cloned cDNA. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portner A., Scroggs R. A., Metzger D. W. Distinct functions of antigenic sites of the HN glycoprotein of Sendai virus. Virology. 1987 May;158(1):61–68. doi: 10.1016/0042-6822(87)90238-8. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Shibuta H. Syncytium formation by recombinant vaccinia viruses carrying bovine parainfluenza 3 virus envelope protein genes. J Virol. 1989 Sep;63(9):3661–3668. doi: 10.1128/jvi.63.9.3661-3668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Kanda T., Hazama A., Adachi A., Matumoto M. Parainfluenza 3 virus: plaque-type variants lacking neuraminidase activity. Infect Immun. 1981 Oct;34(1):262–267. doi: 10.1128/iai.34.1.262-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuta H., Nozawa A., Shioda T., Kanda T. Neuraminidase activity and syncytial formation in variants of parainfluenza 3 virus. Infect Immun. 1983 Aug;41(2):780–788. doi: 10.1128/iai.41.2.780-788.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L. Human parainfluenza virus type 3: messenger RNAs, polypeptide coding assignments, intergenic sequences, and genetic map. J Virol. 1986 Sep;59(3):646–654. doi: 10.1128/jvi.59.3.646-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. Selection of lectin-resistant mutants of animal cells. Methods Enzymol. 1983;96:157–184. doi: 10.1016/s0076-6879(83)96015-9. [DOI] [PubMed] [Google Scholar]
- Tsurudome M., Yamada A., Hishiyama M., Ito Y. Monoclonal antibodies against the glycoproteins of mumps virus: fusion inhibition by anti-HN monoclonal antibody. J Gen Virol. 1986 Oct;67(Pt 10):2259–2265. doi: 10.1099/0022-1317-67-10-2259. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J. Identification of amino acids involved in the sialidase activity of the mumps virus hemagglutinin-neuraminadase protein. Virology. 1988 Nov;167(1):226–232. doi: 10.1016/0042-6822(88)90072-4. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Wolinsky J. S. A fusing mumps virus variant selected from a nonfusing parent with the neuraminidase inhibitor 2-deoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1986 Jun;151(2):286–295. doi: 10.1016/0042-6822(86)90050-4. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Lambert D. M., Galinski M. S., Mink M. A., Rochovansky O., Pons M. W. Immediate persistent infection by human parainfluenza virus 3: unique fusion properties of the persistently infected cells. J Gen Virol. 1987 Jun;68(Pt 6):1737–1748. doi: 10.1099/0022-1317-68-6-1737. [DOI] [PubMed] [Google Scholar]






