Abstract
Aged dogs demonstrate cognitive decline that is linked to brain aging. The purpose of the present study was to examine if a commercially available nutraceutical supplement that may be neuroprotective and contains phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine could improve cognitive function in aged beagles. Nine aged beagles were tested on performance on a delayed-non-matching-to-position task, which is a neuropsychological test of short-term visuospatial memory. All subjects were tested on 5 baseline sessions; then, to assess the supplement, a crossover design was used in which 1 group received the supplement and the other a control substance in the 1st phase, with treatment conditions being reversed in the 2nd phase. Performance accuracy was significantly improved in supplemented dogs compared with control dogs and the effect was long lasting. These findings suggest that the nutraceutical supplement can improve memory in aged dogs.
Résumé
Amélioration de la performance de la mémoire à court terme chez des Beagle âgés par un supplément nutraceutique contenant de la phosphatidylsérine, du Ginkgo biloba, de la vitamine E et de la pyridoxine. Les chiens âgés démontrent un déclin cognitif qui est lié au vieillissement du cerveau. La présente étude avait pour but d’examiner si un supplément nutraceutique disponible dans le commerce, qui peut fournir une neuroprotection et contient de la phosphatidylsérine, du Ginkgo biloba, de la vitamine E et de la pyridoxine, pourrait améliorer la fonction cognitive chez des Beagle âgés. Neuf Beagle âgés ont été évalués pour leur performance lors d’une tâche d’appariement différé sans correspondance, qui est un test neuropsychologique de la mémoire visuospatiale à court terme. Tous les sujets ont été testés en fonction de 5 séances de référence; puis, pour évaluer le supplément, un schéma croisé a été utilisé selon lequel un groupe a reçu le supplément et l’autre une substance contrôlée dans la première phase, avec renversement des conditions de traitement dans la deuxième phase. La performance a été significativement améliorée chez les chiens ayant pris le supplément comparativement aux chiens témoins et l’effet a été de longue durée. Ces constatations suggèrent que le supplément nutraceutique peut améliorer la mémoire chez les chiens âgés.
(Traduit par Isabelle Vallières)
Introduction
With increasing age, dogs develop a form of neurodegenerative disease, which has many similarities to age-related cognitive impairment and Alzheimer’s disease in humans (1,2). Diagnosing cognitive dysfunction syndrome (CDS) in the dog is difficult, because it is primarily dependent on the pet owner reporting signs in specific categories, including disorientation, alterations in social interactions with owners or other pets, alterations in sleep-wake cycles, appearance of house soiling or changes in learned behaviors, and alterations in activity levels. In 1 study that examined the prevalence of cognitive dysfunction in 180 dogs aged 11 y and older with no underlying medical problems, at least 1 category of clinical signs was reported by 28% of owners of dogs aged 11 to 12 y and 68% of owners of dogs aged 15 to 16 y (3). In another study, it was found that when asked, 75% of owners of dogs over 7 y of age reported 1 or more signs, but only 12% of these owners reported the signs spontaneously to their veterinarians (4). In a more recent study of a population of 102 dogs aged 7 y and older, 27 of the dogs experienced successful aging, 42 dogs had alterations in 1 category, and 33 dogs had signs in 2 or more categories (5). Consequently, CDS represents a significant clinical therapeutic target, but only 2 therapeutic agents, selegiline and a prescription diet (Canine b/d; Hill’s Pet Nutrition, Topeka, Kansas, USA), are approved in Canada and the USA.
In contrast to the clinical environment, neuropsychological testing in the laboratory clearly reveals age differences in canine learning and memory (6). These tests provide quantitative and objective measures of cognitive function without relying on subjective owner evaluations. Neuropsychological test results indicate that learning and memory deficits in a test of short-term working memory, the delayed-non-matching-to-position (DNMP) task, can be identified in dogs as early as 6 y of age (7), thereby indicating that the DNMP is particularly sensitive to age. By contrast, clinical signs of cognitive dysfunction may not be recognized by pet owners until 11 y of age or older (8,9). Rather than relying on the subjective assessment of pet owners in populations of clinically affected senior pets, neuropsychological tests can provide objective measurements of therapeutic improvement by using a relatively small number of dogs.
Although the exact cause of cognitive dysfunction is not known, several changes have been identified in the brains of aging dogs and cats, including increased markers of oxidative stress; reduced brain mass; increased ventricular size; meningeal calcification; demyelination; glial changes; a reduction in neurons; neuroaxonal degeneration; and an increase in apoptic bodies, lipofuscin, and beta-amyloid (10–14). Neuropsychological testing suggests that both oxidative damage and increased beta-amyloid load in the canine brain are potential factors leading to cognitive dysfunction (6,12,15,16). Recent studies in dogs with cholinergic drugs also indicate that the early impairments in DNMP is likely linked to age-dependent cholinergic dysfunction, which is also seen in humans (17,18). Because aging is associated with several physiological changes, it is suggested that a synergistic combination of ingredients, such as fatty acids, essential minerals, vitamins, antioxidants, energetic cofactors, and trophic nutrients, rather than monotherapy, may be ideally suited to contribute to brain cell health and memory preservation (19–21). Because oxidative damage, in combination with several other pathological events, accumulates with age in the dog (2,6,12), this multimodal synergistic approach has become a focus of therapeutic interventions in the dog (5,22–26).
The current study examined a proprietary nutraceutical supplement (Senilife; Innovet Italia s.r.l., Milano, Italy) containing phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine. The product is licensed in Italy for treatment of pathophysiological brain aging of elderly cats and dogs and is marketed as a neuroprotective nutritional supplement. In reports of 3 recent clinical trials, it was stated that products containing at least phosphatidylserine, or the specific proprietary supplement mentioned above, improved clinical signs associated with CDS in client-owned pets (5,24–26); however, the effects on cognition have not been investigated in a laboratory setting. Consequently, we examined the effects of this nutraceutical formula on memory performance of aged dogs by using the DNMP, which is highly sensitive to age-associated impairment (7), with the overall purpose of determining if this combination could improve short-term memory and if neuropsychological test protocols in screening interventions that modify cognitive function were able to predict clinical outcomes.
Materials and methods
Subjects
Four male and 5 female beagles, ranging in age from 7 to 12.7 y (mean = 8.2, s&xmacr; = 0.60 y), were used in this study, because, previously, we have found that this age range demonstrates deficits on the DNMP (7). All subjects were part of an aged beagle colony (CanCog Technologies, Toronto, Ontario) that had had previous experience on a variety of cognitive tests and were healthy over the duration of the study. The subjects were housed in pens approximately 1.5 × 4.9 m in groups of 4, based on compatibility, and were provided with toys for enrichment. Daily food rations (Purina Pro Plan Chicken and Rice; Nestle Purina, St. Louis, Missouri, USA) were provided once daily in the morning for a half-hour period prior to testing; water was available ad libitum. Dogs were maintained on a natural light-dark cycle. All procedures were approved by the facility’s Animal Use and Care Committee and were compliant with the guidelines of the Canadian Council on Animal Care (27).
General design
The general design of the experiment is shown in Table 1. At the start of the study, subjects were tested on 5 DNMP sessions. The subjects then were divided into 2 groups, based primarily on DNMP performance, which was done to avoid a performance bias between the 2 groups, and secondarily on gender and age to the extent possible. A blinded crossover design was then used to examine the effects of the supplement. For the 1st phase, 1 of the 2 groups received the supplement in a single meatball (5 subjects), the other group (4 subjects) served as control and received a single meatball without the supplement. The subjects received their respective treatments over a 60-day wash-in and for an additional 10 d on which they were tested on the DNMP, for a total of 70 d on their respective treatment conditions. For the 2nd phase, the groups were reversed, such that the subjects that received a meatball with the supplement during the 1st phase received a meatball without supplement (control) in the 2nd phase and vice versa. In all other respects, the 2nd phase was identical to the 1st. The treatment length was based roughly on the time course of a positive clinical outcome that used the same supplement (5).
Table 1.
General design of the delayed-non-matching-to-position (DNMP) testing schedule
| Test procedure | Test day |
|---|---|
| Baseline DNMP | 1–5 |
| Phase 1: Wash-in | 6–65 |
| Phase 1: DNMP testing | 66–75 |
| Phase 2: Wash-in | 76–135 |
| Phase 2: DNMP testing | 136–145 |
Administration of supplement
A single capsule of the supplement was administered per 5 kg of body weight once daily and all capsules were administered in a single meatball, such that an animal weighing between 5.01 kg and 10.00 kg received 2 capsules; an animal weighing between 10.01 kg and 15.00 kg received 3 capsules; and an animal weighing between 15.01 kg and 20.00 kg received 4 capsules. Each capsule contained 25 mg of phosphatidylserine (from GM-free soya), 50 mg of Ginkgo biloba extract titrated in ginkgosides (24%), 20.5 mg of pyridoxine HCl (vitamin B6), and 33.5 mg of d-alpha-tocopherol (natural vitamin E). Control subjects received only a single meatball. Subjects were weighed every 2 wk and the doses were adjusted accordingly. All dosing occurred in the morning prior to any cognitive testing.
Cognitive testing apparatus
A wooden chamber, previously described (28,29), was used for cognitive testing. Vertical stainless steel bars covering the front of the box provided access to a sliding plastic tray, which contained 1 medial and 2 lateral food wells. The bars were adjustable, allowing the size of the openings to be individually set for each dog. During the test trials, 1 or 2 wells were covered by objects; in the present study, these consisted of yellow blocks. Displacement of any object was considered a response choice. The dogs and the tester were separated by a wooden screen, which had a hinged door at the bottom, to allow presentation of the sliding tray, and a 1-way mirror above, which allowed the subject to view the dog. A 60-watt light, attached to the front of the chamber, served as the only light source. For an illustration of the apparatus see references 8 and 28.
Delayed-non-matching-to-position procedures
The DNMP is a test of short-term memory that is highly sensitive to age and has been discussed and validated extensively (6,7,17,22,30). In the variable form of the task, the delays vary within a session to differentially tax short-term memory. All subjects were previously trained on the task and were performing consistently (baseline performance) prior to the start of the study. Each trial consisted of 2 phases. The initial sample presentation consisted of placing a block in 1 of 3 spatial positions (left, middle, right). The subject was required to displace the block to obtain a hidden food reward. The tray was then removed from view and a delay was initiated. After the delay, on the test phase, the tray was presented with 2 identical blocks covering 2 spatial positions, 1 of which was the position used during the sample phase. To obtain the food reward, the animal was required to remember the location used in the sample phase and displace the block at the other location. Thus, if the initial sample was presented to the left location, and on the test phase, blocks were at the left and right locations, the correct response would be to displace the block at the right location. For each test session, animals received 12 trials during a daily test session, separated by a 30-s interval, using delays of 20 and 90 s equally within the test session. A correction procedure was used in which the subject was allowed to correct its 1st incorrect choice and receive the reward; on all subsequent errors, the subject was not permitted to retrieve the reward.
Data analysis
Statistical analyses were conducted by using commercially available software (Statistica 6.0; StatSoft, Tulsa, Oklahoma, USA), with significance set at P < 0.05. The data all passed the Kolmogorov-Smirnov normality test, and, consequently, were analyzed using parametric statistics. Initially, mean DNMP performance was examined with a repeated-measures analysis of variance (ANOVA), with test order (supplement-control versus control-supplement) serving as a between-subject variable, and with treatment (baseline versus control versus supplement) and delay (20 s versus 90 s) serving as within-subject variables. The data were analyzed subsequently by using a repeated-measures ANOVA with test order as a between-subject variable and treatment (control versus supplement), phase (sessions 1–5 versus sessions 6–10), and delay (20 s versus 90 s) as within-subject variables. Post-hoc tests consisted of ANOVA or Fisher’s least significant difference.
Results
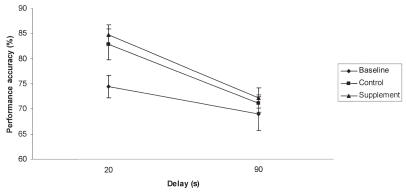
The initial analysis comparing mean performance at each delay among baseline, control, and supplement treatment revealed a significant effect of treatment (P = 0.020) and delay (P < 0.001). Post-hoc Fisher’s least significant difference indicated that performance in subjects receiving the supplement was improved, compared with their baseline performance (P = 0.039) (Figure 1). By contrast, the performance of subjects receiving placebo (control group) did not differ from their baseline. The main effect of delay was due to less accurate performance at the 90 s delay compared with the 20 s delay.
Figure 1.
Mean performance across delays. Subject’s performance was significantly more accurate on the supplement (triangle) compared with the baseline (circle). No difference between control (square) performance and other conditions were found. The supplement contained phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine in a meatball. The control was only a meatball and the baseline consisted of no treatment and was used to group the dogs into 2 groups of similar DNMP performance levels. Error bars represent standard error of the mean.
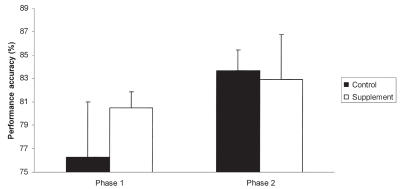
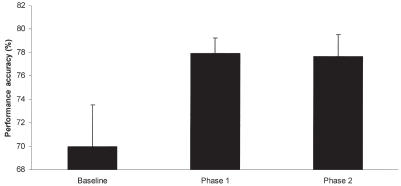
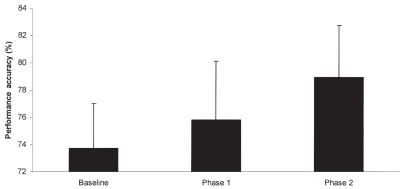
The analysis excluding baseline performance revealed a significant effect of phase (P = 0.008), which was due to improved performance in the 2nd group of 5 sessions compared with the 1st group. Additionally, a significant interaction among treatment, phase, and test order was noted (P = 0.035). The origin of this interaction was based on performance on the 2nd group of 5 test sessions. Post-hoc Fisher’s least significant difference indicated that subjects receiving the supplement following crossover performed significantly better (P = 0.022) on the supplement compared with their control performance in the 1st phase of the crossover (Figure 2). By contrast, no differences were found between control and treatment performance in the group receiving the supplement in the 1st phase of the crossover, indicating a maintained level of performance in those subjects. To better clarify this effect, subjects that received the supplement in the 1st phase of the study showed an increase in performance accuracy compared with baseline that was maintained over the 2nd phase of the study (Figure 3). Subjects that received the supplement in the 2nd phase of the study showed the largest improvement on the 2nd phase of the study (Figure 4). Thus, both groups showed the greatest increase in performance while on the supplement.
Figure 2.
Mean performance accuracy by treatment and order. Subjects receiving control (black bars) followed by the supplement (white bars) showed a significant increase in performance accuracy over on the last 5 DNMP sessions. By contrast, animals that received the supplement prior to control did not show a significant increase in performance despite the additional practice. The supplement contained phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine in a meatball. The control was only a meatball. The 1st phase represents the treatment provided before the crossover and the 2nd phase represents the treatment conditions after crossover. Error bars represent standard error of the mean.
Figure 3.
Mean performance accuracy over the 3 phases of the study in subjects that received the supplement (phase 1) before control (phase 2). Subjects receiving the supplement before control showed an increase in performance accuracy compared to baseline while under the supplement that was maintained during control. The supplement contained phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine in a meatball. The control was only a meatball. The 1st phase represents the treatment provided before the crossover and the 2nd phase represents the treatment conditions after crossover. Error bars represent standard error of the mean.
Figure 4.
Mean performance accuracy over the 3 phases of the study in subjects that received the control (phase 1) before supplement (phase 2). Subjects receiving the supplement after control showed an increase in performance accuracy only while on the supplement. The supplement contained phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine in a meatball. The control was only a meatball. The 1st phase represents the treatment provided before the crossover and the 2nd phase represents the treatment conditions after crossover. Error bars represent standard error of mean.
Discussion
The present results indicate that a nutraceutical supplement containing phosphatidylserine, Ginkgo biloba, vitamin E, and pyridoxine improved canine short-term memory performance when assessed by the DNMP. Increasing delay on the DNMP resulted in lower performance due to increased memory demand, which further supports the use of the DNMP in assessing canine memory function. In the current study, subjects reached their peak performance while on the supplement and their performance was maintained after discontinuation over 70 d, which suggests that the treatment may have long-term benefits. This effect is consistent with the effects of phosphatidylserine in humans (31,32), where beneficial effects may be present up to 12 wk after treatment is discontinued. The current and previous studies did not address the pharmacokinetic to pharmacodynamic relationship of the supplement ingredients. Consequently, the beneficial long-term effect may be a result of either a direct long-acting effect or an indirect carry over effect of cognition enhancement in the previous test phase. Further research is required to determine the mechanism of action of this long-term beneficial effect.
The absence of a difference between placebo and supplemented dogs over the course of this study was likely due to the long lasting effect of the supplement in the subjects that received the nutraceutical treatment in the 1st phase of the study. Alternately, the high levels of performance during the 2nd phase of the study could also be attributed to a practice effect. If this were the sole explanation, we would have expected performance of both groups of subjects to improve in the 2nd phase of the study compared with the 1st, which was not the case. By contrast, a short-term practice effect was found, in that performance of both groups improved significantly in the 2nd group of 5 sessions compared with the 1st group of 5 sessions. This improvement was most dramatic when on the treatment, which suggests that the nutraceutical may have worked by improving animals’ ability to learn to remember. Collectively, this supports the hypothesis that the effect on DNMP performance in the present study was due to treatment and not a practice effect.
A major limitation of the present study was the treatment duration (70 d), which may have provided only a limited ability to address the progressive nature of cognitive dysfunction or possible neuroprotective effects of the treatment. To obviate this difficulty, we selected a task in which dogs show deficits as early as 6 y of age (7). In a longitudinal study examining the effects of an antioxidant and mitochondrial cofactor enriched diet, a treatment effect on the DNMP was not detected until the subjects had been on the diet for 2 y (22), but that study differed from the current study in 3 important aspects: 1) the subjects in the present study were, on average, younger; older dogs have greater levels of brain lesions and generally show greater impairment, both of which may not be readily reversible; 2) the previous study examined relearning of the DNMP at 10 s and DNMP memory capacity, which may not be as robust as the variable-delay paradigm used in the present study; and 3) nutraceuticals can provide larger concentrations of active ingredients compared with dietary supplementation, especially for ingredients that are difficult to formulate into foods. Regardless, it is likely that a larger effect would be evident with a larger sample or an increased treatment duration, because both cognitive decline and brain lesions are progressive and should result in increased impairment in subjects under control conditions.
Although there is no direct correlation between the clinical signs seen in pets and the findings of neuropsychological testing, laboratory studies have documented differences in exploratory behavior, social interactions, and activity between young dogs and those that have learning or memory impairments in neuropsychological testing (33). Overall activity is lowest in aged unimpaired dogs compared with young dogs, but the most severely impaired dogs are significantly more active and display undirected and stereotypical patterns of behavior. Similarly, young dogs interact with novel toys to a greater extent than aged unimpaired dogs, age impaired dogs show the least interest. In a test of social responsiveness to a passive human subject, young dogs are more interactive than age unimpaired dogs, while age impaired dogs show the lowest levels of interaction. Lastly, age impaired dogs show disruption in day-night activity levels (34). All of these, and particularly the last, may represent important behavioral changes with clinical relevance. Both the previously mentioned antioxidant enriched diet and the present nutraceutical treatment have been reported as resulting in improvement of clinical signs associated with canine CDS (5,22–26,35), which further supports the use of the laboratory canine cognitive model in assessing interventions for dogs as well as humans (22,36).
The present study did not examine the mechanism of action of the supplement, but previous research on the individual components of the supplement suggests several possibilities. Phosphatidylserine constitutes a major building block of the cell membrane (37–40), stimulates acetylcholine release in the cerebral cortex of elderly animals (39), modulates acetylcholinesterase activity in the synaptosome of the canine brain (37), and inhibits the age-related loss of muscarinic receptors in the rat brain (40). Ginkgo biloba stimulates several neurotransmitters, including acetylcholine (41–49), exerts antioxidant effects (50,51), and may also protect against beta-amyloid lesions (52,53). Pyridoxine, or vitamin B6, is reported to be a cofactor in the synthesis of several neurotransmitters (serotonin, noradrenaline, dopamine) (54), and vitamin E is a potent antioxidant (55). This combination may, therefore, be of particular benefit to dogs, since cholinergic dysfunction (17,18), beta-amyloid lesions (11,16), and increases in oxidative stress (12,56,57) are all linked to age-related cognitive decline in dogs.
Consistent with the cognitive benefits in the current study, supplementation with phosphatidylserine improves social interactions, memory retrieval, and activity in humans and rodents (54–63) and Ginkgo biloba may promote short-term retention of spatial memory (63) and improve cognitive function in humans (65,66) and aged animals (67). Vitamin E was a key ingredient in the above mentioned antioxidant enriched diet in dogs, and blood levels of vitamin E were correlated with cognitive improvement in canine neuropsychological tests (68). Although the mechanism of action was not addressed in the present study, we can speculate that the treatment may have acted in a multimodal fashion by augmenting cholinergic transmission, increasing antioxidant defense, and possibly reducing amyloid lesions, all of which are known to be modified in canine aging and likely contribute to CDS. The findings, therefore, support the argument that combination interventions should be more effective for treating CDS than single ingredients, because they target several changes that occur with aging (22). The results are also consistent with our previous studies examining the antioxidant enriched diet, in that some improvement in cognitive function may occur soon after the treatment is initiated (22).
Another possible limitation of the current study is that the subjects may be younger than those that generally present with CDS in the clinic. Consequently, supplementation of dogs in this age range may not result in obvious and immediate benefits to the pet owner. However, younger dogs (7 to 10 y) showed greater improvement than older dogs (11 to 16 y) in a clinical trial testing the supplement used in the current study (5,24,25). In human clinical trials, phosphatidylserine improved cognition in patients demonstrating mild cognitive impairment as well as in those with Alzheimer’s disease (58,59). Given the progressive nature of CDS (28), it is likely that prevention earlier in the course of the disease, before the formation of pathological lesions, would be most beneficial (69). Collectively, these and the current results support the use of the supplement prior to the onset of CDS.
Footnotes
Author contributions
Drs. Milgram and Araujo developed the protocol with feedback from Dr. Miolo, conducted the study, and interpreted the data. All authors contributed to the writing of the manuscript.
Disclaimer
This project was sponsored by Innovet Italia s.r.l., which provided both funding and the nutraceutical supplement. The study was conducted under contract by CanCog Technologies Inc.
References
- 1.Head E, McCleary R, Hahn FF, Milgram NW, Cotman CW. Region-specific age at onset of beta-amyloid in dogs. Neurobiol Aging. 2000;21:89–96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- 2.Rofina J, van Andel I, van Ederen AM, van Asten AJAM, Wilhelm J, Gruys E. Canine counterpart of senile dementia of the Alzheimer type: amyloid plaques near capillaries but lack of spatial relationship with activated microglia and macrophages. Amyloid. 2003;10:86–96. doi: 10.3109/13506120309041730. [DOI] [PubMed] [Google Scholar]
- 3.Nielson JC, Hart BL, Cliff KD, Ruehl WW. Prevalence of behavioral changes associated with age-related cognitive cognitive impairment in dogs. J Am Vet Med Assoc. 2001;218:1787–1791. doi: 10.2460/javma.2001.218.1787. [DOI] [PubMed] [Google Scholar]
- 4.Hills Pet Nutrition, U.S. Marketing Research Summary, Omnibus Study on Aging Pets, Nov. 2000.
- 5.Osella MC, Re G, Odore R, et al. Canine cognitive dysfunction syndrome; Prevalence, clinical signs and treatment with a neuroprotective nutraceutical. Appl Anim Behav Sci. 2007;105:297–310. [Google Scholar]
- 6.Adams B, Chan A, Callahan H, Milgram NW. The canine as a model of human cognitive aging: recent developments. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:675–692. doi: 10.1016/s0278-5846(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 7.Studzinski CM, Christie LA, Araujo JA, et al. Visuospatial function in the beagle dog: An early marker of cognitive decline in a model of human cognitive aging and dementia. Neurobiol Learn Mem. 2006;86:197–204. doi: 10.1016/j.nlm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Landsberg G, Hunthausen W, Ackerman L. Handbook of Behavior Problems of the Dog and Cat. 2. Edinburgh: Saunders; 2003. pp. 269–322. [Google Scholar]
- 9.Landsberg G, Ruehl W. Geriatric behavioral problems. Vet Clinics North Am Small Anim Pract. 1997;27:1537–1559. doi: 10.1016/s0195-5616(97)50138-0. [DOI] [PubMed] [Google Scholar]
- 10.Borras D, Ferrer I, Pumarola M. Age related changes in the brain of the dog. Vet Pathol. 1999;36:202–211. doi: 10.1354/vp.36-3-202. [DOI] [PubMed] [Google Scholar]
- 11.Cummings BJ, Satou T, Head E, et al. Diffuse plaques contain c-terminal AB42 and not AB40: Evidence from cats and dogs. Neurobiol Aging. 1996;17:653–659. doi: 10.1016/0197-4580(96)00062-0. [DOI] [PubMed] [Google Scholar]
- 12.Head E, Liu J, Hagen TM, et al. Oxidative damage increases with age in a canine model of human brain aging. J Neurochem. 2002;82:375–381. doi: 10.1046/j.1471-4159.2002.00969.x. [DOI] [PubMed] [Google Scholar]
- 13.Rofina JE, Singh K, Skolumalova-Vesela A, et al. Histochemical accumulation of oxidative damage products is associated with Alzheimer-like pathology in the canine. Amyloid: J Protein Folding Disord. 2004;11:90–100. doi: 10.1080/13506120412331285779. [DOI] [PubMed] [Google Scholar]
- 14.Tapp PD, Siwak CT, Gao FQ, et al. Frontal lobe volume, function, and beta-amyloid pathology in a canine model of aging. J Neurosci. 2004;24:8205–8213. doi: 10.1523/JNEUROSCI.1339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings BJ, Head E, Afagh AJ, Ivy GO, Milgram NW, Cotman CW. β-Amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol Learn Mem. 1996;66:11–23. doi: 10.1006/nlme.1996.0039. [DOI] [PubMed] [Google Scholar]
- 16.Colle MA, Hauw JJ, Crespeau F, et al. Vascular and parenchymal Aβ deposition in the aging dog: Correlation with behaviour. Neurobiol Aging. 2000;21:695–704. doi: 10.1016/s0197-4580(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 17.Araujo JA, Chan AD, Winka LL, Seymour PA, Milgram NW. Dose-specific effects of scopolamine on canine cognition: Impairment of visuospatial memory, but not visuospatial discrimination. Pyschopharmacology. 2004;175:92–98. doi: 10.1007/s00213-004-1777-y. [DOI] [PubMed] [Google Scholar]
- 18.Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Psychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Adams SM, Standridge JB. What should we eat? Evidence from observational studies. South Med J. 2006;99:744–748. doi: 10.1097/01.smj.0000220887.52952.f0. [DOI] [PubMed] [Google Scholar]
- 20.Kidd PM. Neurodegeneration from mitochondrial insufficiency; nutrients stem cells, growth factors, and prospects for brain rebuilding through integrative management. Altern Med Rev. 2005;10:268–293. [PubMed] [Google Scholar]
- 21.Donini LM, De Felice MR, Cannella C. Nutritional status determinants and cognition in the elderly. Arch Gerentol Geriatr. 2007;44:143–153. doi: 10.1016/j.archger.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 22.Araujo JA, Studzinski CM, Head E, Cotman CW, Milgram NW. Assessment of nutritional interventions for modification of age-associated cognitive decline using a canine model of human aging. AGE. 2005;27:27–37. doi: 10.1007/s11357-005-4001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milgram NW, Zicker SC, Head EA, et al. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol Aging. 2002;23:737–745. doi: 10.1016/s0197-4580(02)00020-9. [DOI] [PubMed] [Google Scholar]
- 24.Cena F, Colangeli R, Fassola F, et al. Effect of a combination of phosphatidylserine, Ginkgo biloba, vitamin E and pyridoxine on clinical signs of brain ageing; a pilot multicentric study. Proc 2nd Annu Meet Eur Coll Vet Behav Med Companion Anim & 11th Annu Meet Eur Soc Vet Clin Ethology; 2005. pp. 127–135. [Google Scholar]
- 25.Colangeli R, Antoni M, Cena F, et al. The effect and tolerability of a neuroprotective nutraceutical containing phosphatidylserine and Ginkgo biloba on clinical signs of brain aging in dogs. A pilot multicenter study. Veterinaria. 2005;19:13–18. Italian. [Google Scholar]
- 26.Heath SE, Barabas S, Craze PG. Nutritional supplementation in cases of canine cognitive dysfunction — a clinical trial. Appl Anim Behav Sci. 2007;105:274–283. [Google Scholar]
- 27.Canadian Council on Animal Care. Guide to the Care and Use of Experimental Animals, vols. 1 and 2. Ottawa: Canadian Council on Animal Care; 1993. [Google Scholar]
- 28.Landsberg G, Araujo JA. Behavior problems in geriatric pets. Vet Clinics North Am Small Anim Pract. 2005;35:675–698. doi: 10.1016/j.cvsm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Milgram NW, Head E, Weiner E, Thomas E. Cognitive functions and aging in the dog: Acquisition of nonspatial visual tasks. Behav Neurosci. 1994;108:57–68. doi: 10.1037//0735-7044.108.1.57. [DOI] [PubMed] [Google Scholar]
- 30.Chan AD, Nippak PM, Murphey H, et al. Visuospatial impairments in aged canines (Canis familiaris): the role of cognitive-behavioral flexibility. Behav Neurosci. 2002;116:443–454. [PubMed] [Google Scholar]
- 31.Engel RR, Satzger W, Gunther W, et al. Double-blind cross-over study of phosphatidylserine vs. placebo in patients with early dementia of the Alzheimer type. Eur Neuropsychopharmacol. 1992;2:149–155. doi: 10.1016/0924-977x(92)90025-4. [DOI] [PubMed] [Google Scholar]
- 32.Lombardi GF. Pharmacological treatment with phosphatidylserine of 40 ambulatory patients with senile dementia syndrome. Minerva Med. 1989;80:599–602. [PubMed] [Google Scholar]
- 33.Siwak CT, Tapp PD, Milgram NW. Effect of age and level of cognitive function on spontaneous and exploratory behaviors in the beagle dog. Learn Mem. 2001;8:65–70. doi: 10.1101/lm.41701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siwak CT, Tapp PD, Zicker SC, et al. Locomotor activity rhythms in dogs with age and cognitive status. Behav Neurosci. 2003;117:813–824. doi: 10.1037/0735-7044.117.4.813. [DOI] [PubMed] [Google Scholar]
- 35.Dodd CE, Zicker SC, Jewell DE, Fritsch DA, Lowry SR, Allen TA. Can a fortified food affect the behavioral manifestations of age-related cognitive decline in dogs? Vet Med. 2003;98:396–408. [Google Scholar]
- 36.Studzinski CM, Araujo JA, Milgram NW. The canine model of human cognitive aging and dementia: Pharmacological validity of the model for assessment of human cognitive-enhancing drugs. Prog Neuropharmacol Biol Psychiatry. 2005;29:489–498. doi: 10.1016/j.pnpbp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Tasakiris S, Deconstantinos G. Phosphatidylserine and calmodulin effects on Ca2+-stimulated ATPase and acetylcholinesterase activities in the dog brain synatosomal plasma membranes. Int J Biochem. 1985;17:1117–1119. doi: 10.1016/0020-711x(85)90045-x. [DOI] [PubMed] [Google Scholar]
- 38.Re G, Miolo A, Badino P. Phosphatidylserine for brain aging in dogs and cats: a pharmacological glance. Proc 9th Eur Soc Vet Clin Ethology Meet. 2003:53–57. [Google Scholar]
- 39.Yamatoya H, Sakai M, Kudo S. The effects of soybean transphosphatidylated phosphatidylserine on cholinergic synaptic functions of mice. Jpn J Pharmacol. 2000;84:93–96. doi: 10.1254/jjp.84.93. [DOI] [PubMed] [Google Scholar]
- 40.Gelbmann CM, Muller WE. Chronic treatment with phosphatidylserine restores muscarinic cholinergic receptor deficits in the aged mouse brain. Neurobiol Aging. 1992;13:45–50. doi: 10.1016/0197-4580(92)90007-k. [DOI] [PubMed] [Google Scholar]
- 41.Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chopin P, Briley M. Effects of four non-cholinergic cognitive enhancers in comparison with tacrine and galantamine on scopolamine-induced amnesia in rats. Psychopharmacology. 1992;106:26–30. doi: 10.1007/BF02253584. [DOI] [PubMed] [Google Scholar]
- 43.Huguet F, Drieu K, Piriou A. Decreased cerebral 5-HT1A receptors during ageing: reversal by Ginkgo biloba extract (EGb 761) J Pharm Pharmacol. 1994;46:316–318. doi: 10.1111/j.2042-7158.1994.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 44.Huguet F, Tarrade T. Alpha 2-adrenoceptor changes during cerebral ageing. The effect of Ginkgo biloba extract. J Pharm Pharmacol. 1992;44:24–27. doi: 10.1111/j.2042-7158.1992.tb14357.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee TF, Chen CF, Wang LC. Effect of ginkgolides on beta-amyloid-suppressed acetylcholine release from rat hippocampal slices. Phytother Res. 2004;18:556–560. doi: 10.1002/ptr.1493. [DOI] [PubMed] [Google Scholar]
- 46.Nathan P. Can the cognitive enhancing effects of Ginkgo biloba be explained by its pharmacology? Med Hypotheses. 2000;55:491–493. doi: 10.1054/mehy.2000.1099. [DOI] [PubMed] [Google Scholar]
- 47.Taylor JE. Binding of neuromediators to their receptors in rat brain. Effect of chronic administration of Ginkgo biloba extract. Presse Med. 1986;15:1491–1493. [PubMed] [Google Scholar]
- 48.Wang Y, Wang L, Wu J, Cai C. The in vivo synaptic plasticity mechanism of EGb 761-induced enhancement of spatial learning and memory in aged rats. Br J Pharmacol. 2006;148:147–153. doi: 10.1038/sj.bjp.0706720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams B, Watanabe CM, Schultz PG, Krucker T. Age-related effects of Ginkgo biloba extract on synaptic plasticity and excitability. Neurobiol Aging. 2004;25:955–962. doi: 10.1016/j.neurobiolaging.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging. 2002;23:891–889. doi: 10.1016/s0197-4580(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 51.Sastre J, Pallardo FV, Vina J. Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life. 2000;49:427–435. doi: 10.1080/152165400410281. [DOI] [PubMed] [Google Scholar]
- 52.Yao ZX, Han Z, Drieu K, Papadopoulos V. Ginkgo biloba extract (Egb 761) inhibits beta-amyloid production by lowering free cholesterol levels. J Nutr Biochem. 2004;15:749–756. doi: 10.1016/j.jnutbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Yao Z, Drieu K, Papadopoulos V. The Ginkgo biloba extract EGb 761 rescues the PC12 neuronal cells from beta-amyloid-induced cell death by inhibiting the formation of beta-amyloid-derived diffusible neurotoxic ligands. Brain Res. 2001;889:181–190. doi: 10.1016/s0006-8993(00)03131-0. [DOI] [PubMed] [Google Scholar]
- 54.Dakshinamurti K, Paulose CS, Siow YL. Neurobiology of pyridoxine. In: Reynolds RD, Leklem JE, editors. Vitamin B6: Its Role in Health and Disease. New York: Alan R Liss; 1995. pp. 99–121. [Google Scholar]
- 55.Behl C, Moosmann B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Radic Biol Med. 2002;33:182–191. doi: 10.1016/s0891-5849(02)00883-3. [DOI] [PubMed] [Google Scholar]
- 56.Rofina JE, van Ederen AM, Toussaint MJ, et al. Cognitive disturbances in old dogs suffering from the canine counterpart of Alzheimer’s disease. Brain Res. 2006;1069:216–226. doi: 10.1016/j.brainres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Skoumalova A, Rofina J, Schwippelova Z, Gruys E, Wilhelm J. The role of free radicals in canine counterpart of senile dementia of the Alzheimer type. Exp Gerontol. 2003;38:711–719. doi: 10.1016/s0531-5565(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 58.Crook TH, Petrie W, Wells C, Massari DC. Effects of phosphatidylserine in Alzheimer’s disease. Psychopharmacol Bull. 1992;28:61–66. [PubMed] [Google Scholar]
- 59.Crook TH, Tinklenberg J, Yesavage J, Petrie W, Nunzi MG, Massari DC. Effects of phosphatidylserine in age associated memory impairment. Neurology. 1991;41:644–649. doi: 10.1212/wnl.41.5.644. [DOI] [PubMed] [Google Scholar]
- 60.Cenacchi B, Bertoldin T, Farina C, Fiori MG, Crepaldia G. Cognitive decline in the elderly: a double-blind, placebo-controlled multicenter study on efficacy of phosphatidylserine administration. Aging. 1993;5:123–133. doi: 10.1007/BF03324139. [DOI] [PubMed] [Google Scholar]
- 61.Furushiro M, Suzuki S, Shishido Y, et al. Effects of oral administration of soybean lecithin transphosphatidylated phosphatidylserine on impaired learning of passive avoidance in mice. Jpn J Pharmacol. 1997;75:447–450. doi: 10.1254/jjp.75.447. [DOI] [PubMed] [Google Scholar]
- 62.Kataoka-Kato A, Ukai M, Sakai M, Kudo S, Kameyama T. Enhanced learning of normal adult rodents by repeated oral administration of soybean transphosphatidylated phosphatidylserine. J Pharmacol Sci. 2005;98:307–314. doi: 10.1254/jphs.fp0050366. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki S, Yamatoya H, Sakai M, Kataoka A, Furishiro M, Kudo S. Oral administration of soybean lecithin transphophatidylate phosphatidylserine improves memory impairment in aged rats. J Nutr. 2001;131:2951–2956. doi: 10.1093/jn/131.11.2951. [DOI] [PubMed] [Google Scholar]
- 64.Hoffman JR, Donato A, Robbins SJ. Ginkgo biloba promotes short-term retention of spatial memory in rats. Pharmacol Biochem Behav. 2004;77:533–539. doi: 10.1016/j.pbb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Gertz HJ, Kiefer M. Review about Ginkgo biloba special extract EGb 761 (Ginkgo) Curr Pharm Des. 2004;10:261–264. doi: 10.2174/1381612043386437. [DOI] [PubMed] [Google Scholar]
- 66.Le Bars PL, Velasco FM, Ferguson JM, Dessain EC, Kieser M, Hoerr R. Influence of the severity of cognitive impairment on the effect of the Ginkgo biloba Egb761 in Alzheimer’s disease. Neuropsychobiol. 2002;45:19–26. doi: 10.1159/000048668. [DOI] [PubMed] [Google Scholar]
- 67.Wang SJ, Chen HH. Ginkgolide B, a constituent of Ginkgo biloba, facilitates glutamate exocytosis from rat hippocampal nerve terminals. Eur J Pharmacol. 2005;514:141–149. doi: 10.1016/j.ejphar.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 68.Ikeda-Douglas CJ, Zicker SC, Estrada J, Jewell DE, Zicker NW. Prior experience, antioxidants, and mitochondrial cofactors improve cognitive function in aged beagles. Vet Ther. 2004;5:5–16. [PubMed] [Google Scholar]
- 69.Head E, Thornton PL, Tong L, Cotman CW. Initiation and propagation of molecular cascades in human brain aging: Insight from the canine model to promote successful aging. J Neurochem. 2000;82:375–381. doi: 10.1016/s0278-5846(00)00105-6. [DOI] [PubMed] [Google Scholar]