Abstract
The mammalian ERCC1-XPF endonuclease has a suggested role in the repair of DNA double-strand breaks (DSB) by single-strand annealing (SSA). Here, we investigated the role of ERCC1 in homologous recombination in mammalian cells, and confirm a role of ERCC1 in SSA. Interestingly, we also report an unexpected role for ERCC1 in gene conversion. This provides support that gene conversion in mammalian somatic cells is carried out through synthesis-dependent strand annealing, rather than through a double Holliday Junction mechanism. Moreover, we find low frequencies of SSA and gene conversion in G1-arrested cells, suggesting that SSA is not a frequent DSB repair pathway in G1-arrested mammalian cells, even in the presence of perfect repeats. Furthermore, we find that SSA is not influenced by inhibition of CDK2 (using Roscovitine), ATM (using Caffeine and KU55933), Chk1 (using CEP-3891) or DNA-PK (using NU7026).
INTRODUCTION
The ERCC1 protein exists in a complex with the XPF endonuclease, which specifically cleaves 3′ single-stranded DNA (ssDNA) flaps of double-stranded DNA (dsDNA) (1). The mammalian ERCC1/XPF endonuclease functions in nucleotide excision repair (NER) (1,2), gene targeting (3) and single-strand annealing (SSA) (4). Although ERCC1 plays a major role in NER, it was found not to be associated with the defects in any of the known human NER disorders, such as Xeroderma pigmentosum (XP), Cockayne syndrome and Trichothiodystrophy (5–7). However, a human deficiency in the ERCC1 gene was recently reported (8) and the patient's cells showed only moderate hypersensitivity to UV light and mitomycin C as compared to XP patients. Surprisingly, the clinical features of the ERCC1-defective patient were very severe and were compatible with a diagnosis of cerebro-oculo-facio-skeletal syndrome. The mild repair defect and clinical severity suggest novel functions for ERCC1, in agreement with the observations in ERCC1 knockout mice, which display severe phenotypes compared to other NER deficient mouse models, involving multiorgan failure, severe runting and death before weaning (9–11). These observations indicate additional roles for the ERCC1-XPF endonuclease other than its function in NER.
There is evidence for a role of ERCC1 in homologous recombination (HR) between direct repeats in the adenine phosphoribosyltransferase locus in Chinese hamster cells (12,13). Moreover, ERCC1/XPF was found to be required for targeted gene replacement via recombination in mouse embryonic stem cells (14). SSA is an efficient way to repair 2-ended double-strand breaks (DSB) between repeated sequences (15). SSA requires resected DNA ends, which are believed to be produced by the Mre11/Rad50/Nbs1 complex (16). The combined function of the RAD52 protein, which binds 3′ ssDNA ends, and the RPA protein, which binds tightly to ssDNA, is believed to be sufficient to initiate SSA between repeated sequences, e.g. Alu sequences, in mammalian cells (17–19).
To test the role of ERCC1 in SSA and gene conversion, we introduced the DRneo recombination reporter construct into the ERCC1-defective Chinese hamster cell line UV4, which we then complemented with ERCC1. A DSB was induced into the reporter construct by transfecting with an I-SceI expression plasmid and SSA and gene conversion frequencies were determined. We found reduced levels of both SSA and gene conversion in ERCC1-defective cells, suggesting that ERCC1 is involved in both these recombination pathways in mitotic mammalian cells. Furthermore, we find lower frequencies of SSA in G1-arrested cells, suggesting that this pathway is not a common complement to non-homologous end joining (NHEJ) in repair of 2-ended DSBs in G1-arrested cells.
MATERIALS AND METHODS
Cell lines
The cell lines used are UV4DR7, ERCC1.17, ERCC1.21 and PEF7. The UV4DR7 cell line was created following electroporation of the DRneo vector and isolation of individual clones as described earlier (20). To obtain an ERCC1-complemented UV4DR7 cell line, the ERCC1 gene was cloned into the PEF6-V5-His-Topo vector according to the manufacturer's protocol (Invitrogen). The ERCC1 cDNA was amplified in a two-step RT-PCR with RNA extracted from SPD8 cells using primers; ERCC1-f 5′-CAG ATG GAC CTT GGG AAA GAC-3′ and ERCC1-r 5′-TTA TGA CGC TGT AGC CTC AGC-3′. A total of 5 × 106 cells/ml were electroporated with 30 μg of pEF6-V5-His-ERCC1 or empty control vector (1500 μF capacity, 250 V for 30 s). Blasticidin (5 µg/ml) was added 48 h following electroporation and individual blasticidin resistant colonies isolated and expanded. All cell lines were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal calf serum, penicillin (60 µg/ml), streptomycin (100 µg/ml) at 37°C containing 5% CO2, in a humidified incubator. The medium was supplemented with hygromycin (0.05 mM) in order to maintain the DRneo vector, and Blasticidin (3 µg/ml) to maintain the PEf6-V5-His-ERCC1 and PEF6-V5-His-Topo vector-containing cell lines.
Southern blotting
Genomic DNA was isolated from the UV4DR7 cell line, phenol–chloroform treated and digested with restriction endonucleases. Ten microgram of purified DNA was digested HindIII, HindIII + SacI, HindIII + KpnI and/or HindIII + NcoI was used. To determine that only one integrated copy of DRneo was present in the genome. The HindIII + XhoI was used to determine the size of the DRneo substrate in G418R clones. All DNA was separated by gel electrophoresis and Southern blotting was carried out as previously described using the S2neo fragment as probe (20).
UV survival assay
Five hundred cells from each cell line were seeded over night to be treated with different doses of UV (0, 0.2, 0.5, 1, 2, 5 and 10 J/m2) on the following day. After that they were incubated for 7–14 days in a humidified 5% CO2/37C incubator and then the colonies were fixed and stained with 0.4% methylene blue in methanol and individual colonies (greater than 30 cells) counted.
Recombination assay
To avoid contamination with spontaneous G418 resistant clones in the DRneo recombination assay, 103 cells from each cell lines were separately expanded to confluencency on about 10 different Petri dishes for each cell line (Ø 100 mm). The spontaneous recombination frequency was determined for each cell population by selection of 2 × 105 cells/plate (Ø 100 mm) seeded in G418 (100 μg/ml). Only those cell populations that did not retrieve viable G418R colonies were used for further recombination assays, as they have low background frequency of spontaneous G418R cells.
In the recombination assay, 1.5 × 106 cells of each cell line (UV4DR7, ERCC1.17, ERCC1.21 and PEF7) were seeded over night, and transiently transfected with or without pCMV3xnls-I-SceI expression vector (100 ng) according to manufacturer's protocol (Lipofectamine2000™, Invitrogen, UK, final DNA concentration 10 µg/ml) to create DSBs. After 5 h of incubation in the humidified 5% CO2/37°C incubator, cells were changed to complete medium. Twenty-four hours after transfection, the cells were trypsinized and counted. For recombination frequency, 2 × 105 cells/plate (Ø 100 mm) were seeded and G418 (100 μg/ml) and/or hygromycin (0.5 mg/ml) were added for selection to both treated and untreated plates. A total of 500 cells/plate were seeded to determine the cloning efficiency. Plates were incubated for 7 or 10 days for cloning and selection, respectively, after which they were fixed and stained with 0.4% methylene blue in methanol.
For synchronization of cells in G1 phase of the cell cycle, we plated 1.5 × 106 ERCC1.17 and 2.5 × 106 UV4DR7 cells for 48 h to reach contact inhibition, or 2 × 106 ERCC1.17 and UV4DR7 cells were grown for 48 h in serum-free media. Cells were then transiently transfected with either pCMV3xnls-I-SceI or pEGFP-C2 vectors and then followed the above protocol for cloning and selection, or treated with inhibitors throughout the transfection with 2 mM caffeine, 10 µM KU55933, 10 µM NU7026, 25 µM Roscovitine or 500 nM CEP-3891. All experiments were repeated independently at least three times.
FACS analysis
To determine lipofectamin transfection rates, cells were transiently transfected with a reporter vector, pEGFP-C2 Vector (Invitrogen), using the manufacturer's protocol (Invitrogen) and expression of EGFP was analysed by FACS Sort Vantage (Becton Dickinson, UK).
For cell cycle analysis, arrested cells were harvested, washed twice in PBS and fixed in 70% cold ethanol followed by treatment with 1 mg/ml RnaseA and staining with 100 mg/ml propidium iodide. The proportion of arrested cells in each phase of cell cycle was then determined by FACS Sort Vantage.
Western blotting
A total of 2 × 106 cells was plated onto 100 mm dishes and grown for 24 h. Cells were then lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 0.1 mM PMSF in PBS) in the presence of 1× protease and phosphatase inhibitor cocktails (Sigma, UK). An aliquot of 50 µg total protein was then run on a 10% SDS–PAGE gel and transferred to Hybond C membrane (Amersham Pharmacia, UK) using a semi-dry transfer cell (Bio-Rad). This membrane was blocked in 5% milk for 1 h and immunoblotted with mouse monoclonal antibody ERCC1 vAb-2 (clone 8F1; Labvision), diluted 1:200 in 5% milk in PBS-0.05% Tween and rabbit anti-actin (Sigma) antibody diluted 1:2000 in 5% milk in PBS-0.05% Tween overnight. Anti-mouse peroxidase conjugate (Sigma 1:2000) and anti-rabbit peroxidase conjugate (Cell Signaling 1:1000) were used, respectively, as secondary antibodies and immunoreactive protein was visualized using ECL reagents (Amersham Pharmacia) following manufacturer's instructions.
RESULTS
ERCC1 is involved in SSA and gene conversion
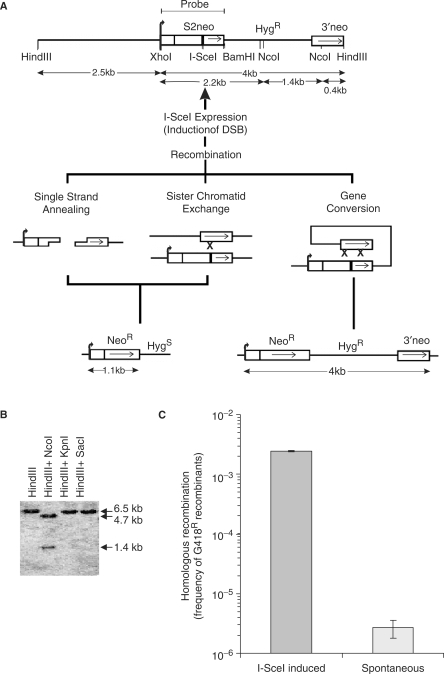
Here, we studied the role of ERCC1 using a recombination reporter construct, DRneo (21), which contains a hygromycin (Hyg) resistance gene and two non-functional copies of the neoR gene. After introduction of a DSB, these non-functional copies revert to a functional neoR gene through either SSA, sister chromatid exchange (SCE) or gene conversion, conferring resistance to geneticin (G418) (Figure 1A). We first stably integrated the DRneo reporter construct into the genome of the UV4 hamster cell line that is deficient in ERCC1 (1), and isolated individual hygromycin-resistant clones. The presence and correct integration of a single DRneo copy into the UV4DR7 clone was confirmed using Southern blotting with S2neo as probe (Figure 1B). HindIII cleavage resulted in a single 6.5 kb band, showing that the DRneo construct is present in one copy (Figure 1B). HindIII and KpnI or SacI cleavage gave the same 6.5 kb band, which implies that KpnI and SacI cleave outside the 6.5 kb region. HindIII and NcoI cleavage produced a 4.7 kb and a 1.4 kb band, showing that the internal structure of DRneo is preserved in the UV4DR7 clone.
Figure 1.
Homologous recombination is induced >100-fold by a single DSB in ERCC1-defective UV4DR7 cells. (A) Structure of the DRneo recombination substrate containing two non-functional copies of the neoR gene. A functional neoR gene can be produced by SSA, SCE or gene conversion upon induction of a DSB following expression of the I-SceI restriction endonuclease. Recombination to a functional neoR gene using SSA or SCE results in loss of the hygR gene, while the hygR gene is preserved following recombination using gene conversion. (B) Southern blot analysis performed on UV4DR7 to confirm the intact DRneo structure in the cell line. The S2neo was used as probe. (C) Recombination frequency in UV4DR7 cells following induction of a DSB using transient transfection with the pCMV3nlsI-SceI vector. The average and standard deviation of at least three experiments is depicted.
The pCMV3xnlsI-SceI vector was transiently transfected into UV4DR7 cells to express the I-SceI endonuclease to induce a DSB in the DRneo substrate. We performed a fluctuation assay to determine the extent of recombination induced and found that a DSB induces HR 103-fold (Figure 1C). This shows that ERCC1-defective cells have no overall defect in HR.
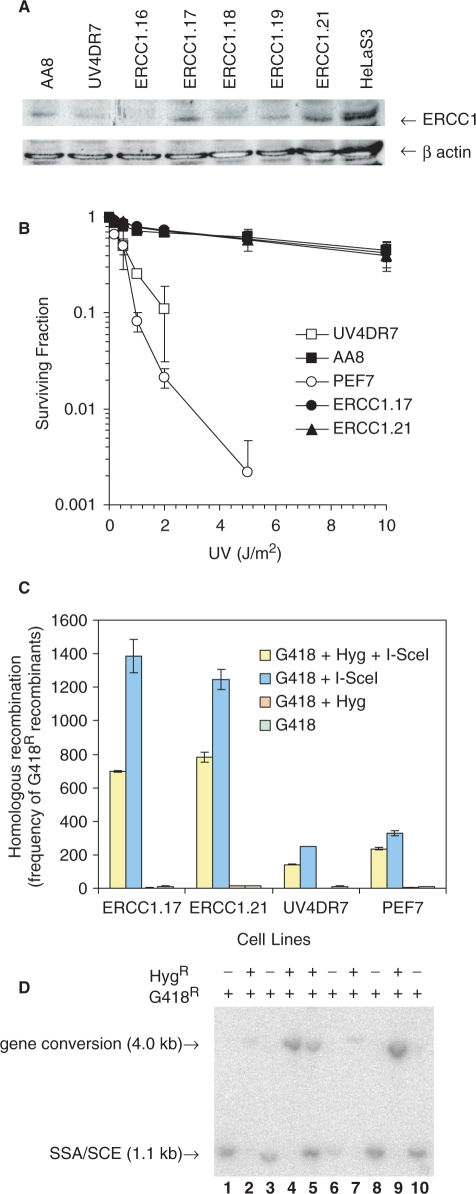
We transfected the UV4DR7 cell line with the pEF6-V5-His vector containing the hamster ERCC1 gene, or with the empty control vector. Individual clones were isolated and the level of ERCC1 expression quantified by western blotting, using extracts from hamster AA8 and human HeLa cells as control (Figure 2A). The ERCC1.17 and ERCC1.21 clones expressed ERCC1 to the same extent as wild-type hamster AA8 cells and fully complemented the sensitivity of ERCC1-defective cells to UV treatments (Figure 2B). In contrast, the clone PEF7 containing the empty control vector was still sensitive to UV as a result of the continued defect in ERCC1. The reason for the slight UV sensitivity difference to UV4DR7 is likely explained by that PEF7 the fact is an isolated clone from UV4DR7.
Figure 2.
ERCC1 is involved in SSA and gene conversion. (A) Western blot analysis showing the expression of ERCC1 protein in individual blasticidin resistant UV4DR7 clones transfected with pEF6-V5-His-ERCC1 vector using a monoclonal ERCC1 antibody and anti-β-actin antibody as a loading control. (B) Surviving fraction of wild-type AA8, UV4DR7, PEF7 and ERCC1-complemented UV4DR7 clones ERCC1.17 and ERCC1.21 following exposure to UV light. The average (symbol) and standard deviation (bars) of at least three experiments is depicted. (C) Recombination frequency to G418 or G418 + Hygromycin (Hyg) resistance following transient transfection of the pCMVI-SceI3xnls vector. The level of resistance to G418 alone reflects a recombination event using either gene conversion or SSA (blue), while frequencies of gene conversion alone are indicated by G418 + Hyg resistance (yellow). The average and standard deviation of at least three experiments is depicted. (D) Southern blot analysis using S2neo as probe on XhoI and HindIII digested DNA isolated from individual recombinant G418R clones. The subsequently found resistance to Hygromycin is indicated.
We investigated HR in the UV4DR7, ERCC1.17, ERCC1.21 and PEF.7 cell lines by transiently transfecting the cells with pCMV3xnlsI-SceI vector to create a DSB in the S2neo fragment of the DRneo reporter construct. Recombination of DRneo to a neoR gene can occur through either gene conversion, SSA or SCE (Figure 1A). If the neoR gene is restored by gene conversion, the hygR gene in between the repeats will be retained, while it will be lost following SSA or SCE (Figure 1A). We selected recombinant clones with G418 or G418 plus hygromycin (G418 + Hyg) to determine the frequency of the different recombination events.
If ERCC1 is exclusively involved in SSA, it would be expected that the levels of G418 resistant, but not G418 + Hyg resistant, clones would be lower in UV4DR7 and PEF7 cells, as those represent the products of SSA. Surprisingly, both the levels of G418 and G418 + Hyg resistant recombinants were reduced in UV4DR7 and PEF7 cells (Figure 2C). The levels of SSA and gene conversion were reduced by 83% and 75% in ERCC1-defective cells, respectively. These data demonstrate that ERCC1 is involved not only in SSA, but also in gene conversion.
We isolated 10 G418-resistant clones and determined the recombination products using Southern blotting and compared the results to their subsequent resistance to hygromycin (Figure 2D). The presence of a 4.0 kb band in the Southern blots correlated with hygromycin resistance. Interestingly, we found a clone with both the 1.1 and 4.0 kb bands, indicating that two DRneo alleles (present after replication) reverted to neoR genes using different mechanisms, which has been reported earlier for a similar recombination substrate (20,22). Altogether, these data show that G418 + Hyg resistance correlates with a gene conversion recombination product, which has also been shown to be the case with the same recombination reported earlier (21).
Suppressed SSA in G1-arrested cells
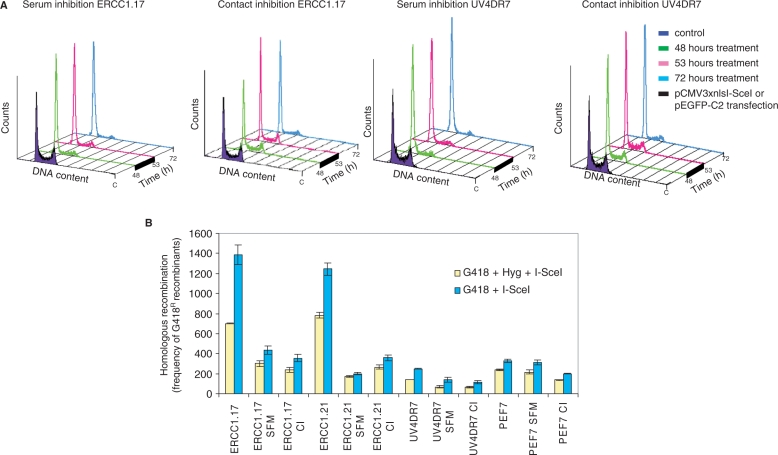
It is well established that HR predominates during and following replication and that NHEJ predominates in the G1 phase of the cell cycle (23,24). As SSA is generally believed to occur also in the absence of a sister chromatid, it is plausible that this could be a frequently used pathway for DSB repair in G1-arrested cells, as a complement to NHEJ. We had the DRneo substrate present in both ERCC1-defective and complemented cells, allowing us to test if SSA is specifically used for DSB repair in G1-arrested cells. We synchronized cells in the G1 phase using either serum starvation (SFM) or contact inhibition (CI) (by growing cells to confluency). We found that a 48-h SFM or CI of UV4DR7 or ERCC1.17 cells accumulated in the G1 phase (Figure 3A). The cells were subsequently transfected with the pCMV3xnlsI-SceI vector for 5 h and kept arrested for an additional 19 h, to ensure expression of the I-SceI restriction endonuclease and DSB formation while cells were still in the G1 phase, before replating for selection in G418 or G418 + Hyg. We have previously reported differential transfection efficiency between cycling and arrested cells (24). Thus, to compensate for differential effects in expression from the pCMV3xnlsI-SceI, we co-transfected cells with the same amount of pEGFP-C2 vector, expressing the green fluorescent protein (GFP) from the same CMV promoter as the one expressed in the pCMV3xnlsI-SceI vector. We found reduced GFP expression in G1-arrested cells (data not shown), in agreement with the previously published results (24). We determined the I-SceI-induced recombination frequencies in G1-arrested and cycling cells and compensated the recombination frequency of the arrested cells for the reduced transfection efficiency. In ERCC1 expressing wild-type cells, we found that both the levels of gene conversion and SSA drop in G1-arrested cells. The gene conversion level (represented by the yellow bar) dropped 2-and 4-fold in serum-starved ERCC1.17 and ERCC1.21 cells, respectively and 3-fold in both cell lines that were contact-inhibited (Figure 3B), in agreement with our earlier report that gene conversion events are reduced in G1-arrested cells (24).
Figure 3.
SSA is suppressed in the G1 phase of the cell cycle. (A) Cell cycle profiles of ERCC1.17 and UV4DR7 cells arrested in the G1 phase by SFM or CI. After 48 h arrest, cells were transfected with either pCMV3xnlsI-SceI or pEGFP-C2 vector for 5 h and then maintained arrested for an additional 19 h before the recombination or GFP expression assays were performed. (B) Homologous recombination induced by an I-SceI-induced DSB in the DRneo construct in asynchronous and G1-arrested cells. The average and standard deviation of at least three experiments is depicted. All values have been corrected for reduced GFP expression in arrested cells. Blue bars indicate gene conversion + SSA (resistance to G418 alone) and yellow bars gene conversion (resistance to both G418 + Hyg).
Interestingly, the SSA levels in ERCC1 proficient cells (represented by blue bar minus yellow bar) dropped 5- to 6-fold in both serum-starved and contact-inhibited cells as compared to dividing control cells (statistically significant P < 0.001). Thus, the efficiency of SSA, even within perfect repeats, is reduced in G1-arrested cells.
The reduction of gene conversion and SSA was not as profound in ERCC1-defective UV4DR7 and PEF7 cells. These data suggest that ERCC1-dependent SSA and gene conversion are more efficient in cycling cells and that ERCC1-independent recombination events contribute to the recombination events that restore a functional neoR gene in G1-arrested cells. Overall, our data suggest that ERCC1-dependent SSA and gene conversion is more efficient following replication in the S/G2 phase of the cell cycle.
SSA is independent of cell cycle checkpoint signalling
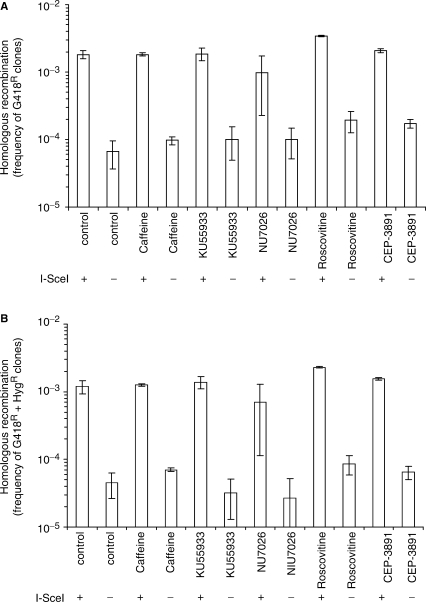
It was reported earlier that HR repair of a DSB depends on the checkpoint proteins Ataxia-Telangiectasia and Rad3-related (ATR) and Chk1 (25), and that resection of DNA ends to facilitate ATR activation and HR relies on cyclin-dependent kinases (CDK) activity (26–28). Thus, one reason for reduced SSA in G1-arrested cells could be the absence of CDK activity in these cells and thus a reduced resection at DSBs. Absence of resection is likely to prevent SSA as no ssDNA homologies would be unveiled. To test the possibility that SSA depends on cell cycle checkpoint signalling, we used various protein kinase inhibitors; Roscovitine inhibits CDK especially CDK2, which plays an essential role in initiating replication (29). Caffeine is a broad range phosphoinositide 3-kinase related kinase inhibitor, inhibiting both ATM (Ataxia-Telangiectasia Mutated) and ATR (30). KU55933 is a specific ATM inhibitor and NU7026 inhibits the DNA-dependent protein kinase (DNA-PK) required for NHEJ (31). CEP-3891 is a specific inhibitor of Chk1 (25). We treated ERCC1.17 cells with the inhibitors during transfection of the pCMV3xnlsI-SceI vector and during the 24 h recovery period before re-plating with selection with G418 or G418 + Hyg. We found no differences in either gene conversion or SSA levels in the presence of any of the inhibitors (Figure 4). No differences in gene conversion or SSA levels were found in the UV4DR7 cell line (data not shown). Others and we have previously reported that HR is indeed inhibited by caffeine, KU55933 and CEP-3891 (25,32,33), using other recombination reporter substrates. The difference in these reporter substrates is that they require strand invasion to recover a neoR gene, and thus SSA does not produce a neoR gene. Overall, our data does not support a role for checkpoint signalling in SSA.
Figure 4.
SSA of a single DSB is independent of inhibition of ATM, DNA-PK, CDK and Chk1 activity. Homologous recombination induced by an I-SceI-induced DSB in the DRneo construct in the ERCC1.17 cell line, co-treated with 2 mM caffeine, 10 µM KU55933, 10 µM NU7026, 25 µM Roscovitine or 500 nM CEP-3891 throughout transfections and 24 h recovery period prior to the recombination assay, using selection with (A) G418 alone (gene conversion + SSA) or (B) G418+Hyg (gene conversion only). The average and standard deviation of at least three experiments is depicted.
DISCUSSION
Here, we investigate the role of ERCC1 in HR using established ERCC1-defective hamster cells (1,34). The ERCC1 protein partners with the XPF endonuclease, and together they act as a heterodimer (35,36). Thus, we are essentially studying the role of the ERCC1/XPF endonuclease in HR. However, it is important to point out that disease connected with an ERCC1 mutation is much more severe than those with XPF mutation (8). Thus, the effects seen here might also be unrelated to XPF. Previously, it was established that ERCC1 has an important role in SSA, interstrand cross-link repair as well as in gene targeting (3,12,37–40). Here, we confirm the role of ERCC1 in SSA and find almost 10-fold reduced levels of SSA in ERCC1-defective cells.
In spite of the SSA defect in ERCC1-defective cells, it is important to note that our results show that HR between repeated sequences is induced >100-fold by a DSB even in absence of ERCC1 (Figure 1C). Thus, there are likely other endonucleases that can compensate for the loss of ERCC1. The recombination reporter used here cannot distinguish between an SSA and SCE event. However, it has been shown earlier that SCEs are very rare products of the repair of 2-ended DSBs produced by I-SceI (20–22). Thus, the 10-fold reduction in recombination rates likely corresponds to a defect in SSA.
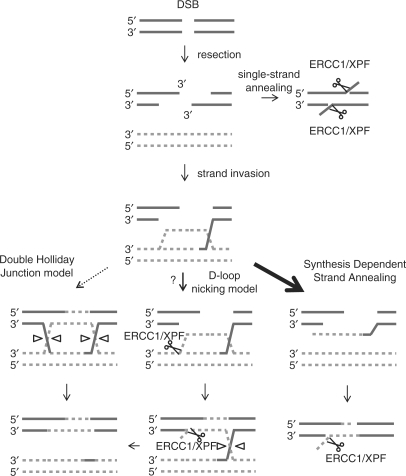
Interestingly, we also find a defect in gene conversion in ERCC1-defective cells. This implicates a role for the ERCC1/XPF endonuclease in gene conversion repair of a 2-ended DSB. Currently, there are two different models suggested for repair of a 2-ended DSB, the double-Holliday Junction (HJ) model (41) and the synthesis-dependent strand annealing (SDSA) model (42). The strand invasion in the SDSA model is reversed, once repair synthesis has progressed beyond the initial breakpoint and the repair is carried out in an SSA step (Figure 5). As the ERCC1/XPF endonuclease is required for efficient SSA, it is expected that the same complex also would carry out cleavage of non-homologous 3′ ends produced also in this model. Thus, our data support the SDSA model for repair of 2-ended DSBs in favour of the double-HJ model as the ERCC1/XPF is likely not a HJ resolvase (43,44). A D-loop nicking model for DSB repair has recently been proposed for meiotic recombination (45). The ERCC1/XPF complex may be an important mediator at several steps in such a pathway (Figure 5), but it is unexplored whether this pathway would be applicable for 2-ended DSB repair in mitosis.
Figure 5.
The role of ERCC1/XPF in gene conversion supports the synthesis-dependent strand annealing model for repair of 2-ended DSBs. A resected 2-ended DSB may be repaired through SSA between two repeat sequences and the non-homologous 3′ DNA ends trimmed away by the ERCC1/XPF endonuclease. A 2-ended DSB may also be repaired by homologous recombination using the intact sister chromatid as donor. There are two major models for repair of 2-ended DSBs by homologous recombination that result in gene conversion: the double-Holliday Junction model (41) and SDSA model (42). Here, we show that ERCC1/XPF is involved in the repair of 2-ended DSBs using gene conversion. A non-homologous 3′ DNA end substrate for ERCC1/XPF is only produced in the SDSA model. Thus, our data suggest that SDSA is the preferred repair pathway for 2-ended DSBs. A third alternative D-loop nicking model for 2-ended DSB repair has recently been proposed for meiotic recombination (45), and is illustrated here to envision how ERCC1/XPF may potentially be involved in such pathway. However, there is so far no evidence for this pathway for mitotic DSB repair. Only non-cross-over resolutions of Holliday Junctions are illustrated, as these are strongly favoured in mitotic mammalian cells (22,48).
It has been argued that SSA, as opposed to homologous recombination, is independent of the cell cycle stage as only a single DNA molecule is used and the sister chromatid is expendable. Here, we find that the level of SSA is reduced 3-fold in G1-arrested cells, which does not support a role for SSA in the G1 phase of the cell cycle. We find a 3-fold reduction in SSA in spite of the fact that perfect repeat sequences are available for repair. There are only limited repeat sequences present in mammalian cells (46) and thus the actual SSA levels in G1-arrested cells are probably even lower than we report here.
Here, we wanted to determine what could be responsible for the suppression of SSA in the G1 phase of the cell cycle. It has been reported earlier that resection at a DSB depends on CDK activity (26–28) and that HR depends on ATM (47) and Chk1 signalling (25). Resection at a DNA end is required for SSA and thus we wanted to test if SSA depends on any of the CDK2, ATM, Chk1 or DNA-PK kinases. Although all inhibitors were used at concentrations that inhibit these enzymes in the same cell type (25,31), we found no effect on the levels of SSA. Overall, this suggests that CDK2, ATM, Chk1 or DNA-PK are not involved in SSA or alternatively that the single DSB produced by the I-SceI endonuclease is not sufficient to trigger a checkpoint response that activates DNA damage signalling.
In conclusion, we show that the ERCC1/XPF endonuclease is involved in both SSA and gene conversion in mammalian cells, which favours the SDSA model for repair of 2-ended DSBs. We find that SSA is suppressed in the G1 phase of the cell cycle and that SSA of a single DSB occurs efficiently also in presence of inhibitors of cell cycle checkpoints and DNA repair.
ACKNOWLEDGEMENTS
We thank Dr Larry Thompson, Dr Maria Jasin, Dr Graeme Smith, Dr Stephen Trusko for materials, Dr Eva Petermann for comments and the Swedish Cancer Society, the Swedish Children's Cancer Foundation, the Swedish Research Council, the Swedish Pain Relief Foundation, the Medical Research Council and Yorkshire Cancer Research for supporting this work financially. Funding to pay the Open Access publication charges for this article was provided by the Swedish Pain Relief Foundation.
Conflict of interest statement. None declared.
REFERENCES
- 1.Biggerstaff M, Szymkowski DE, Wood RD. Co-correction of the ERCC1, ERCC4 and xeroderma pigmentosum group F DNA repair defects in vitro. EMBO J. 1993;12:3685–3692. doi: 10.1002/j.1460-2075.1993.tb06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aboussekhra A, Biggerstaff M, Shivji MK, Vilpo JA, Moncollin V, Podust VN, Protic M, Hubscher U, Egly JM, et al. Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell. 1995;80:859–868. doi: 10.1016/0092-8674(95)90289-9. [DOI] [PubMed] [Google Scholar]
- 3.Adair GM, Rolig RL, Moore-Faver D, Zabelshansky M, Wilson JH, Nairn RS. Role of ERCC1 in removal of long non-homologous tails during targeted homologous recombination. EMBO J. 2000;19:5552–5561. doi: 10.1093/emboj/19.20.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sargent RG, Meservy JL, Perkins BD, Kilburn AE, Intody Z, Adair GM, Nairn RS, Wilson JH. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucleic Acids Res. 2000;28:3771–3778. doi: 10.1093/nar/28.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Duin M, Vredeveldt G, Mayne LV, Odijk H, Vermeulen W, Klein B, Weeda G, Hoeijmakers JH, Bootsma D, et al. The cloned human DNA excision repair gene ERCC-1 fails to correct xeroderma pigmentosum complementation groups A through I. Mutat. Res. 1989;217:83–92. doi: 10.1016/0921-8777(89)90059-1. [DOI] [PubMed] [Google Scholar]
- 6.Wood RD. Nucleotide excision repair in mammalian cells. J. Biol. Chem. 1997;272:23465–23468. doi: 10.1074/jbc.272.38.23465. [DOI] [PubMed] [Google Scholar]
- 7.de Boer J, Hoeijmakers JH. Nucleotide excision repair and human syndromes. Carcinogenesis. 2000;21:453–60. doi: 10.1093/carcin/21.3.453. [DOI] [PubMed] [Google Scholar]
- 8.Jaspers NG, Raams A, Silengo MC, Wijgers N, Niedernhofer LJ, Robinson AR, Giglia-Mari G, Hoogstraten D, Kleijer WJ, et al. First reported patient with human ERCC1 deficiency has cerebro-oculo-facio-skeletal syndrome with a mild defect in nucleotide excision repair and severe developmental failure. Am. J. Hum. Genet. 2007;80:457–466. doi: 10.1086/512486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McWhir J, Selfridge J, Harrison DJ, Squires S, Melton DW. Mice with DNA repair gene (ERCC-1) deficiency have elevated levels of p53, liver nuclear abnormalities and die before weaning. Nat. Genet. 1993;5:217–224. doi: 10.1038/ng1193-217. [DOI] [PubMed] [Google Scholar]
- 10.Weeda G, Donker I, de Wit J, Morreau H, Janssens R, Vissers CJ, Nigg A, van Steeg H, Bootsma D, et al. Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr. Biol. 1997;7:427–439. doi: 10.1016/s0960-9822(06)00190-4. [DOI] [PubMed] [Google Scholar]
- 11.Tian M, Shinkura R, Shinkura N, Alt FW. Growth retardation, early death, and DNA repair defects in mice deficient for the nucleotide excision repair enzyme XPF. Mol. Cell Biol. 2004;24:1200–1205. doi: 10.1128/MCB.24.3.1200-1205.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargent RG, Meservy JL, Perkins BD, Kilburn AE, Intody Z, Adair GM, Nairn RS, Wilson JH. Role of the nucleotide excision repair gene ERCC1 in formation of recombination-dependent rearrangements in mammalian cells. Nucleic Acids Res. 2000;28:3771–3778. doi: 10.1093/nar/28.19.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargent RG, Rolig RL, Kilburn AE, Adair GM, Wilson JH, Nairn RS. Recombination-dependent deletion formation in mammalian cells deficient in the nucleotide excision repair gene ERCC1. Proc. Natl Acad. Sci. USA. 1997;94:13122–13127. doi: 10.1073/pnas.94.24.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niedernhofer LJ, Essers J, Weeda G, Beverloo B, de Wit J, Muijtjens M, Odijk H, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells. EMBO J. 2001;20:6540–6549. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paques F, Haber JE. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tauchi H, Kobayashi J, Morishima K, van Gent DC, Shiraishi T, Verkaik NS, vanHeems D, Ito E, Nakamura A, et al. Nbs1 is essential for DNA repair by homologous recombination in higher vertebrate cells. Nature. 2002;420:93–98. doi: 10.1038/nature01125. [DOI] [PubMed] [Google Scholar]
- 17.Stasiak AZ, Larquet E, Stasiak A, Muller S, Engel A, Van Dyck E, West SC, Egelman EH. The human Rad52 protein exists as a heptameric ring. Curr. Biol. 2000;10:337–40. doi: 10.1016/s0960-9822(00)00385-7. [DOI] [PubMed] [Google Scholar]
- 18.Van Dyck E, Stasiak AZ, Stasiak A, West SC. Binding of double-strand breaks in DNA by human Rad52 protein. Nature. 1999;401:403. doi: 10.1038/19560. [DOI] [PubMed] [Google Scholar]
- 19.Wold MS. Replication protein A: a heterotrimeric, single-stranded DNA-binding protein required for eukaryotic DNA metabolism. Annu. Rev. Biochem. 1997;66:61–92. doi: 10.1146/annurev.biochem.66.1.61. [DOI] [PubMed] [Google Scholar]
- 20.Saleh-Gohari N, Bryant HE, Schultz N, Parker KM, Cassel TN, Helleday T. Spontaneous homologous recombination is induced by collapsed replication forks that are caused by endogenous DNA single-strand breaks. Mol. Cell. Biol. 2005;25:7158–7169. doi: 10.1128/MCB.25.16.7158-7169.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang F, Han M, Romanienko PJ, Jasin M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc. Natl Acad. Sci. USA. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson RD, Jasin M. Sister chromatid gene conversion is a prominent double-strand break repair pathway in mammalian cells. EMBO J. 2000;19:3398–3407. doi: 10.1093/emboj/19.13.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothkamm K, Kruger I, Thompson LH, Lobrich M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 2003;23:5706–5715. doi: 10.1128/MCB.23.16.5706-5715.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh-Gohari N, Helleday T. Conservative homologous recombination preferentially repairs DNA double-strand breaks in the S phase of the cell cycle in human cells. Nucleic Acids Res. 2004;32:3683–3688. doi: 10.1093/nar/gkh703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, Helleday T. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat. Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 26.Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 27.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–1017. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J. 2004;23:4868–4875. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala LA, Scovassi AI, Meijer L, Prosperi E. The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp. Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 30.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 31.Bryant HE, Helleday T. Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res. 2006;34:1685–1691. doi: 10.1093/nar/gkl108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golding SE, Rosenberg E, Neill S, Dent P, Povirk LF, Valerie K. Extracellular signal-related kinase positively regulates ataxia telangiectasia mutated, homologous recombination repair, and the DNA damage response. Cancer Res. 2007;67:1046–1053. doi: 10.1158/0008-5472.CAN-06-2371. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Boecker W, Wang H, Wang X, Guan J, Thompson LH, Nickoloff JA, Iliakis G. Caffeine inhibits homology-directed repair of I-SceI-induced DNA double-strand breaks. Oncogene. 2004;23:824–834. doi: 10.1038/sj.onc.1207168. [DOI] [PubMed] [Google Scholar]
- 34.Hoy CA, Thompson LH, Mooney CL, Salazar EP. Defective DNA cross-link removal in Chinese hamster cell mutants hypersensitive to bifunctional alkylating agents. Cancer Res. 1985;45:1737–1743. [PubMed] [Google Scholar]
- 35.Matsunaga T, Mu D, Park CH, Reardon JT, Sancar A. Human DNA repair excision nuclease. Analysis of the roles of the subunits involved in dual incisions by using anti-XPG and anti-ERCC1 antibodies. J. Biol. Chem. 1995;270:20862–20869. doi: 10.1074/jbc.270.35.20862. [DOI] [PubMed] [Google Scholar]
- 36.Park CH, Sancar A. Formation of a ternary complex by human XPA, ERCC1, and ERCC4(XPF) excision repair proteins. Proc. Natl Acad. Sci. USA. 1994;91:5017–5021. doi: 10.1073/pnas.91.11.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargent RG, Rolig RL, Kilburn AE, Adair GM, Wilson JH, Nairn RS. Recombination-dependent deletion formation in mammalian cells deficient in the nucleotide excision repair gene ERCC1. Proc. Natl Acad. Sci. USA. 1997;94:13122–13127. doi: 10.1073/pnas.94.24.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rolig RL, Lowery MP, Adair GM, Nairn RS. Characterization and analysis of Chinese hamster ovary cell ERCC1 mutant alleles. Mutagenesis. 1998;13:357–365. doi: 10.1093/mutage/13.4.357. [DOI] [PubMed] [Google Scholar]
- 39.Niedernhofer LJ, Essers J, Weeda G, Beverloo B, de Wit J, Muijtjens M, Odijk H, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required for targeted gene replacement in embryonic stem cells. EMBO J. 2001;20:6540–6549. doi: 10.1093/emboj/20.22.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, et al. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 42.Nassif N, Penney J, Pal S, Engels WR, Gloor GB. Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair. Mol. Cell. Biol. 1994;14:1613–1625. doi: 10.1128/mcb.14.3.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y, Masson JY, Shah R, O’Regan P, West SC. RAD51C is required for Holliday junction processing in mammalian cells. Science. 2004;303:243–246. doi: 10.1126/science.1093037. [DOI] [PubMed] [Google Scholar]
- 44.Constantinou A, Chen XB, McGowan CH, West SC. Holliday junction resolution in human cells: two junction endonucleases with distinct substrate specificities. EMBO J. 2002;21:5577–5585. doi: 10.1093/emboj/cdf554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cromie GA, Hyppa RW, Taylor AF, Zakharyevich K, Hunter N, Smith GR. Single Holliday junctions are intermediates of meiotic recombination. Cell. 2006;127:1167–1178. doi: 10.1016/j.cell.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstock DM, Richardson CA, Elliott B, Jasin M. Modeling oncogenic translocations: distinct roles for double-strand break repair pathways in translocation formation in mammalian cells. DNA Repair. 2006;5:1065–1074. doi: 10.1016/j.dnarep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Golding SE, Rosenberg E, Khalil A, McEwen A, Holmes M, Neill S, Povirk LF, Valerie K. Double strand break repair by homologous recombination is regulated by cell cycle-independent signaling via ATM in human glioma cells. J. Biol. Chem. 2004;279:15402–15410. doi: 10.1074/jbc.M314191200. [DOI] [PubMed] [Google Scholar]
- 48.Richardson C, Moynahan ME, Jasin M. Double-strand break repair by interchromosomal recombination: suppression of chromosomal translocations. Genes Dev. 1998;12:3831–3842. doi: 10.1101/gad.12.24.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]