For human immunodeficiency virus type 1 (HIV-1) vaccine development, a central focus is developing a vaccine that primarily drives strong and broad CD8+ cytotoxic T lymphocytes (CTLs). This focus is largely due to the difficulty in generating cross-neutralizing antibodies against the diverse array of HIV viral envelopes (9, 40, 78, 139). The ability of CD8+ T cells to impact or control viral replication is supported by model primate studies and is reiterated by studies of immune control in infected individuals. For example, Cao et al. (28) reported that HIV-1-infected long-term nonprogressors exhibited high levels of HIV-specific CTLs. In addition, HIV-1-infected patients with high levels of virus-specific CTLs exhibited lower viral loads, slower CD4+ T-cell declines as measured in peripheral blood, and relatively stable clinical statuses (23, 81, 108, 112). Preclinical studies conducted with simian immunodeficiency virus (SIV)-infected monkeys showed that upon their in vivo depletion of CD8+ T cells, a rapid and dramatic increase in viremia was observed (70, 131). While no correlates of immunity for protection against HIV-1 have been concretely established, these data as well as those of others (6, 13, 137) strongly suggest that CD8+ T cells are important in controlling viral infection and may be an important component of an effective HIV-1 vaccine. In this review, we summarize recent advances in HIV vaccine development utilizing various platforms that target the induction of cellular immunity. In particular, we draw inferences, as appropriate, for the induction of cellular immune responses at mucosal as well as systemic sites.
CTL-BASED VACCINES: STEPPED ON?
The discontinuation of the Merck recombinant adenovirus type 5 (rAd5) phase II proof-of-concept STEP study is undoubtedly a significant setback for the field of HIV vaccine development. In the study, a trivalent, three-vector rAd5 (Gag, Pol, Nef) vaccine was tested in two cohorts of 1,500 individuals at high risk for HIV infection. The vaccine was scheduled for three immunizations of 1.5 × 1010 viral genomes of each rAd5 vector encoding Gag, Pol, or Nef administered on day 1, month 1, and month 6. There are concerns that preexisting vector serology can mute the effectiveness of a CTL vaccine approach. Accordingly, the first cohort was made up of 1,500 individuals with low levels of preexisting antibodies against Ad5, with titers of ≤1:200, while the second cohort of 1,500 included participants with anti-Ad5 titers of greater than 1:200. However, the STEP study was prematurely stopped by the data- and safety-monitoring board because it was clear that even in the cohort with low titers of preexisting antibodies against Ad5, there was no evidence of efficacy. Table 1 summarizes the results from the cohort with titers of ≤1:200, comparing the rates of infection and viral loads of the vaccinated versus placebo groups. This was the best-response group in earlier clinical studies, eliciting CTL responses in over 60% of vaccinees. As shown in Table 1, in the subset of individuals that received at least two immunizations, there was a trend toward more HIV infections in the rAd5-vaccinated (19/672) group than in those in the placebo (11/691) group. The difference in viral loads 12 to 18 weeks postacquisition of the infection between the vaccinated and placebo groups (40,000 versus 37,000 copies per ml, respectively) were not statistically significant. Since this rAd5 trivalent vaccine did not prevent infection or decrease viral replication in vaccinated individuals, the implications for CTL-based vaccines remain unclear, except that future studies should test hypotheses and concepts that are not identical to the Merck approach. Furthermore, this study draws a line in the sand in that the following CTL-based approach should induce higher levels of CTL responses and induce a different immune phenotype. The inclusion of different antigens or prime-boost strategies in future studies with defined immune endpoints will be important. We will consider many of these in the following sections.
TABLE 1.
Ad5 STEP study trial design and results available to date
| Treatment | No. of patientsa who received indicated no. of immunizations with indicated result:
|
Viral loadb (copies/ml) | |||
|---|---|---|---|---|---|
| ≤1 immunization
|
≤2 immunizations
|
||||
| Infected | Uninfected | Infected | Uninfected | ||
| Placebo | 21 | 762 | 11 | 691 | 37,000 |
| Vaccinated | 24 | 741 | 19 | 672 | 40,000 |
The participants included 1,150 women and 1,850 men.
Determined 8 to 12 weeks after diagnosis of infection.
THE IMPORTANCE OF MUCOSAL RESPONSES
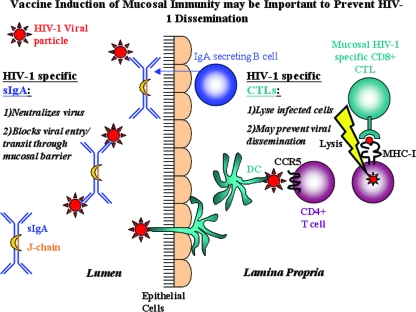
The hallmark of HIV-1 infection is the rapid depletion of CD4+ T cells from the peripheral blood during acute infection. More recently, the ablation of CD4+ CCR5+ T cells in the gut was noted as a dramatic consequence of both SIV (87, 99, 156) and HIV (27, 54, 102, 103) infections. The majority of HIV-1 infections occur across the mucosal barrier, and the mucosal tissues are an active site for viral replication of SIV (87, 155) and HIV (134, 169). The induction of both antigen-specific immunoglobulin A (IgA) antibodies and CTLs at these critical sites may provide a first line of defense with immediate effector activity (Fig. 1). Antigen-specific CTLs in the mucosa could potentially eliminate infected cells within the mucosa and subsequently prevent the systemic spread of the virus. It has been shown in murine (16, 17) and macaque (18) models that mucosal, not systemic, CTLs are necessary to protect against mucosally transmitted virus. Clearly, the generation of CTLs that can target mucosal sites may be an important facet of a successful HIV-1 vaccine. Overall, there is a great need for analyses of mucosal immune responses in preclinical and clinical trials, as the currently available data are minimal.
FIG. 1.
Vaccine induction of mucosal immunity may be important to prevent HIV-1 dissemination. HIV-1 is primarily transmitted through the mucosal surfaces. HIV-1-specific IgA antibodies could potentially neutralize the virus or block viral adherence or entry to mucosal tissues. CTLs specific for HIV-1 could lyse infected cells that present viral antigens via MHC-I, thereby preventing their entry into the systemic compartments. sIgA, secretory IgA.
VIRAL VECTORS
The use of viral vectors as vaccines for HIV-1 has been reviewed elsewhere (135). Viral vectors have been generally studied for gene therapy applications but have also been adapted for vaccine development based upon the intrinsic immunity elicited by their infection of the host. Generally, recombinant viral vectors are constructed by removing gene sequences encoding crucial viral proteins, rendering the resulting vector replication defective, and using the space generated by the removal of these genes for vaccine antigens of interest. As expression systems, recombinant viral vectors can be engineered to transduce a wide variety of cell types, including antigen-presenting cells (APCs), and, subsequently, to express HIV-1-encoded antigens in the cytoplasm of the target cell. Intracellular expression allows for the efficient priming of CD8+ T cells by the presentation of antigenic peptides through major histocompatibility complex class I (MHC-I). In addition, transduced cells serve as antigen factories, which can leak antigens for MHC-II presentation. Through cross-presentation, nontransduced APCs can take up and present extracellular antigens through MHC-I. APCs can “bite” off pieces of transduced cells (58) as well as phagocytosing apoptotic cells that contain transduced antigen (4, 15, 68, 148). It is also possible that pieces of, or whole, antigenic proteins can be transferred from transduced cells to APCs (125) and presented to CTLs through cross-presentation. In addition, viral vectors can activate innate immune responses through the pathogen-associated molecular patterns of Toll-like receptors on APCs (reviewed in reference 170).
Adenovirus.
Adenovirus is a nonenveloped DNA virus that is typically acquired by humans in childhood. Clinical manifestations model the common cold and include respiratory, gastrointestinal, and ocular infections. While symptoms are transient, some serotypes are capable of persisting for much longer (months to years) in mucosal tissues and continuing to shed virus. Adenovirus has been studied extensively as a vaccine platform due to its high transduction efficiency, ease of manipulation, and ability to quickly grow to high viral titers in culture. In addition, recombinant adenoviral vectors can potentially carry large transgene inserts (up to 8 kb) when regions of the viral genome dispensable for viral growth (i.e., E1, E3) are deleted (21, 74). rAd5 is rendered replication deficient by deletion of the E1 region, which is essential for adenovirus replication as well as expression of downstream viral genes. The antigenic transgene is inserted in place of the E1 region under the control of a promiscuous promoter like cytomegalovirus and flanked by a bovine growth hormone polyadenylation terminus signal. Upon administration of a rAd5 vector plus an antigenic insert, the recombinant viral vector binds cells expressing the coxsackievirus and adenovirus receptor, thus facilitating its internalization. As a result, antigen synthesis occurs intracellularly, which leads to efficient processing and presentation of the antigen by MHC-I (reviewed in reference 11). A robust cellular and humoral immune response against the viral vector and transgene insert is observed, in part due to its intrinsic hexon protein-adjuvanting properities (104).
Of the 51 currently known serotypes, rAd5 has received the most attention for use as a vaccine vector and is currently in several clinical trials. However, the potent immune response generated against the Ad5 vector that makes it a promising HIV-1 vaccine candidate also creates one of the most difficult challenges for its development. The prevalence of preexisting anti-Ad5 immunity is astoundingly high among populations that are in desperate need of an HIV-1 vaccine, including many regions of sub-Saharan Africa (12, 80, 110, 158). In fact, Ad5 seroprevalence is greater than 90% in some regions of Africa, with much of the population exhibiting high titers of neutralizing antibody. Ad-specific immunity resulting from natural Ad5 infections decreases the immunogenicity generated against the antigen insert in the recombinant Ad5 vectors (143). In a phase I clinical trial carried out by Merck using rAd5-Gag, the vaccine elicited cellular immune responses in just 28% of subjects that had moderate levels of preexisting Ad5 neutralizing-antibody titers (>200), whereas Gag-specific cellular immune responses were observed in 82% of subjects with no Ad5 immunity (reviewed in reference 12). However, the trivalent vaccine was more effective at an increased dosage (36). Clearly, the significance of developing a vaccine to avoid preexisting antibody responses may be important, and studies using chemical alterations, chimeric Ad vectors, chimpanzee Ad vectors, and rare Ad serotypes found in humans are in development. Chemical modifications to aid Ad5 vectors in immune escape include the addition of microparticles (128) or polyethylene glycol (38) to reduce neutralizing-antibody recognition and increase the effectiveness of multiple immunizations. Another strategy is the construction of chimeric Ad vectors, which are created by swapping regions of the Ad5 fiber or hexon proteins with the corresponding regions of a different serotype. Chimeric Ad hexon vectors are capable of partially evading the antibody response against Ad5; however, they can be difficult to construct due to the preservation of low-titer neutralizing-antibody epitopes (50, 150), low-titer vector replication (126), and the induction of cross-reactive CTLs to other proteins of the virus (167). Barouch's group constructed a rAd5/Ad48 chimeric vector that replaces Ad5 neutralizing-antibody targets with Ad48 surface loops and has been shown to be immunogenic in rhesus macaques despite anti-Ad5 immunity (122). This important modified vector is moving forward into phase I clinical trials in collaboration with the International AIDS Vaccine Initiative (IAVI) (69). Novel rAd5 vectors are also being constructed using nonhuman Ads from ovine, porcine, bovine, and chimpanzee sources (45, 65, 119, 120, 154, 161). In preclinical trials, chimp vectors appear to be highly immunogenic, with T-cell responses against transgenes marginally affected by preexisting antibodies to human Ad5 (46, 117). In addition, chimp vectors elicit antibodies against the transgene in the presence of preexisting antibodies against human Ad5, whereas antibodies against the encoded transgene product are completely inhibited when delivered by the human Ad5 (100). Another strategy is the use of less-prominent adenovirus serotype antibodies found in humans, such as Ad6 (reviewed in reference 12), Ad35 (158), Ad11 (67), and Ad26 (1). Specifically, it has been reported that Ad35 is immunogenic and not inhibited by preexisting anti-Ad5 antibodies in mice (12); however, Ad35 elicits less-potent antitransgene responses than recombinant Ad5 vectors (11, 136). Ad35 is in clinical development by the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), and GenVec. Based upon the safety data for Ad35 from 15 volunteers, a second part of the trial will pursue the delivery of Ad35 in combination with Ad5 (69). Ad26 is another adenovirus serotype, less prevalent in humans, that will be moving into clinical trials (69). In rhesus macaques, rAd26 elicited strong cellular and humoral immune responses similar to those observed with rAd5 (1). In addition, an rAd26 prime-rAd5 boost regimen elicited cellular responses 10 times that observed with a homologous rAd5 immunization and partially controlled viremia following a SIVmac251 challenge (84). Recombinant adenovirus vectors are also attractive vaccines for HIV-1 because they can be administered at mucosal sites, both intranasally and orally. Furthermore, they have an intrinsic tropism for mucosal tissues, where they can persist for some time. In mice, adenoviral vectors have been shown to elicit cellular immune responses in the mucosa (92, 147); however, there is limited mucosal data on the use of these vectors in nonhuman primates, and it will be important to further characterize these responses.
ONE STEP FORWARD: LESSONS LEARNED FROM NONHUMAN PRIMATE STUDIES
Studies comparing cellular responses induced by recombinant adenovirus, poxvirus, alphavirus, and plasmid DNA vaccines, all expressing SIV Gag in macaques, supported that rAd5 elicited the highest levels of antigen-specific CTLs, supporting its further clinical advance (136, 137). The results of studies with rAd5 in animal models highlight considerations for evaluation of candidate vaccines. The rAd5 vaccine never protected against infection: regardless of the monkey haplotype or challenge virus studied, all monkeys became infected following challenge, leading to the unrealistic expectation that vaccination with the rAd5 trivalent vaccine would protect against infection in humans. There was a clear hierarchy of control of viral replication by rAd5 vaccines in primate challenge models. In hindsight, the “easiest” challenge model, A01-positive (A01+) macaques challenged with simian-human immunodeficiency virus (SHIV) 89.6p, produced a clear 3-log reduction in viral loads compared to those of the control (137). Challenge with the more-virulent strain SIVmac239 resulted in a sustained 1.5-log decrease in the viral loads of rAd5-immunized A01+ monkeys (101). It is clear that Mamu A01+ rhesus macaques are useful models for observing antigen-specific responses by using tetramer staining, but it is questioned whether they are accurate models for predicting vaccine efficacy in humans. In preclinical studies with the rAd5 vaccine testing outbread macaques challenged with SHIV 89.6, a more-variable 2- to 3-log reduction was observed (89). However, when outbred macaques were challenged with SIVmac239 post-rAd5 immunization, no significant reduction in viral load was seen in vaccinated monkeys compared to that of the control (29). This hierarchy establishes that the use of outbred macaques and challenge with SIV should be considered the most-stringent vaccine model and be a focus for future studies evaluating HIV vaccine efficacy. However, based on a lack of evidence of protection competency in this model, it is clear that all primate studies are still at-risk studies without known correlates or clear immune targets and without a guarantee that protection in stringent SIV challenges guarantees similar protection in humans.
ONE STEP BACK: IMPLICATIONS FOR CTL-BASED VACCINES
Merck, the HIV Vaccines Trial Network (HVTN), and the Division of AIDS, NIH, should be commended for their valiant effort, creativity, organization, and management of this important efficacy trial. The bar for the STEP study was set high, as rAd5 was the most-promising CTL platform at the time, based upon clinical and preclinical data for rhesus macaques. In earlier clinical trials, significant response rates to all three HIV antigens (73% to Gag, 59% to Pol, and 68% to Nef) among individuals with low seropositivity to Ad5 who were vaccinated three times with the trivalent Ad5 were observed at week 30. The reported geomean of responders as measured by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays were 205, 342, and 177 spot-forming units (SFU) to Gag, Pol, and Nef, respectively (37). Response rates were lower at the same timepoint for Ad5-seropositive individuals with high virus titers, with 69% responding to Gag (406 SFU), 38% to Pol (834 SFU), and 54% to Nef (428 SFU) (37). The majority of responders responded to only one eptitope in the Gag, Pol, or Nef peptide pools, with approximately 30% of responders recognizing three or more epitopes (37). Although the trial consisted primarily of men who have sex with men, it is interesting that one woman in the placebo group was infected. This critically important observation highlights the importance of the clinic as a teaching ground for HIV vaccine development. This observation has implications for HIV transmission between men who have sex with men versus heterosexual transmission that must be considered in future trial development. With this in mind, the analysis of the responder data from the STEP trial will be critical in determining a relationship among vaccine responders, the levels of the response, and the resulting incidence of infection. In addition, we anxiously await the data analysis from the high-titer vector-seropositive cohort as well as the Phambilli study to see if these results reflect an increased trend for infectious compared to low-titer Ad5-seropositive cohorts. The increased trend toward infection is not likely a general trend for all vaccines that elicit T cells. The Vaxgen protein trial, which mobilized B and T helper cells, did not show a similar trend, nor did the ALVAC trials, which mobilized weak CD8+ T cells. It should be noted that this trend is being debated at this time, and therefore its importance to vaccines under development remains speculative.
Unanswered questions include the following. Are the response levels (on average about 400+ SFU) observed in responders high enough? Are the vaccine targets the most relevant? Would polyfunctional immune responses or some other T-cell phenotype change vaccine efficacy? Do the responses need to be directed against a broader number of epitopes to be effective? What animal models are most important as predictors of human vaccine efficacy? What do the mucosal responses in Ad5-vaccinated people look like, and would such responses change the impact of the vaccine? Why are the acquisition rates different between men and women?
ADENO-ASSOCIATED VIRUS
Adeno-associated virus (AAV) is a helper-dependent parvovirus that has been well characterized as a gene therapy vector (reviewed in reference 144). The generation of the recombinant vector entails removing all viral genes except the sequences that encode the GC-rich inverted terminal repeats (ITRs). The entire genome is a mere 4.7 kb, which limits the size of the antigenic insert (between the ITRs) to roughly 3.8 kb, excluding the promoter and poly(A) tail. The removal of viral genes reduces the intrinsic immunogenicity of AAV; however, rAAV remains an attractive vaccine candidate due to the ability to produce it on a commercial scale (34), its outstanding clinical safety profile for nearly a decade (reviewed in reference 72), the ease with which it can be administered intramuscularly, and the high transduction efficiency of myofibers (reviewed in reference 72). Johnson et al. (72) demonstrated the efficacy of rAAV for delivery of SIV antigens (SIVsm/E660) in an intravenous challenge model. A portion of their study included two injections of three rAAV vectors encoding SIV genes at doses measured by DNase-resistant particles, which are equivalent to encapsulated vector genomes. The three rAAV vectors contained fragments of (i) SIV Gag, protease, and reverse transcriptase; (ii) SIV Rev and Env; and (iii) reverse transcriptase and integrase at 1013, 5 × 1012, and 1013 DNase-resistant particles, respectively. The results showed robust cellular (tetramer-specific CTLs) and humoral (serum antibody) immune responses after a single injection. In fact, after a virulent intravenous challenge, immunized monkeys exhibited significant control of SIV replication, with viral loads almost 2 logs lower than those of the control group. The protection from challenge may be attributed to CTLs, as there were no detectable neutralizing antibodies against the SIV challenge stock observed in the sera of vaccinated animals. Interestingly, there was no increase in the observed immune response after the second immunization, suggesting the generation of antivector antibodies. In fact, the preexisting or vaccine vector-induced immunity to the capsid is still a major concern for this platform. For example, recombinant AAV type 2 (AAV2) is the most-studied serotype to date, and it has been shown that 27% of the population has neutralizing antibodies directed against AAV2 (162). Similar to strategies being employed with recombinant adenoviral vectors, less-prevalent rAAV serotypes found naturally in humans, including AAV1 and AAV5, are being examined to circumvent this issue. Alternating rAAV capsid serotypes from one immunization to the next is being explored. A phase II clinical trial organized by Targeted Genetics and IAVI utilizing rAAV encoding HIV Gag, Pol, and Δ reverse transcriptase (tgAAC09) is under way in Zambia and has been reported safe and well tolerated thus far. While initial clinical studies using rAAV appear to be overwhelmingly safe, recently a patient participating in a phase I/II gene therapy trial for the treatment of rheumatoid arthritis died after receiving a rAAV vector encoding the soluble tumor necrosis factor alpha receptor tgAAC94. The patient was also taking a tumor necrosis factor alpha blocker, Humira, which is immune suppressive. While there has been no report of a definitive determination of the cause of the patient's death, it appears likely that infection with a fungus, Histoplasma capsulatum, could be the cause (75). The role of the AAV treatment in this patient's outcome is not clear. Importantly, this study highlights the potential pitfalls of performing trials in immunosuppressed individuals whose responses are clearly different from those of immunocompetent people. The immunogenicity of rAAV vectors clearly needs improvment, and the use of molecular adjuvants and prime-boost strategies will undoubtedly be tested and will be discussed in future sections. The published data on the generation of mucosal immune responses by rAAV is limited, although it is an interesting vector due to its stability at a wide range of pH values and high temperatures.
ALPHAVIRUSES AND RHABDOVIRUSES
Alphaviruses are positive-stranded RNA viruses with a broad host range that cause a variety of mosquito-transmitted diseases, including Eastern equine encephalitis, Western equine encephalitis, and Venezuelan equine encephalitis (VEE), in the United States. While the majority of alphaviral infections are asymptomatic in humans, flu-like symptoms, including fever, headaches, and body aches, can occur. The virus is also capable of invading the central nervous system, leading to encephalitis and perhaps even death. Modifications to the virus for the generation of vaccine vectors has been well studied (reviewed in references 118 and 130). To generate vaccine replicon particles, the nonstructural protein genes and natural subgenomic promoter (26S) are retained, while the structural protein genes are replaced with the antigenic gene of interest. Current alphavirus replicon particle vaccines have been derived from the Sindbis virus, Semliki Forest virus (91), and VEE virus. This process creates a single-cycle replication vector that is translated upon entry into the cytoplasm of the target cell, allowing for intracellular processing and subsequent efficient presentation by MHC-I. The cytoplasmic amplification of these vectors includes a double-stranded RNA intermediate that activates both the innate and adaptive immune systems, strengthening this platform as a vaccine candidate delivery system (85). Indeed, the replication of alphavirus replicons results in extremely high levels of antigen expression (157, 166, 171); however, this replication is generally toxic to the infected cell, thus preventing long-term antigen expression (48). The antivector immune response against alphavirus replicons has so far been minimal, allowing for readministration, a major benefit for a vaccine (118). Similarly, seroprevalence is low in humans, and the replicons can be engineered to be lymph node trophic, enhancing antigen presentation through efficient targeting to, and expression in, the immune inductive site. A possible drawback to the alphavirus platform includes the requirement of the Rep protein for high-level antigen expression. This antigen may be prone to immune surveillance by CTLs. However, this platform has many positive features. For example, previous studies report that the primary cellular targets of replicon particles have been shown to be those of the CD14+ monocyte lineage in rhesus macaques (55) and dendritic cells in human peripheral blood mononuclear cells (51). In nonhuman primates, vaccination with 2 × 108 PFU of chimeric SINenv and VEErep alphavirus replicon particles expressing SIVp55Gag and/or HIVΔV2gp140 alone resulted in high titers of anti-HIV neutralizing antibodies, while moderate anti-Env IFN-γ ELISPOT responses were also observed (163). In a separate study, 1 × 107 infectious units of each of three VEE virus replicon particles expressing the SIVsmH-4 matrix capsid region of Gag, gp160, or unanchored gp140 were administered three times to rhesus macaques, followed by a mucosal challenge with SIVsmE660 (73). Macaques immunized with SIV-VEE virus replicon particles produced neutralizing antibodies and had reduced peak viral loads (1 log) postchallenge. In correlation with the decreased viral loads was an increase in the peripheral CD4+ T-cell counts in immunized macaques, observed at the viral set point. While not measured in this study, immune responses at mucosal sites, namely the preservation of CD4+ T cells, will be interesting to analyze. Overall, however, this platform has progressed very slowly. To date, there is a limited amount of clinical data, and studies to support the efficiency of this platform are remain important.
VESICULAR STOMATITIS VIRUS
A member of the Rhabdoviridae family, vesicular stomatitis virus (VSV) is a negative-stranded RNA virus that primarily infects livestock. In most of the world, infection of humans is rare and asymptomatic but can cause flu-like symptoms. VSV is an attractive platform for clinical use, as it can be delivered at mucosal sites, carry multiple transgenes, is easily manufactured, and has low seroprevalence in humans (reviewed in references 88 and 121). One potential drawback to VSV vaccine vectors is the development of antibodies against VSV surface glycoproteins in immunized hosts after one immunization. Therefore, effective boosting may require substituting a glycoprotein from a different VSV serotype. In nonhuman primates, immunization with rVSV vectors encoding HIV Env and SIV Gag protected against disease after challenges with SHIV 89.6p (41, 43). Furthermore, the direct comparison of intramuscular versus intranasal administrations of equal doses of HIV Env and SIV Gag (5 × 106 PFU each) to rhesus macaques demonstrated that intranasal vaccination elicited higher cellular immune responses, as measured by IFN-γ ELISPOT assays, Cr release assays, and tetramer analysis (43). Interestingly, there was no difference in the humoral immune responses elicited by either vaccination strategy at systemic or mucosal sites (43). The intranasal delivery of VSV vectors does not seem to cause neurovirulence in nonhuman primates (71). It will be interesting to see if the mucosal administration of this promising platform can elicit cellular immune responses at mucosal sites.
POXVIRUS VECTORS
Poxviruses are large DNA viruses that evolved as vectors based on experience with the smallpox vaccine (reviewed in reference 93). As vaccine vectors, they are able to carry multiple and large genes (∼25 kb) (97) that are stably expressed. These vectors are also capable of inducing immune responses similar to those observed with the pathogen. Several studies have shown that vaccinia virus vectors can be pathogenic in immunocompromised people, thereby refocusing efforts on developing a more-attenuated, modified vaccinia virus Ankara (MVA) as a vector system (105). MVA has been shown to be safe for mice, monkeys, and humans, as it cannot complete an entire life cycle in humans or primates (41) while still being capable of inducing strong cellular and humoral immune responses in mice and primates. Numerous poxvirus-based vaccines have been tested in rhesus macaques after they were challenged with SIV, SHIV, and HIV-2 isolates (2, 7, 19, 47, 57, 59-62, 114) and have produced various degrees of cell-mediated immune responses and protection against viral challenge.
In a study conducted with rhesus macaques, SHIV DNA plasmid-producing noninfectious viral particles were delivered intranasally, with a rMVA SIV Gag-Pol and HIV Env boost. Coimmunization with an interleukin-2 (IL-2)/Ig plasmid elicited both mucosal and systemic humoral and cellular anti-SHIV immune responses (22). Intranasal immunization with other platforms had previously been reported to be the only route of mucosal vaccination that results in disseminated humoral and cellular immune responses (82, 83) and increases the level of vaginal responses compared to that for rectal immunization (20, 83). Indeed, as nasal immunization also induced greater systemic antibody responses and thus generated both systemic and mucosal immunity, the intranasal route may precede other mucosal routes (83). Mucosal SHIV-specific responses were detected in the IL-2/Ig group, as measured by the levels of IgA in rectal secretions and tetramer-positive T cells in rectal biopsies. Upon rectal challenge with SHIV 89.6p, all of the animals became infected, but the group coimmunized with IL-2/Ig was protected from CD4+ T-cell loss and disease progression to AIDS. Interestingly, a different study showed that tetramer-positive CD8+ T-cell responses were induced at mucosal sites by live attenuated poxvirus NYVAC encoding SIV Gag, Pol, and Env, whether it was administered by the intranasal, intramuscular, or intrarectal route (140).
A recombinant canarypox vector (ALVAC 1452) has been tested in multiple clinical trials with different doses, formulations, and prime-boost regimens by the HVTN and others. The results of a phase II study of ALVAC and VaxGen's gp120 protein (HVTN 203), demonstrated by IFN-γ ELISPOT assays, were observed in less than 36% of the vaccinees; therefore, the planned phase III trial (HVTN 501) was cancelled. Results from another phase II trial, outside of the United States, which tested ALVAC with or without a rgp120 booster (HVTN 026), did not show a difference in cellular immunity between the vaccinated and placebo groups, as measured by IFN-γ ELISPOT assays, lymphocyte proliferation assays, or Cr release assays (35). Increasing the dose of the ALVAC vector (HVTN 039) or the addition of lipopeptides (HVTN 042) did not enhance the number of responders, 5 of 52 and 3 of 77, respectively (37). A phase III clinical trial in Thailand involving the same ALVAC vector expressing subtypes E and B Env (gp41), Gag, protease plus gp160, or Chiron gp120B/E boosting is currently under way (76). In this study, CTL responses have been observed in less than 30% of the recipients, and antibodies, but not broadly neutralizing antibodies, have been observed. This finding may have been expected, as the preceding phase I/II study results showed no statistical difference between cellular responses in the ALVAC prime plus gp120 or gp160 protein boost group and the placebo group (149). Improving the immunogenicity of these experienced vectors is a major focus of the poxvirus field.
HERPESVIRUS
Unlike the viral vectors described above, herpes simplex virus type 1 (HSV-1) is able to persist for essentially the lifetime of the infected host. Active HSV-1 infection occurs at mucosal surfaces, including the mouth and genitals, and the virus remains latent in the nervous system, where it can be periodically reactivated. In addition, both cellular and humoral immune responses are generated in response to HSV-1 infection. These features of HSV-1 highlight its potential utility as a vaccine vector in regard to long-term antigenic expression, immunogenicity, and tropism for mucosal surfaces. Both replication-competent and replication-defective recombinant HSV-1 vectors have been studied as vaccines for HIV-1 in nonhuman primate models. In a study by Murphy et al. (107), the replication capability of HSV-1 did not elicit statistically different results, as measured by antibody levels, CTL activity, or protection against challenge. In fact, two rhesus macaques were protected against a SIVmac239 rectal challenge, one immunized with a replication-competent, and the other a replication-deficient, HSV-1 encoding SIV Env and Nef. However, the replication-competent group had peak viral loads 1.2 logs lower than those of the infected control animals. Clearly, the protection against mucosal challenge and the tropism of HSV-1 for mucosal surfaces warrant further examination of immune responses elicited by the mucosal delivery using this platform.
BACTERIAL VECTORS
The use of recombinant bacteria as vectors for gene delivery has been extensively reviewed elsewhere (94). Here we will touch on two particular platforms that have been used for HIV-1 vaccines in nonhuman primate models, Listeria monocytogenes and Salmonella.
Listeria monocytogenes.
A recombinant strain of Listeria monocytogenes has many promising features as a vaccine vector, including a natural tropism for mucosal tissues, an ability to infect APCs, a high level of CD8+ T-cell induction, a low prevalence of preexposure in humans, and ease of manipulation and production in culture (reviewed in reference 90). Two independent studies performed with rhesus macaques illustrated the induction of mucosal cellular immune responses with a DNA prime and a Listeria boost, both encoding SIV Gag and Env (26, 109). In a study performed by Boyer et al. (26), the macaques that received the DNA prime-Listeria boost had better protection against an intrarectal challenge of SIV239, measured by control of viral loads for a longer period of time. Neeson et al. (109) further examined the induction of a mucosal SIV Gag-specific cellular immune response by the DNA prime-Listeria boost by demonstrating that Gag-specific CD8+ T cells in the peripheral blood coexpress the gut-homing marker B7 in the peripheral blood and home to gut mucosal tissues.
Salmonella.
Like Listeria monocytogenes, the natural route of infection by Salmonella species is oral, and therefore, use of the recombinant bacteria to induce mucosal immunity is promising. Recombinant Salmonella vectors have been engineered to utilize the bacterial type III secretion system in order to more efficiently present antigenic peptides via MHC-I and, subsequently, prime CD8+ CTLs (127). Priming the immune response with an intragastric administration of the modified Salmonella vector expressing HIV-1 Gag in combination with a MVA Gag booster elicited mucosal immune responses in rhesus macaques (44). This combination, in particular, elicited SIV Gag-specific CD8+ T cells that expressed α4β7 and homed to the colonic mucosa. While such responses were unable to confer protection against an intrarectal challenge with SIV239, the elicitation of mucosal cellular responses (albeit minimal) is noteworthy. Improving the immune potency of this platform is important.
NUCLEIC ACIDS
While RNA vaccines have been important in the area of immune therapy for cancer, work in the particular area of an HIV-1 vaccine has been limited. Accordingly, we focus here on the DNA vaccine approach.
DNA has been studied as a vaccine platform for over 15 years. Studies with mice immunized with plasmid antigens yielded promising levels of immunity that, unfortunately, have not been well translated in initial clinical studies. Currently, there is a significant amount of work in the field devoted to improving this platform, as it is appealing on many levels, including large-scale vaccine production, safety, repeat administration (no preexisting or vaccine-induced vector serology), and storage, as a cold chain is not required. DNA vaccines for HIV-1 have been reviewed elsewhere (66). Here we will focus primarily on the optimization of future DNA vaccines.
Genetic level.
The optimization of plasmids has yielded a substantial increase in the immunogenicity of plasmid-encoded antigens via enhanced levels of gene expression on a per-cell basis. For example, codon optimization improves expression by adapting the codon usage to the bias of the target species, thereby enhancing transcription through more-abundant tRNA pools (8, 39, 159, 172). Additional modifications, including eliminating mRNA instability and mRNA inhibitory elements, may also be made to the encoded gene sequence to increase the stability of the mRNA (133). The inclusion of a constitutive transport element can enhance RNA stability and the export of RNA from the nucleus. The addition of IgE leader sequences also facilitates expression by improving the loading of mRNA onto the ribosome (159, 164). By removing these negative sequences, avoiding splice sites, removing double-stranded pairing/secondary-structure formation, and including the above sequences, mRNA structures that are more stable and more efficiently transported to the ribosome can be created. As a result, the mRNA is translated more effectively and gene expression is enhanced on a per-cell basis, which can improve immunogenicity.
Molecular adjuvants.
As a vaccine strategy, the use of DNA has been suboptimal in producing immunogenicity. In addition to genetic modifications enhancing the expression of the antigenic plasmid, there is a plethora of work on improving the potency of DNA vaccines. One promising technique involves the incorporation of plasmids carrying immunomodulatory genes. Molecular adjuvants reported to induce mucosal immunity include the chemokines CCL19, CCL21, and macrophage inflammatory protein 1α and the cytokines granulocyte-macrophage colony-stimulating factor, IL-2, IL-12, and IL-18 (152). Two recent nonhuman primate studies conducted by Wyeth included codelivery of the plasmid-encoded cytokines IL-12 (129) and/or IL-15 (33) with an SIV Gag DNA construct. The IL-12 plasmid was the best adjuvant, substantially enhancing both cellular (ELISPOT) and humoral (enzyme-linked immunosorbent assay) immunity against HIV-1 Gag. Coimmunization with both IL-12 and IL-15 enhanced humoral, but not cellular, responses, and this version of the IL-15 plasmid did not statistically enhance the responses observed with the antigen alone. Upon challenge with the pathogenic SHIV 89.6p, macaques coimmunized with IL-12 had lower viral loads and a trend toward the preservation of peripheral blood CD4+ T cells, although the groups were too small for statistical significance. Similarly, coimmunization with the IL-12 plasmid augmented cellular immune responses, as measured by IFN-γ and granzyme B levels in cynomologus macaques (24). In a separate study, cynomologus macaques coimmunized with plasmid-encoded SIV Gag and a highly optimized macaque IL-15 had significantly lower viral loads than the antigen-only group following challenge with SHIV 89.6p, even though coimmunization with IL-15 did not significantly enhance the IFN-γ response compared to that of the antigen alone (25). This study, as well as others, highlights the necessity of supplementing the ELISPOT assay with multiparameter immune response analysis, as well as other assays of T-cell function. Another adjuvant, IL-2, has shown encouraging potential. Coimmunization of rhesus macaques with plasmid-encoded Gag and Env and an IL-2/Ig fusion construct resulted in a potent CTL response and subsequently reduced viral loads, maintained stable CD4+ T-cell counts, and prevented disease progression to AIDS (13). Overall, these primate data are exciting. The clinical studies of such approaches are anxiously being followed. In addition to cytokines being codelivered with an antigenic plasmid, other immunomodulatory genes such as costimulatory molecules, including CD40L and glucocorticoid-induced tumor necrosis factor receptor, are being tested in macaques (141, 142), and granulocyte-macrophage colony-stimulating factor (14), B7.1, LFA-3, and ICAM-1 (165) are being tested in mice. Whether there are mucosal benefits to DNA vaccination remains to be seen.
Improvements in delivery.
Strategies to improve the dosing, delivery, longevity, and transfection efficiency of DNA vaccines are receiving a great deal of attention. One method of facilitating delivery of plasmid DNA is through formulations that include incorporation of liposomes or polymers to extend plasmid longevity following injection and thereby enhance uptake over time and, subsequently, expression (5, 32, 53, 111). Improved delivery methods include gene gun (49, 145, 153), BioJet (3, 56, 153), laser (168), ultrasound (146), and electroporation (63, 151, 160) approaches to enhance physical delivery. The gene gun is advantageous in that it requires very low amounts of DNA. This method of plasmid delivery has an apparent ability to drive antibody responses in both nonhuman primates and humans, although improving CTLs is an important goal of this technology. Another method, the BioJet, is a needle-free jet injection device that may reduce the variability of responses in primates and humans (3, 56, 153). The use of laser, ultrasound, and electroporation are all based on the permeabilization of cell membranes at the injection site through light, sound, and electric current, respectively.
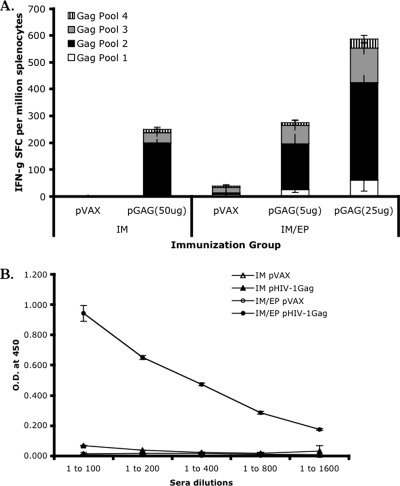
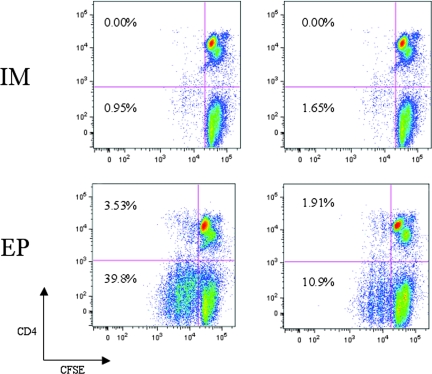
Various types of in vivo electroporation have also recently been employed to increase the transfection efficiency of plasmid DNA. This technique involves applying a specific electric current to the target, either the muscle or skin, based upon the type of immunization delivered, intramuscular or intradermal. Enhanced delivery is observed and is likely due to the formation of transient pores within the cell membrane, allowing for more cellular uptake of plasmid DNA. Concurrently, an inflammatory response is induced and likely enhances the observed immune response as well (10). In a comparative study of intramuscularly delivered plasmid DNA (IM) versus electroporation, electroporated mice were given 2- to 10-fold less DNA than the IM group. As shown in Fig. 2, the electroporated mice immunized with 25 μg of DNA had levels of IFN-γ secretion twofold higher than the IM group immunized with 50 μg of DNA without electroporation (approximately 550 versus 250 spot forming cells [SFC], respectively). Furthermore, the electroporated mice immunized with only 5 μg of DNA had levels of IFN-γ secretion similar to those of mice immunized IM with 50 μg and elicited significantly higher anti-Gag antibody levels than the IM group without electroporation (Fig. 2). In rhesus macaques immunized with plasmid DNA versus electroporation with one-fifth of the DNA, the cellular immune responses of the electroporated group were enhanced by 10- to 40-fold, with antibody titers 2.5 logs greater than those immunized with DNA alone (96). The results of this study put plasmid DNA vaccines for HIV-1 on the potency map. The benefits observed in the primate model include enhanced plasmid delivery, resulting in increased gene expression, and enhanced immunogenicity (64, 113). The combination of electroporation with novel formulations is exciting to consider. Our laboratory has shown a significant enhancement of T-cell proliferation in rhesus macaques that were given an intramuscular DNA immunization with electroporation versus an intramuscular immunization alone (64a). However, there are obvious concerns with this technology versus the standard needle and syringe delivery method, including increased pain, more-complex technology to establish, and requirement of a machine and training for the clinical staff who administer the vaccine. The overall goal will be to decrease the required dose and voltage and, subsequently, to increase the tolerability without sacrificing effectiveness. The inclusion of cytokines (Fig. 3) appears to further lower the dose while simultaneously enhancing the potency of the vaccine and may allow for a decrease in the number of injections required to achieve useful clinical potency.
FIG. 2.
Electroporation enhances both cellular (A) and humoral (B) immune responses, using less amounts of DNA than intramuscular immunizations alone. BALB/c mice were immunized in the muscle 2 weeks apart with or without electroporation. The group given the intramuscular injection alone (IM) received 50 μg of plasmid DNA, and the electroporated group (IM/EP) received 5 μg or 25 μg per immunization. The mice were sacrificed 1 week following the final immunization. (A) ELISPOT assay with the splenocytes of mice immunized with empty plasmid (pVAX) or pHIV-1 Gag DNA (pGAG). HIV-1 Gag-specific IFN-g secretion is shown as SFC per million. Splenocytes were stimulated with four overlapping peptide pools spanning HIV-1 Gag. (B) Antibodies against p24 HIV-1 Gag, as measured by enzyme-linked immunosorbent assay. Sera were collected from immunized mice at the time of sacrifice. O. D. at 450, optical density at 450 nm.
FIG. 3.
Electroporation increases CD4 and CD8 T-cell proliferation. Rhesus macaques were immunized three times with 1.0 mg each of plasmid-encoded consensus HIV Gag and rhesus IL-12 by intramuscular injection (IM) immunization alone (n = 2), or electroporation (EP; n = 3). Peripheral blood mononuclear cells were isolated from samples taken 2 weeks after the third immunization, and the proliferative capacities of HIV Gag-specific CD4 and CD8 T cells were determined by carboxyfluoroscein succinimidyl ester (CFSE) assay. Representative dot plots for two animals from each group are shown. Percentages represent Gag-specific proliferation with background proliferation (medium control) subtracted..
Prime-boost protocols.
Heterologous prime-boost protocols are particularly beneficial for recombinant viral vector approaches, as antivector immune responses can prevent the readministration of the homologous vector. DNA is a conceptually weak delivery system that is uniquely specific for the antigen. Most studies have shown that DNA can prime efficiently for a protein or recombinant viral vector boost.
DNA/VECTOR PRIME-PROTEIN BOOST
The administration of a DNA prime and a subsequent protein boost effectively induces both cellular and humoral immunity. In rhesus macaques, a polyvalent DNA encoding Env from multiple subtypes and a Gag gene prime plus a homologous gp120 protein boost elicited modest cellular (around 500 SFC) and robust humoral (∼106 SFC) responses (116). Upon rectal challenge with SHIV-Ba-L, the majority of immunized macaques were protected from infection, while the rest had lower viral loads than the unvaccinated group. A similar human ALVAC-HIV-1 recombinant vaccine expressing Gag, Pol, and gp120 or a Gag, Pol, and gp160 prime plus a homologous Env protein boost resulted in decreased viral load levels in the blood and in mucosal sites while simultaneously protecting macaques from peripheral CD4+ T-cell loss after challenge with SHIVKU2 (115). This approach is being studied in a phase I clinical trial by S. Lu (95) and colleagues, using five different gp120 isolates and one Gag DNA prime plus a boost with heterologous gp120 protein. Interestingly, anti-gp120 antibody titers comparable to those of patients with chronic HIV-1 infection have been reported. In addition, anti-Env cellular responses were induced by this combination.
DNA PRIME-rAd BOOST
rAd5 is also being used as a boost for a unique DNA prime in large-scale clinical trials being conducted by the NIH and VRC. In a phase I trial, DNA and Ad5 were administered separately to 86 recipients at increasing doses. The vast majority of recipients had an anti-HIV-1 T-cell response, the first high responses in published reports (30, 52). Four DNA constructs, clade B Gag-Pol-Nef fusion protein, and clades A, B, C Env glycoproteins (31, 79) elicited an antibody and long-lasting Env-specific CD4+ and CD8+ T cells. Comparatively, a higher percentage of responders with a greater magnitude of immune responses was observed in recipients of rAd5 (clade B Gag-Pol fusion protein and clades A, B, C Env glycoproteins). Although impressive levels of cellular and humoral immunity were observed in the majority of subjects, preexisting immunity to Ad5 lowered T-cell responses approximately threefold compared to those of seronegative subjects. Based on the safety of, and encouraging results from, this study, a phase II trial has been initiated (124). Recent studies have shown a dramatic increase in HIV-1-specific immune responses with the combination of these two platforms. The DNA prime with a rAd5 boost resulted in a greater-than-1,000-fold increase in antibody and a greater-than-5-fold increase in T-cell responses against Env (124). Data from macaques immunized with this strategy and subsequently challenged with SIV show long-term preservation of the total number and function of central memory CD4+ T cells in the peripheral blood as a result of vaccine-generated protection against high levels of virus replication (86, 98). Efficacy trials of this approach are being planned through a HVTN/VRC collaboration.
DNA PRIME-rAAV BOOST
In the previously mentioned rAAV nonhuman primate study by Johnson et al. (72), a DNA prime-rAAV boost protocol elicited potent responses compared to those of other regimens using rAAV vectors alone. The study utilized either a DNA or rAAV prime with a mixture of three plasmids encoding the same SIV subgenomic fragments of (i) Gag, protease, and reverse transcriptase, (ii) reverse transciptase and integrase, and (iii) Rev and Env. Both groups received the same rAAV Rev-Env boost. Despite the weakly immunogenic DNA prime, the monkeys with the lowest viral loads and therefore the best control of SIV replication postchallenge were in the DNA prime and rAAV boost group.
DNA PRIME-VSV BOOST
While preexisting immunity to VSV is not an obstacle for humans, the ability to boost with the same rVSV strain is limited due to antivector immune responses. Therefore, the use of VSV in a heterologous prime-boost regimen with plasmid DNA is an attractive strategy for eliciting more-potent immune responses. In A01-negative rhesus macaques, intramuscular immunization with 5 mg of each DNA plasmid encoding SIV Gag and the cytokine adjuvant IL-12, followed by intranasal delivery of 5 × 106 PFU of each rVSV encoding HIV Env (gp120) and a SIV Gag booster elicited both cellular and humoral immune responses greater than either platform alone (42). In particular, following a high-dose (300 monkey infectious doses) intravenous SHIV 89.6p challenge, this prime-boost regimen resulted in a 2.4-log reduction in viral loads compared to that for the DNA prime (1.5 log) or rVSV boost (1.1 log) given alone (42). In addition to there being no clinical disease observed postchallenge (42), there was enhanced preservation of CD4+ T cells in the peripheral blood of these animals.
DNA PRIME-MVA BOOST
One of the most-studied prime-boost regimens is a DNA prime and a poxvirus boost (123, 132). Interestingly, the T-cell responses generated with this heterologous strategy produce immune responses 10 times higher than either platform given separately (106). In fact, an important clinical trial by Robinson and colleagues is under way through the HVTN, utilizing a multivalent DNA (Gag, Pol, Env, Tat, Rev, Vpu) prime followed by a MVA boost. Initial results for humans show exceptional safety and tolerability but undetectable HIV-1-specific cellular (responders = 0/28, as determined by IFN-γ ELISPOT assay, and 0/24 by Cr release assay [37]) or humoral responses after a DNA prime (106). However, based upon data for rhesus macaques, the animals with minimal responses detected after a DNA prime were able to generate potent immune responses post-MVA boost (138), suggesting that the same may be true for humans. Preliminary reports suggest that the poxviral component increases the levels of immunity observed in these studies. In addition, data from another rhesus macaque study using Gag, Pol, and Env DNA prime plus MVA boost resulted in the control of viremia after a mucosal challenge with SHIV 89.6p (6).
DNA PRIME-HSV-1 BOOST
A recent study with rhesus macaques compared the immune responses elicited by immunization with recombinant DNA and HSV-1-encoding SIV genes in a heterologous DNA prime-HSV-1 boost strategy to those from homologous HSV-1 immunizations (77). Despite the robust cellular and modest humoral immune responses observed in the immunized macaques, all became infected upon an intravenous challenge with SIV239. Interestingly, the macaques immunized with the DNA prime-HSV-1 boost regimen had greater levels of T-cell responses than those of macaques immunized with HSV-1 alone. The observed levels of IFN-γ secretion for the prime-boost group were greater than or equal to those observed for unvaccinated macaques infected with wild-type SIV. In fact, while the DNA prime-HSV-1 boost regimen also produced a higher percentage of tetramer-postive IFN-γ-secreting cells, the group that received the homologous HSV-1 immunizations had more α4β7-positive cells, a marker of T cells homing to the mucosa. We look forward to future studies addressing the ability of HSV-1 to induce mucosal immune responses.
CONCLUSIONS
It is a tumultuous time for HIV vaccine development. There are few promising leads in the vaccine toolbox for antibody-based approaches. The STEP study has thrown cold water on the cadre of CTL-based platforms. These approaches (summarized in Table 2) provide the broadest array of T-cell-driving vectors in the history of vaccinology. In the near future, additional prime-boost studies and the use of adjuvanted DNA, designer Ad vectors, and DNA electroporation strategies will be expanded in the clinic. The world's first glimpse of the lack of efficacy of a pure T-cell-based approach supports new research goals for the CTL-based HIV vaccine field. (i) More-stringent primate models for determining vaccine efficacy in decision studies should be encouraged. (ii) We should demand greater CTL responses in humans, greater than an average of 300 to 400 106 peripheral blood mononuclear cells, from next-generation is approaches. (iii) The induction of T-helper responses T-cell desirable and likely important, but we need to learn a great deal more about their contributions to immune control. (iv) The importance of polyfunctional immune responses, in addition to the proliferative capacity of vaccine-elicited T-cell responses, should be studied, and benchmarked as they relate to vaccine outcome in macaques and immune control in humans. (v) Novel and clean hypotheses regarding vectors entering clinical trials should be encouraged, and immune evaluations should be designed on the basis of these hypotheses and serve as benchmarks for progress in the clinic. (vi) Most importantly, rather than redividing the limited DAIDS funding pie, thus depriving important programs of needed resources, Congress should support the NIH by increasing funding for basic HIV vaccine research and pilot programs for early clinical testing. New ideas and concepts cannot be advanced effectively based on the current levels of funding for R01's, P01's, and developmental resources.
TABLE 2.
Summary of the advantages, disadvantages, and strategies for improvement of the reviewed HIV-1 vaccine platforms
| Vaccine platform | Advantages | Disadvantages | Strategies for improvement |
|---|---|---|---|
| Poxvirus (MVA) | Ability to carry large antigenic inserts | Low immunogenicity in humans | Remove antigenic vector sequences |
| Can be delivered mucosally and elicit mucosal immune responses | Use priming/boosting regimens | ||
| Safe | |||
| Thermostable | |||
| Adenovirus | Elicits best cellular and humoral immune responses | Preexisting immunity to most efficacious serotypes in humans | Use chimp and other mammalian vectors |
| in humans | Antivector immune responses develop | Use chimeric vectors, swap hexons | |
| High transduction efficiency | Readministration difficult | Use rare human serotypes | |
| Produces high titers quickly in culture | Use priming/boosting regimens | ||
| Use higher doses | |||
| Easily manipulated | |||
| Antigenic inserts of up to 8 kb | |||
| Adeno-associated virus | Safe | Preexisting neutralizing antibodies | Use priming/boosting regimens |
| Efficiently transduces myofibers | Small transgene capability (3.8 kb) | Use new serotypes | |
| Poor immunogenicity | Use adjuvants | ||
| Alphavirus replicons | Wide range of host cell types | Transient expression due to replication-induced cytotoxicity | Remove antigenic vector sequences |
| Capable of transducing DCs | |||
| Produce large amounts of antigenic protein | |||
| Lack of preexisting immunity in humans | |||
| Induce innate and adaptive immunity | |||
| Vesicular stomatitis virus | Infects mucosal surfaces | Antivector immune responses develop | Use priming/boosting regimens |
| Mucosal delivery | Swap glycoproteins | ||
| Low seroprevalence in humans | |||
| Herpes simplex virus | Infects mucosal surfaces | Preexisting immunity | Use priming/boosting regimens |
| Long-term persistence | Safety concerns | ||
| Bacteria (Salmonella, L. monocytogenes) | Mucosa tropic | Poor immunogenicity in humans | Increase antigen stability |
| Infect APCs | Improve vector design | ||
| Inexpensive to manufacture | Use priming/boosting regimens | ||
| DNA | Safe | Poor immunogenicity in humans | Make genetic modifications |
| Thermostable | Low antibody titer | Use different formulations | |
| Easy to manufacture | Use different delivery mechanisms | ||
| Can boost indefinitely | |||
| Use molecular adjuvants |
The Merck STEP study should be a catalyst for propelling the next generation of vaccines forward by raising the bar for what we expect from them. Such an occurrence will firmly add the CTL platform with all its potential promise to the armitarium of vaccinologists and, with support, hard work, and luck, welcome in the heyday of the age of designer vaccine platforms.
Acknowledgments
K.A.S. acknowledges the support of NIH grant 1-T32-A107632. D.B.W. acknowledges support from the NIH, including HIVRAD funding.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 814654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abimiku, A. G., G. Franchini, J. Tartaglia, K. Aldrich, M. Myagkikh, P. D. Markham, P. Chong, M. Klein, M. P. Kieny, E. Paoletti, et al. 1995. HIV-1 recombinant poxvirus vaccine induces cross-protection against HIV-2 challenge in rhesus macaques. Nat. Med. 1321-329. [DOI] [PubMed] [Google Scholar]
- 3.Aguiar, J. C., R. C. Hedstrom, W. O. Rogers, Y. Charoenvit, J. B. Sacci, Jr., D. E. Lanar, V. F. Majam, R. R. Stout, and S. L. Hoffman. 2001. Enhancement of the immune response in rabbits to a malaria DNA vaccine by immunization with a needle-free jet device. Vaccine 20275-280. [DOI] [PubMed] [Google Scholar]
- 4.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 39286-89. [DOI] [PubMed] [Google Scholar]
- 5.Alpar, H. O., S. Somavarapu, K. N. Atuah, and V. W. Bramwell. 2005. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 57411-430. [DOI] [PubMed] [Google Scholar]
- 6.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 29269-74. [DOI] [PubMed] [Google Scholar]
- 7.Andersson, S., B. Makitalo, R. Thorstensson, G. Franchini, J. Tartaglia, K. Limbach, E. Paoletti, P. Putkonen, and G. Biberfeld. 1996. Immunogenicity and protective efficacy of a human immunodeficiency virus type 2 recombinant canarypox (ALVAC) vaccine candidate in cynomolgus monkeys. J. Infect. Dis. 174977-985. [DOI] [PubMed] [Google Scholar]
- 8.André, S., B. Seed, J. Eberle, W. Schraut, A. Bültmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 721497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apetrei, C., P. A. Marx, and S. M. Smith. 2004. The evolution of HIV and its consequences. Infect. Dis. Clin. N. Am. 18369-394. [DOI] [PubMed] [Google Scholar]
- 10.Babiuk, S., M. E. Baca-Estrada, M. Foldvari, M. Storms, D. Rabussay, G. Widera, and L. A. Babiuk. 2002. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine 203399-3408. [DOI] [PubMed] [Google Scholar]
- 11.Barouch, D. H., and G. J. Nabel. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16149-156. [DOI] [PubMed] [Google Scholar]
- 12.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 1726290-6297. [DOI] [PubMed] [Google Scholar]
- 13.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290486-492. [DOI] [PubMed] [Google Scholar]
- 14.Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168562-568. [DOI] [PubMed] [Google Scholar]
- 15.Bellone, M., G. Iezzi, A. Martin-Fontecha, L. Rivolta, A. A. Manfredi, M. P. Protti, M. Freschi, P. Dellabona, G. Casorati, and C. Rugarli. 1997. Rejection of a nonimmunogenic melanoma by vaccination with natural melanoma peptides on engineered antigen-presenting cells. J. Immunol. 158783-789. [PubMed] [Google Scholar]
- 16.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8+ cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Investig. 1022072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belyakov, I. M., M. A. Derby, J. D. Ahlers, B. L. Kelsall, P. Earl, B. Moss, W. Strober, and J. A. Berzofsky. 1998. Mucosal immunization with HIV-1 peptide vaccine induces mucosal and systemic cytotoxic T lymphocytes and protective immunity in mice against intrarectal recombinant HIV-vaccinia challenge. Proc. Natl. Acad. Sci. USA 951709-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belyakov, I. M., Z. Hel, B. Kelsall, V. A. Kuznetsov, J. D. Ahlers, J. Nacsa, D. I. Watkins, T. M. Allen, A. Sette, J. Altman, R. Woodward, P. D. Markham, J. D. Clements, G. Franchini, W. Strober, and J. A. Berzofsky. 2001. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat. Med. 71320-1326. [DOI] [PubMed] [Google Scholar]
- 19.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIVmac251: dependence on route of challenge exposure. J. Virol. 724170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergquist, C., E. L. Johansson, T. Lagergard, J. Holmgren, and A. Rudin. 1997. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 652676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berkner, K. L. 1992. Expression of heterologous sequences in adenoviral vectors. Curr. Top. Microbiol. Immunol. 15839-66. [DOI] [PubMed] [Google Scholar]
- 22.Bertley, F. M., P. A. Kozlowski, S. W. Wang, J. Chappelle, J. Patel, O. Sonuyi, G. Mazzara, D. Montefiori, A. Carville, K. G. Mansfield, and A. Aldovini. 2004. Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates. J. Immunol. 1723745-3757. [DOI] [PubMed] [Google Scholar]
- 23.Betts, M. R., J. F. Krowka, T. B. Kepler, M. Davidian, C. Christopherson, S. Kwok, L. Louie, J. Eron, H. Sheppard, and J. A. Frelinger. 1999. Human immunodeficiency virus type 1-specific cytotoxic T lymphocyte activity is inversely correlated with HIV type 1 viral load in HIV type 1-infected long-term survivors. AIDS Res. Hum. Retrovir. 151219-1228. [DOI] [PubMed] [Google Scholar]
- 24.Boyer, J. D., T. M. Robinson, M. A. Kutzler, R. Parkinson, S. A. Calarota, M. K. Sidhu, K. Muthumani, M. Lewis, G. Pavlakis, B. Felber, and D. Weiner. 2005. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J. Med. Primatol. 34262-270. [DOI] [PubMed] [Google Scholar]
- 25.Boyer, J. D., T. M. Robinson, M. A. Kutzler, G. Vansant, D. A. Hokey, S. Kumar, R. Parkinson, L. Wu, M. K. Sidhu, G. N. Pavlakis, B. K. Felber, C. Brown, P. Silvera, M. G. Lewis, J. Monforte, T. Waldmann, J. Eldridge, and D. B. Weiner. 2007. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques following coimmunization with SHIV antigen and IL-15 plasmid. Proc. Natl. Acad. Sci. USA 10418648-18653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyer, J. D., T. M. Robinson, P. C. Maciag, X. Peng, R. S. Johnson, G. Pavlakis, M. G. Lewis, A. Shen, R. Siliciano, C. R. Brown, D. B. Weiner, and Y. Paterson. 2005. DNA prime Listeria boost induces a cellular immune response to SIV antigens in the rhesus macaque model that is capable of limited suppression of SIV239 viral replication. Virology 33388-101. [DOI] [PubMed] [Google Scholar]
- 27.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao, Y., L. Qin, L. Zhang, J. Safrit, and D. D. Ho. 1995. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N. Engl. J. Med. 332201-208. [DOI] [PubMed] [Google Scholar]
- 29.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 7915547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catanzaro, A. T., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, L. Gu, J. E. Martin, L. Novik, B. K. Chakrabarti, B. T. Butman, J. G. Gall, C. R. King, C. A. Andrews, R. Sheets, P. L. Gomez, J. R. Mascola, G. J. Nabel, and B. S. Graham. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J. Infect. Dis. 1941638-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakrabarti, B. K., W. P. Kong, B. Y. Wu, Z. Y. Yang, J. Friborg, X. Ling, S. R. King, D. C. Montefiori, and G. J. Nabel. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 765357-5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, W. C., and L. Huang. 2005. Non-viral vector as vaccine carrier. Adv. Genet. 54315-337. [DOI] [PubMed] [Google Scholar]
- 33.Chong, S. Y., M. A. Egan, M. A. Kutzler, S. Megati, A. Masood, V. Roopchard, D. Garcia-Hand, D. C. Montefiori, J. Quiroz, M. Rosati, E. B. Schadeck, J. D. Boyer, G. N. Pavlakis, D. B. Weiner, M. Sidhu, J. H. Eldridge, and Z. R. Israel. 2007. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV89.6P challenge in rhesus macaques. Vaccine 254967-4982. [DOI] [PubMed] [Google Scholar]
- 34.Clark, K. R. 2002. Recent advances in recombinant adeno-associated virus vector production. Kidney Int. 61(Suppl. 1)9-15. [DOI] [PubMed] [Google Scholar]
- 35.Cleghorn, F., J. W. Pape, M. Schechter, C. Bartholomew, J. Sanchez, N. Jack, B. J. Metch, M. Hansen, M. Allen, H. Cao, D. C. Montefiori, G. D. Tomaras, S. Gurunathan, D. J. Eastman, R. F. do Lago, S. Jean, J. R. Lama, D. N. Lawrence, and P. F. Wright. 2007. Lessons from a multisite international trial in the Caribbean and South America of an HIV-1 canarypox vaccine (ALVAC-HIV vCP1452) with or without boosting with MN rgp120. J. Acquir. Immune Defic. Syndr. 46222-230. [DOI] [PubMed] [Google Scholar]
- 36.Cohen, P. 2006. Immunity's yin and yang. A successful vaccine must first avoid being eliminated by pre-existing immunity before it can promote a protective immune response. IAVI Rep. 101-5. [PubMed] [Google Scholar]
- 37.Corey, L. 2006. Vaccines to induce CTL responses to HIV in immunocompetent individuals: state of the field. 13th Conf. Retrovir. Opportun. Infect., Denver, Colorado, 5 to 8 February 2006. http://www.retroconference.org/AbstractSearch/Default.aspx?Conf=is.
- 38.Croyle, M. A., N. Chirmule, Y. Zhang, and J. M. Wilson. 2002. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum. Gene Ther. 131887-1900. [DOI] [PubMed] [Google Scholar]
- 39.Deml, L., A. Bojak, S. Steck, M. Graf, J. Wild, R. Schirmbeck, H. Wolf, and R. Wagner. 2001. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J. Virol. 7510991-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drosopoulos, W. C., L. F. Rezende, M. A. Wainberg, and V. R. Prasad. 1998. Virtues of being faithful: can we limit the genetic variation in human immunodeficiency virus? J. Mol. Med. 76604-612. [DOI] [PubMed] [Google Scholar]
- 41.Earl, P. L., J. L. Americo, L. S. Wyatt, L. Anne Eller, D. C. Montefiori, R. Byrum, M. Piatak, J. D. Lifson, R. Rao Amara, H. L. Robinson, J. W. Huggins, and B. Moss. 2007. Recombinant modified vaccinia virus Ankara provides durable protection against disease caused by an immunodeficiency virus as well as long-term immunity to an orthopoxvirus in a non-human primate. Virology 36684-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan, M. A., S. Y. Chong, S. Megati, D. C. Montefiori, N. F. Rose, J. D. Boyer, M. K. Sidhu, J. Quiroz, M. Rosati, E. B. Schadeck, G. N. Pavlakis, D. B. Weiner, J. K. Rose, Z. R. Israel, S. A. Udem, and J. H. Eldridge. 2005. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retrovir. 21629-643. [DOI] [PubMed] [Google Scholar]
- 43.Egan, M. A., S. Y. Chong, N. F. Rose, S. Megati, K. J. Lopez, E. B. Schadeck, J. E. Johnson, A. Masood, P. Piacente, R. E. Druilhet, P. W. Barras, D. L. Hasselschwert, P. Reilly, E. M. Mishkin, D. C. Montefiori, M. G. Lewis, D. K. Clarke, R. M. Hendry, P. A. Marx, J. H. Eldridge, S. A. Udem, Z. R. Israel, and J. K. Rose. 2004. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retrovir. 20989-1004. [DOI] [PubMed] [Google Scholar]
- 44.Evans, D. T., L. M. Chen, J. Gillis, K. C. Lin, B. Harty, G. P. Mazzara, R. O. Donis, K. G. Mansfield, J. D. Lifson, R. C. Desrosiers, J. E. Galan, and R. P. Johnson. 2003. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 772400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 7511603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 1701416-1422. [DOI] [PubMed] [Google Scholar]
- 47.Franchini, G., M. Robert-Guroff, J. Tartaglia, A. Aggarwal, A. Abimiku, J. Benson, P. Markham, K. Limbach, G. Hurteau, J. Fullen, et al. 1995. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res. Hum. Retrovir. 11909-920. [DOI] [PubMed] [Google Scholar]
- 48.Frolov, I., and S. Schlesinger. 1994. Comparison of the effects of Sindbis virus and Sindbis virus replicons on host cell protein synthesis and cytopathogenicity in BHK cells. J. Virol. 681721-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fynan, E. F., R. G. Webster, D. H. Fuller, J. R. Haynes, J. C. Santoro, and H. L. Robinson. 1993. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. USA 9011478-11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gall, J. G., R. G. Crystal, and E. Falck-Pedersen. 1998. Construction and characterization of hexon-chimeric adenoviruses: specification of adenovirus serotype. J. Virol. 7210260-10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardner, J. P., I. Frolov, S. Perri, Y. Ji, M. L. MacKichan, J. zur Megede, M. Chen, B. A. Belli, D. A. Driver, S. Sherrill, C. E. Greer, G. R. Otten, S. W. Barnett, M. A. Liu, T. W. Dubensky, and J. M. Polo. 2000. Infection of human dendritic cells by a Sindbis virus replicon vector is determined by a single amino acid substitution in the E2 glycoprotein. J. Virol. 7411849-11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Graham, B. S., R. A. Koup, M. Roederer, R. T. Bailer, M. E. Enama, Z. Moodie, J. E. Martin, M. M. McCluskey, B. K. Chakrabarti, L. Lamoreaux, C. A. Andrews, P. L. Gomez, J. R. Mascola, and G. J. Nabel. 2006. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J. Infect. Dis. 1941650-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenland, J. R., and N. L. Letvin. 2007. Chemical adjuvants for plasmid DNA vaccines. Vaccine 253731-3741. [DOI] [PubMed] [Google Scholar]
- 54.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 7711708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta, S., F. Zhou, C. E. Greer, H. Legg, T. Tang, P. Luciw, J. zur Megede, S. W. Barnett, J. J. Donnelly, D. T. O'Hagan, J. M. Polo, and M. Vajdy. 2006. Antibody responses against HIV in rhesus macaques following combinations of mucosal and systemic immunizations with chimeric alphavirus-based replicon particles. AIDS Res. Hum. Retrovir. 22993-997. [DOI] [PubMed] [Google Scholar]
- 56.Haensler, J., C. Verdelet, V. Sanchez, Y. Girerd-Chambaz, A. Bonnin, E. Trannoy, S. Krishnan, and P. Meulien. 1999. Intradermal DNA immunization by using jet-injectors in mice and monkeys. Vaccine 17628-638. [DOI] [PubMed] [Google Scholar]
- 57.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 737524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harshyne, L. A., S. C. Watkins, A. Gambotto, and S. M. Barratt-Boyes. 2001. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J. Immunol. 1663717-3723. [DOI] [PubMed] [Google Scholar]
- 59.Hel, Z., J. Nacsa, B. Kelsall, W. P. Tsai, N. Letvin, R. W. Parks, E. Tryniszewska, L. Picker, M. G. Lewis, Y. Edghill-Smith, M. Moniuszko, R. Pal, L. Stevceva, J. D. Altman, T. M. Allen, D. Watkins, J. V. Torres, J. A. Berzofsky, I. M. Belyakov, W. Strober, and G. Franchini. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J. Virol. 7511483-11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 1694778-4787. [DOI] [PubMed] [Google Scholar]
- 61.Hel, Z., W. P. Tsai, E. Tryniszewska, J. Nacsa, P. D. Markham, M. G. Lewis, G. N. Pavlakis, B. K. Felber, J. Tartaglia, and G. Franchini. 2006. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J. Immunol. 17685-96. [DOI] [PubMed] [Google Scholar]
- 62.Hel, Z., D. Venzon, M. Poudyal, W. P. Tsai, L. Giuliani, R. Woodward, C. Chougnet, G. Shearer, J. D. Altman, D. Watkins, N. Bischofberger, A. Abimiku, P. Markham, J. Tartaglia, and G. Franchini. 2000. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat. Med. 61140-1146. [DOI] [PubMed] [Google Scholar]
- 63.Heller, L. C., and R. Heller. 2006. In vivo electroporation for gene therapy. Hum. Gene Ther. 17890-897. [DOI] [PubMed] [Google Scholar]
- 64.Hirao, L. A., L. Wu, A. S. Khan, A. Satishchandran, R. Draghia-Akli, and D. B. Weiner. 2008. Intradermal/subcutaneous immunization by electroporation improves plasmid vaccine delivery and potency in pigs and rhesus macaques. Vaccine 26440-448. [DOI] [PubMed] [Google Scholar]
- 64a.Hirao, L., L. Wu, A. S. Khan, D. A. Hokey, J. Yian. A. Dai, M. R. Betts, R. Draghia-Akli, and D. B. Weiner. Vaccine, in press. [DOI] [PMC free article] [PubMed]
- 65.Hofmann, C., P. Loser, G. Cichon, W. Arnold, G. W. Both, and M. Strauss. 1999. Ovine adenovirus vectors overcome preexisting humoral immunity against human adenoviruses in vivo. J. Virol. 736930-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hokey, D. A., and D. B. Weiner. 2006. DNA vaccines for HIV: challenges and opportunities. Springer Semin. Immunopathol. 28267-279. [DOI] [PubMed] [Google Scholar]
- 67.Holterman, L., R. Vogels, R. van der Vlugt, M. Sieuwerts, J. Grimbergen, J. Kaspers, E. Geelen, E. van der Helm, A. Lemckert, G. Gillissen, S. Verhaagh, J. Custers, D. Zuijdgeest, B. Berkhout, M. Bakker, P. Quax, J. Goudsmit, and M. Havenga. 2004. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J. Virol. 7813207-13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Inaba, K., S. Turley, F. Yamaide, T. Iyoda, K. Mahnke, M. Inaba, M. Pack, M. Subklewe, B. Sauter, D. Sheff, M. Albert, N. Bhardwaj, I. Mellman, and R. M. Steinman. 1998. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1882163-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.International AIDS Vaccine Initiative. 2007. Vaccine briefs. IAVI Rep. 1115. [Google Scholar]
- 70.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson, J. E., F. Nasar, J. W. Coleman, R. E. Price, A. Javadian, K. Draper, M. Lee, P. A. Reilly, D. K. Clarke, R. M. Hendry, and S. A. Udem. 2007. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology 36036-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson, P. R., B. C. Schnepp, M. J. Connell, D. Rohne, S. Robinson, G. R. Krivulka, C. I. Lord, R. Zinn, D. C. Montefiori, N. L. Letvin, and K. R. Clark. 2005. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J. Virol. 79955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnston, R. E., P. R. Johnson, M. J. Connell, D. C. Montefiori, A. West, M. L. Collier, C. Cecil, R. Swanstrom, J. A. Frelinger, and N. L. Davis. 2005. Vaccination of macaques with SIV immunogens delivered by Venezuelan equine encephalitis virus replicon particle vectors followed by a mucosal challenge with SIVsmE660. Vaccine 234969-4979. [DOI] [PubMed] [Google Scholar]
- 74.Jolly, D. 1994. Viral vector systems for gene therapy. Cancer Gene Ther. 151-64. [PubMed] [Google Scholar]
- 75.Kaiser, J. 2007. Gene therapy. Questions remain on cause of death in arthritis trial. Science 3171665. [DOI] [PubMed] [Google Scholar]
- 76.Kantakamalakul, W., M. De Souza, C. Karnasuta, A. Brown, S. Gurunathan, D. Birx, P. Thongcharoen, and T. Taveg. 2004. Enhanced sensitivity of detection of cytotoxic T lymphocyte responses to HIV type 1 proteins using an extended in vitro stimulation period for measuring effector function in volunteers enrolled in an ALVAC-HIV phase I/II prime boost vaccine trial in Thailand. AIDS Res. Hum. Retrovir. 20642-644. [DOI] [PubMed] [Google Scholar]
- 77.Kaur, A., H. B. Sanford, D. Garry, S. Lang, S. A. Klumpp, D. Watanabe, R. T. Bronson, J. D. Lifson, M. Rosati, G. N. Pavlakis, B. K. Felber, D. M. Knipe, and R. C. Desrosiers. 2007. Ability of herpes simplex virus vectors to boost immune responses to DNA vectors and to protect against challenge by simian immunodeficiency virus. Virology 357199-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keulen, W., M. Nijhuis, R. Schuurman, B. Berkhout, and C. Boucher. 1997. Reverse transcriptase fidelity and HIV-1 variation. Science 275229-231. [PubMed] [Google Scholar]
- 79.Kong, W. P., Y. Huang, Z. Y. Yang, B. K. Chakrabarti, Z. Moodie, and G. J. Nabel. 2003. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J. Virol. 7712764-12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kostense, S., W. Koudstaal, M. Sprangers, G. J. Weverling, G. Penders, N. Helmus, R. Vogels, M. Bakker, B. Berkhout, M. Havenga, and J. Goudsmit. 2004. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS 181213-1216. [DOI] [PubMed] [Google Scholar]
- 81.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kozlowski, P. A., S. Cu-Uvin, M. R. Neutra, and T. P. Flanigan. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 651387-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kozlowski, P. A., S. B. Williams, R. M. Lynch, T. P. Flanigan, R. R. Patterson, S. Cu-Uvin, and M. R. Neutra. 2002. Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: influence of the menstrual cycle. J. Immunol. 169566-574. [DOI] [PubMed] [Google Scholar]
- 84.Kresge, K. J. 2007. What next? IAVI Rep. 1113-14. [PubMed] [Google Scholar]
- 85.Leitner, W. W., L. N. Hwang, M. J. deVeer, A. Zhou, R. H. Silverman, B. R. Williams, T. W. Dubensky, H. Ying, and N. P. Restifo. 2003. Alphavirus-based DNA vaccine breaks immunological tolerance by activating innate antiviral pathways. Nat. Med. 933-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 3121530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 88.Li, S., E. Locke, J. Bruder, D. Clarke, D. L. Doolan, M. J. Havenga, A. V. Hill, P. Liljestrom, T. P. Monath, H. Y. Naim, C. Ockenhouse, D. C. Tang, K. R. Van Kampen, J. F. Viret, F. Zavala, and F. Dubovsky. 2007. Viral vectors for malaria vaccine development. Vaccine 252567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang, X., D. R. Casimiro, W. A. Schleif, F. Wang, M. E. Davies, Z. Q. Zhang, T. M. Fu, A. C. Finnefrock, L. Handt, M. P. Citron, G. Heidecker, A. Tang, M. Chen, K. A. Wilson, L. Gabryelski, M. McElhaugh, A. Carella, C. Moyer, L. Huang, S. Vitelli, D. Patel, J. Lin, E. A. Emini, and J. W. Shiver. 2005. Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys. J. Virol. 7912321-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lieberman, J., and F. R. Frankel. 2002. Engineered Listeria monocytogenes as an AIDS vaccine. Vaccine 202007-2010. [DOI] [PubMed] [Google Scholar]
- 91.Liljestrom, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 91356-1361. [DOI] [PubMed] [Google Scholar]
- 92.Lin, S. W., A. S. Cun, K. Harris-McCoy, and H. C. Ertl. 2007. Intramuscular rather than oral administration of replication-defective adenoviral vaccine vector induces specific CD8+ T cell responses in the gut. Vaccine 252187-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu, M. A. 1998. Vaccine developments. Nat. Med. 4515-519. [DOI] [PubMed] [Google Scholar]
- 94.Loessner, H., and S. Weiss. 2004. Bacteria-mediated DNA transfer in gene therapy and vaccination. Expert Opin. Biol. Ther. 4157-168. [DOI] [PubMed] [Google Scholar]
- 95.Lu, S. 2006. Combination DNA plus protein HIV vaccines. Springer Semin. Immunopathol. 28255-265. [DOI] [PubMed] [Google Scholar]
- 96.Luckay, A., M. K. Sidhu, R. Kjeken, S. Megati, S. Y. Chong, V. Roopchand, D. Garcia-Hand, R. Abdullah, R. Braun, D. C. Montefiori, M. Rosati, B. K. Felber, G. N. Pavlakis, I. Mathiesen, Z. R. Israel, J. H. Eldridge, and M. A. Egan. 2007. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 815257-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mackett, M., G. L. Smith, and B. Moss. 1982. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc. Natl. Acad. Sci. USA 797415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 2031533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 100.McCoy, K., N. Tatsis, B. Korioth-Schmitz, M. O. Lasaro, S. E. Hensley, S. W. Lin, Y. Li, W. Giles-Davis, A. Cun, D. Zhou, Z. Xiang, N. L. Letvin, and H. C. Ertl. 2007. Effect of preexisting immunity to adenovirus human serotype 5 antigens on the immune responses of nonhuman primates to vaccine regimens based on human- or chimpanzee-derived adenovirus vectors. J. Virol. 816594-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McDermott, A. B., D. H. O'Connor, S. Fuenger, S. Piaskowski, S. Martin, J. Loffredo, M. Reynolds, J. Reed, J. Furlott, T. Jacoby, C. Riek, E. Dodds, K. Krebs, M. E. Davies, W. A. Schleif, D. R. Casimiro, J. W. Shiver, and D. I. Watkins. 2005. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J. Virol. 7915556-15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehandru, S., M. A. Poles, K. Tenner-Racz, V. Manuelli, P. Jean-Pierre, P. Lopez, A. Shet, A. Low, H. Mohri, D. Boden, P. Racz, and M. Markowitz. 2007. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol. 81599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molinier-Frenkel, V., R. Lengagne, F. Gaden, S. S. Hong, J. Choppin, H. Gahery-Segard, P. Boulanger, and J. G. Guillet. 2002. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 76127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 9311341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mulligan, M. J., N. D. Russell, C. Celum, J. Kahn, E. Noonan, D. C. Montefiori, G. Ferrari, K. J. Weinhold, J. M. Smith, R. R. Amara, and H. L. Robinson. 2006. Excellent safety and tolerability of the human immunodeficiency virus type 1 pGA2/JS2 plasmid DNA priming vector vaccine in HIV type 1 uninfected adults. AIDS Res. Hum. Retrovir. 22678-683. [DOI] [PubMed] [Google Scholar]
- 107.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 747745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Musey, L., J. Hughes, T. Schacker, T. Shea, L. Corey, and M. J. McElrath. 1997. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N. Engl. J. Med. 3371267-1274. [DOI] [PubMed] [Google Scholar]
- 109.Neeson, P., J. Boyer, S. Kumar, M. G. Lewis, L. Mattias, R. Veazey, D. Weiner, and Y. Paterson. 2006. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response characterized by high levels of α4β7 integrin and an effector memory phenotype. Virology 354299-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nwanegbo, E., E. Vardas, W. Gao, H. Whittle, H. Sun, D. Rowe, P. D. Robbins, and A. Gambotto. 2004. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 11351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O'Hagan, D. T., and M. Singh. 2003. Microparticles as vaccine adjuvants and delivery systems. Expert Rev. Vaccines 2269-283. [DOI] [PubMed] [Google Scholar]
- 112.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 2792103-2106. [DOI] [PubMed] [Google Scholar]
- 113.Otten, G. R., M. Schaefer, B. Doe, H. Liu, J. Z. Megede, J. Donnelly, D. Rabussay, S. Barnett, and J. B. Ulmer. 2006. Potent immunogenicity of an HIV-1 gag-pol fusion DNA vaccine delivered by in vivo electroporation. Vaccine 244503-4509. [DOI] [PubMed] [Google Scholar]
- 114.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 742740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pal, R., D. Venzon, S. Santra, V. S. Kalyanaraman, D. C. Montefiori, L. Hocker, L. Hudacik, N. Rose, J. Nacsa, Y. Edghill-Smith, M. Moniuszko, Z. Hel, I. M. Belyakov, J. A. Berzofsky, R. W. Parks, P. D. Markham, N. L. Letvin, J. Tartaglia, and G. Franchini. 2006. Systemic immunization with an ALVAC-HIV-1/protein boost vaccine strategy protects rhesus macaques from CD4+ T-cell loss and reduces both systemic and mucosal simian-human immunodeficiency virus SHIVKU2 RNA levels. J. Virol. 803732-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pal, R., S. Wang, V. S. Kalyanaraman, B. C. Nair, S. Whitney, T. Keen, L. Hocker, L. Hudacik, N. Rose, A. Cristillo, I. Mboudjeka, S. Shen, T. H. Wu-Chou, D. Montefiori, J. Mascola, S. Lu, and P. Markham. 2005. Polyvalent DNA prime and envelope protein boost HIV-1 vaccine elicits humoral and cellular responses and controls plasma viremia in rhesus macaques following rectal challenge with an R5 SHIV isolate. J. Med. Primatol. 34226-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pinto, A. R., J. C. Fitzgerald, W. Giles-Davis, G. P. Gao, J. M. Wilson, and H. C. Ertl. 2003. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J. Immunol. 1716774-6779. [DOI] [PubMed] [Google Scholar]
- 118.Rayner, J. O., S. A. Dryga, and K. I. Kamrud. 2002. Alphavirus vectors and vaccination. Rev. Med. Virol. 12279-296. [DOI] [PubMed] [Google Scholar]
- 119.Reddy, P. S., Y. Chen, N. Idamakanti, C. Pyne, L. A. Babiuk, and S. K. Tikoo. 1999. Characterization of early region 1 and pIX of bovine adenovirus-3. Virology 253299-308. [DOI] [PubMed] [Google Scholar]
- 120.Reddy, P. S., N. Idamakanti, Y. Chen, T. Whale, L. A. Babiuk, M. Mehtali, and S. K. Tikoo. 1999. Replication-defective bovine adenovirus type 3 as an expression vector. J. Virol. 739137-9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 733723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441239-243. [DOI] [PubMed] [Google Scholar]
- 123.Robinson, H. L., D. C. Montefiori, R. P. Johnson, M. L. Kalish, S. L. Lydy, and H. M. McClure. 2000. DNA priming and recombinant pox virus boosters for an AIDS vaccine. Dev. Biol (Basel) 10493-100. [PubMed] [Google Scholar]
- 124.Robinson, H. L., and K. J. Weinhold. 2006. Phase 1 clinical trials of the National Institutes of Health Vaccine Research Center HIV/AIDS candidate vaccines. J. Infect. Dis. 1941625-1627. [DOI] [PubMed] [Google Scholar]
- 125.Rock, K. L. 1996. A new foreign policy: MHC class I molecules monitor the outside world. Immunol. Today 17131-137. [DOI] [PubMed] [Google Scholar]
- 126.Roy, S., P. S. Shirley, A. McClelland, and M. Kaleko. 1998. Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 726875-6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rüssmann, H., H. Shams, F. Poblete, Y. Fu, J. E. Galan, and R. O. Donis. 1998. Delivery of epitopes by the Salmonella type III secretion system for vaccine development. Science 281565-568. [DOI] [PubMed] [Google Scholar]
- 128.Sailaja, G., H. HogenEsch, A. North, J. Hays, and S. K. Mittal. 2002. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 91722-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schadeck, E. B., M. Sidhu, M. A. Egan, S. Y. Chong, P. Piacente, A. Masood, D. Garcia-Hand, S. Cappello, V. Roopchand, S. Megati, J. Quiroz, J. D. Boyer, B. K. Felber, G. N. Pavlakis, D. B. Weiner, J. H. Eldridge, and Z. R. Israel. 2006. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 244677-4687. [DOI] [PubMed] [Google Scholar]
- 130.Schlesinger, S. 2001. Alphavirus vectors: development and potential therapeutic applications. Expert Opin. Biol. Ther. 1177-191. [DOI] [PubMed] [Google Scholar]
- 131.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 132.Schneider, J., S. C. Gilbert, C. M. Hannan, P. Degano, E. Prieur, E. G. Sheu, M. Plebanski, and A. V. Hill. 1999. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol. Rev. 17029-38. [DOI] [PubMed] [Google Scholar]
- 133.Schneider, R., M. Campbell, G. Nasioulas, B. K. Felber, and G. N. Pavlakis. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 714892-4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schneider, T., H. U. Jahn, W. Schmidt, E. O. Riecken, M. Zeitz, and R. Ullrich. 1995. Loss of CD4 T lymphocytes in patients infected with human immunodeficiency virus type 1 is more pronounced in the duodenal mucosa than in the peripheral blood. Gut 37524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schnell, M. J. 2001. Viral vectors as potential HIV-1 vaccines. FEMS Microbiol. Lett. 200123-129. [DOI] [PubMed] [Google Scholar]
- 136.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55355-372. [DOI] [PubMed] [Google Scholar]
- 137.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 138.Smith, J. M., R. R. Amara, H. M. McClure, M. Patel, S. Sharma, H. Yi, L. Chennareddi, J. G. Herndon, S. T. Butera, W. Heneine, D. L. Ellenberger, B. Parekh, P. L. Earl, L. S. Wyatt, B. Moss, and H. L. Robinson. 2004. Multiprotein HIV type 1 clade B DNA/MVA vaccine: construction, safety, and immunogenicity in macaques. AIDS Res. Hum. Retrovir. 20654-665. [DOI] [PubMed] [Google Scholar]
- 139.Spira, S., M. A. Wainberg, H. Loemba, D. Turner, and B. G. Brenner. 2003. Impact of clade diversity on HIV-1 virulence, antiretroviral drug sensitivity and drug resistance. J. Antimicrob. Chemother. 51229-240. [DOI] [PubMed] [Google Scholar]
- 140.Stevceva, L., X. Alvarez, A. A. Lackner, E. Tryniszewska, B. Kelsall, J. Nacsa, J. Tartaglia, W. Strober, and G. Franchini. 2002. Both mucosal and systemic routes of immunization with the live, attenuated NYVAC/simian immunodeficiency virus SIVgpe recombinant vaccine result in Gag-specific CD8+ T-cell responses in mucosal tissues of macaques. J. Virol. 7611659-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stone, G. W., S. Barzee, V. Snarsky, K. Kee, C. A. Spina, X.-F. Yu, and R. S. Kornbluth. 2006. Multimeric soluble CD40 ligand and GITR ligand as adjuvants for human immunodeficiency virus DNA vaccines. J. Virol. 801762-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stone, G. W., S. Barzee, V. Snarsky, C. A. Spina, J. D. Lifson, V. K. Pillai, R. R. Amara, F. Villinger, and R. S. Kornbluth. 2006. Macaque multimeric soluble CD40 ligand and GITR ligand constructs are immunostimulatory molecules in vitro. Clin. Vaccine Immunol. 131223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sumida, S. M., D. M. Truitt, M. G. Kishko, J. C. Arthur, S. S. Jackson, D. A. Gorgone, M. A. Lifton, W. Koudstaal, M. G. Pau, S. Kostense, M. J. Havenga, J. Goudsmit, N. L. Letvin, and D. H. Barouch. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol. 782666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun, J. Y., V. Anand-Jawa, S. Chatterjee, and K. K. Wong. 2003. Immune responses to adeno-associated virus and its recombinant vectors. Gene Ther. 10964-976. [DOI] [PubMed] [Google Scholar]
- 145.Tang, D. C., Z. Shi, and D. T. Curiel. 1997. Vaccination onto bare skin. Nature 388729-730. [DOI] [PubMed] [Google Scholar]
- 146.Taniyama, Y., K. Tachibana, K. Hiraoka, M. Aoki, S. Yamamoto, K. Matsumoto, T. Nakamura, T. Ogihara, Y. Kaneda, and R. Morishita. 2002. Development of safe and efficient novel nonviral gene transfer using ultrasound: enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 9372-380. [DOI] [PubMed] [Google Scholar]
- 147.Tatsis, N., S. W. Lin, K. Harris-McCoy, D. A. Garber, M. B. Feinberg, and H. C. Ertl. 2007. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology 367156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Théry, C., L. Zitvogel, and S. Amigorena. 2002. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2569-579. [DOI] [PubMed] [Google Scholar]
- 149.Thongcharoen, P., V. Suriyanon, R. M. Paris, C. Khamboonruang, M. S. de Souza, S. Ratto-Kim, C. Karnasuta, V. R. Polonis, L. Baglyos, R. E. Habib, S. Gurunathan, S. Barnett, A. E. Brown, D. L. Birx, J. G. McNeil, and J. H. Kim. 2007. A phase 1/2 comparative vaccine trial of the safety and immunogenicity of a CRF01_AE (subtype E) candidate vaccine: ALVAC-HIV (vCP1521) prime with oligomeric gp160 (92TH023/LAI-DID) or bivalent gp120 (CM235/SF2) boost. J. Acquir. Immune Defic. Syndr. 4648-55. [DOI] [PubMed] [Google Scholar]
- 150.Thorner, A. R., A. A. Lemckert, J. Goudsmit, D. M. Lynch, B. A. Ewald, M. Denholtz, M. J. Havenga, and D. H. Barouch. 2006. Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity. J. Virol. 8012009-12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Titomirov, A. V., S. Sukharev, and E. Kistanova. 1991. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta 1088131-134. [DOI] [PubMed] [Google Scholar]
- 152.Toka, F. N., C. D. Pack, and B. T. Rouse. 2004. Molecular adjuvants for mucosal immunity. Immunol. Rev. 199100-112. [DOI] [PubMed] [Google Scholar]
- 153.Trimble, C., C. T. Lin, C. F. Hung, S. Pai, J. Juang, L. He, M. Gillison, D. Pardoll, L. Wu, and T. C. Wu. 2003. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine 214036-4042. [DOI] [PubMed] [Google Scholar]
- 154.Tuboly, T., and E. Nagy. 2001. Construction and characterization of recombinant porcine adenovirus serotype 5 expressing the transmissible gastroenteritis virus spike gene. J. Gen. Virol. 82183-190. [DOI] [PubMed] [Google Scholar]
- 155.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280427-431. [DOI] [PubMed] [Google Scholar]
- 156.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 7457-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vignuzzi, M., S. Gerbaud, S. van der Werf, and N. Escriou. 2001. Naked RNA immunization with replicons derived from poliovirus and Semliki Forest virus genomes for the generation of a cytotoxic T cell response against the influenza A virus nucleoprotein. J. Gen. Virol. 821737-1747. [DOI] [PubMed] [Google Scholar]
- 158.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 778263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang, S., D. J. Farfan-Arribas, S. Shen, T. H. Chou, A. Hirsch, F. He, and S. Lu. 2006. Relative contributions of codon usage, promoter efficiency and leader sequence to the antigen expression and immunogenicity of HIV-1 Env DNA vaccine. Vaccine 244531-4540. [DOI] [PubMed] [Google Scholar]
- 160.Widera, G., M. Austin, D. Rabussay, C. Goldbeck, S. W. Barnett, M. Chen, L. Leung, G. R. Otten, K. Thudium, M. J. Selby, and J. B. Ulmer. 2000. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 1644635-4640. [DOI] [PubMed] [Google Scholar]
- 161.Xiang, Z., G. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 762667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xiao, W., N. Chirmule, S. C. Berta, B. McCullough, G. Gao, and J. M. Wilson. 1999. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 733994-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu, R., I. K. Srivastava, C. E. Greer, I. Zarkikh, Z. Kraft, L. Kuller, J. M. Polo, S. W. Barnett, and L. Stamatatos. 2006. Characterization of immune responses elicited in macaques immunized sequentially with chimeric VEE/SIN alphavirus replicon particles expressing SIVGag and/or HIVEnv and with recombinant HIVgp140Env protein. AIDS Res. Hum. Retrovir. 221022-1030. [DOI] [PubMed] [Google Scholar]
- 164.Yang, J. S., J. J. Kim, D. Hwang, A. Y. Choo, K. Dang, H. Maguire, S. Kudchodkar, M. P. Ramanathan, and D. B. Weiner. 2001. Induction of potent Th1-type immune responses from a novel DNA vaccine for West Nile virus New York isolate (WNV-NY1999). J. Infect. Dis. 184809-816. [DOI] [PubMed] [Google Scholar]
- 165.Yang, S., J. W. Hodge, D. W. Grosenbach, and J. Schlom. 2005. Vaccines with enhanced costimulation maintain high avidity memory CTL. J. Immunol. 1753715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ying, H., T. Z. Zaks, R. F. Wang, K. R. Irvine, U. S. Kammula, F. M. Marincola, W. W. Leitner, and N. P. Restifo. 1999. Cancer therapy using a self-replicating RNA vaccine. Nat. Med. 5823-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Youil, R., T. J. Toner, Q. Su, M. Chen, A. Tang, A. J. Bett, and D. Casimiro. 2002. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 13311-320. [DOI] [PubMed] [Google Scholar]
- 168.Zeira, E., A. Manevitch, A. Khatchatouriants, O. Pappo, E. Hyam, M. Darash-Yahana, E. Tavor, A. Honigman, A. Lewis, and E. Galun. 2003. Femtosecond infrared laser-an efficient and safe in vivo gene delivery system for prolonged expression. Mol. Ther. 8342-350. [DOI] [PubMed] [Google Scholar]
- 169.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 2861353-1357. [DOI] [PubMed] [Google Scholar]
- 170.Zhong, B., P. Tien, and H. B. Shu. 2006. Innate immune responses: crosstalk of signaling and regulation of gene transcription. Virology 35214-21. [DOI] [PubMed] [Google Scholar]
- 171.Zhou, X., P. Berglund, G. Rhodes, S. E. Parker, M. Jondal, and P. Liljestrom. 1994. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine 121510-1514. [DOI] [PubMed] [Google Scholar]
- 172.zur Megede, J., M. C. Chen, B. Doe, M. Schaefer, C. E. Greer, M. Selby, G. R. Otten, and S. W. Barnett. 2000. Increased expression and immunogenicity of sequence-modified human immunodeficiency virus type 1 gag gene. J. Virol. 742628-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]