Abstract
The BCL-2 family of proteins is composed of both pro- and antiapoptotic regulators, although its most critical biochemical functions remain uncertain. The structural similarity between the BCL-XL monomer and several ion-pore-forming bacterial toxins has prompted electrophysiologic studies. Both BAX and BCL-2 insert into KCl-loaded vesicles in a pH-dependent fashion and demonstrate macroscopic ion efflux. Release is maximum at ≈pH 4.0 for both proteins; however, BAX demonstrates a broader pH range of activity. Both purified proteins also insert into planar lipid bilayers at pH 4.0. Single-channel recordings revealed a minimal channel conductance for BAX of 22 pS that evolved to channel currents with at least three subconductance levels. The final, apparently stable BAX channel had a conductance of 0.731 nS at pH 4.0 that changed to 0.329 nS when shifted to pH 7.0 but remained mildly Cl− selective and predominantly open. When BAX-incorporated lipid vesicles were fused to planar lipid bilayers at pH 7.0, a Cl−-selective (PK/PCl = 0.3) 1.5-nS channel displaying mild inward rectification was noted. In contrast, BCL-2 formed mildly K+-selective (PK/PCl = 3.9) channels with a most prominent initial conductance of 80 pS that increased to 1.90 nS. Fusion of BCL-2-incorporated lipid vesicles into planar bilayers at pH 7.0 also revealed mild K+ selectivity (PK/PCl = 2.4) with a maximum conductance of 1.08 nS. BAX and BCL-2 each form channels in artificial membranes that have distinct characteristics including ion selectivity, conductance, voltage dependence, and rectification. Thus, one role of these molecules may include pore activity at selected membrane sites.
The BCL-2 family of proteins is composed of both antiapoptotic and proapoptotic members that function in a distal apoptotic pathway common to all multicellular organisms. The ratio of death antagonists (BCL-2, BCL-XL, MCL-1, A1) to agonists (BAX, BCL-XS, BAK, BAD, BIK, BID) determines the response to an apoptotic stimulus (1, 2). Family members with a hydrophobic C-terminal signal anchor sequence are intracellular integral membrane proteins most convincingly localized to mitochondria, endoplasmic reticulum, and nuclear membrane (3–6). BCL-2 appears to function upstream of a family of caspases (cysteine proteases with a P1 specificity for aspartic acid) in that BCL-2 expression prevents the activation of proteases such as caspase-3 (7–9). Moreover, the BCL-2 family represents an active checkpoint in the death pathway in that induction of BAX or BAK will initiate death in the absence of any additional signal (10, 11). BAX induces a downstream program of mitochondrial dysfunction as well as the activation of caspases.
A striking characteristic of the BCL-2 family is its propensity to form both homodimers and heterodimers, the latter often between anti- and proapoptotic molecules such as BCL-2/BAX (1). Mutational analysis of BCL-2 and BCL-XL identified key residues within BH1 and BH2 domains required for both heterodimerization with BAX and repression of cell death (12, 13). However, other mutants that lost dimerization with BAX still retain death-repressor activity, suggesting these two functions are separable (14). Another domain, BH3, has proven essential for the proapoptotic molecules such as BAK, BAX, BID, and BAD to bind antagonists like BCL-2 or BCL-XL and to function in promoting death (15–18). A genetic approach utilized gain- and loss-of-function murine models to assess whether death agonists (Bax) or antagonists (Bcl-2) were dominant in regulating apoptosis. Despite the evidence for in vivo competition between the molecules, BCL-2 and BAX each proved capable of regulating apoptosis independent of the other product (19).
The multidimensional NMR and x-ray crystallographic structure of a BCL-XL monomer indicated that BH1–4 domains corresponded to α helices 1–7. The α helices contributed by BH1–3 are closely juxtaposed to form a hydrophobic pocket (20). A detailed NMR analysis of wild-type and mutant peptides of the BH3 amphipathic α2-helix of BAK indicated it formed critical interactions with this pocket (21). Moreover, the α helical structure of BCL-XL proved similar to the ion pore-forming toxins of colicin and diphtheria toxin B fragment. Of particular note are the two central hydrophobic helices α5 and α6 of 30-Å length reminiscent of membrane insertion domains in the bacterial toxins. This observation has prompted a series of electrophysiologic studies by others (22–24) and ourselves to examine the capacity of BCL-2 family members to form ion channels in artificial lipid membranes. Here we compare the distinct ion conductive channels of proapoptotic BAX with antiapoptotic BCL-2.
MATERIALS AND METHODS
Plasmid Preparation and Protein Purification.
Murine BAX and BCL-2 lacking the C-terminal hydrophobic region (BAXΔC19, amino acids 1–173; BCL-2ΔC21, amino acids 1–218) were cloned into pGEX-KG (2, 3). GST-BAXΔC19 and GST-BCL-2ΔC21 fusion proteins were induced in XL-1 by 0.1 mM IPTG. The bacterial pellets were resuspended in lysis buffer (0.5 mM EDTA/1 mM DTT/1% Triton X-100/0.1 mg/ml PMSF/2 μg/ml aprotinin/2 μg/ml leupeptine/1 μg/ml pepstatin A in PBS) and sonicated. After centrifugation at 20,000 × g for 20 min, the supernatant was applied to glutathione-agarose beads (Sigma). The beads were washed with buffer and treated with 10 units of thrombin per original liter. Cleaved BAXΔC19 and BCL-2ΔC21 were eluted from beads and the cleavage reaction was terminated by adding 80 μg of Nα-p-tosyl-l-lysine choloromethyl ketone (TLCK). The cleaved eluent was dialyzed against buffer (20 mM Tris pH 8.5/5 mM EDTA/1 mM DTT/0.1% Triton X-100). To remove the GST protein and incompletely cleaved fusion proteins, the dialyzed preparation was further purified on a monoQ column and the proteins were eluted with a NaCl gradient. Experiments utilized three independent protein preparations of BAX and BCL-2.
Release of KCl from Synthetic Lipid Vesicles.
Unilamellar vesicles composed of 40% 1,2-dioleoyl phosphatidyl glycerol and 60% 1,2-dioleoyl phosphatidyl choline (Avanti Polar Lipids) were prepared in 100 mM KCl/2 mM CaNO3/10 mM dimethylglutarate, pH 5.0, as previously described (25). The resulting liposomes were diluted 200-fold to a concentration of 0.05 mg/ml in 100 mM KNO3/2 mM CaNO3/10 mM dimethylglutarate titrated to the appropriate pH with NaOH or with acetic acid. BAXΔC19 and BCL-2ΔC21 were added at a concentration of 500 ng/ml. Triton X-100 (0.1%) was added to release the total encapsulated Cl−. The total amount of Cl− released was quantitated by a calibration curve produced by successive additions of 25 μM KCl. Cl− efflux was measured with a Cl− combination ion-selective electrode (Accumet, Hudson, MA).
Low pH Insertion of BAX and BCL-2 into Planar Lipid Bilayers.
Planar lipid bilayers were prepared from soybean lipids by chloroform extraction of Azolectin Type II (10–20% choline, 80–90% negatively charged lipids) (Sigma). The chloroform was removed with a stream of nitrogen, and the lipids were stored under N2 until dissolved in decane at 30 mg/ml. This preparation was then stored under nitrogen. The 0.25-mm orifice of a polystyrene cuvette (Warner Instruments, Hamden, CT) was pretreated with 2 μl of the decane lipid solution, and the solvent was allowed to evaporate. The cuvette was then placed into a bilayer chamber and connected to a Bilayer Clamp BC525-a (Warner Instruments) by Ag/AgCl electrodes via agar bridges. Data were collected using axoscope (Axon Instruments, Foster City, CA), archived on videotape using a Neurocorder DR-484 (Neuro Data Instruments, Delewar Water Gap, PA), and analyzed using origin (Microcal, Amherst, MA) and pclamp6 (Axon Instruments). Slope conductance was calculated by the method of least squares, and the variance is given. Ion selectivities were calculated using the reversal potential and the Goldman equation. Bilayers were formed by spreading with a polished glass rod and allowed to thin to a capacitance of 0.4 μF/cm2, at which point the noise was typically 0.2 pA and the leak conductance was 20 pS. The salt concentrations were initially 450 mM KCl in the cis chamber (1.0 ml) and 150 mM KCl in the trans chamber (0.5 ml) to permit identification of spontaneous initial currents. Outward (positive) currents were defined as K+ moving cis to trans. All solutions were buffered to pH 4.0 with 10 mM K-acetate. Protein (≈1 μg) was added to the cis chamber with mixing. After initial currents were identified the soluble protein was usually removed by exchanging the cis chamber contents with aliquots of buffer.
Reconstitution of BAX and BCL-2 into Liposomes and Fusion with Planar Lipid Bilayers.
The preparation of Azolectin vesicles for fusion with planar lipid bilayer was as follows. Purified protein (0.25 μg) was added to 50 mg lipid in 20 μl of KCl buffer (150 mM KCl/10 mM Hepes, pH 7.0). This was placed into a dialysis bag (cutoff of 12 kDa). After 16 hr of dialysis in 3,000 volumes of 150 mM KCl (10 mM Hepes, pH 7.0) the vesicle suspension was removed and placed on ice for immediate use. The bilayer was set up as described for pH 4.0 insertion to identify the ion selectivity of the initial currents. Fusion was initiated by adding 5–20 μl of lipid vesicles (only 5–20 ng of protein) to the cis bilayer chamber with mixing. Vesicles were removed and solutions were exchanged as described above.
RESULTS
pH Dependence of BAX- and BCL-2-Mediated Release of Ions from Lipid Vesicles.
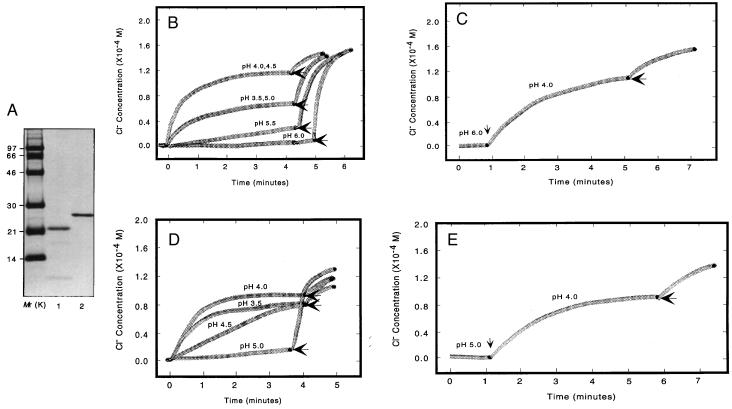
Recombinant BCL-2 and BAX that were lacking the C-terminal hydrophobic region (BCL-2ΔC21, amino acids 1–218; BAXΔC19, amino acids 1–173) were purified to homogeneity and verified by SDS/PAGE followed by Western blot analysis (not shown) or Coomassie blue staining (Fig. 1A). The C-terminal signal-anchor membrane-targeting sequences were removed to better assess the capacity of internal α helices to mediate insertion (5). The pH dependence of BAX- and BCL-2-mediated release of Cl− from KCl-loaded lipid vesicles was compared (Fig. 1 B–E). The maximum release of Cl− by BAX occurs at pH 4.0–4.5, decreasing to 50% at pH 3.5 or 5.0 and to less than 10% at a pH of 5.5 (Fig. 1B). When BAX added to vesicles at pH 6.0 was subsequently shifted to pH 4.0, a rapid release of Cl− resulted, indicating the reversibility of the pH influence (Fig. 1C). BCL-2 displays a more narrow pH dependence of Cl− release, with complete inactivation occurring by pH 5.0 (Fig. 1D). Once again, shifting from pH 5.0 to 4.0 activated the release of Cl− by BCL-2 (Fig. 1E). Thus, both purified BAX and BCL-2 proteins are capable of pH-dependent macroscopic ion release that requires activity of the bulk population of BCL-2 and BAX proteins.
Figure 1.
BAX and BCL-2 induced release of ions from synthetic lipid vesicles. (A) An analytical gel of purified recombinant BAX and BCL-2 preparations used in this study. Recombinant murine BAXΔC19 and BCL-2ΔC21, prepared as described in Materials and Methods, were separated on a 12% SDS polyacrylamide gel and stained with Coomassie blue. Markers (Mr) are in the left lane. Lane 1, recombinant BAXΔC19; lane 2, recombinant BCL-2ΔC21. Graphs of BAX-induced (B) or BCL-2-induced (D) Cl− efflux from synthetic lipid vesicles are shown. KCl-loaded, negatively charged, unilamellar vesicles were added to 10 mM dimethylglutarate buffer at the indicated pH values, resulting in a 200-fold inside:outside KCl gradient. BAX or BCL-2 was added (time = 0) and ion efflux was measured using a Cl−-sensitive electrode. Triton X-100 was added (horizontal arrow) to release all encapsulated Cl−. Graphs show BAX-induced (C) or BCL-2-induced (E) Cl− efflux from vesicles in buffer at pH 6.0 or pH 5.0, respectively, which subsequently shifted to pH 4.0 (vertical arrow).
Low pH Formation of BAX Channels in Planar Lipid Bilayers.
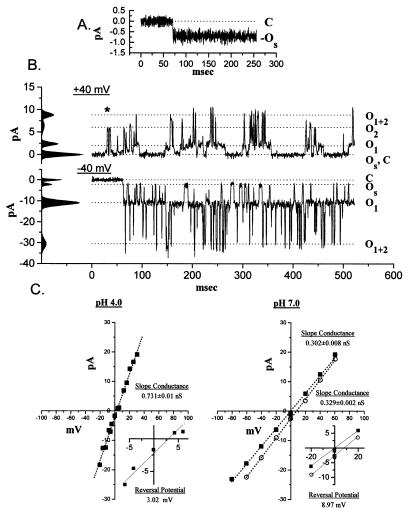
One microgram of purified, soluble BAX protein was added to the cis chamber of an established planar lipid bilayer in a 450-/150-mM (cis-to-trans) KCl gradient at pH 4.0. The initial, inward current appeared spontaneously (OS), reflecting Cl− moving down the KCl gradient (Fig. 2A). In multiple experiments the initial current was always inward, usually appeared within 10 min following BAX addition, and had a conductance of 22 (SD 5, n = 5) pS.
Figure 2.
Insertion of soluble BAX into planar lipid bilayers. (A) Purified BAX (≈1 μg) was added to the cis chamber of a 450-/150-mM KCl gradient, and the first spontaneous current (OS; V = 0 mV) was anionic with a slope conductance of 22 ± 5 pS. (B) Tracings of a well defined intermediate stage that appeared during pH 4.0 BAX insertions. The records are at +40 mV (Upper) and −40 mV (Lower) with a 450-/150-mM KCl gradient, which accounts for the asymmetry in the current amplitudes. The difference in opening kinetics is a voltage-dependent effect seen at this intermediate stage of channel maturation. ∗ denotes direct C−OS-to-O2 transitions. (C) The current–voltage plots for the open pore at pH 4.0 and after a shift to pH 7.0 in the presence of a 450-/150-mM KCl gradient (○) or in symmetric 150-mM KCl (▪). The region of reversal potential is expanded in Inset.
Characteristic patterns of BAX currents were observed ranging from OS to a large, open pore. Fig. 2 shows the initial spontaneous current (OS) (Fig. 2A), a complex multiconductance state (Fig. 2B), and a simple open pore (Fig. 2C). The multiconductance state was most clear at ±40 mV. At +40 mV four current levels were noted: 0 pA (C and OS), 2.36 pA (O1), 6.4 pA (O2), and 8.81 pA (O1+2) (Fig. 2B Upper). The largest current appears to be the sum of the two smaller levels (O1 and O2). Direct transitions between these levels, which occur in both directions, suggest a random mechanism for movement between the open states (Fig. 2B). At −40 mV there were also four levels: 0 pA (C), −2.14 pA (OS), −10.9 pA (O1), and −30.5 pA (O1+2), but no transitions from OS to O2 were observed (Fig. 2B Lower). Hyperpolarization to −40 mV shifted the most common state to O1, demonstrating voltage-dependent behavior. Based on reversal potential the Er for each of these current levels gives a substantial Cl− selectivity that averages PK/PCl ≅ 0.10. The third state of the BAX channel at pH 4.0 was Cl− selective, with a slope conductance of 0.731 ± 0.01 nS (Fig. 2C Left), and was characteristically open. Typically, BAX currents progressed through these states after the addition of protein to the cis chamber. Increasing the pH to 7.0 altered the conductance of BAX to 0.329 ± 0.002 nS in a 450-/150-mM KCl gradient and 0.302 ± 0.008 nS when both chambers were 150 mM KCl (Fig. 2C Right). The BAX channel was observed in this activity state at pH 7.0 for prolonged periods. At pH 7.0 the BAX pore had linear voltage relationship and retained a mild selectivity for Cl− (PK/PCl = 0.5).
Incorporation of BAX into Planar Lipid Bilayers at pH 7.0 by Fusion of Reconstituted Proteoliposomes.
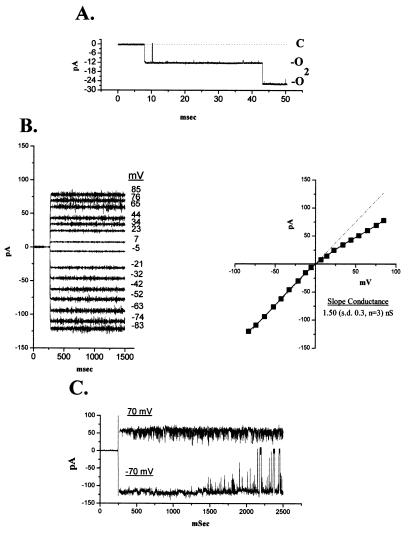
Purified BAX protein incorporated into lipid vesicles was added to the cis chamber of an established lipid bilayer with a 450-/150-mM KCl gradient to permit identification of initial currents. The inward (PK < PCl) current observed when liposomes containing reconstituted BAX fused to the planar lipid bilayers at pH 7.0 had a large open time (PO > 0.95) and a large conductance (1.5 ± 0.3 nS, n = 3, Fig. 3B). Transitions between current levels (O → O2, Fig. 3A) that were identical in amplitude suggested that O represented the single-channel conductance (Fig. 3A). The voltage dependence of the current was determined when one channel was present (Fig. 3B Left). Mild outward rectification is evident in symmetrical KCl concentrations. A mild Cl− selectivity (PK/PCl = 0.32) was calculated from the reversal potential when cis-to-trans KCl was 450/150 mM. At positive voltages (+70 mV) the rapid flickering (Fig. 3C) was consistent with channel block as a mechanism for the rectification seen in B. At −70 mV there was no flickering, but periodic closing of 20- to 30-msec duration was observed (Fig. 3C), which is similar to that reported for porin-type channels (26).
Figure 3.
Proteoliposome insertion of BAX into planar lipid bilayer membranes. (A) Proteoliposomes with incorporated BAX were added to the cis chamber after a bilayer with 0.4-μF capacitance was obtained. A large Cl−-selective pore (O) appears in the presence of a 450-/150-mM KCl gradient (V = 0 mV). The size of the current labeled O2 that appears after the initial activity (O) implies the presence of two channels. (B) Voltage dependence of the current in symmetrical 150 mM KCl. (C) Longer tracing of currents demonstrates rectification at +70 mV and closures at −70 mV.
Low pH Formation of BCL-2 Channels in Planar Lipid Bilayers.
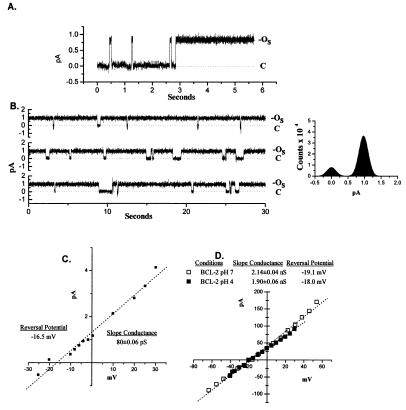
Purified soluble BCL-2 protein was added to the cis chamber of an established bilayer in a 450-/150-mM KCl gradient at pH 4.0. An initial outward (PK/PCl = 3.9) current (OS) consistently appeared within 5 min in multiple experiments (Fig. 4A). The magnitude of the current was 0.85 ± 0.06 pA, which flickered open and shut in the first few seconds and subsequently remained open. Under these conditions the BCL-2 channel had a conductance of 80.3 ± 0.06 pS (Fig. 4C).
Figure 4.
Insertion of soluble BCL-2 into planar lipid bilayers. (A) Purified BCL-2 (≈1 μg) was added to the cis chamber of a 450-/150-mM KCl gradient, and in 5–20 min an outward K+ current was consistently observed. (B) Long recordings of the channel transitions between the open (OS) and closed (C) state. A histogram of the distribution of amplitudes over 120 sec is shown. (C) A current–voltage plot of the BCL-2 channel from B. (D) A current–voltage plot of the large pore that formed over time at pH 4.0 and its current–voltage plot following the shift to pH 7.0.
Subsequently this channel remained open for long periods (5–10 sec) with brief closures (Fig. 4B). This initial state of the BCL-2 channel was present for only 2–5 min under these conditions. BCL-2 progressed to a stable open pore at pH 4.0 with a 1.90 ± 0.06 nS conductance (Fig. 4D). Shifting the pH to 7.0 maintained a large pore with a 2.14 ± 0.04 nS conductance and K+ selectivity of PK/PCl = 6.5 (Fig. 4D).
Incorporation of BCL-2 into Planar Lipid Bilayers by Fusion of Reconstituted Proteoliposomes.
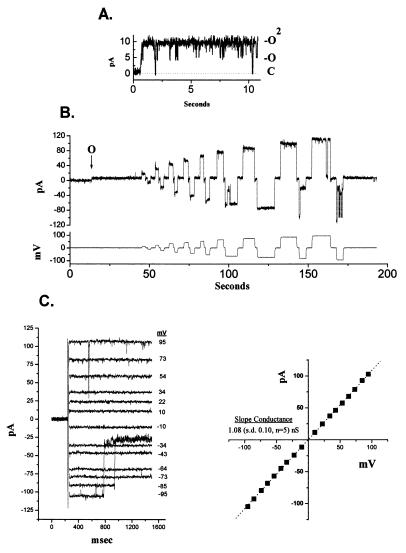
Purified BCL-2 protein incorporated into lipid vesicles was added to the cis chamber of an established bilayer with a 450-/150-mM KCl gradient. In multiple experiments reconstituted BCL-2 always resulted in an outward (K+) current (Fig. 5A). Fig. 5A shows a channel that initially flickers between multiple levels (O, O2). When a series of voltage steps were applied to a single channel established in the bilayer, channel closures were observed at voltages greater than ±50 mV (Fig. 5B). These closures became more frequent as the voltage increased. The two plots in Fig. 5C show the voltage dependence of the open channel current in symmetrical 150-mM KCl as linear with a slope conductance of 1.08 (SD 0.10, n = 5) nS. However, with hyperpolarization to more than −70 mV, partial channel closures occur (Fig. 5C Left). The reversal potential in KCl gradients indicated a mild K+ selectivity (PK/PCl = 2.4). The small channels and time-dependent changes noted when soluble BCL-2 was inserted into planar lipid bilayers were not observed when reconstituted proteoliposomes containing BCL-2 were fused to planar lipid bilayers.
Figure 5.
Proteoliposome insertion of BCL-2 into planar lipid bilayer membranes. (A) Proteoliposomes with incorporated BCL-2 were added to the cis chamber after a bilayer with 0.4 μF capacitance was obtained. In multiple determinations an initial K+ channel was always obtained. In the preparation shown, two BCL-2 conductance levels (O, O2) appear simultaneously in the bilayer (V = 0 mV). (B) A series of voltage steps (indicated in the lower tracing) was applied to an established single channel (indicated by O) in a 450-/150-mM KCl gradient. The resultant continuous tracing of channel current is shown in the upper tracing. (C) The open channel currents in symmetric 150-mM KCl and the current–voltage plot for the BCL-2 pore.
DISCUSSION
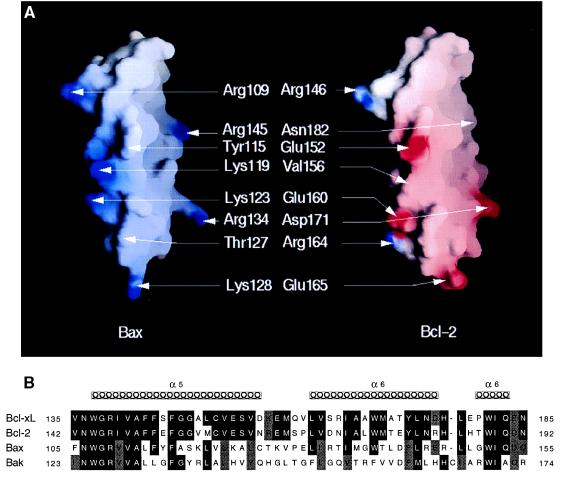
The structure of BCL-XL, in which two central hydrophobic helices, α5 and α6, are surrounded by four amphipathic helices, is similar to the T8 and T9 helices of diphtheria toxin fragment B, which is important for insertion into membranes at low pH (20, 27, 28). Mutational studies that replace acidic with basic residues in T8/T9 prevented the insertion at low pH, which is consistent with a role for their protonation in membrane fusion (29). The analogy with pore-forming toxins was extended by the demonstration that acid pH facilitates the insertion of BCL-XL into lipid bilayers (22). We found that both BAX and BCL-2 initiate rapid release of ions from liposomes when added at low pH. However, BAX demonstrated a broader pH optimum, retaining activity as high as pH 5.5. This might reflect the higher α5-helix pI of 10.64 for BAX versus 4.55 for BCL-2 (Fig. 6). The observation that deletion of the α5 and α6 helices of BCL-2 altered its characteristics emphasizes their importance (23). If the insertion of the putative transmembrane α5 and α6 helices of these apoptotic regulators benefits from charge reduction, the lower pH requirement for BCL-2 may reflect glutamic acid residues that would be prone to ionization with increasing pH in contrast to the presence of lysine and arginine residues in BAX (Fig. 6A).
Figure 6.
Comparison of the charged amino acids in the putative membrane penetrating α-5 and α-6 helices of BAX and BCL-2. (A) Views of the positively charged surface of the α-5 and α-6 helices of BAX (Left) and of the negatively charged surface of the same region of BCL-2 (Right), calculated and displayed using grasp (40). The surfaces are colored deep blue (15 kBT) in the most positively charged regions and deep red (−15 kBT) in the most negative, with linear interpolation for values in between. Both models were generated using insightii (BiosymTechnologies, San Diego) from the crystallographic model of BCL-XL (PDB entry 1MAZ). (B) Sequence alignment of α-5 and α-6 helices in two antiapoptotic molecules (BCL-XL and BCL-2) and in two proapoptotic molecules (BAX and BAK).
The ion channels formed by BAX and BCL-2 in planar lipid bilayers have characteristics that depend in part on the method of incorporation. When soluble BAX or BCL-2 was inserted into bilayers at low pH the initial currents were small with conductances of 22 and 80 pS, respectively. Like BCL-XL (22) and other observations on BCL-2 (23), we also observed a mild cation selectivity for antiapoptotic BCL-2; however, we noted that the proapoptotic molecule BAX has a consistent anion selectivity. If the α5 and α6 helices contribute to the channel these selectivities may reflect the positively charged residues of BAX and negatively charged residues of BCL-2 (Fig. 6A). Although modest differences in ion selectivity are unlikely to be the sole explanation for opposite influences on apoptosis, these charge reversals appear to be consistent in the α5 and α6 helices of anti- versus proapoptotic members (Fig. 6B). BAX channels respond to shifting the pH to 7.0 after insertion at pH 4.0 consistent with previous observations on toxins and porins (30, 31). The changes we noted could also relate to pH dependent ionization of charged residues in these channels.
A striking progression of the BAX channel in planar bilayers occurred within 2–4 min of its initial appearance. This included (i) an early Cl−-selective small channel, (ii) a transition phase with multiple subconductance levels and moderate Cl− selectivity, and (iii) an apparently stable ohmic pore of large conductance that is mildly Cl− selective and open continuously (Fig. 2). The BCL-2 channel activity also progressed from an early K+-selective small channel that opened and closed spontaneously to a large ohmic pore (Fig. 4). Removal of protein from the chamber and alteration of salt concentrations did not prevent this transition, which may represent intramembranous organization of BCL-2 or BAX into its mature form. Of note, shifting from pH 4.0 to 7.0 altered the conductance and selectivity of BAX but not BCL-2. In contrast, the overnight reconstitution of BAX or BCL-2 into lipid vesicles that were subsequently fused to planar bilayers resulted immediately in large, open pores (Figs. 3 B and 5C). These observations support the mutational and genetic analyses that argue that BCL-2 and BAX can each function independently. However, it remains uncertain as to whether monomers, dimers, or higher-order structures of these molecules are the active subunit. We cannot firmly exclude the possibility that the progression of BCL-2 and BAX currents reflects a nonphysiologic aggregation of these molecules. Alternatively, the consistency of these transitions indicates they may result from oligomerization, perhaps dimerization, that might also occur in vivo. The large-conductance pores noted for the BCL-2 family share some characteristics with the porins of bacteria, yeast, and mammals (26). The closest structural homologs, diphtheria toxin and colicin, are also capable of forming large ion-conductive pores (27, 28).
How might ion-conductive channels regulate apoptosis? The selective targeting of the BCL-2 family to the outer mitochondrial membrane, nuclear membrane, and endoplasmic reticulum appears important (32–35). Although the differences in ion selectivity are moderate for BCL-2 and BAX, these channels do display other unique characteristics including conductance, voltage dependence, and rectification. BCL-2 and BAX might regulate an electrochemical gradient, osmotic balance, or transport critical substrates residing in the intermembrane space including cytochrome c (36). Upon subcellular fractionation, BCL-2 was retained in mitoplasts (3) and, by immunoelectron microscopy, was clustered (6), indicating that, in part, it localizes to contact points between inner and outer membranes. Apoptosis is accompanied by a permeability transition pore (PTP) that ensures an electrochemical collapse of mitochondria (37, 38). Patch-clamp studies of mitoplasts identified a 1.3-nS megachannel (MMC) that was considered to represent the PTP (39). BCL-2 and BAX become candidates for regulating or being components of the PTP. Alternatively, the BCL-2 family could modify other ion channels or transport molecules not yet tested. BCL-2 apoptotic regulators join bacterial toxins, perforin and complement as pore-forming proteins, suggesting a conserved component of cell death from bacteria to mammals.
Acknowledgments
We thank Colin G. Nichols and Jim Huettner for valuable advice and Mary Pichler for preparation of the manuscript. A.G. is supported by a fellowship from the European Molecular Biology Organization. K.Y. is a fellow of the National Cancer Institute–Japanese Foundation for Cancer Research Research Training Program. P.H.S. is supported by Alzheimer’s Disease Research Center Grant AG05681-14.
References
- 1.Farrow S N, Brown R. Curr Opin Gen Dev. 1996;6:45–49. doi: 10.1016/s0959-437x(96)90009-x. [DOI] [PubMed] [Google Scholar]
- 2.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 3.Hockenbery D, Nunez G, Milliman C, Schreiber R D, Korsmeyer S J. Nature (London) 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 4.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 5.Nguyen M, Millar D G, Yong V W, Korsmeyer S J, Shore G C. J Biol Chem. 1993;268:25265–25268. [PubMed] [Google Scholar]
- 6.de Jong D, Prins F A, Mason D Y, Reed J C, van Ommen G B, Kluin P M. Cancer Res. 1994;54:256–260. [PubMed] [Google Scholar]
- 7.Boulakia C A, Chen G, Ng F W H, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- 8.Armstrong R C, Aja T, Xiang J, Gaur S, Krebs J F, Hoang K, Bai X, Korsmeyer S J, Karanewsky D S, Fritz L C, Tomaselli K J. J Biol Chem. 1996;271:16850–16855. doi: 10.1074/jbc.271.28.16850. [DOI] [PubMed] [Google Scholar]
- 9.Chinnaiyan A M, Orth K, Orourke K, Duan H J, Poirier G G, Dixit V M. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 10.Xiang J, Chao D T, Korsmeyer S J. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy N J, Whyte M K, Gilbert C S, Evan G I. J Cell Biol. 1997;136:15–27. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin X M, Oltvai Z N, Korsmeyer S J. Nature (London) 1994;369:321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 13.Sedlak T W, Oltvai Z N, Yang E, Wang K, Boise L H, Thompson C B, Korsmeyer S J. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng E H, Levine B, Boise L H, Thompson C B, Hardwick J M. Nature (London) 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 15.Chittenden T, Flemington C, Houghton A B, Ebb R G, Gallo G J, Elangovan B, Chinnadurai G, Lutz R J. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter J J, Parslow T G. J Biol Chem. 1996;271:8521–8524. doi: 10.1074/jbc.271.15.8521. [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Yin X-M, Chao D T, Milliman C L, Korsmeyer S J. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 18.Zha, J. P., Harada, H., Osipov, K., Jockel, J., Waksman, G. & Korsmeyer, S. J. (1997) J. Biol. Chem., in press. [DOI] [PubMed]
- 19.Knudson C M, Korsmeyer S J. Nat Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- 20.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S, Ng S, Fesik S W. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 21.Sattler M, Liang H, Nettesheim D, Meadows R P, Harlan J E, Eberstadt M, Yoon H S, Shuker S B, Chang B S, Minn A J, Thompson C B, Fesik S W. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 22.Minn A J, Velez P, Schendel S L, Liang H, Muchmore S W, Fesik S W, Fill M, Thompson C B. Nature (London) 1997;385:353–357. doi: 10.1038/385353a0. [DOI] [PubMed] [Google Scholar]
- 23.Schendel S L, Xie Z, Montal M O, Matsuyama S, Montal M, Reed J C. Proc Natl Acad Sci USA. 1997;94:5113–5118. doi: 10.1073/pnas.94.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonsson B, Conti F, Ciavatta A M, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod J-J, Mazzei G, Maundrell K, Gambale R S, Martinou J-C. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 25.Peterson A A, Cramer W A. J Membr Biol. 1987;99:197–204. doi: 10.1007/BF01995700. [DOI] [PubMed] [Google Scholar]
- 26.Benz R. Biochim Biophys Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 27.Choe S, Bennett M J, Fujii G, Curmi P M, Kantardjieff K A, Collier R J, Eisenberg D. Nature (London) 1994;357:216–222. doi: 10.1038/357216a0. [DOI] [PubMed] [Google Scholar]
- 28.Stroud R. Curr Opin Struct Biol. 1995;5:514–520. doi: 10.1016/0959-440x(95)80037-9. [DOI] [PubMed] [Google Scholar]
- 29.Silverman J, Mindell J, Finkelstein A, Shen W, Collier R. J Biol Chem. 1994;269:22524–22532. [PubMed] [Google Scholar]
- 30.Mindell J, Silverman J, Collier R, Finkelstein A. Biophys J. 1992;62:41–44. doi: 10.1016/S0006-3495(92)81772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todt J, Rocque W, McGroarty E. Biochem. 1992;31:10471–10478. doi: 10.1021/bi00158a009. [DOI] [PubMed] [Google Scholar]
- 32.Hockenbery D M, Oltvai Z N, Yin X-M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen M, Branton P E, Walton P A, Oltvai Z N, Korsmeyer S J, Shore G C. J Biol Chem. 1994;269:16521–16524. [PubMed] [Google Scholar]
- 34.Tanaka S, Saito K, Reed J C. J Biol Chem. 1993;268:10920–10926. [PubMed] [Google Scholar]
- 35.Zhu W, Cowie A, Wasfy G W, Penn L Z, Leber B, Andrews D W. EMBO J. 1996;15:4130–4141. [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X S, Kim C N, Yang J, Jemmerson R, Wang X D. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 37.Zamzami N, Susin S A, Marchetti P, Hirsch T, Gomez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoratti M, Szabo I. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 39.Szabo I, Zoratti M. J Biol Chem. 1991;266:3376–3379. [PubMed] [Google Scholar]
- 40.Nicholls A, Sharp K A, Honig B. Protein Struct Funct Genet. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]