Abstract
The active absorption of fluid from the airspaces of the lung is important for the resolution of clinical pulmonary edema. Although ENaC channels provide a major route for Na+ absorption, the route of Cl− transport has been unclear. We applied a series of complementary approaches to define the role of Cl− transport in fluid clearance in the distal airspaces of the intact mouse lung, using wild-type and cystic fibrosis ΔF508 mice. Initial studies in wild-type mice showed marked inhibition of fluid clearance by Cl− channel inhibitors and Cl− ion substitution, providing evidence for a transcellular route for Cl− transport. In response to cAMP stimulation by isoproterenol, clearance was inhibited by the CFTR inhibitor glibenclamide in both wild-type mice and the normal human lung. Although isoproterenol markedly increased fluid absorption in wild-type mice, there was no effect in ΔF508 mice. Radioisotopic clearance studies done at 23°C (to block active fluid absorption) showed ∼20% clearance of 22Na in 30 min both without and with isoproterenol. However, the clearance of 36Cl was increased by 47% by isoproterenol in wild-type mice but was not changed in ΔF508 mice, providing independent evidence for involvement of CFTR in cAMP-stimulated Cl− transport. Further, CFTR played a major role in fluid clearance in a mouse model of acute volume-overload pulmonary edema. After infusion of saline (40% body weight), the lung wet-to-dry weight ratio increased by 28% in wild-type versus 64% in ΔF508 mice. These results provide direct evidence for a functionally important role for CFTR in the distal airspaces of the lung.
Keywords: pulmonary edema, cystic fibrosis, lung epithelium, cAMP, lung fluid balance
INTRODUCTION
The mechanisms that regulate the removal of salt and water from the distal airspaces of the lung are relevant to understanding the resolution of clinical pulmonary edema. Most experimental studies have attributed a primary role for active sodium transport in the vectorial transport of salt and water from the apical to the basal surface of the alveolar epithelium. Several in vivo studies have demonstrated that inhibition of sodium uptake by amiloride, or one of its analogues, reduces the rate of vectorial salt and water transport in the sheep, rat, rabbit, mouse, and human lung (Berthiaume et al., 1987; Effros et al., 1989; Matthay et al., 1996). In vitro studies support a role for sodium in driving salt transport across cultured alveolar epithelial type II cells (Cheek et al., 1989; Matalon and O'Brodovich, 1999; Jain et al., 2001). Also, the α subunit of the apical epithelial sodium channel (ENaC)* is essential for the perinatal removal of alveolar fluid in the mouse lung (Hummler et al., 1996).
The contribution of chloride transport to the isosmolar reabsorption of fluid from the distal airspaces of the lung is less clear. Measurements on cultured alveolar epithelial type II cells suggested that cAMP mediated apical uptake of sodium may be driven by an increase in chloride conductance (Jiang et al., 1998). However, the results were considered inconclusive, partly because the experiments were done using cultured alveolar epithelial cells of uncertain phenotype (Lazrak et al., 2000; Widdicombe, 2000). Furthermore, studies of isolated alveolar epithelial type II cells do not address the possibility that vectorial fluid transport may be mediated by several different epithelial cell types including alveolar epithelial type I cells (Borok et al., 2002; Johnson et al., 2002) as well as distal airway epithelial cells (Folkesson et al., 1996). Studies in several species have indicated that the distal airway epithelium is capable of ion transport (Ballard et al., 1992; Al-Bazzaz, 1994). Both ENaC and the CFTR are expressed in distal airway as well as alveolar epithelia (Engelhardt et al., 1994; Rochelle et al., 2000).
We reasoned that intact lung studies were required to define the role of chloride and CFTR in active salt and water transport across the distal airspaces. Several strategies were used. Inhibition and ion substitution experiments indicated an important role for transcellular chloride transport. Experiments in wild-type mice and the ex vivo human lung demonstrated that isoproterenol-stimulated fluid absorption was inhibited by glibenclamide, suggesting a role for CFTR. To test the role of CFTR directly, cystic fibrosis mice (ΔF508) were studied. Both fluid absorption and 36Cl uptake from the distal airspaces were stimulated by isoproterenol in wild-type, but not in ΔF508 mice. Finally, the significance of the impaired fluid transport in ΔF508 mice was tested in a model of acute hydrostatic pulmonary edema. The impaired fluid clearance in ΔF508 mice resulted in high lung water and alveolar edema. These studies provide the first direct evidence for a major function of CFTR in the distal epithelium of the lung.
MATERIALS AND METHODS
Transgenic Mice
ΔF508 mice on a C57BL6/J-C3H/HeJ hybrid genetic background were provided by the CFRDP animal core at the University of California, San Francisco. Heterozygous offspring, which appeared phenotypically normal, were intercrossed to generate homozygous mutant ΔF508 mice. Genotype analysis of tail DNA was done by PCR at 10 d of age. The wild-type and heterozygous mice were fed a standard diet and the ΔF508 mice a liquid diet as recommended (Kent et al., 1996). The ΔF508 mice show pathological and electrophysiological changes consistent with a CF phenotype (Colledge et al., 1995). Measurements were done in litter-matched mice (8–12 wk of age). The investigators were blinded to genotype information for all comparative transport measurements. Protocols were approved by the University of California at San Francisco Committee on Animal Research.
Measurements of Fluid Clearance in Mice
Mice were killed using intraperitoneal pentobarbital (200 mg/kg). A tracheostomy was rapidly done with a 20-gauge angiocatheter. Lungs were inflated with 100% oxygen at 4 cm H2O continuous positive airway pressure throughout the experiment. In these in situ experiments, body temperature was maintained at 37–38°C using an infrared lamp and intra-abdominal monitoring thermister. In in situ perfused experiments, the pulmonary artery was cannulated with polyethylene PE-20 tubing and the left atrium was transected to permit fluid exit. The pulmonary artery was gravity perfused at 5 cm H2O pressure, and the perfusate was maintained at 37°C as described previously (Bai et al., 1999; Ma et al., 2000).
To measure fluid clearance from the distal airspaces, 10 ml/kg of instillate was delivered to both lungs over 30 s through the tracheal cannula. The instillate consisted of Ringer's lactate ([in mM] 102.6 NaCl, 4.02 KCl, 1.36 CaCl2, and 28 sodium lactate) containing 5% BSA and [131I]albumin (0.1 μCi) adjusted to 325 mOsm with NaCl and pH 7.4 to match the mouse serum osmolarity. At the end of the experimental time period, a fluid sample (50–100 μl) was aspirated with a 1-ml syringe connected directly into the catheter. The aspirate was weighed and assayed for 131I radioactivity. The percent fluid absorption at 15 min was computed from the ratio of instillate and aspirate radioactivities as described previously (Fukuda et al., 2000; Ma et al., 2000).
For the ion substitution experiments, perfusion was started 10 min before the airspace solution was instilled. The perfusate was identical to the instillate except for the absence of the volume marker [131I]albumin. In some studies, 1 mM amiloride, 0.1 mM NPPB, 0.1 mM ouabain, 0.1 mM glibenclamide, 0.1 mM isoproterenol, or 0.1 mM each of forskolin + IBMX was added to the instillate. The ion substitution solution was as follows: “100% NaCl” ([in mM] 162 NaCl, 0.9 CaCl2, and 1.5 KH2PO4), “50% Na+/choline+” ([in mM] 81 NaCl, 81 choline Cl, 0.9 CaCl2, 1.5 KH2PO4), “50% Cl−/NO3” ([in mM] 81 NaCl, 81 NaNO3, 0.9 CaCl2, and 1.5 KH2PO4), and “50% Cl−/gluconate−” ([in mM] 81 NaCl, 81 sodium gluconate, 0.9 mM CaCl2, and 1.5 KH2PO4). All solutions were adjusted to 325 mOsm and pH 7.4.
Measurement of Fluid Clearance in Human Lung
The ex vivo human lung study was done with the approval of Human Research Committee at UCSF. Human lungs were obtained from 42 human lung donors whose lungs were rejected for transplantation. As previously described (Sakuma et al., 1994, 1996), a segmental bronchus was occluded by a balloon catheter. Through the catheter, the lung was inflated with 8 cm H2O airway pressure with 100% oxygen and placed in a plastic bag and a humidified incubator at 37°C for 3–4 h to warm the lung. Next, 60–120 ml of isosmolar 5% human albumin solution containing 5 μCi [131I]albumin warmed at 37°C was instilled into the occluded segment followed by 40 ml of air to advance the instilled albumin solution into the distal airspaces. 1 h after instillation, alveolar fluid was aspirated. The aspirate sample was assayed for 131I radioactivity and fluid absorption calculated. In some experiments, 0.1 mM terbutaline and/or 0.1 mM glibenclamide were added to the instillate.
Uptake of 22Na and 36Cl in the Mouse Lung
These studies were done in the in situ perfused mouse lung at room temperature (23°C). Identical solutions (102.6 mM NaCl, 4.02 mM KCl, 1.36 mM CaCl2, 28 mM sodium lactate, 5% albumin, and 325 mOsm, pH 7.4) were used in the perfusate and instillate, except for the presence of tracer quantities of 22Na and 36Cl in the airspace instillate. 22Na was measured using a γ counter, and 36Cl by a scintillation counter (with correction for 22Na counts). A sample of the instilled fluid was obtained at 1 and 30 min (1 min was taken as 0 point because the instillate may be diluted initially). In some experiments, 0.1 mM isoproterenol and/or 0.1 mM glibenclamide was added to the instillate and perfusate. Albumin concentrations were measured at 1 and 30 min to confirm that there was no net fluid clearance from the airspaces of the lung, as we and others have reported previously that room temperature abolishes active fluid clearance (Matthay et al., 1996). In some experiments [14C]mannitol was instilled as a paracellular permeability marker.
Hydrostatic Volume-overload Studies in Mice
A standard model of acute hydrostatic edema was used (Broaddus et al., 1990; Frank et al., 2000). Mice were anesthetized (ketamine 80 mg/kg and xylazine 12 mg/kg) and ventilated with a constant volume ventilator (Harvard Apparatus) with a tidal volume of 8 ml/kg, a positive end–expiratory pressure of 3 cm H2O, and 100% oxygen. A catheter was inserted into the left carotid artery to obtain blood samples and infuse fluid. The respiratory rate was adjusted to maintain the PaCO2 at 30–40 mmHg. The mice were monitored by electrocardiography. After a 20-min baseline period, an intra-arterial infusion of saline was given by an infusion pump over 2 h (total volume = 40% of body weight, with 40% of the total volume given over the first 20 min, the remaining 60% volume administered over 100 min). In some experiments, propranolol was given at escalating dose (5–21 μg/kg/min) before volume overload. At 2 h, the mice were killed by exsanguination, a blood sample was obtained for measurement of hemoglobin concentration and the wet-to-dry weight ratio of blood. The lungs were removed and homogenized for measurement of the wet-to-dry weight ratio using standard methods (Berthiaume et al., 1987; Fukuda et al., 2000). Histopathology was done as previously described (Kaner et al., 2000): lungs were inflated to total lung capacity, and the tracheas were ligated. Lungs were placed in 300 ml PBS heated to 60°C for 3 min in a microwave oven and transferred to 4% paraformaldehyde overnight. The lungs were embedded with paraffin and sections were cut at 4-μm thickness stained with hematoxylin and eosin.
Statistics
Data are summarized as mean ± SEM. Analysis of variance was used to compare the different animal groups. Where appropriate, an unpaired t test was used. P < 0.05 was taken as statistically significant.
RESULTS
Role of Transcellular Sodium and Chloride Transport
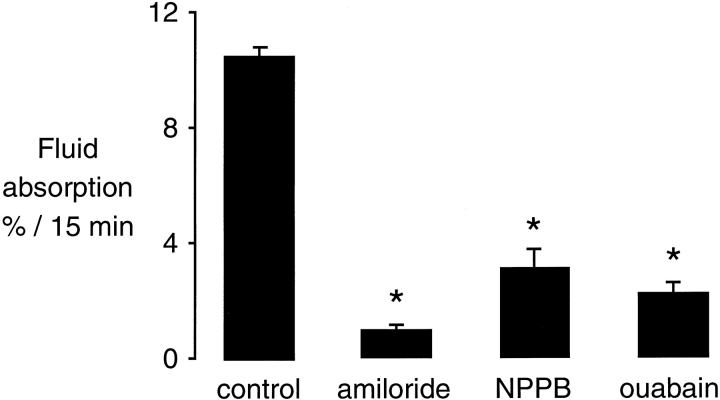
Isosmolar fluid absorption, measured initially in the in situ nonperfused mouse lung, was reduced by 70–80% with amiloride or NPPB (Fig. 1), indicating that inhibition of sodium or chloride transport can prevent basal vectorial fluid transport across the distal pulmonary epithelium. These results provide evidence that transcellular fluid transport probably occurs for both sodium and chloride. As expected, inhibition of Na+/K+-ATPase by ouabain markedly inhibited fluid absorption.
Figure 1.
Effect of amiloride, NPPB, and ouabain on isosmolar fluid clearance at 37°C in the in situ nonperfused lung of wild-type mice. Fluid clearance is expressed as the percent fluid absorption at 15 min (n = 6–8 mice in each group). Where indicated, the instillate contained 1 mM amiloride, 0.1 mM NPPB, or 0.1 mM ouabain. *P < 0.05 compared with control, data as mean ± SEM.
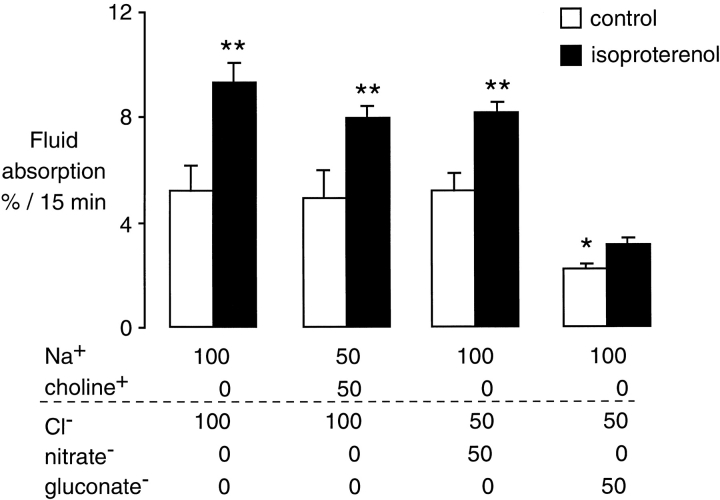
To assess qualitatively the relative contributions of sodium and chloride to fluid absorption, isosmolar ion substitution studies were performed in the in situ perfused mouse lung. In the in situ perfused model, the basal fluid clearance rates are ∼50% of those in the nonperfused in situ lung (Ma et al., 2000). The same concentration of solutes on both sides of the distal pulmonary epithelium was achieved by using the same solution for both the perfusate and the instillate in the airspaces. This approach avoids the problem of solute imbalance that can occur with ion substitution experiments that change solute concentrations on only one side of the transporting epithelium. A reduction in [Na+] to 50% by the substitution of choline+ had little effect on basal fluid clearance (Fig. 2, open bars). However, reduction in [Cl−] to 50% by the substitution of gluconate- inhibited distal airspace fluid clearance by ∼50%. Reduction in the concentration of [Cl−] to 50% by substitution of nitrate−, an anion that can generally substitute for Cl− in Cl− channels, had no effect on basal fluid clearance. Also, fluid absorption after cAMP agonists was significantly lower with a 50% reduction of [Cl−] than with a 50% reduction of [Na+] (Fig. 2, closed bars).
Figure 2.
Effect of ion substitution on isosmolar fluid clearance from the distal airspaces. Experiments were done in the in situ perfused lung at 37°C in wild-type mice. The x-axis indicates the composition of the test solutions. Measurements were done under basal (open bars, n = 6 mice in each group) and isoproterenol stimulated (closed bars, n = 6 in each group) conditions. *P < 0.05 compared with all other control conditions; **P < 0.05 compared with basal in each group, data as mean ± SEM.
The results suggest that chloride can be rate limiting in isosmolar fluid transport under both basal and isoproterenol-stimulated conditions. However, substitution of Cl− for gluconate− may depolarize the apical membrane potential and could reduce the driving force for Na+ transport. Alternatively, the low free–ionized calcium in the gluconate solutions may reduce possible calcium-dependent chloride permeability. Therefore, the results of these studies provided suggestive, but not conclusive, evidence for a role of chloride in transcellular epithelial transport. Additional experiments were performed to assess the role of chloride transport by CFTR, using both pharmacologic inhibition of CFTR and cystic fibrosis mice.
Role of CFTR in cAMP-stimulated Fluid Absorption
Two strategies were used to test the potential role of CFTR in fluid absorption in the distal airspaces of the lung. The first approach was to inhibit chloride transport with glibenclamide, a relatively selective inhibitor of CFTR (Schultz et al., 1999). The second approach was to measure fluid absorption in ΔF508 mice that lack functional CFTR in the cell plasma membrane (Clarke et al., 1992). Studies were done under both basal- and cAMP-stimulated conditions.
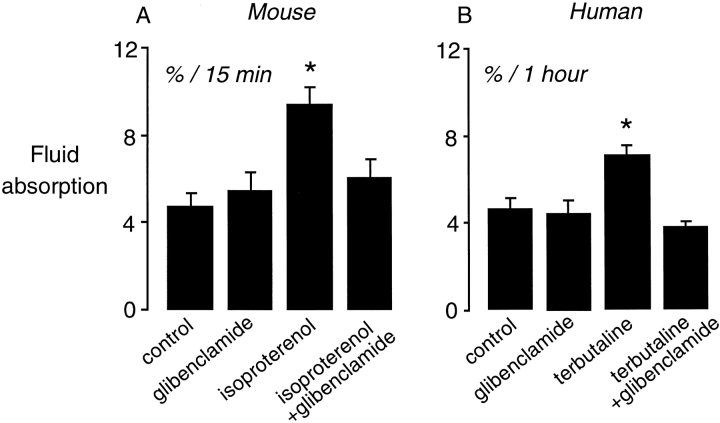
The initial experiments showed that glibenclamide had no effect on basal clearance (Fig. 3 A). Isoproterenol stimulated basal fluid clearance, as previously reported (Bai et al., 1999; Fukuda et al., 2000), but glibenclamide prevented the cAMP-induced increase in fluid clearance (Fig. 3 A). These results provided support for the hypothesis that CFTR may mediate the cAMP stimulated increase in fluid clearance.
Figure 3.
Effect of glibenclamide on fluid clearance in mouse and human lung. (A) Fluid clearance in the in situ perfused lung of wild-type mice at 37°C. Fluid clearance is expressed as the percent absorption at 15 min under control conditions (n = 12), glibenclamide (0.1 mM, n = 6), isoproterenol (0.1 mM, n = 18), and isoproterenol + glibenclamide (n = 6). *P < 0.05 compared with control, data as mean ± SEM. (B) Measurements of fluid clearance in rewarmed ex vivo human lung at 37°C. Fluid clearance is expressed as the percent absorption at 1 h under control conditions (n = 23), glibenclamide (0.1 mM, n = 5), terbutaline (0.1 mM, n = 8), and terbutaline + glibenclamide (n = 6). *P < 0.05 compared with control, data as mean ± SEM.
To determine if CFTR inhibition by glibenclamide would also impair cAMP-stimulated clearance in the human lung, an ex vivo human lung preparation was used (Sakuma et al., 1998). Glibenclamide alone had no effect on basal fluid clearance in the ex vivo human lung (Fig. 3 B). cAMP stimulation with terbutaline increased fluid clearance. cAMP-stimulated fluid clearance was prevented by glibenclamide, which is similar to the studies in the mouse lung.
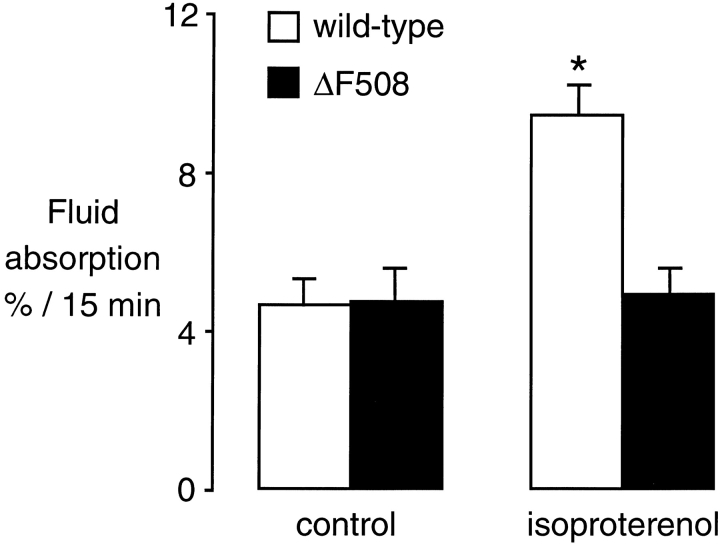
To directly test the role of CFTR in isosmolar fluid clearance in the in situ mouse lung, we used cystic fibrosis mice. Studies of fluid absorption in wild-type and ΔF508 mice showed no difference in basal isosmolar fluid clearance (Fig. 4, open bars), which is consistent with the observation that glibenclamide did not impair basal clearance in the human or mouse lung. In the presence of isoproterenol, fluid clearance was markedly increased in the wild-type mice but not changed in the ΔF508 mice (Fig. 4, closed bars). To be certain that the lack of response to isoproterenol was not due to downregulation of β receptors, additional studies were done with forskolin/IBMX (0.1 mM each, n = 10 wild-type and 6 ΔF508 mice). There was a 57 ± 7% increase in fluid clearance in the wild-type mice, but no change in fluid clearance in the ΔF508 mice. The data support the conclusion that CFTR is required for cAMP-mediated upregulation of fluid clearance, but is not necessary for basal fluid absorption.
Figure 4.
Fluid clearance from the distal airspaces of wild-type (open bars) and ΔF508 (closed bars) mice. Measurements were done in the in situ perfused lung at 37°C under basal conditions (n = 24 wild-type, n = 7 ΔF508) and in the presence of 0.1 mM isoproterenol (n = 9 wild-type, n = 6 ΔF508). *P < 0.05 compared with control group, data as mean ± SEM.
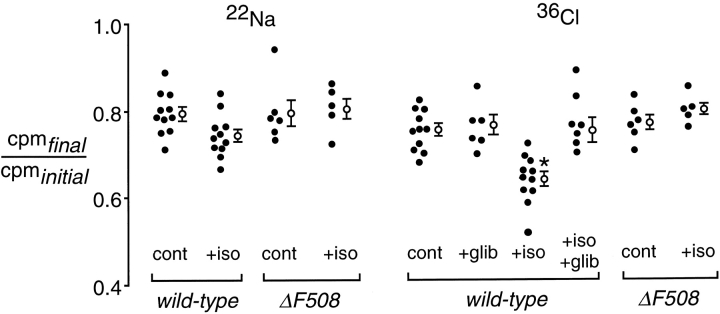
22Na and 36Cl Uptake in Mouse Lung under Isotopic Conditions
Active transport across the distal lung epithelium at 37°C couples salt (sodium and chloride) and water to maintain isosmolar conditions (Serikov et al., 1993). Thus, it is not possible to study the separate transport of sodium and chloride. Experiments were designed to measure the passive transport of tracer 22Na and 36Cl in in situ perfused mouse lungs at room temperature (23°C). Since room temperature abolishes active ion transport, isotopic transport of 22Na and 36Cl from the distal airspaces of the lung occurs without a change in the net air space fluid volume, and can occur by an exchange mechanism without obligate counterion cotransport. Measurement of alveolar protein concentration confirmed there was no net clearance of distal airspace fluid during these experiments, since the concentration of albumin was the same at 1 and 30 min after instillation. Under basal conditions, 22Na and 36Cl loss from the air spaces was similar, ∼20% in 30 min (Fig. 5). In the presence of isoproterenol, 36Cl removal was accelerated significantly, whereas 22Na removal was not changed. The isoproterenol-induced increase in 36Cl transport was inhibited by glibenclamide, providing evidence that the cAMP stimulated uptake of 36Cl under isotopic conditions may be mediated by CFTR.
Figure 5.
Isotopic 22Na and 36Cl transport from the airspace compartment of wild-type and ΔF508 mice. Measurements were done in the in situ perfused lung preparation at 23°C. The y-axis is the ratio of final (30 min after instillation) to initial (1 min after instillation) 22Na or 36Cl radioactivities in fluid sampled from the distal airspaces. Individual (closed circles) and averaged (mean ± SEM) values are shown. Where indicated, the instillate contained 0.1 mM isoproterenol (iso), 0.1 mM glibenclamide (glib), and 0.1 mM isoproterenol + 0.1 mM glibenclamide. *P < 0.05 compared with all other groups.
To determine the contribution of CFTR in mediating cAMP-induced 36Cl transport from the distal air spaces, similar studies were performed in ΔF508 mice. The loss of 22Na and 36Cl in ΔF508 mice was not affected by isoproterenol (Fig. 5). These results provide direct evidence for a role of CFTR in cAMP-stimulated Cl− transport in the distal airway epithelium. [14C]mannitol loss from the airspaces was the same under basal conditions and with isoproterenol stimulation, thus excluding an effect of cAMP agonists stimulation on paracellular epithelial permeability.
Hydrostatic Volume-overload Model of Pulmonary Edema
The previous experiments established a role for CFTR in cAMP stimulated fluid clearance from the distal airspaces of the lung. The final set of experiments were designed to test the contribution of CFTR to fluid clearance using a model of hydrostatic volume overload. Previous studies established that endogenous release of epinephrine stimulates fluid clearance from the airspaces of the lung during a hydrostatic stress (Campbell et al., 1999). These experiments were done to test the hypothesis that the lack of functional CFTR in ΔF508 mice would limit their capacity to remove alveolar edema.
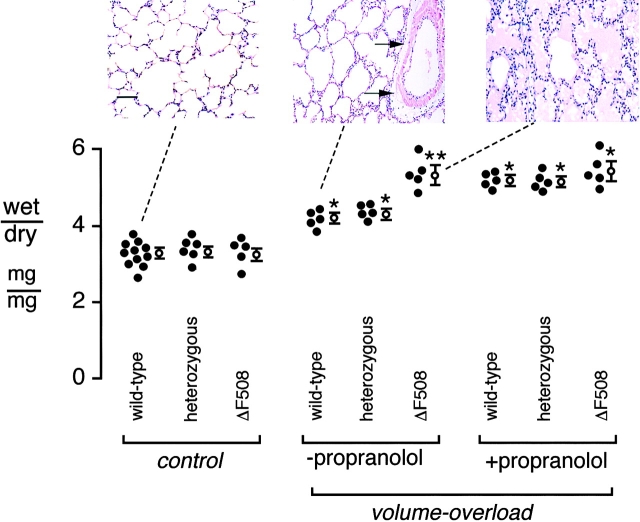
Hydrostatic pulmonary edema was induced in ventilated mice using a standard preparation of acute intravascular volume expansion. After volume overload by saline infusion, there was 27% and 31% increase in the lung wet-to-dry weight ratio in wild-type and heterozygous mice, respectively (Fig. 6). In the ΔF508 mice, the lung wet-to-dry weight ratio increased by 64% (P < 0.05). Lung histology showed moderate interstitial edema with perivascular fluid cuffs in wild-type mice without alveolar flooding, but marked alveolar edema was present in ΔF508 mice (Fig. 6, insets).
Figure 6.
Effect of acute volume overload on the lung wet-to-dry weight ratio in wild-type, heterozygous, and ΔF508 mice. 40% of body weight fluid was infused over 2 h (materials and methods). The y-axis is the lung wet-to-dry weight ratio. Individual (closed) and averaged (mean ± SEM) values are shown for indicated genotypes of mice. In the volume-overload group, where indicated, measurements were performed with and without propranolol (0.1 mM) pretreatment. (*P < 0.05 compared with basal control; **P < 0.05 compared with wild-type and heterozygous mice in the same group. [Insets] Micrographs show typical lung histopathology from three different groups, as indicated with the dashed lines). Normal distal airway and alveolar structure in control ventilated wild-type mice not subjected to volume overload (left). Thickening of interstitial space and perivascular fluid cuffs (arrow) in volume-overloaded wild-type mice (middle). Alveolar edema in most sections of volume-overloaded ΔF508 mice (right). Bar, 30 μm.
If the higher lung water and alveolar edema in the ΔF508 mice were explained by the inability of elevated endogenous catecholamines to stimulate cAMP-dependent fluid clearance from the distal airspaces in the ΔF508 mice, blockade of endogenous catecholamines in the wild-type and heterozygous mice should produce a similar increase in lung water. Therefore, the effect of the β antagonist propranolol was tested. Blockade of endogenous catecholamines with propranolol resulted in similar increases in lung wet-to-dry weight ratio in the wild–type and heterozygous mice to the level measured in the ΔF508 mice. Thus, the impaired capacity to remove edema fluid from the distal airspaces of the lung in ΔF508 mice resulted in a significant increase in extravascular lung water in the presence of hydrostatic pulmonary edema.
DISCUSSION
These experiments provide new information regarding the role of chloride and CFTR in the isosmolar reabsorption of fluid from the distal airspaces of the lung. Pharmacologic, ion substitution, isotopic ion transport, and gene knockout experiments indicated that cAMP-dependent fluid absorption from the distal airspaces of the lung involves chloride transport by CFTR. A potential role for chloride in cAMP-mediated fluid transport has been suggested in studies of cultured alveolar type II cells (Jiang et al., 2001), but the lack of intact lung studies has made it difficult to evaluate the role of chloride under conditions that are germane to in vivo fluid absorption. There are several cell types that may participate in salt and water transport from the distal airspaces of the lung including alveolar type I cells (Ding et al., 1997; Dobbs et al., 1998; Borok et al., 2002; Johnson et al., 2002), alveolar type II cells (Matalon and O'Brodovich, 1999), and distal airway epithelial cells (Ballard et al., 1992; Al-Bazzaz, 1994). The primary goal for these studies was to test the role of chloride and CFTR in the intact lung where the normal in vivo tissue morphology and driving forces for ion and water transport are present. Experiments in the intact lung also made it possible to study the role of chloride transport and CFTR in the pathophysiology of pulmonary edema.
The majority of the studies reported here were done in the intact mouse lung, a species that has a maximal rate of alveolar fluid clearance (Fukuda et al., 2000; Ma et al., 2000) that is similar to the rate of maximal alveolar fluid clearance measured during the resolution of alveolar edema in the human lung in patients with pulmonary edema (Verghese et al., 1999; Ware and Matthay, 2001). Pharmacologic studies were also done in an ex vivo human lung to confirm the relevance of findings in the mouse to the human lung.
The studies with glibenclamide, an inhibitor of CFTR, provided pharmacologic evidence that CFTR may be important in cAMP-stimulated fluid absorption in mouse lung as well as in the human lung. Because of the imperfect specificity of glibenclamide (Schultz et al., 1999), experiments also were done in homozygous ΔF508 mutant mice. In contrast to wild-type mice, neither isoproterenol nor forskolin increased fluid absorption. Also, isoproterenol did not increase 36Cl uptake in the isotopic studies in the ΔF508 mice. Although CFTR is necessary for cAMP-unregulated fluid clearance, basal clearance did not depend on CFTR, as demonstrated by normal rates of fluid clearance and 36Cl uptake in the ΔF508 mice and the lack of effect of glibenclamide on basal fluid clearance in the human or mouse lung. These studies indicate that basal fluid clearance in the mouse is CFTR- independent while cAMP stimulated fluid transport is CFTR-dependent.
The involvement of chloride transport and CFTR in lung fluid absorption were tested using an established mouse model of acute hydrostatic pulmonary edema that is associated with an increase in endogenous catecholamine levels. An acute increase in endogenous catecholamines is normally associated with a compensatory increase in the rate of distal epithelial fluid clearance that can protect against alveolar edema and reduce the quantity of edema formation in the lung (Pittet et al., 1994). A hydrostatic stress with volume overload resulted in significantly more pulmonary edema ΔF508 mice than in wild-type ΔF508 heterozygous mice. Alveolar edema was detected only in the ΔF508 mice. To confirm that the wild-type and heterozygous mice were protected by upregulated cAMP-stimulated fluid transport, the effect of endogenous catecholamines was inhibited by β blockade, as reported previously (Pittet et al., 1994, 1996). β blockade produced similar degrees of pulmonary edema in wild-type and ΔF508 mice, supporting the conclusion that cAMP stimulated CFTR activity plays an important role in the clearance of edema fluid from the distal airspaces of the lung.
There are several implications of these experiments. Since basal alveolar fluid clearance is rapid in the mouse and the human lung, the lack of CFTR would not be expected to prevent the normal clearance of perinatal fluid at the time of birth. This conclusion fits well with the observation that the lack of CFTR does not increase the risk of acute respiratory failure at birth in humans with cystic fibrosis nor in ΔF508 mice. However, the lack of CFTR in the adult lung could impair clearance of fluid from the distal airspaces of the lung under some pathological conditions that may be relevant to human cystic fibrosis. The most common cause of acute respiratory failure in cystic fibrosis is advanced obstructive airway disease, which is often complicated by bacterial pneumonia (Boucher et al., 2000). We previously reported that the removal of excess fluid from the distal airspaces of the lung is an important protective mechanism in P. aeruginosa pneumonia in rats (Rezaiguia et al., 1997), and cAMP fluid dependent clearance is important in minimizing alveolar edema in septic and hypovolemic shock (Pittet et al., 1994, 1996). In addition, in patients with pulmonary edema from several different etiologies, the inability to generate maximal alveolar fluid clearance is associated with a longer duration of mechanical ventilation and a higher mortality (Ware and Matthay, 2001).
Finally, although the functional importance of CFTR is well recognized in the pathophysiology of cystic fibrosis in the proximal airways of the lung, it has been proposed without direct evidence that CFTR may have important functional significance in the distal lung (Boucher et al., 2000). These studies provide the first evidence for a functional role of CFTR in the distal pulmonary epithelium. Because the alveolar epithelium comprises the vast majority of the surface area of the lung (Weibel, 1989), previous estimates have discounted a significant role for the distal airway epithelium in the reabsorption of pulmonary edema. However, the findings of these studies indicate that distal airway epithelium may play an important role, partly because the expression of CFTR is greater in distal airway epithelium than in the alveoli (Engelhardt et al., 1994; Rochelle et al., 2000). Recent studies have shown that water channel AQP4 (expressed in airway, but not alveolar, epithelia) plays a small but significant role in osmotically driven lung fluid transport (Song et al., 2001). cAMP fluid transport through CFTR also may occur across the alveolar epithelium based on evidence of expression of CFTR and β receptors in type I and II alveolar epithelial cells (Carstairs et al., 1985; Engelhardt et al., 1994; Rochelle et al., 2000; Liebler et al., 2001). Thus, the resolution of airspace edema is likely to depend on vectorial salt and water transport at the level of both the distal airway and alveolar epithelium, although further studies are needed to define the exact contributions of alveolar versus distal airway epithelium to the removal of distal airspace edema fluid.
Acknowledgments
This study was supported by grants HL 51854 and HL51856 (to M.A. Matthay) and HL60288 (to A.S. Verkman) from the National Institutes of Health and the transgenic mouse core of CFRDP grant R613 from the Cystic Fibrosis Foundation.
Drs. Fang and Fukuda contributed equally to the study.
Footnotes
Abbreviation used in this paper: ENaC, epithelial sodium channel.
References
- Al-Bazzaz, F.J. 1994. Regulation of Na and Cl transport in sheep distal airways. Am. J. Physiol. 267:L193–L198. [DOI] [PubMed] [Google Scholar]
- Bai, C., N. Fukuda, Y. Song, T. Ma, M.A. Matthay, and A.S. Verkman. 1999. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Invest. 103:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, S.T., S.M. Schepens, J.C. Falcone, G.A. Meininger, and A.E. Taylor. 1992. Regional bioelectric properties of porcine airway epithelium. J. Appl. Physiol. 73:2021–2027. [DOI] [PubMed] [Google Scholar]
- Berthiaume, Y., N.C. Staub, and M.A. Matthay. 1987. β-Adrenergic agonists increase lung liquid clearance in anesthetized sheep. J. Clin. Invest. 79:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borok, Z., J. Liebler, R. Lubman, M. Foster, B. Zhou, X. Li, S. Zabski, and E.D. Crandall. 2002. Sodium transport proteins are expressed by rat alveolar epithlelial type I cells. Am. J. Physiol. Lung. In press. [DOI] [PubMed] [Google Scholar]
- Boucher, R.C., M.R. Knowles, and J.R. Yanaskas. 2000. Cystic fibrosis. Textbook of Respiratory Medicine. J.F. Murray, and J.A. Nadel, editors. W.B. Saunders & Co., Philadephia. 1291–1323.
- Broaddus, V.C., J.P. Wiener-Kronish, and N.C. Staub. 1990. Clearance of lung edema into the pleural space of volume-loaded anesthetized sheep. J. Appl. Physiol. 68:2623–2630. [DOI] [PubMed] [Google Scholar]
- Campbell, A.R., H.G. Folkesson, Y. Berthiaume, J. Gutkowska, S. Suzuki, and M.A. Matthay. 1999. Alveolar epithelial fluid clearance persists in the presence of moderate left atrial hypertension in sheep. J. Appl. Physiol. 86:139–151. [DOI] [PubMed] [Google Scholar]
- Carstairs, J.R., A.J. Nimmo, and P.J. Barnes. 1985. Autoradiographic visualization of beta-adrenoreceptor subtypes in human lung. Am. J. Respir. Crit. Care Med. 132:541–547. [DOI] [PubMed] [Google Scholar]
- Cheek, J.M., K.J. Kim, and E.D. Crandall. 1989. Tight monolayers of rat alveolar epithelial cells: bioelectric properties and active sodium transport. Am. J. Physiol. 256:C668–C693. [DOI] [PubMed] [Google Scholar]
- Clarke, L.L., B.R. Grubb, S.E. Gabriel, O. Smithies, B.H. Koller, and R.C. Boucher. 1992. Defective epithelial chloride transport in a gene-targeted mouse model of cystic fibrosis. Science. 257:1125–1128. [DOI] [PubMed] [Google Scholar]
- Colledge, W.H., B.S. Abella, K.W. Southern, R. Ratcliff, C. Jiang, S.H. Cheng, L.J. Macvinish, J.R. Anderson, A.W. Cuthbert, and M.J. Evans. 1995. Generation and characterization of a ΔF508 cystic fibrosis mouse model. Nat. Genet. 10:445–452. [DOI] [PubMed] [Google Scholar]
- Ding, C., E.D. Potter, W. Qiu, S.L. Coon, M.A. Levine, and S.E. Guggino. 1997. Cloning and widespread distribution of the rat rode-type cyclic nucleotide-gated cation channel. Am. J. Physiol. 272:C1335–C1344. [DOI] [PubMed] [Google Scholar]
- Dobbs, L.G., R. Gonzalez, M.A. Matthay, E.P. Carter, L. Allen, and A.S. Verkman. 1998. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspaces and vasculature in rat lung. Proc. Natl. Acad. Sci. USA. 95:2991–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros, R.M., G.R. Mason, J. Hukkanen, and P. Silverman. 1989. New evidence for active sodium transport from fluid-filled rat lungs. J. Appl. Physiol. 66:906–919. [DOI] [PubMed] [Google Scholar]
- Engelhardt, J.F., M. Zepeda, J. Cohn, J. Yankaskas, and J.M. Wilson. 1994. Expression of the cystic fibrosis gene in adult human lung. J. Clin. Invest. 93:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson, H.G., M.A. Matthay, A. Frigeri, and A.S. Verkman. 1996. Transepithelial water permeability in microperfused distal airways: evidence for channel-mediated water transport. J. Clin. Invest. 97:664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, J.A., Y. Wang, O. Oscar, and M.A. Matthay. 2000. β-Adrenergic agonist therapy accelerates the resolution of hydrostatic pulmonary edema in sheep and rats. J. Appl. Physiol. 89:1255–1265. [DOI] [PubMed] [Google Scholar]
- Fukuda, N., H.G. Folkesson, and M.A. Matthay. 2000. Relationship of interstitial fluid volume to alveolar fluid clearance in mice: ventilated vs. in situ studies. J. Appl. Physiol. 89:672–679. [DOI] [PubMed] [Google Scholar]
- Hummler, E., P. Barker, J. Gatzy, F. Beermann, C. Verdumo, A. Schmidt, R. Boucher, and B.C. Rossier. 1996. Early death due to defective neonatal lung liquid clearance in α-ENaC–deficient mice. Nat. Genet. 12:325–328. [DOI] [PubMed] [Google Scholar]
- Jain, L., X. Chen, S. Ramosevac, L.A. Brown, and D.C. Eaton. 2001. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. Am. J. Physiol. 280:L646–L658. [DOI] [PubMed] [Google Scholar]
- Jiang, X., D.H. Ingbar, and S.M. O'Grady. 1998. Adrenergic stimulation of Na+ transport across alveolar epithelial cells involves activation of apical Cl− channels. Am. J. Physiol. 275:C1610–C1620. [DOI] [PubMed] [Google Scholar]
- Jiang, X., D.H. Ingbar, and S.M. O'Grady. 2001. Adrenergic regulation of ion transport across adult alveolar epithelial cells: Effects on Cl− channel activation and transport functions in cultures with apical air interface. J. Membr. Biol. 181:195–204. [DOI] [PubMed] [Google Scholar]
- Johnson, M., J. Widdicombe, L. Allen, P. Barbry, and L. Dobbs. 2002. Alveolar epithelial type I cells contain transport proteins and transport sodium, supporting an active role for type I cells in regulation of lung liquid homeostasis. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner, R.J., J.V. Ladetto, R. Singh, N. Fukuda, M.A. Matthay, and R.G. Crystal. 2000. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 22:657–664. [DOI] [PubMed] [Google Scholar]
- Kent, G., M. Oliver, J.K. Foskett, H. Frndova, P. Durie, J. Forstner, G.G. Forstner, J.R. Riordan, D. Percy, and M. Buchwald. 1996. Phenotypic abnormalities in long-term surviving cystic fibrosis mice. Pediatr. Res. 40:233–241. [DOI] [PubMed] [Google Scholar]
- Lazrak, A., V.G. Nielsen, and S. Matalon. 2000. Mechanisms of increased sodium transport in alveolar type II cells by cAMP: we agree or disagree to do more experiments. Am. J. Physiol. 278:L233–L238. [DOI] [PubMed] [Google Scholar]
- Liebler, J.M., Z. Borok, K.J. Kim, and E.D. Crandall. 2001. Immunoreactive β2 adrenergic receptors are expressed in rat alveolar epithelial type I cells. Am. J. Respir. Crit. Care Med. 163:570a. (Abstr.) [Google Scholar]
- Ma, T., N. Fukuda, Y. Song, M.A. Matthay, and A.S. Verkman. 2000. Lung fluid transport in aquaporin-5 knock out mice. J. Clin. Invest. 105:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon, S., and H. O'Brodovich. 1999. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu. Rev. Physiol. 61:627–661. [DOI] [PubMed] [Google Scholar]
- Matthay, M.A., H.G. Folkesson, and A.S. Verkman. 1996. Salt and water transport across alveolar and distal airway epithelia in the adult lung. Am. J. Physiol. 270:L487–L503. [DOI] [PubMed] [Google Scholar]
- Pittet, J.F., J.P. Wiener-Kronish, M.C. McElroy, H.G. Folkesson, and M.A. Matthay. 1994. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J. Clin. Invest. 94:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet, J.F., T.J. Brenner, K. Modelska, and M.A. Matthay. 1996. Alveolar liquid clearance is increased by endogenous catecholamines in hemorrhagic shock in rats. J. Appl. Physiol. 81:830–837. [DOI] [PubMed] [Google Scholar]
- Rezaiguia, S., C. Garat, C. Delclaux, M. Meignan, J. Fleury, P. Legrand, M.A. Matthay, and C. Jayr. 1997. Acute bacterial pneumonia in rats increases alveolar epithelial fluid clearance by a tumor necrosis factor-α-dependent mechanism. J. Clin. Invest. 99:325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochelle, L.G., D. Li, H. Ye, E. Lee, C.R. Talbot, and R.C. Boucher. 2000. Distribution of ion transport mRNAs throughout murine nose and lung. Am. J. Physiol. 279:L14–L24. [DOI] [PubMed] [Google Scholar]
- Sakuma, T., G. Okaniwa, T. Nakada, S. Nishimura, S. Fujimura, and M.A. Matthay. 1994. Alveolar fluid clearance in the resected human lung. Am. J. Respir. Crit. Care Med. 150:305–310. [DOI] [PubMed] [Google Scholar]
- Sakuma, T., S. Suzuki, K. Usuda, M. Handa, G. Okaniwa, S. Nakada, S. Fujimura, and M.A. Matthay. 1996. Alveolar epithelial fluid transport mechanisms are preserved in the rewarmed human lung following severe hypothermia. J. Appl. Physiol. 80:1681–1686. [DOI] [PubMed] [Google Scholar]
- Sakuma, T., K. Takahashi, N. Ohya, T. Nakada, and M.A. Matthay. 1998. Effects of ATP-sensitive potassium channel opener on potassium transport and alveolar fluid clearance in the resected human lung. Pharmacol. Toxicol. 83:16–22. [DOI] [PubMed] [Google Scholar]
- Schultz, B.D., A.K. Singh, D.C. Devor, and R.J. Bridges. 1999. Pharmacology of CFTR channel activity. Physiol. Rev. 79:S109–S144. [DOI] [PubMed] [Google Scholar]
- Serikov, V.B., M. Grady, and M.A. Matthay. 1993. Effect of temperature on alveolar liquid and protein clearance in an in situ perfused goat lung. J. Appl. Physiol. 75:940–947. [DOI] [PubMed] [Google Scholar]
- Song, Y., S. Jayaraman, B. Yang, M.A. Matthay, and A.S. Verkman. 2001. Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J. Gen. Physiol. 117:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese, G.M., L.B. Ware, B.A. Matthay, and M.A. Matthay. 1999. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J. Appl. Physiol. 87:1301–1312. [DOI] [PubMed] [Google Scholar]
- Ware, L.B., and M.A. Matthay. 2001. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 163:1293–1294. [DOI] [PubMed] [Google Scholar]
- Weibel, E.R. 1989. Lung morphometry and models in respiratory physiology. Respiratory Physiology. H.K. Chang, and M. Pavla, editors. Marcel Dekker Inc., New York. 1–56.
- Widdicombe, J.H. 2000. How does cAMP increase active Na+ absorption across alveolar epithelium? Am. J. Physiol. 278:L231–L232. [DOI] [PubMed] [Google Scholar]