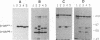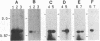Abstract
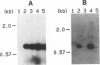
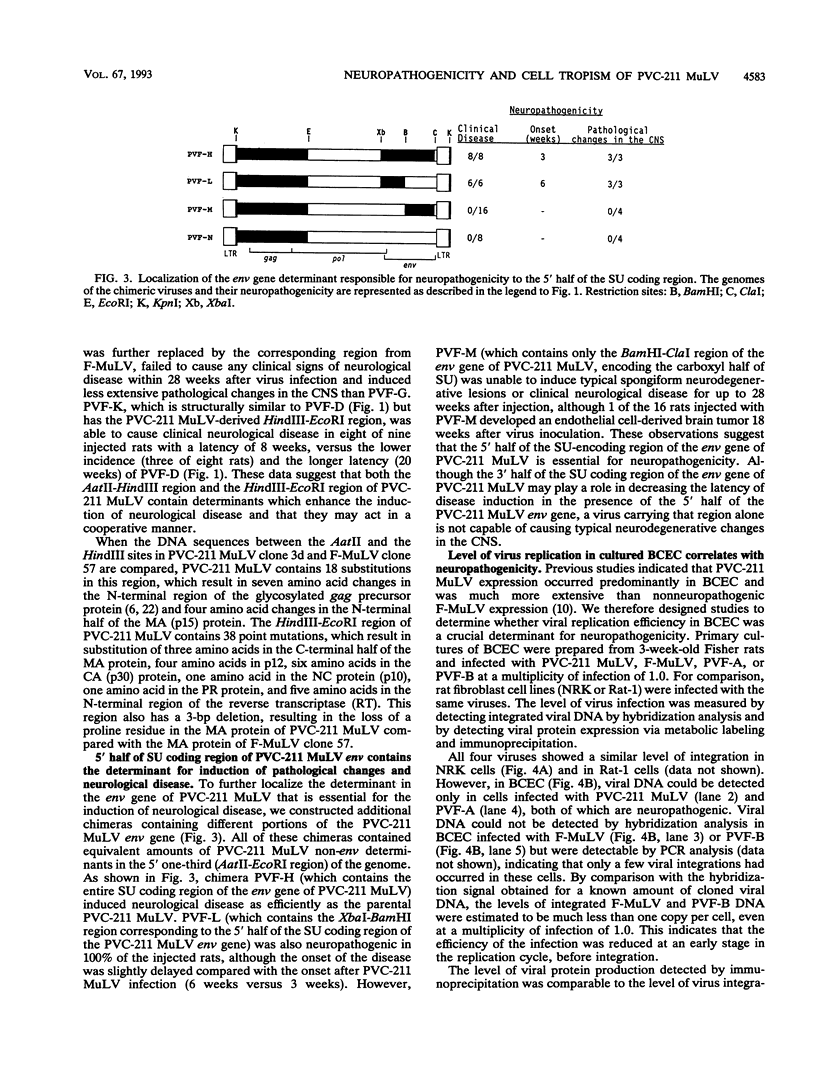
PVC-211 murine leukemia virus (MuLV) is a neuropathogenic, weakly leukemogenic variant of the nonneuropathogenic, highly leukemogenic Friend MuLV (F-MuLV). Chimeric viruses constructed from PVC-211 MuLV clone 3d and F-MuLV clone 57 indicate that the env gene of PVC-211 MuLV contains the determinant(s) responsible for pathological changes in the central nervous system. However, sequences within the 5' one-third (AatII-EcoRI region) of the PVC-211 MuLV genome, which include the 5' leader sequence, the gag gene, and the 5' quarter of the pol gene, are also needed in conjunction with the env gene determinant(s) to cause clinically evident neurological disease in the majority of virus-infected animals after a short latency. In the presence of the AatII-EcoRI region of the PVC-211 MuLV genome, the PVC-211 MuLV env gene sequences encoding the amino-terminal half of the SU protein, which contains the receptor-binding region of the protein, were sufficient to cause rapidly progressive neurological disease. When PVC-211 MuLV, F-MuLV, and various chimeric viruses were tested for their ability to replicate in cultured brain capillary endothelial cells (BCEC), the primary site of PVC-211 MuLV replication within the central nervous system, there was a direct correlation between the replication efficiency of a virus in BCEC in vitro and its ability to cause neurological disease in vivo. This observation indicates that the sequences in PVC-211 MuLV that render it neuropathogenic affect its replication in BCEC and suggests that rapid and efficient replication of the virus in BCEC is crucial for the pathological changes in the central nervous system that result in development of neurological disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Andersson P., Goldfarb M. P., Weinberg R. A. A defined subgenomic fragment of in vitro synthesized Moloney sarcoma virus DNA can induce cell transformation upon transfection. Cell. 1979 Jan;16(1):63–75. doi: 10.1016/0092-8674(79)90188-0. [DOI] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. Mutations in gag proteins P12 and P15 of Moloney murine leukemia virus block early stages of infection. J Virol. 1984 Mar;49(3):909–917. doi: 10.1128/jvi.49.3.909-917.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Dresler S., Kabat D. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J Virol. 1977 Dec;24(3):865–874. doi: 10.1128/jvi.24.3.865-874.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Chute H., Chao E., Feuerman M. Construction and characterization of Moloney murine leukemia virus mutants unable to synthesize glycosylated gag polyprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5965–5969. doi: 10.1073/pnas.80.19.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Heard J. M., Danos O. An amino-terminal fragment of the Friend murine leukemia virus envelope glycoprotein binds the ecotropic receptor. J Virol. 1991 Aug;65(8):4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Cimino E. F., Robbins D. S., Broadwell R. D., Powers J. M., Ruscetti S. K. Cellular tropism and localization in the rodent nervous system of a neuropathogenic variant of Friend murine leukemia virus. Lab Invest. 1992 Sep;67(3):314–321. [PubMed] [Google Scholar]
- Kai K., Furuta T. Isolation of paralysis-inducing murine leukemia viruses from Friend virus passaged in rats. J Virol. 1984 Jun;50(3):970–973. doi: 10.1128/jvi.50.3.970-973.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay D. G., Gravel C., Robitaille Y., Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1281–1285. doi: 10.1073/pnas.88.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Nowinski R. C., Eisenman R. N. Biosynthesis and metabolism of viral proteins expressed on the surface of murine leukemia virus-infected cells. Virology. 1978 Nov;91(1):116–129. doi: 10.1016/0042-6822(78)90360-4. [DOI] [PubMed] [Google Scholar]
- Masuda M., Remington M. P., Hoffman P. M., Ruscetti S. K. Molecular characterization of a neuropathogenic and nonerythroleukemogenic variant of Friend murine leukemia virus PVC-211. J Virol. 1992 May;66(5):2798–2806. doi: 10.1128/jvi.66.5.2798-2806.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perryman S. M., McAtee F. J., Portis J. L. Complete nucleotide sequence of the neurotropic murine retrovirus CAS-BR-E. Nucleic Acids Res. 1991 Apr 11;19(7):1707–1707. doi: 10.1093/nar/19.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perryman S., Nishio J., Chesebro B. Complete nucleotide sequence of Friend murine leukemia virus, strain FB29. Nucleic Acids Res. 1991 Dec 25;19(24):6950–6950. doi: 10.1093/nar/19.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts O. M., Powers J. M., Bilello J. A., Hoffman P. M. Ultrastructural changes associated with retroviral replication in central nervous system capillary endothelial cells. Lab Invest. 1987 Apr;56(4):401–409. [PubMed] [Google Scholar]
- Portis J. L., Perryman S., McAtee F. J. The R-U5-5' leader sequence of neurovirulent wild mouse retrovirus contains an element controlling the incubation period of neurodegenerative disease. J Virol. 1991 Apr;65(4):1877–1883. doi: 10.1128/jvi.65.4.1877-1883.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., De Billy G., Wang P., Darlix J. L. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J Mol Biol. 1989 Jan 20;205(2):363–372. doi: 10.1016/0022-2836(89)90347-1. [DOI] [PubMed] [Google Scholar]
- Remington M. P., Hoffman P. M., Ruscetti S. K., Masuda M. Complete nucleotide sequence of a neuropathogenic variant of Friend murine leukemia virus PVC-211. Nucleic Acids Res. 1992 Jun 25;20(12):3249–3249. doi: 10.1093/nar/20.12.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Ball J. K., Wong P. K. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990 Feb;64(2):467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary J. F., Knupp C. J., Wong P. K. Noninflammatory spongiform polioencephalomyelopathy caused by a neurotropic temperature-sensitive mutant of Moloney murine leukemia virus TB. Am J Pathol. 1986 Sep;124(3):457–468. [PMC free article] [PubMed] [Google Scholar]