Abstract
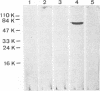
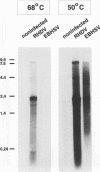
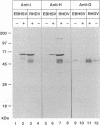
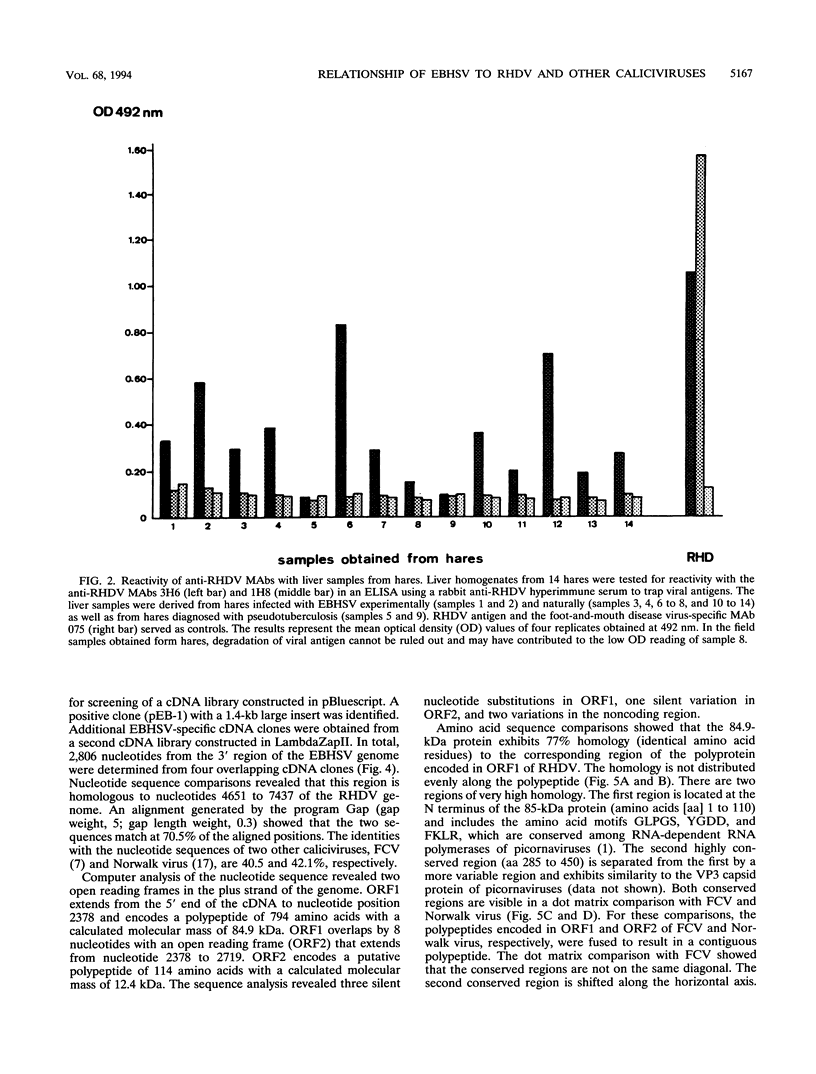
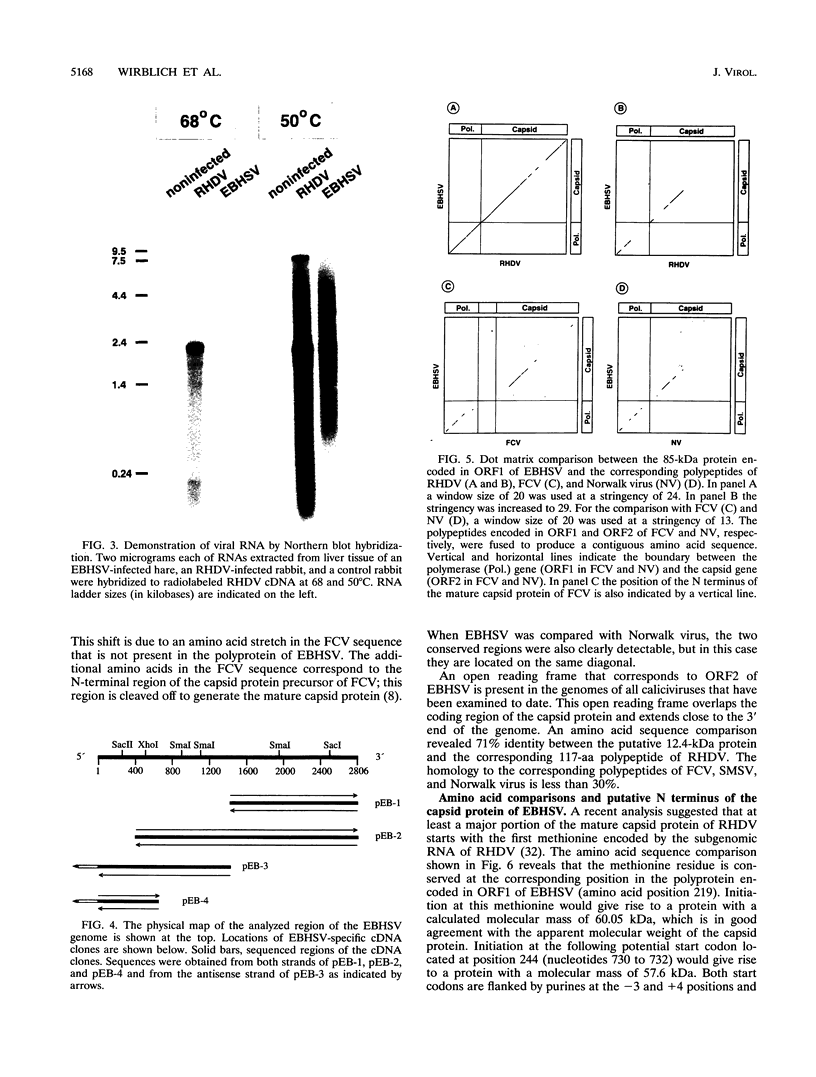
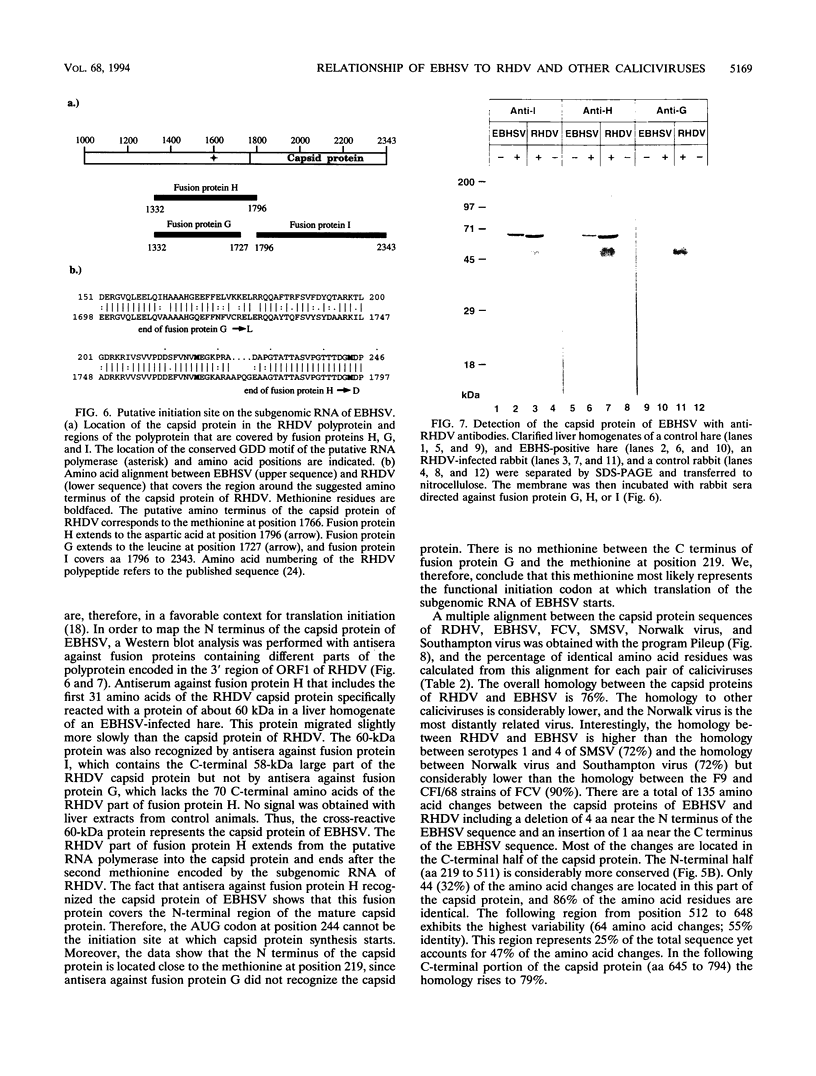
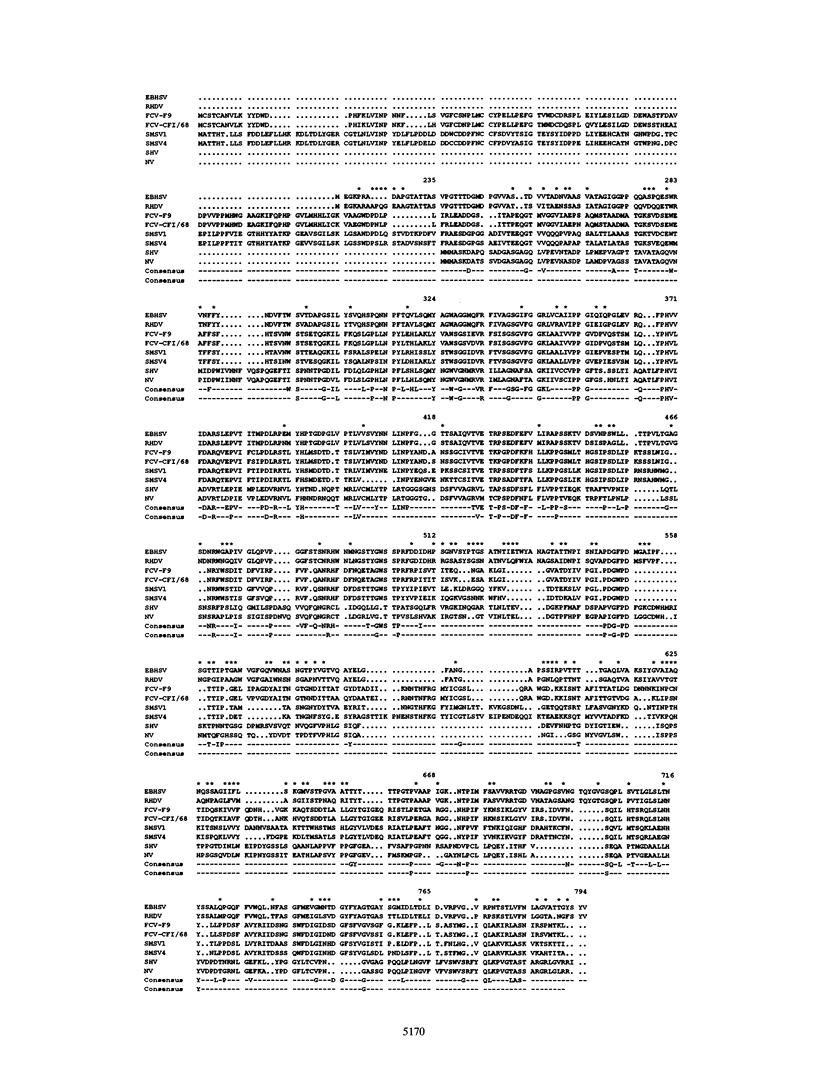
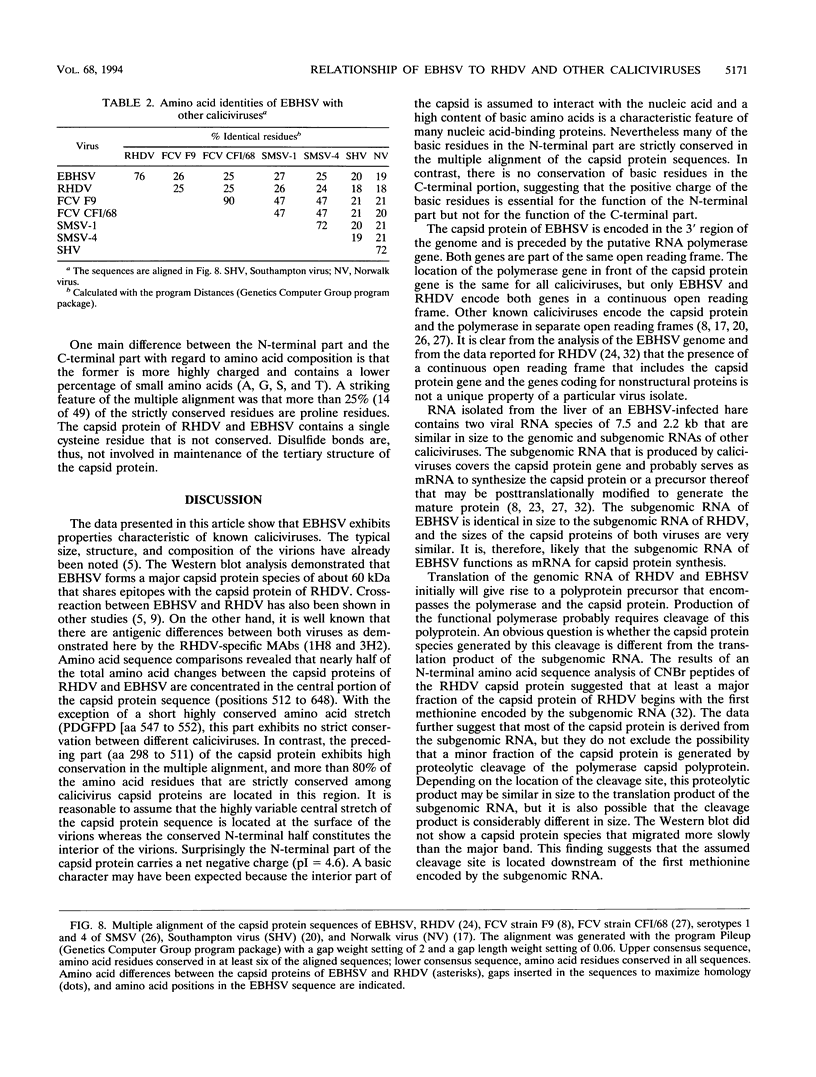
Monoclonal antibodies directed against the capsid protein of rabbit hemorrhagic disease virus (RHDV) were used to identify field cases of European brown hare syndrome (EBHS) and to distinguish between RHDV and the virus responsible for EBHS. Western blot (immunoblot) analysis of liver extract of an EBHS virus (EBHSV)-infected hare revealed a single major capsid protein species of approximately 60 kDa that shared epitopes with the capsid protein of RHDV. RNA isolated from the liver of an EBHSV-infected hare contained two viral RNA species of 7.5 and 2.2 kb that comigrated with the genomic and subgenomic RNAs of RHDV and were recognized by labeled RHDV cDNA in Northern (RNA) hybridizations. The nucleotide sequence of the 3' 2.8 kb of the EBHSV genome was determined from four overlapping cDNA clones. Sequence analysis revealed an open reading frame that contains part of the putative RNA polymerase gene and the complete capsid protein gene. This particular genome organization is shared by RHDV but not by other known caliciviruses. The deduced amino acid sequence of the capsid protein of EBHSV was compared with the capsid protein sequences of RDDV and other caliciviruses. The amino acid sequence comparisons revealed that EBHSV is closely related to RHDV and distantly related to other caliciviruses. On the basis of their genome organization, it is suggested that caliciviruses be divided into three groups.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biermann U., Krauss H. Detection of virus in connection with "European brown hare syndrome" in Hesse, F.R.G. Zentralbl Veterinarmed B. 1991 Feb;38(1):21–24. doi: 10.1111/j.1439-0450.1991.tb00841.x. [DOI] [PubMed] [Google Scholar]
- Boga J. A., Marín M. S., Casais R., Prieto M., Parra F. In vitro translation of a subgenomic mRNA from purified virions of the Spanish field isolate AST/89 of rabbit hemorrhagic disease virus (RHDV). Virus Res. 1992 Oct;26(1):33–40. doi: 10.1016/0168-1702(92)90144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capucci L., Scicluna M. T., Lavazza A. Diagnosis of viral haemorrhagic disease of rabbits and the European brown hare syndrome. Rev Sci Tech. 1991 Jun;10(2):347–370. doi: 10.20506/rst.10.2.561. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Milton I. D., Meanger J., Bennett M., Gaskell R. M., Turner P. C. The complete nucleotide sequence of a feline calicivirus. Virology. 1992 Sep;190(1):443–448. doi: 10.1016/0042-6822(92)91231-i. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Milton I. D., Turner P. C., Meanger J., Bennett M., Gaskell R. M. Identification and sequence determination of the capsid protein gene of feline calicivirus. Arch Virol. 1992;122(3-4):223–235. doi: 10.1007/BF01317185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasey D., Lucas M., Westcott D., Williams M. European brown hare syndrome in the U.K.; a calicivirus related to but distinct from that of viral haemorrhagic disease in rabbits. Arch Virol. 1992;124(3-4):363–370. doi: 10.1007/BF01309816. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Forss S., Strebel K., Beck E., Schaller H. Nucleotide sequence and genome organization of foot-and-mouth disease virus. Nucleic Acids Res. 1984 Aug 24;12(16):6587–6601. doi: 10.1093/nar/12.16.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Henriksen P., Gavier D., Elling F. Acute necrotising hepatitis in Danish farmed hares. Vet Rec. 1989 Nov 4;125(19):486–487. doi: 10.1136/vr.125.19.486. [DOI] [PubMed] [Google Scholar]
- Jiang X., Wang M., Graham D. Y., Estes M. K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992 Nov;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Wang K., Estes M. K. Sequence and genomic organization of Norwalk virus. Virology. 1993 Jul;195(1):51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Caul E. O., Ashley C. R., Clarke I. N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993 Jan 22;259(5094):516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- Meyers G., Tautz N., Dubovi E. J., Thiel H. J. Viral cytopathogenicity correlated with integration of ubiquitin-coding sequences. Virology. 1991 Feb;180(2):602–616. doi: 10.1016/0042-6822(91)90074-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G., Wirblich C., Thiel H. J. Genomic and subgenomic RNAs of rabbit hemorrhagic disease virus are both protein-linked and packaged into particles. Virology. 1991 Oct;184(2):677–686. doi: 10.1016/0042-6822(91)90437-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers G., Wirblich C., Thiel H. J. Rabbit hemorrhagic disease virus--molecular cloning and nucleotide sequencing of a calicivirus genome. Virology. 1991 Oct;184(2):664–676. doi: 10.1016/0042-6822(91)90436-f. [DOI] [PubMed] [Google Scholar]
- Neill J. D. Nucleotide sequence of a region of the feline calicivirus genome which encodes picornavirus-like RNA-dependent RNA polymerase, cysteine protease and 2C polypeptides. Virus Res. 1990 Nov;17(3):145–160. doi: 10.1016/0168-1702(90)90061-f. [DOI] [PubMed] [Google Scholar]
- Neill J. D. Nucleotide sequence of the capsid protein gene of two serotypes of San Miguel sea lion virus: identification of conserved and non-conserved amino acid sequences among calicivirus capsid proteins. Virus Res. 1992 Jul;24(2):211–222. doi: 10.1016/0168-1702(92)90008-w. [DOI] [PubMed] [Google Scholar]
- Neill J. D., Reardon I. M., Heinrikson R. L. Nucleotide sequence and expression of the capsid protein gene of feline calicivirus. J Virol. 1991 Oct;65(10):5440–5447. doi: 10.1128/jvi.65.10.5440-5447.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlinger V. F., Haas B., Meyers G., Weiland F., Thiel H. J. Identification and characterization of the virus causing rabbit hemorrhagic disease. J Virol. 1990 Jul;64(7):3331–3336. doi: 10.1128/jvi.64.7.3331-3336.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra F., Boga J. A., Marin M. S., Casais R. The amino terminal sequence of VP60 from rabbit hemorrhagic disease virus supports its putative subgenomic origin. Virus Res. 1993 Mar;27(3):219–228. doi: 10.1016/0168-1702(93)90034-k. [DOI] [PubMed] [Google Scholar]
- Parra F., Prieto M. Purification and characterization of a calicivirus as the causative agent of a lethal hemorrhagic disease in rabbits. J Virol. 1990 Aug;64(8):4013–4015. doi: 10.1128/jvi.64.8.4013-4015.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rümenapf T., Meyers G., Stark R., Thiel H. J. Hog cholera virus--characterization of specific antiserum and identification of cDNA clones. Virology. 1989 Jul;171(1):18–27. doi: 10.1016/0042-6822(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Beck E., Strohmaier K., Schaller H. Characterization of foot-and-mouth disease virus gene products with antisera against bacterially synthesized fusion proteins. J Virol. 1986 Mar;57(3):983–991. doi: 10.1128/jvi.57.3.983-991.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]