Abstract
Loss of circulating CD4+ T cells in HIV-1 disease is balanced by CD8+ lymphocytosis to maintain normal CD3+ T cell counts [blind T cell homeostasis (TCH)]. However, for unknown reasons TCH generally fails 1.5−2.5 years before clinically defined AIDS. We investigated whether TCH failure was associated with changes in thymic production of T cells. Using specimens stored prospectively in the Multicenter AIDS Cohort Study (MACS), we measured expression of signal-joint T cell receptor excision circles (sjTRECs), a marker for thymic T cell production, and the fraction of proliferating naive and memory T cells during a 6−8 year period bracketing TCH failure. Segmented regression modeling assessed (1) rates of change in TREC levels before and after TCH failure, and (2) whether these were affected by cellular proliferation, which may dilute sjTREC levels. TCH failure was associated with a large decline in sjTREC (median 1109-fold, p = 0.028); the rate of this decline was only slightly affected when increased proliferation of naive T cells or other peripheral lymphocytes was taken into account. Preferential loss of naive CD4+ T cells was also noted before TCH failure, as has been seen in other studies. These results suggest that deficits in de novo T cell production, either through the decline of thymic function or the destruction of naive T cells, are likely to play an important role in TCH failure and progression of HIV-1 disease.
INTRODUCTION
Chronic HIV-1 disease is characterized by progressively declining numbers of CD4+ T cells, yet normal total (CD3+) T cell counts are maintained for many years.1–4 Approximately 1.5−2.5 years (on average) before the onset of AIDS, a marked and sustained decline in total T cell counts begins, which reflects a loss of homeostatic control of T cell levels.5–7 The time when this marked decline begins can be defined in the large majority of HIV-infected individuals who develop clinically defined AIDS6,7 and its onset predicts the development of AIDS independently of the CD4+ T cell count.8
Little is known about the mechanisms responsible for the loss of T cell homeostasis (TCH) as HIV disease progresses. In theory, either increased destruction or decreased production of T cells could explain it, and evidence for both exists.9–16 In particular, the presence of circulating T cells expressing signal joint (sj) T cell receptor excision circles (TRECs) suggests that thymic production of T cells contributes to maintenance of T cell counts throughout much of the course of HIV infection. Moreover, naive T cells, which are necessary for maintenance of the memory T cell pool, are depleted as HIV disease progresses.17,18
Based on these data, we hypothesized that the onset of CD3+ T cell loss preceding AIDS is associated with a depletion of naive T cells. To address this hypothesis, we performed longitudinal measurements of the number of sjTRECs, and both the number and proportion of naive and memory T cells, in HIV-infected men in whom the onset of CD3+ T cell decline was clearly defined. Because sjTREC levels can be reduced simply by dilution among dividing naive and memory T cells,19 we also measured the proliferation fractions of these cells throughout the study period. The results revealed a dramatic decline in sjTREC levels coincident with the onset of CD3+ T cell decline (TCH failure). Relatively modest increases in cellular proliferation occurred over the course of the study period; however, these increases were not sufficient to explain the large decline in sjTRECs levels.
MATERIALS AND METHODS
Study participants
The Multicenter AIDS Cohort Study (MACS) consists of 5622 homosexual and bisexual men recruited between 1984 and 1990 and followed semiannually20 with laboratory studies [including T cell counts by flow cytometry,21 as well as storage of pelleted and cryopreserved peripheral blood mononuclear cells (PBMC) from each study visit at −70°C and −135°C, respectively22]. The present study included MACS participants who had (1) TCH failure, defined as a stable CD3+ T cell count for >5 years followed by a decline of >10% per year before AIDS,7 (2) no use of HAART, and (3) availability of stored PBMC at more than six study visits, including at least three from before and three from after the time of TCH failure. The time of TCH failure for each individual was determined using published methods.7
Quantification of sjTRECs
sjTRECs were measured in duplicate in DNA isolated from three million PBMC as described,23 using PBMC pellets from 8 participants and cryopreserved PBMC from 11 others. Replicate differences were always <15% (median = 4.2%). To control for variability in sample preparation and lysis efficiency, sjTREC values were normalized to the number of albumin genes present in the sample, as determined by comparison with standards (plasmids containing a partial albumin gene, Megabases, Chicago, IL) ranging from 2.4 × 100 to 5 × 106 plasmids/liter. Albumin replicates were always within 10% (median = 1.4%). The assay had a lower limit of detectability of 200 TRECs/106 cells.
Measurement of proliferating lymphocytes
Cryopreserved PBMC were stained for surface markers and then the intracellular proliferation marker Ki-6724 as follows. Cells were incubated for 20 min with fluorochrome-conjugated monoclonal antibodies: anti-CD45RA-phycoerythrin (BD-Pharmingen, Torreyana, CA), and either anti-CD4-ECD (Beckman-Coulter, Miami, FL) or anti-CD8-ECD (Beckman-Coulter). 7-Amino actinomycin D (Pharmingen) was used to distinguish viable from nonviable cells.25 (Viability of cryopreserved cells was usually greater than 95% and was never less than 85%.) Finally, cells were permeabilized and fixed with Ortho PermeaFix Solution (Ortho Diagnostic Systems, Raritan, NJ) according to the manufacturer's instructions, and then incubated for 40 min with anti-Ki-67 (fluorescein isothiocyanate, FITC) or IgG1-FITC isotype control (Pharmingen) to identify proliferating cells (Ki-67+) cells.24 (In preliminary experiments, Ki-67 was equivalently expressed in fresh and cryopreserved cells.) Samples were washed, resuspended in 500 μL phosphate-buffered saline (PBS) containing sodium azide, and analyzed immediately (Beckman-Coulter XL flow cytometer).
At least 1000 naive (staining brightly for CD45RA) and memory (CD45RA–) CD4+ and CD8+ cells were analyzed, except in rare populations (e.g., naive CD4+ T cells in subjects with advanced HIV-1 infection), where >200 cells were analyzed. Proper classification of naive and memory cells was confirmed in 41 of 176 patient visits, where individuals with >5% CD4+ T cells (nearly all study participants) had essentially equivalent proportions of naive and memory cells based on measurement of the CD45RAbright phenotype or the CD45RA+ CD45RO– phenotype.
To validate Ki-67 as a marker of proliferating T cells, Ki-67 expression was compared with in vivo cellular proliferation fractions measured directly by deuterium labeling as described26 in 14 HIV+ and 4 HIV– individuals (recruited from the MACS and other ongoing cohort studies). Proliferation fractions obtained by the two methods were significantly correlated (r = 0.58, p = 0.001), and this was not significantly affected under the conservative assumption that all contaminating cells in the naive cell preparations were memory cells (r = 0.62, p = 0.001). Thus, for the longitudinal studies of patients with TCH failure on the 19 study participants, measurements of T cell proliferation fractions were performed using Ki-67.
Statistical analysis
sjTREC values were logarithmically transformed to improve the fit of linear regression models. Overall sjTREC levels before and after TCH failure were compared using Wilcoxon rank-sum tests. A more refined analysis of the temporal trends in sjTRECs was performed using segmented regression models with random effects that account for within-individual correlation. The segmented regression component included independent terms that represented the slopes of sjTREC before and after TCH failure. The statistical significance of these slopes (as compared to 0.0) and the comparison of pre- versus post-TCH failure slopes were determined using standard regression methods. To investigate the effect of cellular proliferation on sjTREC levels, the fractions of proliferating cells before and after the time of TCH failure were entered as mediating variables in the multivariate regression model, yielding adjusted estimates of the slopes.
RESULTS
Changes in sjTREC levels
In the 19 individuals studied, sjTRECs were always detectable while TCH was intact (median = 6236.5/106 PBMC, range = 364 to 1.44 × 105 per 106 PBMC), but rapidly declined after TCH failure (median post-TCH failure = 334/106 PBMC, p = 0.001). Within-individual declines ranged from approximately 3- to 80,000-fold, with a median decline of 1109-fold. The smaller decreases reflected lower TREC levels before TCH failure, relative to the limit of detection of the assay.
Figure 1 shows sjTREC levels normalized by the within-individual median values. The average slope of TREC levels before TCH failure was not significantly different from zero (p = 0.189), but a highly significant decline of 46% per year began at the time the total T cell count began to decline (p = 0.028; Table 1). This steep decline was not due to the expansion of non-T cell populations within PBMC, since the proportion of T cells within PBMCs changed only slightly with TCH failure (median CD3+ cell percentages before and after failure were 63% and 58%, respectively). Moreover, in a subset of individuals for whom the numbers of naive and memory T cells were also measured (n = 8), the rate of sjTREC decline was similar in both PBMC and naive T cells (45% and 47% per year, respectively), suggesting that the decrease in sjTRECs could not be attributed to a dilution caused by a relative expansion of memory T cells over naive cells.
FIG. 1.
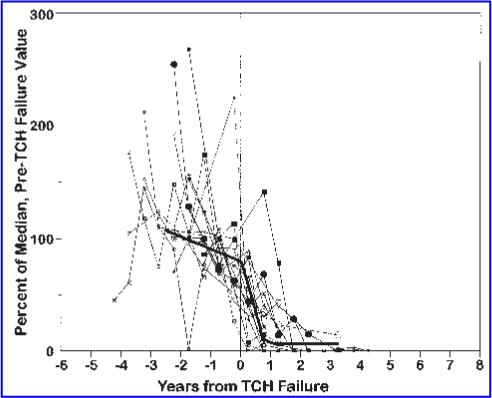
Changes in relative sjTREC levels in relation to TCH failure. To normalize for inter-individual variation, TREC levels for each participant were expressed relative to the mean of the participant's measurement before failure of TCH. Segmented regression analysis showed that TRECs did not change significantly before TCH failure, but declined substantially (at a rate of 46% per year) after TCH failure (thick black trend line).
Table 1.
Changes in Levels of sjTRECs and T Cell Subsets in Relation to T Cell Homeostasis (TCH) Failure
|
Median absolute cell count (cells/μl) |
Percent change relative to TCH failure |
Median annual decline relative to TCH failure (percent)a |
||||
|---|---|---|---|---|---|---|
| Phenotype | Before TCH failure | After TCH failure | p value | Before | After | |
| CD3+ | 1526 | 733 | −52 | 0.002 | 3 | 23 |
| TREC (/106 PBMC) | 6236 | 334 | −94.7 | 0.001 | 0 | 46 |
| CD4+ Total | 430 | 127.5 | −70.3 | 0.016 | 22 | 22 |
| Dividing | 32 | 15 | −53.1 | 0.001 | 13 | 13 |
| Resting | 388.7 | 110.5 | −71.6 | 0.025 | 23 | 23 |
| Total naive CD4+ | 106 | 22.8 | −78.5 | 0.014 | 48 | 48 |
| Dividing | 3 | 1 | −66.7 | 0.002 | 30 | 30 |
| Resting | 102.5 | 22.6 | −78 | 0.087 | 46 | 46 |
| Total memory CD4+ | 218.9 | 84.6 | −61.4 | 0.0001 | 31 | 31 |
| Dividing | 24 | 12 | −50 | 0.003 | 70 | 70 |
| Resting | 189.8 | 71.1 | −62.5 | 0.006 | 30 | 30 |
| CD8+ total | 899 | 554.3 | −38.3 | 0.016 | 0 | 13 |
| Dividing | 60.4 | 59.3 | −1.8 | 0.421 | 0 | 0 |
| Resting | 831 | 500.4 | −39.8 | 0.016 | 0 | 15 |
| Total naive CD8+ | 380.1 | 218.9 | −42.4 | 0.003 | 0 | 16 |
| Dividing | 7.8 | 7.2 | −7.7 | 0.637 | 0 | 0 |
| Resting | 376.2 | 169.6 | −54.9 | 0.071 | 0 | 19 |
| Total memory CD8+ | 434.2 | 291.4 | −32.9 | 0.004 | 13 | 13 |
| Dividing | 35.1 | 40.9 | +16.5 | 0.749 | 0 | 0 |
| Resting | 425.9 | 270.5 | −36.5 | 0.005 | 16 | 16 |
Rates of decline that differ before and after TCH failure were statistically significant (p < 0.05) by regression analysis; rates that were not significant are shown as the same before and after TCH failure.
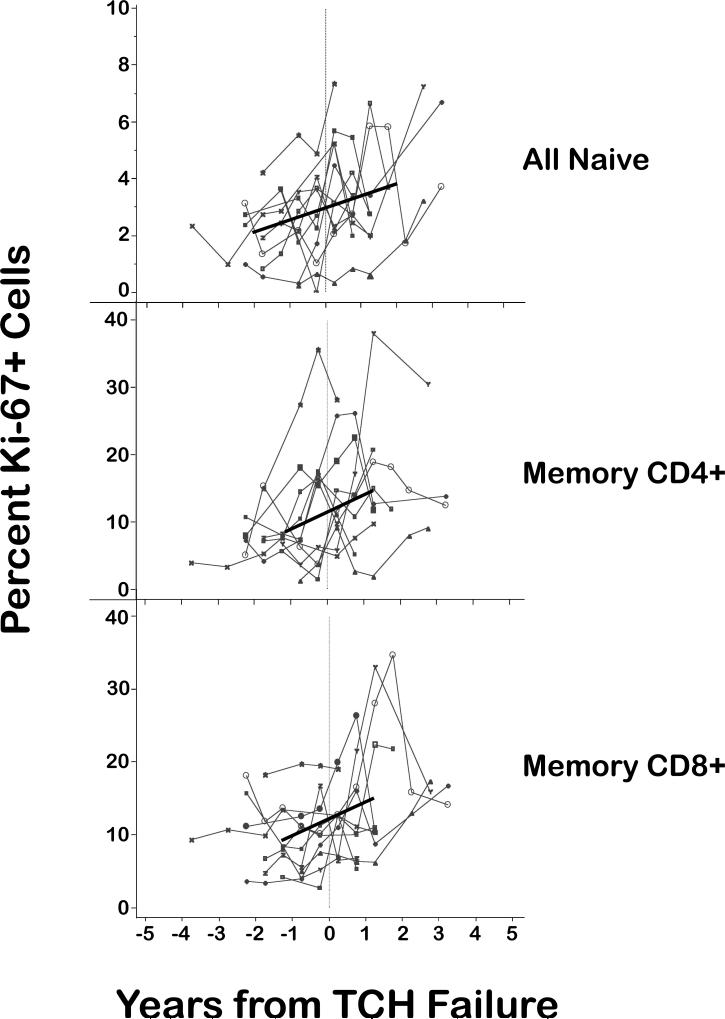
Further evidence that the decline in TREC levels after TCH failure was not due to increased proliferation of T cells came from 11 study participants in whom both the fractions of proliferating (Ki-67+) naive and memory T cells and the numbers of sjTRECs were quantified. In general, the fraction of proliferating T cells increased monotonically, i.e., without inflection at the time of TCH failure (Fig. 2), and therefore was unlikely to account for the sharp decline in TRECs at the time of TCH failure. Small increases in the fraction of proliferating naive cells could, in theory, reduce TREC levels substantially; however, adjustment of the slopes of sjTRECs before and after TCH failure to take into account increases in the fractions of proliferating naive cells had only a minimal effect on the slope of sjTRECs after T cell homeostasis failure (unadjusted = 48%/year, adjusted 46% = per year). Adjustment of sjTRECs for the increases in memory cell proliferation had no statistically significant effect, presumably because these increases occurred more than 1 year after the time of TCH failure, by which time the sjTREC had already declined substantially. Thus, increases in the fraction of proliferating T cells were not sufficient to account for the decline in sjTREC after TCH failure.
FIG. 2.
Changes in the percentage of proliferating (Ki-67+) cells over the study period. The proportion of Ki-67+ cells increased over time for all cell types examined (black trend lines); however, the rates of increase were similar before and after TCH failure. Therefore, these rates of proliferation were unlikely to account for the sharp decline in TREC levels after TCH failure, as confirmed by segmented regression analysis (see text).
Changes in T cell subsets
Naive CD4+ T cells declined as a fraction of lymphocytes during the 3 years before TCH failure, when TRECs per 106 PBMC were not declining significantly. Naive CD4+ T cells declined significantly faster than memory/effector (CD45RA–) CD4+ T cells (median declines of 48.1% and 31.3% per year, respectively, p = 0.019). Very few naive CD4+ T cells remained at the time of TCH failure (estimated mean = 41 cells/μl), suggesting that depletion of these cells may contribute to TCH failure. Of note, the absolute decline in naive CD4+ T cells over the study period was not nearly as great as that in TREC levels (Table 1). Among CD8+ T cells, both naive and memory cells changed little during the 4 years preceding TCH failure (data not shown); however, after TCH failure, naive CD8+ T cells declined significantly (median rate = 16% per year; p = 0.006; Table 1).
As mentioned, the proportion of Ki-67+ cells was higher in all cell types after TCH failure (Fig. 2). For example, in a representative study participant, the proportion of Ki-67+ cells within the total (CD4+ and CD8+) naive cell population increased from 2.76% before TCH failure (median of all time points before failure, range: 2.2−3.3%) to 4.61% after TCH failure (range: 2.74−5.76%). However, the absolute number of proliferating T cells after TCH failure was either lower (for naive and memory CD4+ cells) or unchanged (for naive and memory CD8+ T cells) relative to pre-TCH failure (Table 1). Thus, in absolute terms, the number of proliferating CD4+ and CD8+ T cells after TCH failure did not increase to compensate for the loss of these cells. For this reason, the depletion of CD8+ T cells after TCH failure was due almost entirely to the loss of nondividing cells (19%/year for naive cells and 16%/year for memory CD8+ T cells).
Overall, the proportion of naive cells within the T cell compartment [calculated as (naive CD4+ T cell count naive CD8+ T cell count) divided by the total T cell count] did not change significantly in the 4 years before TCH failure, but fell by 7% per year thereafter (p = 0.013).
DISCUSSION
In most cases, progression of HIV to AIDS is preceded by approximately 2 years of substantial decline in circulating counts of CD3+ T cells. The onset of this decline, termed TCH failure, appears to be an important milestone in the progression of HIV-1 disease to AIDS; however, its cause is not known. Therefore, we performed the most comprehensive longitudinal assessment of naive and memory T cell numbers, sjTREC levels, and T cell proliferation to date. The data obtained, coupled with recent studies validating sjTREC levels as a measure of thymic output, provide new insight into the mechanisms involved in TCH failure. In addition, segmented regression modeling allowed the first adjustment of sjTREC data for the possible effect of changes in directly measured T cell proliferation fractions. Like previous studies,27–29 we found lower levels of circulating sjTREC-bearing T cells with disease progression. We extended these studies, which were almost exclusively cross-sectional, by analyzing the timing of sjTREC loss. The most striking finding was the close temporal relationship between a very large decline (median 1109-fold) in circulating sjTRECs and the failure of T cell homeostasis.
Declines in sjTREC levels may be precipitated by multiple factors, acting alone or in combination. Chief among these are (1) increased cellular proliferation, (2) increased sequestration of sjTREC+ cells, (3) declines in overall T cell counts, (4) decreased production of new T cells by the thymus, and (5) decreased survival of sjTREC+ cells. Our data, and previous reports, argue against some of these possibilities as major mechanisms for the decline in sjTREC observed with TCH failure.
The possibility that proliferation of TREC-bearing cells contributed to the reduction in circulating TRECs19,30 should be considered, but several considerations argue that this mechanism is not a major contributor to our findings. In the present study, only a small fraction (4%) of the decline in sjTREC observed at TCH failure was accounted for by naive cell proliferation. This finding is consistent with most studies that have looked specifically at this question. Many studies found only weak correlations between expression of proliferation markers (such as Ki-67) and levels of circulating sjTRECs.19,29,31,32 Moreover, Dion, et al.31 recently described trends in two species of TREC (sjTRECs, which are produced late in thymic development, and βTRECs, which are formed earlier in thymic development) that were not consistent with a dilution effect caused by enhanced division of naive cells. In that study, people who became infected with HIV exhibited a significant decrease in the ratio of sjTRECs to βTRECs, indicating decreased proliferation of thymocytes, only a few months after infection.
It is true that some patients in our study exhibited substantially increased cellular proliferation after TCH failure. However, these large increases are unlikely to have influenced our findings, for at least four reasons. First, as Fig. 1 shows, most of the loss of sjTRECs occurred within the first year after TCH failure. During this time, changes in the proportion of proliferating cells were generally small. Changes in proliferating cells after this time, though large in a few cases, were therefore too late to account for the loss of sjTRECs. Second, the large increases in proliferation were confined to memory cell subsets, which contain very low levels of TRECs. Thus, changes in memory cell proliferation are unlikely to contribute substantially to the massive change in total TREC levels that we observed. To confirm this, we compared the TREC decline in total PBMC to that in naive cells, and found that they were the same. This finding supports the interpretation that the overall TREC decline is not attributable to high levels of memory cell proliferation. Third, when all cellular proliferation data were included in a statistical model, only a small effect of proliferation on TREC levels was found. Finally, some of the increase in the percentage of Ki-67+ cells (i.e., the fraction of proliferating cells) was due to the declines in absolute numbers of CD4+ or CD8+ T cells (the denominators from which the proliferation fractions were calculated). In absolute terms, the number of proliferating cells did not increase over the study period. Taken together, the evidence from this and previous studies suggests that it is unlikely that increases in cellular proliferation are sufficient to account for the large decline in sjTREC at the time of TCH failure.
Increased sequestration of TREC+ cells in the lymph nodes of HIV+ individuals has been suggested by reports of higher TREC levels in the lymph nodes compared to the peripheral blood.33,34 However, there is no evidence that such sequestration increases with disease progression, and a recent study of SIV-infected animals indicated that T cell dynamics in blood and lymph node mirror each other.35 Moreover, the observation that proliferating lymphocytes labeled with bromodeoxyuridine or deuterium rapidly equilibrate between the blood and lymph nodes36 also argues strongly against the sequestration of TREC-bearing cells as an explanation for our data.
It is also unlikely that declines in sjTREC merely reflect a general decline in total T cell or naive T cell numbers, because the loss of sjTREC at the time of TCH failure was much more rapid than the loss of either CD3+ or naive T cells. One might wonder if the rapid decline in TRECs we observed is plausible, given the long half-life and low turnover of naive T cells.37 In fact, a precedent for the occurrence of large changes in TREC levels over the course of a few weeks to months, as we found, is provided by Dion, et al.31 An additional explanation for the rapid loss of TREC-bearing T cells is that most sjTRECs are contained in the T cell population that has recently emerged from the thymus, and this population turns over much more rapidly than the general naive T cell pool.38–40
Therefore, although the decline of sjTRECs may be partially caused by some combination of the factors discussed above, we favor the interpretation that sjTREC decline at the time of TCH failure is largely attributable to decreased survival of TREC-bearing cells and/or decreased thymic production of T cells. Decreased survival of TREC-bearing cells might involve the emergence of X4 strains of HIV, which use the CXCR4 coreceptor and have long been associated with accelerated progression of HIV disease.41 Of the four study participants for whom data are available, two individuals experienced a switch from CCR5-tropic to CXCR4-tropic viruses (0.25 and 0.75 years before T cell homeostasis failure); the remaining two participants did not42 (Shepherd and Margolick, unpublished observations). However, in larger and more definitive studies, we previously presented evidence that X4 viruses become abundant, on average, about 0.6 years before the failure of TCH42,43 and are present in the vast majority of HIV-infected people who reach this stage of disease progression.42 X4 viruses might contribute to the depletion of TRECs through both thymic and extrathymic mechanisms, since both thymocytes and naive T cells express CXCR4 more than CCR5.44–46 In the thymus, X4 strains could infect (or kill by other means) double-positive thymocytes, thus reducing production of naive T cells expressing TRECs. In the peripheral blood, X4 strains could kill both CD4+ and CD8+ T cells, although depletion of TREC-bearing cells might not be as selective if the mechanism of cytotoxicity depends on CXCR4 expression on mononuclear phagocytes rather than on T cells.47
Decreased thymic output might be attributed to other mechanisms, as well. For example, Dion et al.31 observed higher TREC levels in the peripheral blood of people with early HIV infection, compared to HIV-negative donors, possibly reflecting compensation for the loss of sjTREC-bearing cells. They reasoned that one or more of the following mechanisms could cause increased levels of early thymocytes: (1) release of more stem cells from the bone marrow, (2) enhanced survival of thymocytes, or (3) enhanced survival of peripheral recent thymic emigrants. According to this line of reasoning, failure of one (or more) of these mechanisms, coupled with the rapid turnover of TREC-bearing naive T cells relative to other naive T cells, could account for the rapid loss of TRECs that accompanied progression to AIDS in the present study.
It remains unclear to what extent primary thymic failure accounts for loss of T cells and failure of T cell homeostasis in late-stage HIV disease. The fact that naive T cells, TREC levels in PBMC, and thymic size all rose after institution of highly active antiretroviral therapy (HAART), even in people with CD4+ lymphocyte counts <200/μl,48 suggests that at least some thymic function remains even in people with very low T cell counts and (presumably) T cell homeostasis failure. On the other hand, the clinical benefit of HAART is reduced when it is initiated at CD4+ lymphocyte counts <200/μl,49 consistent with at least some irreversible thymic damage late in HIV disease.14–16 Thus, measurements of TRECs may be helpful in defining more precisely the stage of HIV disease by which HAART should, optimally, be initiated.
ACKNOWLEDGMENTS
We thank Brenna Hill, Stacey Meyerer, and Denise Cesar for expert technical assistance. This work was presented in part at the XIV International AIDS Conference, Barcelona, Spain. Supported by Grants AI 37984, M01-RR00052 (GCRC), AI 35039, AI 35040, AI 35041, AI 35042 (MACS), NI 35043, AI 42532 (AIEDRP), DA 04334 (ALIVE), AI 42855 (CFAR), AI 41401, and AI 43866.
REFERENCES
- 1.Margolick JB, Donnenberg AD, Muñoz A, et al. Changes in T and non-T lymphocyte subsets following seroconversion to HIV-1: Stable CD3+ and declining CD3– populations suggest regulatory responses linked to loss of CD4 lymphocytes. J Acquir Immune Defic Syndr. 1993;6:153–161. [PubMed] [Google Scholar]
- 2.Adleman LM, Wofsy D. T-cell homeostasis: Implications in HIV infection. J Acquire Immune Defic Syndr. 1993;6:144–152. [PubMed] [Google Scholar]
- 3.Margolick JB, Muñoz A, Donnenberg AD, et al. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. Nature Med. 1995;1:674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 4.Margolick JB, Donnenberg AD. T-cell homeostasis in HIV-1 infection. Semin Immunol. 1997;9:381–388. doi: 10.1006/smim.1997.0096. [DOI] [PubMed] [Google Scholar]
- 5.Galai N, Margolick JB, Astemborski J, Vlahov D. Existence and failure of T-cell homeostasis prior to AIDS onset in HIV-infected injection drug users. Clin Immunol Immunopathol. 1996;79:134–141. doi: 10.1006/clin.1996.0060. [DOI] [PubMed] [Google Scholar]
- 6.Maas J, Gange SJ, Schuitemaker H, et al. Strong association between failure of T-cell homeostasis and the syncytium-inducing phenotype among HIV-1 infected men in the Amsterdam Cohort Study. AIDS. 2000;14:1155–1161. doi: 10.1097/00002030-200006160-00012. [DOI] [PubMed] [Google Scholar]
- 7.Gange SJ, Muñoz A, Chmiel JS, et al. Identification of inflections in T-cell counts among HIV-1 infected individuals and relationship with progression to clinical AIDS. Proc Natl Acad Sci USA. 1998;95:10848–19853. doi: 10.1073/pnas.95.18.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margolick JB, Donnenberg AD, Chu C, et al. Decline in total T cell count is associated with onset of AIDS, independent of CD4+ lymphocyte count: Implications for AIDS pathogenesis. Clin Immunol Immunopathol. 1998;88:256–263. doi: 10.1006/clin.1998.4577. [DOI] [PubMed] [Google Scholar]
- 9.McCune JM. Thymic function in HIV-1 disease. Semin Immunol. 1997;9:397–404. doi: 10.1006/smim.1997.0098. [DOI] [PubMed] [Google Scholar]
- 10.McCune JM, Loftus R, Schmidt K, et al. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei X, Ghosh SK, Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 12.Ho DD, Neumann AU, Perelson AS, et al. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 13.Douek DC, Koup RA. Evidence for thymic function in the elderly. Vaccine. 2000;18:1638–1641. doi: 10.1016/s0264-410x(99)00499-5. [DOI] [PubMed] [Google Scholar]
- 14.Steffens CM, Smith KY, Landay A, et al. T cell receptor excision circle (TREC) content following maximum HIV suppression is equivalent in HIV-infected and HIV-uninfected individuals. AIDS. 2001;15:1757–1764. doi: 10.1097/00002030-200109280-00003. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS. 2001;15:1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 16.Markert ML, Alvarez-McLeod AP, Sempowski GD, et al. Thymopoiesis in HIV-infected adults after highly active antiretroviral therapy. AIDS Res Hum Retroviruses. 2001;17:1635–1643. doi: 10.1089/088922201753342040. [DOI] [PubMed] [Google Scholar]
- 17.Rabin RL, Roederer M, Maldonado Y, Petru A, Herzenberg LA. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Invest. 1995;95:2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazenberg Md, Otto SA, Cohen Stuart JW, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med. 2000;6:1036–1042. doi: 10.1038/79549. [DOI] [PubMed] [Google Scholar]
- 20.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126:310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 21.Schenker EL, Hultin LE, Bauer KD, et al. Evaluation of a dual-color flow cytometry immunophenotyping panel in a multicenter quality assurance program. Cytometry. 1993;14:307–317. doi: 10.1002/cyto.990140311. [DOI] [PubMed] [Google Scholar]
- 22.Kleeberger CA, Lyles RH, Margolick JB, et al. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to twelve years in a multicenter study. Clin Diagn Lab Immunol. 1999;6:14–19. doi: 10.1128/cdli.6.1.14-19.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 24.Endl E, Gerdes J. The Ki-67 protein: Fascinating forms and an unknown function. Exp Cell Res. 2000;257:231–237. doi: 10.1006/excr.2000.4888. [DOI] [PubMed] [Google Scholar]
- 25.Schmid I, Krall WJ, Uittenbogaart CH, Braun J, Giorgi JV. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 26.Macallan DC, Fullerton CA, Neese RA, et al. Measurement of cell proliferation by labeling of DNA with stable isotope-labeled glucose: Studies in vitro, animals, and in humans. Proc Natl Acad Sci USA. 1998;95:708–713. doi: 10.1073/pnas.95.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson MW, Sverstiuk AE, Gracely EJ, et al. T-cell receptor excision circles (TREC) and maintenance of long-term non-progression status in HIV-1 infection. AIDS. 2003;17:915–929. doi: 10.1097/00002030-200304110-00018. [DOI] [PubMed] [Google Scholar]
- 28.Hatzakis A, Touloumi G, Karanicolas R, et al. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet. 2000;355:599–604. doi: 10.1016/S0140-6736(99)10311-8. [DOI] [PubMed] [Google Scholar]
- 29.Nobile M, Correa R, Borghans JA, et al. De novo T-cell generation in patients at different ages and stages of HIV-1 disease. Blood. 2004;104:470–477. doi: 10.1182/blood-2003-12-4265. [DOI] [PubMed] [Google Scholar]
- 30.Ye P, Kirschner DE. Reevaluation of T cell receptor excision circles as a measure of human recent thymic emigrants. J Immunol. 2002;168:4968–4979. doi: 10.4049/jimmunol.168.10.4968. [DOI] [PubMed] [Google Scholar]
- 31.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167:6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 33.Nokta MA, Li X-D, Al-Harthi L, et al. Entrapment of recent thymic emigrants in lymphoid tissues from HIV-infected patients: Association with HIV cellular viral load. AIDS. 2003;16:2119–2127. doi: 10.1097/00002030-200211080-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz M, Douek DC, Valdez H, et al. T cells containing T cell receptor excision circles are inversely related to HIV replication and are selectively and rapidly released into circulation with antiretroviral treatment. AIDS. 2003;17:1145–1149. doi: 10.1097/00002030-200305230-00005. [DOI] [PubMed] [Google Scholar]
- 35.Mattapallil JJ, Douek DC, Hill B, et al. Massive infection and loss of memory CD4(+) T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1080–1081. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 36.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–1741. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellerstein MK, Hoh RA, Hanley MB, et al. Subpopulations of long-lived and short-lived T cells in advanced HIV-1 infection. J Clin Invest. 2003;112:956–966. doi: 10.1172/JCI17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2002;167:6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 39.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 40.Berzins SP, Godfrey DI, Miller JF, Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koot M, Keet IPM, Vos AHV, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 42.Shankarappa R, Margolick JB, Gange SJ, et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol. 1999;73:10489–10502. doi: 10.1128/jvi.73.12.10489-10502.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maas JJ, Gange SJ, Schuitemaker H, et al. Strong association between failure of T cell homeostasis and the syncytium-inducing phenotype among HIV-1-infected men in the Amsterdam Cohort Study. AIDS. 2000;14:1155–1161. doi: 10.1097/00002030-200006160-00012. [DOI] [PubMed] [Google Scholar]
- 44.Zamarchi R, Allavena P, Borsetti A, et al. Expression and functional activity of CXCR-4 and CCR-5 chemokine receptors in human thymocytes. Clin Exp Immunol. 2002;127:321–330. doi: 10.1046/j.1365-2249.2002.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkowitz RD, Beckerman KP, Schall TJ, McCune JM. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 46.Kitchen SG, Uittenbogaart CH, Zack JA. Mechanism of human immunodeficiency virus type 1 localization in CD4-negative thymocytes: Differentiation from a CD4-positive precursor allows productive infection. J Virol. 1997;71:5713–5722. doi: 10.1128/jvi.71.8.5713-5722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Herbein G, Mahlknecht U, Batliwalla F, et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 48.Franco JM, Rubio A, Martinez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99:3702–3706. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 49.Ahdieh-Grant L, Yamashita TE, Phair JP, et al. When to initiate highly active antiretroviral therapy: A cohort approach. Am J Epidemiol. 2003;157:738–746. doi: 10.1093/aje/kwg036. [DOI] [PubMed] [Google Scholar]