Abstract
The RNA silencing pathway mediated by small interfering RNAs (siRNAs) plays an important antiviral role in eukaryotes. To counteract this defense barrier, a large number of plant viruses express proteins with RNA silencing suppression activity. Recently, it was reported that the ipomovirus Cucumber vein yellowing virus (CVYV), which lacks the typical silencing suppressor of members of the family Potyviridae, i.e., HCPro, has a duplicated P1 coding sequence and that the downstream P1 copy, named P1b, has silencing suppression activity. In this study, we provide experimental evidence that P1b is a serine protease that self-cleaves at its C terminus but that its proteolytic activity is not essential for silencing suppression. In contrast, a putative zinc finger and a conserved basic motif in the N-terminal region of the protein are required for efficient silencing suppression. In vitro gel filtration-fast protein liquid chromatography and in vivo bimolecular fluorescence complementation assays showed that P1b binds itself to form oligomeric structures and that the zinc finger-like motif is essential for the self interaction. Moreover, we observed that CVYV P1b forms complexes with synthetic siRNAs, and this ability correlated with both silencing suppression activity and enhancement of Potato virus X pathogenicity in a mutational analysis. Together, these results suggest that CVYV P1b resembles potyviral HCPro and other viral proteins in interfering RNA silencing by preventing siRNA loading into the RNA-induced silencing complex.
RNA silencing is a generic term that refers to a group of homology-dependent mechanisms of gene inactivation regulated by small RNAs of 21 to 30 nucleotides (2, 5). Usually, these small RNAs derive from the digestion of perfectly or partially paired RNA duplexes by RNase III-like nucleases called Dicer or Dicer-like (DCL) proteins, although alternative biosynthetic pathways have also been suggested (6). One strand of the small RNA duplex is incorporated into a silencing effector complex, guiding it, by sequence complementarity, to degrade mRNA, inhibit mRNA translation, or interfere with transcription by chromatin rearrangements (53, 66, 81).
The RNA silencing pathway mediated by small interfering RNAs (siRNAs), a kind of small RNAs, is active in the cytoplasm and plays an important antiviral role in plants, insects, nematodes, and perhaps other eukaryotes, since viral siRNAs are generated by the hierarchical action of DCL proteins on double-stranded RNA (dsRNA) replication intermediates, fold-back structures within viral mRNA, or dsRNA products derived from the copy of viral RNA by cellular RNA-dependent RNA polymerases (7, 18, 26, 47, 68). Although Dicer processing might have a direct antiviral activity, RNA silencing appears to disturb virus infection mainly by the slicing activity of RNA-induced silencing complexes (RISC) programmed with viral siRNAs (49, 50).
Viruses need to develop strategies to evade the RNA silencing-mediated defense barrier, and the production of silencing suppressors appears to be among the most successful (12, 39, 56, 61, 82, 86). A large number of viral RNA silencing suppressors have been identified. They are extremely diverse in sequence, structure, and mechanism of action. Given the key role played by siRNAs and long dsRNAs in the silencing pathways, it has been proposed that sequestering these molecules could be a general strategy used by viral silencing suppressors (65). Supporting this hypothesis, recent results have demonstrated that tombusvirus P19, closterovirus P21, and potyvirus HCPro bind siRNAs and prevent their loading into RISC (37, 38), and some other silencing suppressors bind dsRNA without size constraints, which could interfere with Dicer activity (13, 18, 40, 45, 46). However, other viral suppressors appear to block RNA silencing by a variety of quite different mechanisms (14, 52, 71, 85, 89).
HCPro is the typical silencing suppressor of viruses of the family Potyviridae (3, 9, 33). This family consists of five genera with monopartite plus-strand RNA genomes, namely, Potyvirus, Rymovirus, Macluravirus, Ipomovirus, and Tritimovirus, and the genus Bymovirus, which has a bipartite genome (41). It was recently reported that the ipomovirus Cucumber vein yellowing virus (CVYV), which lacks HCPro (32), has a duplicated P1 coding sequence, and the downstream P1 copy, P1b, is a second RNA silencing suppressor in the Potyviridae family (76). In silico analysis suggested that both P1 copies, P1a and P1b, are serine proteinases (75), and the enzymatic activity of P1a was verified experimentally (76). In the present study, we demonstrate the protease activity of P1b, which, unlike a putative zinc finger and a conserved motif located in the N-terminal region of the protein, was not essential for RNA silencing suppression activity. We also show the ability of P1b to self-interact and to bind siRNAs and the functional relevance of these interactions.
MATERIALS AND METHODS
Plasmids.
Gateway technology (Invitrogen) was applied to construct plasmids expressing different tagged forms of wild-type and mutant CVYV P1b, using pDONR-207 (Invitrogen) as the donor vector and pDEST-TH1 (provided by Helena Berglund, Karolinska Institutet, Stockholm, Sweden) (28), pNTAPi and pCTAPi (provided by Michael Fromm, University of Nebraska) (60), pBIFP2 and pBIFP3 (provided by François Parcy, Laboratoire Physiologie Cellulaire Végétale, Grenoble, France), and pGWC-PVX (a Gateway-adapted plasmid derived from pGR106) (B. García et al., manuscript in preparation) as destination vectors. p35S-P1b has been described previously (76). Site-directed mutagenesis of P1b was carried out by two PCR steps, as described by Herlitze and Koenen (30). Primers and templates used for PCR amplification and site-directed mutagenesis of P1b sequences to generate the different entry vectors are listed in Tables S1 and S2 in the supplemental material.
Expression vectors producing wild-type and mutant NTAP-P1b (p35S-NTAP-P1b and its derivatives), NYFP-P1b (p35S-NYFP-P1b and its derivatives), CYFP-P1b (p35S-CYFP-P1b and its derivatives), and maltose binding protein (MBP)-P1b (pMBP-P1b) were constructed by LR clonase reactions between pDONR-nonAUGP1b entry vectors (see Table S1 in the supplemental material) and the destination vectors pNTAPi, pBIFP2, pBIFP3, and pDEST-TH1, respectively. Expression vectors producing wild-type and mutant CTAP-P1b (p35S-CTAP-P1b and its derivatives) and Potato virus X (PVX)-P1b chimeric viruses (p35S-PVX-P1b and its derivatives) were constructed by LR clonase reactions between pDONR-P1bcut entry vectors (see Table S1 in the supplemental material) and the destination vectors pCTAPi and pGWC-PVX, respectively.
Agrobacterium tumefaciens strain C58C1 carrying p35S:GFP (29) plus pCH32 (27) and strain GV3101 carrying pJIC_SA Rep, as well as plasmids p35S:GF-IR (63), pBIN61:P19 (84), and pGR106 (42), were kindly provided by David Baulcombe (Sainsbury Laboratory, United Kingdom).
Sequence analysis.
Sequence alignment of P1 sequences from CVYV (GenBank accession no. AY578085), Sweet potato mild mottle virus (SPMMV; GenBank accession no. Z73124), Oat necrotic mottle virus (GenBank accession no. AY377938), Brome streak mosaic virus (GenBank accession no. Z48506), and Wheat streak mosaic virus (WSMV; GenBank accession no. AF057533) was carried out by using the DNASTAR MegAlign program and refined by manual editing. Plots of charge density at pH 7 were made with the DNASTAR Protean program, with a window size of five residues.
Purification of recombinant MBP-P1b and production of anti-P1b sera.
Escherichia coli DH5α was transformed with pMBP-P1b and used to produce CVYV P1b fused to MBP as previously described (21), except that bacteria were lysed by sonication. MBP-P1b (500 μg) was emulsified in Freund's complete (for initial immunization) or incomplete (for subsequent boosters) adjuvant and injected into rabbits. Sera were collected and used without further purification.
Agroinfiltration and fluorescence imaging.
Nicotiana benthamiana plants were infiltrated with A. tumefaciens strain C58C1 carrying the indicated plasmids, as previously described (76). Green fluorescent protein (GFP) fluorescence was observed under long-wavelength UV light (Black Ray model B 100 AP) and photographed by using a Nikon D1X digital camera with a 62E 022 filter. For yellow fluorescent protein (YFP) imaging in bimolecular fluorescence complementation (BiFC) assays, little pieces of agroinfiltrated leaves were examined with a Leica DMR epifluorescence microscope, using excitation and barrier filters at 450/490 nm and 500/550 nm, respectively, and then photographed with an Olympus DP70 digital camera.
PVX and CVYV infection.
A. tumefaciens strain GV3101 containing the helper plasmid pJIC SA_Rep was transformed with pGR106 and p35S-PVX-P1b or its derivatives. PVX inoculation of N. benthamiana and Nicotiana clevelandii plants was carried out by infiltration with the resulting Agrobacterium strains, as previously described (1). CVYV-infected cucumber tissue was kindly provided by Dirk Janssen, IFAPA, Almería, Spain.
Western blot analysis.
Preparation of protein samples, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electroblotting were done as previously described (76). Specific proteins were detected using the peroxidase-antiperoxidase soluble complex (PAP; Sigma) or different combinations of the following primary and secondary reagents: anti-CVYV P1b or anti-PVX capsid protein (CP) (PVAS643; American Type Culture Collection) polyclonal serum with horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (Jackson), a mixture of two anti-GFP monoclonal antibodies (Roche) with HRP-conjugated sheep anti-mouse IgG (Sigma), or biotinylated calmodulin (Calbiochem) with streptavidin-HRP (Amersham). The immunostained proteins were visualized by enhanced chemiluminescence detection with a LifeABlot kit (Euroclone) according to the manufacturer's instructions. Ponceau red staining was used to check the global protein content of the samples.
RNA extraction and Northern blot analysis.
Samples of large and small RNAs were prepared from agroinfiltrated leaf tissue and subjected to Northern blot analysis as previously described (76).
Purification of TAP-tagged P1b proteins.
N. benthamiana leaves were infiltrated with A. tumefaciens carrying p35S-NTAP-P1b or with a mixture of two A. tumefaciens strains, one carrying a mutated p35S-NTAP-P1b derivative and the other carrying pBIN61:P19, which expresses the silencing suppressor P19 from Tomato bushy stunt virus (TBSV). Agroinfiltrated patches were harvested at 6 days postinfiltration (dpi), ground to a fine powder under liquid nitrogen, and stored at −80°C until use. This powder was incubated with extraction buffer consisting of 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM Mg(CH3COO)2, 4 mM CaCl2, 2 mM β-mercaptoethanol, 0.1% NP-40, 10 μM leupeptin, 1 μM pepstatin, 1 μM aprotinin, 1 mM phenylmethylsulfonyl fluoride, and 10 μM E-64 (2 ml/mg) for 30 min at 4°C, and cell debris was removed by centrifugation at 18,000 × g at 4°C for 10 min. The supernatant was diluted 10 times with extraction buffer lacking the protease inhibitors, filtered through a 0.45-μm nitrocellulose membrane (Millipore), and loaded onto an Econo-column (Bio-Rad) packed with 1 ml of calmodulin-Sepharose beads (Amersham). After being washed with 20 ml of a buffer containing 20 mM Tris-HCl, pH 7.5, and 150 mM NaCl, the bound proteins were eluted with washing buffer supplied with 2 mM EGTA. Tandem affinity purification (TAP) tag removal from the purified proteins was carried out with Tobacco etch virus (TEV) AcTEV protease (Invitrogen) according to the manufacturer's instructions.
Gel filtration-FPLC.
Affinity-purified TAP-tagged P1b proteins, either intact or proteolytically processed by AcTEV protease, were analyzed by gel filtration, using a fast protein liquid chromatography system (FPLC) (ÁKTA-Prime; Pharmacia) with a Hi-Load 16/60 Superdex-200 column (Pharmacia) at 4°C. Column equilibration and chromatography were performed at a flow rate of 0.5 ml/min in a buffer consisting of 20 mM Tris-HCl, pH 7.5, and 150 mM NaCl. Fractions were collected every 2.5 ml and subjected to Western blot analysis.
The column was calibrated with catalase (158 kDa), serum albumin (68 kDa), ovalbumin (50 kDa), and chymotrypsinogen A (21 kDa), using the same chromatography protocol.
Electrophoretic mobility shift assay (EMSA).
Synthetic double-stranded siRNAs with 2-deoxynucleotide 3′ overhangs (5′ CUUACGCUGAGUACUUCGATT 3′ and 5′ UCGAAGUACUCAGCGUAAGTT 3′) (Sigma) were labeled with [γ-32P]ATP by using T4 polynucleotide kinase (Promega).
Crude protein extracts for binding reactions were prepared by homogenizing agroinfiltrated tissue powdered under liquid nitrogen in binding buffer (83 mM Tris-HCl, pH 7.5, 0.8 mM MgCl2, 66 mM KCl, 100 mM NaCl, 2 mM β-mercaptoethanol) (4 ml/mg) and were clarified by centrifugation at 18,000 × g at 4°C for 15 min.
Labeled siRNAs (0.5 nM) were incubated for 30 min at room temperature with different amounts of either affinity chromatography-purified NTAP-P1b proteins or crude protein extracts from agroinfiltrated tissue in reaction mixtures (20 μl) containing binding buffer and 16 U of RNase inhibitor (Takara). After incubation, protein-RNA complexes were resolved in 5% polyacrylamide-containing 0.5× Tris-borate-EDTA gels. The gels were dried and exposed to X-ray-sensitive films. For supershift assays, various amounts of PAP complex were included in the binding mixtures.
RESULTS
P1b is a serine protease.
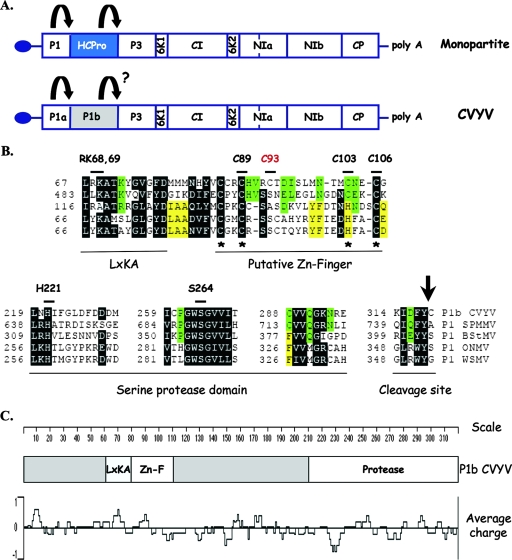
Two serine protease domains have been identified in the P1 region of the ipomovirus CVYV, suggesting the existence of two P1 copies, named P1a and P1b (75). CVYV P1a clustered with P1s from viruses of the genera Potyvirus and Rymovirus in a phylogenetic analysis (75), and its proteolytic activity was demonstrated experimentally (76). In contrast, CVYV P1b was more closely related to P1s from members of the genera Ipomovirus and Tritimovirus (75). Amino acid alignment of P1b-like proteins from ipomoviruses and tritimoviruses showed a well-conserved C-terminal region, which corresponds to the serine protease domain, with the catalytic triad formed, in the case of CVYV P1b, by H221, D229, and S264 (75) (Fig. 1). In addition, this analysis also revealed the presence of two conserved motifs located upstream of the protease domain, namely, a putative zinc finger and an LXKA conserved motif (75) (Fig. 1).
FIG. 1.
Conserved domains present in P1b-like proteins. (A) Genome maps of monopartite viruses from the family Potyviridae. The arrows represent protease activities. The question mark indicates that this proteolytic activity had not been demonstrated experimentally prior to this work. (B) Partial amino acid alignment of P1b of the ipomovirus CVYV (considered to start at amino acid 526 of the polyprotein) and P1s of the ipomovirus SPMMV and the tritimoviruses Brome streak mosaic virus (BStMV), Oat necrotic mottle virus (ONMV), and WSMV. Boxed amino acids are identical or chemically similar between the two ipomoviral sequences (green boxes), between the three tritimoviral sequences (yellow boxes), or between at least four of the aligned sequences (black boxes). Dashes represent gaps. The conserved domains are indicated below the sequence alignment. The position of the first residue of each aligned segment is indicated on the left side of the sequence. Cys and His residues predicted to form a zinc finger domain are marked with asterisks. The arrow indicates the predicted autocatalytic cleavage site. The residues that were mutated in this work and their positions in the P1b sequence are indicated above the alignment. (C) Schematic representation of CVYV P1b showing the locations of conserved domains and a plot of charge density along the protein.
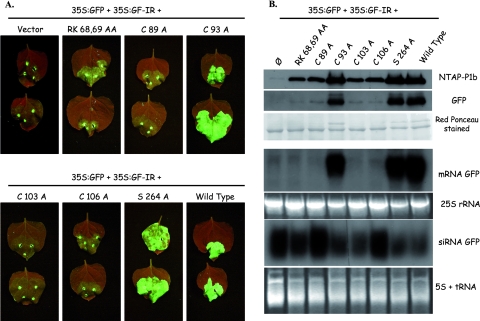
In order to verify the predicted protease activity of CVYV P1b, reporter constructs that coded for fusion products consisting of P1b, the first P3 residue, and a C-terminal TAP tag (p35S-P1b-CTAP) (Fig. 2A) were expressed in planta by A. tumefaciens infiltration. For simplicity, in this work, we refer to each A. tumefaciens strain by the plasmid it carries. Western blot analysis specific for the TAP tag showed accumulation of a protein of ∼22 kDa, the size of TAP, in N. benthamiana leaves infiltrated with wild-type p35S-P1b-CTAP, suggesting that P1b self-cleaves at its C end in P1b-CTAP (Fig. 2B, lane 5). In order to verify whether the serine protease domain identified in silico was involved in the generation of the TAP-related 22,000-molecular-weight (22K) product, we expressed mutated versions of P1b-CTAP in which either of two of the residues of the predicted catalytic triad, H221 and S264, was replaced by alanine. The 22K protein was not detected in the TAP-specific Western blot analysis of leaves infiltrated with p35S-P1b-CTAP H221A or S264A, but a protein of ∼58 kDa, the size expected for noncleaved P1b-CTAP, was shown to accumulate in these samples (Fig. 2B, lanes 3 and 4). The most likely interpretation of this result is that P1b indeed cleaves itself in P1b-CTAP and that the H221A and S264A mutations abolish the P1b proteolytic activity. To investigate the possible relevance of other P1b regions for protease activity, mutations affecting the LXKA motif and the putative zinc finger, i.e., RK68,69AA and C89A mutations, respectively, were introduced into p35S-P1b-CTAP. Neither of these mutations affected accumulation of the 22K protein (Fig. 2B, lanes 1 and 2), suggesting that the mutated domains were not involved in P1b proteolysis.
FIG. 2.
CVYV P1b cleaves at its C terminus and accumulates in CVYV-infected cucumber plants. (A) Schematic representation of the N terminus of the CVYV polyprotein and the C-terminally TAP-tagged P1b reporter. Letters inside the boxes represent either conserved amino acids or conserved domains. LXKA, positively charged conserved motif; Zn-F, putative zinc finger; H D S, H221, D229, and S264 predicted to constitute the protease catalytic triad; and YC, amino acids at the predicted P1b-P3 junction. (B) Western blot analysis with PAP complex of extracts of leaf patches of N. benthamiana plants infiltrated with agrobacteria carrying empty pBin19 (lane 6), p35S-P1b-CTAP (lane 5), or derivatives of this plasmid with the indicated mutations (lanes 1 to 4), collected at 3 dpi. (C) Western blot analysis with anti-P1b serum of extracts of cucumber leaves systemically infected with CVYV (lane 1) or equivalent leaves of mock-inoculated cucumber plants (lane 2) and extracts of leaf patches of N. benthamiana plants infiltrated with agrobacteria carrying p35S-P1b (lane 3) or empty pBin19 (lane 4). The positions of prestained molecular mass markers (in kilodaltons; New England Biolabs) run in the same gels are indicated to the right of the panels. Blots stained with Ponceau red are shown at the bottom as a loading control.
This finding and the previous demonstration of the proteinase activity of CVYV P1a (76) strongly suggest that free P1b could be produced in CVYV infection by proteolytic processing of the viral polyprotein. To verify this prediction, anti-P1b serum was produced and used in a Western blot analysis to detect P1b-related polypeptides in infected cucumber plants, the natural host of CVYV. In agreement with the agroinfiltration results, the anti-P1b serum revealed the accumulation of a protein of ∼36 kDa, the size expected for free P1b, in CVYV-infected cucumber leaves, which was absent in healthy cucumber leaves (Fig. 2C, lanes 1 and 2). This protein had the same electrophoretic mobility as P1b expressed in N. benthamiana leaves by agroinfiltration (Fig. 2C, lanes 3 and 4).
The putative zinc finger and the LXKA motif, but not protease activity, are essential for the RNA silencing suppression activity of CVYV P1b.
A mutational approach was followed to determine protein domains involved in the RNA silencing suppression activity of CVYV P1b. Mutations were introduced into p35S-NTAP-P1b, which encodes an N-terminal TAP-tagged P1b protein. Unlike CTAP tagging, which disturbs the silencing suppression activity of P1b, NTAP tagging, while facilitating protein detection and purification, has no appreciable effect on this activity (data not shown). The effects of the mutations were assessed in a dsRNA-triggered silencing assay (Fig. 3). In N. benthamiana leaves agroinfiltrated with p35S:GFP (expressing GFP mRNA) and p35S:GF-IR (expressing an inverted repeat which generates GFP dsRNA), GF-IR directed a fast and strong silencing of GFP mRNA, and as a consequence, very weak green fluorescence was detected in infiltrated patches at 7 dpi (Fig. 3A). Consistent with this fact, Northern blot and Western blot analyses showed very low accumulation levels of GFP mRNA and protein, respectively, in these agroinfiltrated leaves (Fig. 3B). Coagroinfiltration with wild-type p35S-NTAP-P1b prevented the induction of silencing, and strong fluorescence and high accumulation levels of GFP and GFP mRNA were detected at 7 dpi in leaves agroinfiltrated with the three plasmids (Fig. 3). Patches infiltrated with p35S:GFP, p35S:GF-IR, and either the C93A (affecting a nonconserved cysteine) or S264A (affecting the protease active center) mutant version of p35S-NTAP-P1b also showed high GFP expression levels, indicating that these mutations did not affect the silencing suppression activity of P1b (Fig. 3). In contrast, mutations affecting the LXKA conserved motif (RK68,69AA) or the putative zinc finger (C89A, C103A, and C106A) abolished silencing suppression activity, and leaves infiltrated with p35S:GFP, p35S:GF-IR, and p35S-NTAP-P1b with any of these mutations expressed GFP at very low levels, similar to those of leaves infiltrated with p35S:GFP, p35S:GF-IR, and the empty vector pBin19 (Fig. 3). dsRNA-triggered silencing of GFP expression was associated with an accumulation of specific siRNAs. In agreement with previous results (76), silencing suppression by wild-type P1b or P1b C93A and S264A mutants caused only a slight decrease in GFP siRNA levels (Fig. 3B). We observed some differences in the amounts of siRNAs accumulated in the presence of the different P1b mutants. However, these observations were not exactly reproduced in repetitions of the experiment, suggesting that other unknown factors might affect the siRNA levels, resulting in a certain degree of fluctuation in the analysis (data not shown).
FIG. 3.
Effects of mutations in CVYV P1b conserved domains on silencing suppression activity. N. benthamiana plants were coinfiltrated with agrobacteria carrying p35S:GFP and p35S:GF-IR plus empty pBin19 (vector), p35S-NTAP-P1b (wild type), or derivatives of this plasmid with the indicated mutations. (A) GFP fluorescence pictures taken under a UV lamp at 7 dpi. (B) Western blot analysis with PAP complex and anti-GFP antibodies (upper panels) and Northern blot analysis of GFP mRNA and siRNA (middle and bottom panels) of infiltrated leaves harvested at 7 dpi. A protein blot stained with Ponceau red and RNA agarose (25S rRNA) and polyacrylamide (5S + tRNA) gels stained with ethidium bromide are shown as loading controls.
RNA silencing also affected the expression of the inactive P1b mutants, and TAP-specific Western blot analysis revealed less accumulation of these proteins than of wild-type P1b or the functional C93A and S264A mutants (Fig. 3B). However, at 2 dpi, although the weak sense RNA-triggered silencing affecting p35S-NTAP-P1b expression was still not very effective and all mutant P1b proteins accumulated at similar levels to that of wild-type P1b, dsRNA-triggered silencing already disturbed GFP expression in the absence of P1b or in the presence of P1b with the RK68,69AA, C89A, C103A, or C106A mutation (see Fig. S1 in the supplemental material), further supporting the specific effect of these mutations in RNA silencing suppression activity.
P1b self-interacts in vivo.
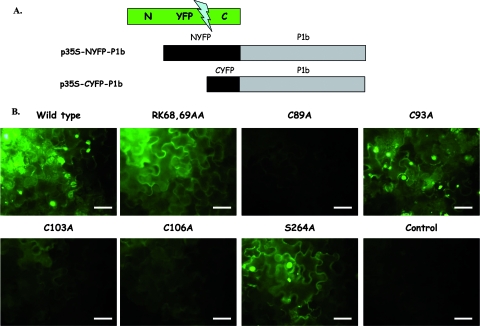
Previous structural studies of different RNA silencing suppressors revealed self interactions driving oligomeric conformations (13, 40, 54, 62, 79, 88). In order to assess whether P1b can also interact with itself, we made use of the BiFC technique. BiFC reveals in vivo interactions between two proteins by reconstitution of a fluorescing complex from two defective fragments of a fluorescent protein, with each one fused to one of the binding proteins (31). We used a simplification of the method involving just P1b fused to either of two fragments of YFP (NYFP and CYFP), which were transiently expressed by agroinfiltration in N. benthamiana leaves (Fig. 4). In order to have uniformly high expression levels, agrobacteria expressing the TBSV P19 silencing suppressor were included in all infiltration mixtures. No fluorescence was detected in cells expressing each P1b fusion product (p35S-NYFP-P1b or p35S-CYFP-P1b) independently or in combination with the complementary YFP fragment fused to a naïve protein (Fig. 4 and data not shown). In contrast, strong fluorescence was detected at 3 and 6 dpi under UV light in leaf patches coexpressing p35S-NYFP-P1b and p35S-CYFP-P1b (Fig. 4 and data not shown).
FIG. 4.
Analysis of CVYV P1b self interaction by BiFC assay. N. benthamiana plants were coinfiltrated with agrobacteria carrying pBin61:P19 plus p35S-NYFP-P1b (wild type or the indicated mutants) plus p35S-CYFP-P1b (wild type or the indicated mutants). Plants coinfiltrated with agrobacteria carrying pBin61:P19 plus p35S-CYFP-P1b were used as a negative control (control). (A) Schematic representation of plasmids used in the assay. (B) YFP fluorescence pictures taken under a fluorescence microscope at 6 dpi. Bars, 50 μm.
P1b mutants were also expressed as NYFP and CYFP fusion proteins and tested for interaction by BiFC. Mutations in the protease active center (S264A), the LXKA motif (RK68,69AA), and a nonconserved cysteine (C93A) did not affect the ability of P1b to self-interact, and fusion proteins with these mutations reconstituted fluorescent YFP with similar efficiencies to that of the wild-type proteins (Fig. 4). However, patches expressing P1b proteins with mutations in the zinc finger motif (C89A, C103A, and C106A) displayed no fluorescence at 3 dpi (data not shown) and just a very weak signal at 6 dpi (Fig. 4), suggesting that the predicted zinc finger plays an important role in P1b self interaction in vivo.
P1b forms homodimers in solution.
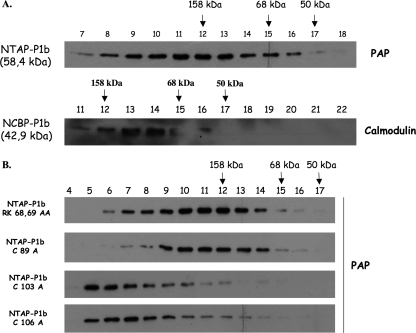
The self interaction of P1b revealed by the BiFC assays suggested that this protein could form oligomeric structures. To test this possibility, we partially purified NTAP-P1b expressed in N. benthamiana leaves by agroinfiltration, using affinity chromatography with calmodulin-Sepharose, and analyzed the purified protein by FPLC-gel filtration. TAP-specific Western blot analysis of the collected fractions showed that NTAP-P1b migrated as a single peak close to a molecular mass marker of 158 kDa, which is much larger than the molecular mass of monomeric NTAP-P1b (58.4 kDa), suggesting that NTAP-P1b was present in an oligomeric form (Fig. 5A). To rule out possible structural effects of the TAP tag, NTAP-P1b was trimmed by proteolytic processing with the AcTEV protease (60). This treatment removes the protein A domain of the TAP tag (15.4 kDa), leaving just the calmodulin binding protein domain (CBP; 6.8 kDa) fused to P1b (NCBP-P1b; 43 kDa). This sample was also analyzed by FPLC-gel filtration. Western blot analysis with biotinylated calmodulin revealed that NCBP-P1b eluted as a single peak with an apparent molecular mass of ∼100 kDa (Fig. 5A), which is approximately double the predicted size for the NCBP-P1b monomer, further supporting the conclusion that P1b could acquire a homodimeric conformation.
FIG. 5.
Oligomerization of CVYV P1b in solution. N-terminally tagged CVYV P1b proteins purified by affinity chromatography were analyzed by gel filtration-FPLC. (A) Elution fractions of NTAP-P1b, intact or digested with acTEV protease (NCBP-P1b), were subjected to Western blot analysis with PAP complex or biotinylated calmodulin, respectively. (B) Elution fractions of NTAP-P1b mutants were subjected to Western blot analysis with PAP complex. Arrows indicate the elution positions of the following molecular mass markers: aldolase (158 kDa), serum albumin (68 kDa), and ovalbumin (50 kDa).
NTAP-P1b mutants with defects in RNA silencing suppression activity were also analyzed by gel filtration-FPLC (Fig. 5B). As expected, the NTAP-P1b RK68,69AA mutant, which was observed to self-interact in vivo in the BiFC assay, also appeared to homodimerize like the wild-type protein (Fig. 5B). However, C103A and C106A mutations at the zinc finger motif appeared to have a drastic effect on P1b conformation, since NTAP-P1b with either of these mutations eluted in the first gel filtration-FPLC fractions, indicative of nonspecific aggregation (Fig. 5B). These data are consistent with the results of the BiFC assay and suggest a structural role for the predicted zinc finger. The gel filtration elution profile of NTAP-P1b C89A, which resembled the other zinc finger mutants in the inability to self-interact efficiently in vivo in the BiFC assay, was usually similar to those of wild-type NTAP-P1b and the RK68,69AA mutant (Fig. 5B), although some NTAP-P1b C89A nonspecific aggregation was also observed in some purification experiments (data not shown).
P1b is a siRNA binding protein.
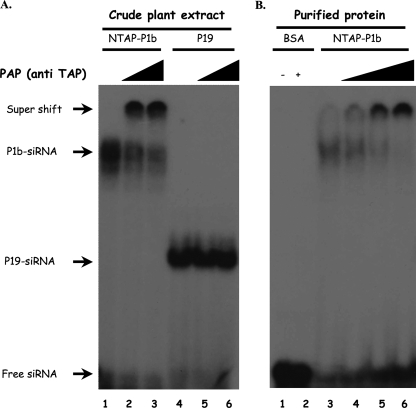
CVYV P1b closely resembled P1-HCPro of the potyvirus Plum pox virus (PPV) in different RNA silencing suppression assays, suggesting that both viral suppressors could target the same step(s) of the silencing pathway (76). Although the mechanism that potyviral HCPro uses to interfere with RNA silencing has still not been unraveled completely, recent data suggest that the activity of HCPro, as well as that of tombusvirus P19 and other silencing suppressors, involves direct sequestering of double-stranded siRNAs (37, 45). The possibility that siRNA binding could also play a role in the RNA silencing suppression activity of P1b was assessed by EMSA. Crude extracts from N. benthamiana leaves agroinfiltrated with p35S-NTAP-P1b or pBIN61:P19, which expresses TBSV P19 and was used as a positive control, were incubated with 32P-labeled synthetic double-stranded siRNAs, and the resulting complexes were resolved by gel electrophoresis (Fig. 6A). As expected, P19 caused a shift in siRNA mobility. Interestingly, an siRNA complex with less mobility than that of the P19-siRNA complex was formed by the NTAP-P1b extract, suggesting that NTAP-P1b may interact with siRNAs or induce siRNA interaction with another protein in the plant extract. To discriminate between these two possibilities, we carried out a supershift assay using the PAP complex, which interacts specifically with TAP. PAP had no effect on the mobility of free siRNA or of P19-siRNA complexes but caused a further band shift of siRNA complexes formed by the NTAP-P1b extract, indicating that NTAP-P1b was a component of these siRNA complexes (Fig. 6A).
FIG. 6.
CVYV P1b binds siRNA in vitro. (A) Crude protein extract (2 μl) from agroinfiltrated leaves expressing NTAP-P1b (lanes 1 to 3) or TBSV P19 (lanes 2 to 5) and harvested at 3 dpi was incubated with 32P-labeled double-stranded siRNAs. The PAP complex was included in the binding mixtures loaded in lanes 2 and 5 (0.1 μl) and 3 and 6 (0.2 μl). (B) NTAP-P1b purified by affinity chromatography (250 nM) (lanes 3 to 6) or control bovine serum albumin (BSA; 250 nM) (lanes 1 and 2) was incubated with siRNAs. The PAP complex was included in the binding mixtures loaded in lanes 4 (0.02 μl), 5 (0.1 μl), and 2 and 6 (0.2 μl). Protein-siRNA complexes were resolved in polyacrylamide gels and revealed by autoradiography.
To further confirm the NTAP-P1b-siRNA interaction, NTAP-P1b partially purified by affinity chromatography was subjected to EMSA (Fig. 6B). Purified NTAP-P1b formed an siRNA complex with the same mobility as that formed by the crude extracts of leaves expressing NTAP-P1b. This complex also suffered a supershift when it was incubated with the PAP reagent (Fig. 6B).
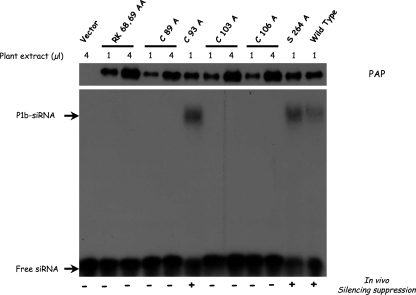
Crude extracts of leaves agroinfiltrated with plasmids expressing NTAP-tagged P1b mutants were also subjected to EMSA (Fig. 7). Whereas C93A and S264A mutants, which are active silencing suppressors, bound siRNAs like wild-type P1b, no siRNA binding was detected for any inactive mutant, even when the dose of extract was increased to have an excess of mutant protein with respect to wild-type P1b (Fig. 7). These results demonstrate that P1b rather than the TAP tag is responsible for siRNA binding of NTAP-P1b and highlight the relevance of this interaction for the RNA silencing suppression activity of P1b.
FIG. 7.
Null silencing suppression CVYV P1b mutants are unable to bind siRNAs. Crude protein extract (1 or 4 μl) from leaves infiltrated with agrobacteria carrying p35S-NTAP-P1b (wild type or the indicated mutants) or empty pBin19 (vector) and harvested at 3 dpi was incubated with 32P-labeled double-stranded siRNAs. Protein-siRNA complexes were resolved in polyacrylamide gels and revealed by autoradiography. The amount of NTAP-P1b protein present in the crude extracts was estimated by Western blot analysis with PAP complex (top panel). The silencing suppression activity of each NTAP-P1b mutant is indicated at the bottom.
Expression of CVYV P1b enhances PVX pathogenicity.
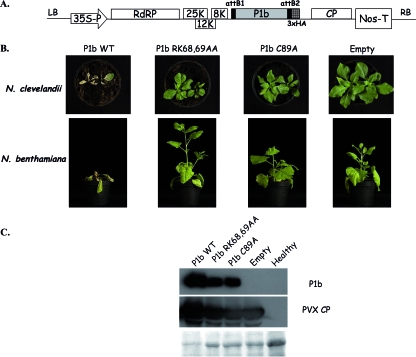
In order to assess the relevance of the strong silencing suppression activity of CVYV P1b in the course of a virus infection, sequences coding for wild-type or mutated P1b were cloned into a PVX-derived vector (Fig. 8A). Both N. benthamiana and N. clevelandii plants were inoculated with the PVX-derived viruses by agroinfiltration. Systemic symptoms were detected at 5 dpi for plants infected with empty PVX or with PVX expressing the CVYV P1b proteins. However, whereas plants infected with empty PVX and PVX expressing either P1b RK68,69AA or P1b C89A, lacking silencing suppression activity, developed relatively mild symptoms, plants infected with PVX carrying wild-type P1b suffered a generalized necrosis, and they died at 15 to 21 dpi (Fig. 8B). Although the extreme necrotic damage of leaves infected with PVX expressing wild-type P1b precluded a precise quantitative assessment, Western blot analysis revealed more accumulation of PVX CP in plants infected with PVX-P1b than in those infected with empty PVX, PVX-P1b-RK68,69AA, or PVX-P1b-C89A (Fig. 8C). Moreover, the Western blot analysis also showed the presence of P1b in plants infected with PVX-P1b-RK68,69AA and PVX-P1b-C89A, but again the accumulation levels were lower than those in plants infected with PVX-P1b (Fig. 8C). Together, these results show that P1b, like other viral silencing suppressors (55), exacerbates PVX infection, and this pathogenicity enhancement is likely the result of a counterdefense activity of P1b, which correlates with silencing suppression activity and siRNA binding capacity.
FIG. 8.
CVYV P1b enhances PVX pathogenicity. (A) Schematic representation of Gateway-adapted T-DNA of pGWC-PVX with the CVYV P1b coding sequence inserted in its cloning site. (B) Symptoms of infection with wild-type PVX (empty) or PVX expressing CVYV P1b (wild type or the indicated mutants) in N. clevelandii and N. benthamiana plants. Photographs were taken at 21 dpi. (C) Western blot analysis with anti-CVYV P1b and anti-PVX CP sera of extracts of N. benthamiana leaves systemically infected with wild-type PVX (empty) or PVX expressing CVYV P1b (wild type or the indicated mutants). Healthy N. benthamiana leaves were also analyzed. The leaves were harvested at 10 dpi. A blot stained with Ponceau red is shown at the bottom as a loading control.
DISCUSSION
Two P1 serine proteases are placed at the N terminus of the CVYV polyprotein.
A previous in silico analysis identified two homologous serine protease domains at the N-terminal region of the CVYV polyprotein, suggesting that the initially proposed long P1 protein was formed of two independent proteins, P1a and P1b (75). Transient in planta expression of the complete P1 region by agroinfiltration revealed the predicted proteolytic activity of P1a (76). We have now followed a similar approach to show the proteolytic activity of P1b, which was abolished by site-directed mutagenesis at H221 and S264 (Fig. 1 and 2), confirming the in silico identification of these residues as components of the catalytic active site of the protein. Cleavages splitting P1a from P1b (76) and P1b from downstream sequences (Fig. 2B) appeared to be very efficient in the agroinfiltration system and also in CVYV-infected cucumber plants (Fig. 2C). Thus, although CVYV lacks an HCPro cysteine proteinase, its strategy of genome expression is quite similar to that of the remaining viruses of the family Potyviridae, involving polyprotein processing by three virus-encoded proteinases. Further research will be required to ascertain specific details of CVYV polyprotein maturation and the possible functional relevance of P1a-P1b and other partially processed products.
P1b protease activity is not necessary for RNA silencing suppression.
P1b CVYV has been shown to have RNA silencing suppression activity similar to that observed for potyviral HCPro (76). Given the proteinase activity of P1b, the possibility that this protein suppressed RNA silencing by promoting specific proteolytic degradation of a component of the silencing pathway was appealing. However, we observed that the point mutation S264A at the proteinase catalytic active site, which completely abolished the protease activity (Fig. 2), did not affect the RNA silencing suppression activity of P1b (Fig. 3). This result suggests that P1b has a direct interfering effect on the silencing machinery, as shown for HCPro, whose proteinase activity is also dispensable for its function as an RNA silencing suppressor (34).
The zinc finger and LXKA motifs of P1b are relevant for its RNA silencing suppression activity.
Sequence alignment of P1b-like proteins revealed the presence of two conserved motifs upstream of the proteinase domain. We have not found matches for the first motif, which is in a highly basic region and has an LXKA signature (Fig. 1), in protein domain databases. The second motif is characterized by several conserved cysteines which are arranged as zinc fingers of the CX2CXnCX2C type (43). Point mutations in the putative zinc finger and in the LXKA motif abolished the silencing suppression activity of P1b, suggesting that these motifs are functionally relevant (Fig. 3). The conservation of these motifs in the P1 proteins of the ipomovirus SPMMV and of three tritimoviruses (Fig. 1) suggests that these proteins might also have RNA silencing suppression activity. Interestingly, these viruses, unlike CVYV, have conserved HCPro coding sequences, although a mutant of the tritimovirus WSMV with a complete HCPro deletion was viable for systemic infection (67). It would be very interesting to find out the viral factor(s) responsible for silencing suppression in viruses with HCPro and a P1b-like P1 protein.
By using BiFC assays, we demonstrated that CVYV P1b binds itself in planta (Fig. 4), and results of gel filtration-FPLC suggested that this protein could form dimers in solution (Fig. 5). P1b mutational analysis suggested that the LXKA motif was not involved in its self interaction (Fig. 4 and 5). In contrast, efficient P1b self interaction appeared to require preservation of the zinc finger domain. P1b proteins with mutations affecting the cysteines predicted to form the zinc finger displayed a very weak interaction in the BiFC assay (Fig. 4), which correlated with a nonspecific aggregation pattern observed by FPLC in the case of the C103A and C106A mutants (Fig. 5). The fact that the C89A mutant usually showed an FPLC elution profile similar to that of the wild-type protein (Fig. 5) suggests that the deleterious effect of the C89A point mutation is less severe than those of the C103A and C106A mutations, and in consequence, it was clearly detected only in planta. A possible explanation for this apparently milder effect of the C89A mutation is that H90 and C93, which are located very close to C89 (Fig. 1), could partially substitute for this residue in the zinc finger configuration.
Zinc fingers were initially identified as protein motifs involved in nucleic acid recognition (36), but it is now known that they are also involved in protein-protein interactions (17, 22). Our results suggest that a zinc finger formed by C86, C89, C103, and C106 is involved in dimerization of CVYV P1b and that disturbance of this interaction causes nonspecific aggregation of the protein. A similar overall destabilization of the protein structure caused by disruption of zinc finger-mediated self interactions has been described previously (51). Interestingly, zinc finger motifs are present in a number of silencing suppressors of plant viruses, including potyviral HCPro (8, 15, 45, 58, 73, 78, 90), and in a subset of P1a-like P1 proteins (75), but the specific relevance of these motifs is still unclear (see below).
CVYV P1b could suppress RNA silencing by siRNA sequestration.
siRNAs are key universal players in the RNA silencing-mediated antiviral defense mechanism, so it is not unexpected that these small RNAs could be the targets of viral counterdefense strategies. In this regard, it has been reported that several viral silencing suppressors interfere with RNA silencing by binding to siRNAs, which prevents the assembly of functional RISC (37, 45). Our results showed that CVYV P1b, either in a crude plant extract or partially purified, can bind siRNAs in vitro (Fig. 6). Moreover, our mutational analysis revealed a positive correlation between the ability to bind siRNA in vitro and the capacity to suppress RNA silencing in vivo (Fig. 7), strongly supporting the suggestion that CVYV P1b uses the strategy of siRNA sequestration to interfere with viral RNA degradation.
Sequence analysis of CVYV P1b did not identify any canonical RNA binding domain, as is also the case for other viral silencing suppressors with siRNA binding activity (16). However, in P1b we found two positively charged regions upstream of the zinc finger motif (Fig. 1C). Basic residues often contribute to RNA binding by directly interacting with the negatively charged ribose-phosphate backbone (11), and this has been shown to take place in binding of several silencing suppressors to RNA (10, 13, 20, 25, 40, 77, 79, 87, 88). Interestingly, one of the CVYV P1b basic domains is part of the LXKA conserved motif (Fig. 1C), and while the RK68,69AA mutation, which drastically reduces the positive charge of this region, did not affect P1b self interaction, this mutation abolished the ability of P1b to cause a siRNA band shift in the gel retardation assay (Fig. 7), suggesting that the LXKA motif could be involved directly in the P1b-siRNA interaction. Mutations affecting the zinc finger structure also disturbed the siRNA binding activity of P1b (Fig. 7), although this disturbance could be an indirect consequence of the effects of the zinc finger mutations on the overall conformation of the P1b dimer (Fig. 4 and 5). The combination of a basic motif followed by a zinc ribbon could have general relevance, since similar arrangements have been shown to be conserved in known and suggested silencing suppressors from different filamentous plant RNA viruses of three genera, including P10 from Grapevine virus A, which resembles CVYV P1b in being able to bind siRNAs (15, 90).
The silencing suppression activity of CVYV enhances viral pathogenicity.
The unavailability of an infectious CVYV cDNA clone precluded a reverse genetic analysis of the role of P1b in CVYV infection. For this reason, we made use of a heterologous system based on PVX-derived recombinant viruses, which has been used previously to study the ability of RNA silencing suppressors to stimulate virus infection (55). We observed that CVYV P1b, like potyviral HCPro and other silencing suppressors, drastically enhanced PVX symptoms, and this enhancement appeared to be the result of a more efficient virus infection (Fig. 8). RK68,69AA and C89A P1b mutants, which are unable to bind siRNAs and to suppress RNA silencing, did not affect PVX pathogenicity (Fig. 8), suggesting that suppression of virus-induced RNA silencing by siRNA binding is the activity of P1b that stimulates virus infection.
P1b versus HCPro.
HCPro was the first viral silencing suppressor identified (3, 9, 33), and it is produced by all members of the family Potyviridae sequenced to date, except for the ipomovirus CVYV, whose silencing suppressor activity appears to be provided by the P1b protein. This is not the only case in which RNA viruses of the same family use different proteins to suppress RNA silencing. In the family Tombusviridae, two unrelated silencing suppressors have been identified, namely, P19 in tombusviruses (83) and CP in carmoviruses (44, 57, 69). Interestingly, the mechanisms of silencing suppression of these proteins are quite different. While tombusvirus P19 sequesters viral siRNAs, precluding their loading into RISC (38, 48), the p38 CP of the carmovirus Turnip crinkle virus interferes with siRNA synthesis by DCL4 and with the activity of siRNAs made by DCL2 (18), and the CP of another carmovirus, Hibiscus chlorotic ringspot virus, affects an early step of RNA silencing preceding dsRNA formation (44). In contrast, the two silencing suppressors of the family Potyviridae, in spite of showing no significant sequence similarity, appear to use similar mechanisms to suppress silencing. Both HCPro and P1b are placed at the same position of the viral polyprotein and are proteases that cleave at their C ends, although their proteolytic activities are not required for silencing suppression (Fig. 2 and 3) (34). Moreover, HCPro and P1b are alike in their ability to self-interact (Fig. 4 and 5) (23, 54, 62, 70, 74), to bind siRNAs (Fig. 6 and 7) (37), and to enhance PVX pathogenicity (Fig. 8) (9, 55). HCPro resembles P1b in having a conserved Cys-rich region, although the spacing of the Cys residues is different in the two proteins (Fig. 1) (59), and this region appeared to be especially relevant for HCPro self interaction in a yeast two-hybrid assay (73). The Cys-rich region is placed in the N-terminal region of HCPro, which has been shown to be dispensable for TEV (19) and Lettuce mosaic virus (54) viability and for dimer formation of Lettuce mosaic virus HCPro in solution (54). Moreover, it has been shown that the Cys-rich region is not necessary for the activity of TEV HCPro as an enhancer of PVX pathogenicity (64), an activity that is supposed to depend on the silencing suppression ability of HCPro. However, deletions in the N-terminal region of HCPro significantly reduced the efficiency of replication of TEV (19) and appeared not to be tolerated by Tobacco vein mottling virus (4) and PPV (24). In addition, some insertions in the N-terminal region of PPV HCPro notably affected the silencing suppression activity of the protein (80). Thus, a more detailed and specific experimental approach will be required to assess the possible contribution of the Cys-rich motif of potyviral HCPro to its silencing suppression activity.
Two regions of the HCPro protein from the potyvirus Potato virus Y have been suggested to be involved in RNA binding (72), although their possible involvement in siRNA binding has not been approached experimentally. In contrast with the LXKA motif of CVYV P1b, these HCPro regions are located downstream of the Cys-rich domain of the protein, and the second one appears to contain a ribonucleoprotein (RNP) motif typical of a large family of RNA binding proteins (11). These data suggest that HCPro and P1b might use different structural elements to interact with siRNAs, although definitive conclusions await high-resolution information about the three-dimensional structures of these proteins.
The extremely high level of divergence between the different silencing suppressors of plant viruses suggests that they derive from independent and recent evolutionary events. Many known viral silencing suppressors are encoded by out-of-frame overlapping genes, suggesting that they may have been created by overprinting on a more ancient gene (39), an evolutionary strategy largely used to expand the coding capacity of genomes (35). In contrast, the silencing suppressor P1b arose in the Potyviridae lineage leading to CVYV by another strategy of gene expansion, gene duplication (75). It is tempting to speculate that silencing suppression activity was incorporated into HCPro, which probably already had its present vector transmission function, only in the evolution of Potyviridae lineages that did not suffer P1 duplication. If this hypothesis is correct, it would be expected that in ipomoviruses and tritimoviruses, which have P1b-like proteins, silencing suppression activity relies on these proteins rather than on HCPro. In agreement with this, HCPro is dispensable for the systemic spread of the tritimovirus WSMV (67). In this scenario, the HCPro coding sequence could have been lost during adaptation of CVYV to whitefly transmission because HCPro was not necessary for interaction with the new vector. Of course, with the present data, the possibility that two silencing suppressors might have developed to act either independently or coordinately, depending on the specific Potyviridae lineage, cannot be ruled out. Another interesting question to be approached in the future is whether RNA silencing suppression is the only function of CVYV P1b or if this protein resembles HCPro in being multifunctional and plays a role in vector transmission or other viral processes. Answering these questions could help us to understand not only the evolution of the family Potyviridae but also that of the antiviral defense mediated by RNA silencing and its suppression by virus factors.
Supplementary Material
Acknowledgments
We thank Juan José López Moya for helpful comments and suggestions, José M. Casasnovas and Florencia Pratto for valuable assistance with FPLC and EMSA experiments, and Dirk Janssen for infected cucumber leaves. We are also grateful to David Baulcombe for providing P19, GFP, and PVX expression vectors; Helena Berglund for providing pDEST-TH1; Michael Fromm for providing pNTAPi and pCTAPi; and François Parcy for providing pBIFP2 and pBIFP3.
This work was supported by grants BIO2004-02687 and BIO2007-67283 from Spanish MEC, CPE03-022-C5-3 from INIA, SAL/0185/2006 from Comunidad de Madrid, and SP22-CT-2004 from the European Union. A.V. was a recipient of an I3P fellowship from CSIC-Fondo Social Europeo.
Footnotes
Published ahead of print on 7 November 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Alamillo, J. M., W. Monger, I. Sola, B. Garcia, Y. Perrin, M. Bestagno, O. R. Burrone, P. Sabella, J. Plana-Duran, L. Enjuanes, G. P. Lomonossoff, and J. A. Garcia. 2006. Use of virus vectors for the expression in plants of active full-length and single chain anti-coronavirus antibodies. Biotechnol. J. 11103-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambros, V., and X. M. Chen. 2007. The regulation of genes and genomes by small RNAs. Development 1341635-1641. [DOI] [PubMed] [Google Scholar]
- 3.Anandalakshmi, R., G. J. Pruss, X. Ge, R. Marathe, A. C. Mallory, T. H. Smith, and V. B. Vance. 1998. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 9513079-13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atreya, C. D., and T. P. Pirone. 1993. Mutational analysis of the helper component-proteinase gene of a potyvirus: effects of amino acid substitutions, deletions, and gene replacement on virulence and aphid transmissibility. Proc. Natl. Acad. Sci. USA 9011919-11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baulcombe, D. 2002. RNA silencing. Curr. Biol. 12R82-R84. [DOI] [PubMed] [Google Scholar]
- 6.Baulcombe, D. C. 2007. Amplified silencing. Science 315199-200. [DOI] [PubMed] [Google Scholar]
- 7.Blevins, T., R. Rajeswaran, P. V. Shivaprasad, D. Beknazariants, A. Si-Ammour, H. S. Park, F. Vazquez, D. Robertson, F. Meins, Jr., T. Hohn, and M. M. Pooggin. 2006. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 346233-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragg, J. N., and A. O. Jackson. 2004. The C-terminal region of the Barley stripe mosaic virus γb protein participates in homologous interactions and is required for suppression of RNA silencing. Mol. Plant Pathol. 5465-481. [DOI] [PubMed] [Google Scholar]
- 9.Brigneti, G., O. Voinnet, W. X. Li, L. H. Ji, S. W. Ding, and D. C. Baulcombe. 1998. Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 176739-6746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Bucher, E., H. Hemmes, P. de Haan, R. Goldbach, and M. Prins. 2004. The influenza A virus NS1 protein binds small interfering RNAs and suppresses RNA silencing in plants. J. Gen. Virol. 85983-991. [DOI] [PubMed] [Google Scholar]
- 11.Burd, C. G., and G. Dreyfuss. 1994. Conserved structures and diversity of functions of RNA-binding proteins. Science 265615-621. [DOI] [PubMed] [Google Scholar]
- 12.Carrington, J. C., K. D. Kasschau, and L. K. Johansen. 2001. Activation of suppression of RNA silencing by plant viruses. Virology 2811-5. [DOI] [PubMed] [Google Scholar]
- 13.Chao, J. A., J. H. Lee, B. R. Chapados, E. W. Debler, A. Schneemann, and J. R. Williamson. 2005. Dual modes of RNA-silencing suppression by flock house virus protein B2. Nat. Struct. Mol. Biol. 12952-957. [DOI] [PubMed] [Google Scholar]
- 14.Chellappan, P., R. Vanitharani, and C. M. Fauquet. 2005. MicroRNA-binding viral protein interferes with Arabidopsis development. Proc. Natl. Acad. Sci. USA 10210381-10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba, M., J. C. Reed, A. I. Prokhnevsky, E. J. Chapman, M. Mawassi, E. V. Koonin, J. C. Carrington, and V. V. Dolja. 2006. Diverse suppressors of RNA silencing enhance agroinfection by a viral replicon. Virology 3467-14. [DOI] [PubMed] [Google Scholar]
- 16.Collins, R. E., and X. Cheng. 2005. Structural domains in RNAi. FEBS Lett. 5795841-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox, E. H., and G. L. McLendon. 2000. Zinc-dependent protein folding. Curr. Opin. Chem. Biol. 4162-165. [DOI] [PubMed] [Google Scholar]
- 18.Deleris, A., J. Gallego-Bartolome, J. S. Bao, K. D. Kasschau, J. C. Carrington, and O. Voinnet. 2006. Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 31368-71. [DOI] [PubMed] [Google Scholar]
- 19.Dolja, V. V., K. L. Herndon, T. P. Pirone, and J. C. Carrington. 1993. Spontaneous mutagenesis of a plant potyvirus genome after insertion of a foreign gene. J. Virol. 675968-5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fenner, B. J., W. Goh, and J. Kwang. 2007. Dissection of double-stranded RNA binding protein B2 from betanodavirus. J. Virol. 815449-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández, A., S. Laín, and J. A. García. 1995. RNA helicase activity of the plum pox potyvirus CI protein expressed in Escherichia coli. Mapping of an RNA binding domain. Nucleic Acids Res. 231327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gamsjaeger, R., C. K. Liew, F. E. Loughlin, M. Crossley, and J. P. Mackay. 2007. Sticky fingers: zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 3263-70. [DOI] [PubMed] [Google Scholar]
- 23.Guo, D. Y., A. Merits, and M. Saarma. 1999. Self-association and mapping of interaction domains of helper component-proteinase of potato A potyvirus. J. Gen. Virol. 801127-1131. [DOI] [PubMed] [Google Scholar]
- 24.Guo, H. S., J. J. López-Moya, and J. A. García. 1998. Susceptibility to recombination rearrangements of a chimeric plum pox potyvirus genome after insertion of a foreign gene. Virus Res. 57195-207. [DOI] [PubMed] [Google Scholar]
- 25.Haasnoot, J., W. de Vries, E. J. Geutjes, M. Prins, P. de Haan, and B. Berkhout. 2007. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog. 3e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton, A. J., and D. C. Baulcombe. 1999. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286950-952. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton, C. M., A. Frary, C. Lewis, and S. D. Tanksley. 1996. Stable transfer of intact high molecular weight DNA into plant chromosomes. Proc. Natl. Acad. Sci. USA 939975-9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammarström, M., N. Hellgren, S. van den Berg, H. Berglund, and T. Härd. 2002. Rapid screening for improved solubility of small human proteins produced as fusion proteins in Escherichia coli. Protein Sci. 11313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haseloff, J., K. R. Siemering, D. C. Prasher, and S. Hodge. 1997. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 942122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herlitze, S., and M. Koenen. 1990. A general and rapid mutagenesis method using polymerase chain reaction. Gene 91143-147. [DOI] [PubMed] [Google Scholar]
- 31.Hu, C. D., and T. K. Kerppola. 2003. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat. Biotechnol. 21539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen, D., G. Martín, L. Velasco, P. Gómez, E. Segundo, L. Ruiz, and I. M. Cuadrado. 2005. Absence of a coding region for the helper component-proteinase in the genome of cucumber vein yellowing virus, a whitefly-transmitted member of the Potyviridae. Arch. Virol. 1501439-1447. [DOI] [PubMed] [Google Scholar]
- 33.Kasschau, K. D., and J. C. Carrington. 1998. A counterdefensive strategy of plant viruses: suppression of posttranscriptional gene silencing. Cell 95461-470. [DOI] [PubMed] [Google Scholar]
- 34.Kasschau, K. D., and J. C. Carrington. 2001. Long-distance movement and replication maintenance functions correlate with silencing suppression activity of potyviral HC-Pro. Virology 28571-81. [DOI] [PubMed] [Google Scholar]
- 35.Keese, P. K., and A. Gibbs. 1992. Origins of genes: “big bang” or continuous creation? Proc. Natl. Acad. Sci. USA 899489-9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klug, A., and D. Rhodes. 1987. ‘Zinc fingers’: a novel protein motif for nucleic acid recognition. Trends Biochem. Sci. 12464-469. [Google Scholar]
- 37.Lakatos, L., T. Csorba, V. Pantaleo, E. J. Chapman, J. C. Carrington, Y. P. Liu, V. V. Dolja, L. F. Calvino, J. J. López-Moya, and J. Burgyán. 2006. Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J. 252768-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lakatos, L., G. Szittya, D. Silhavy, and J. Burgyan. 2004. Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J. 23876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, F., and S. W. Ding. 2006. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu. Rev. Microbiol. 60503-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lingel, A., B. Simon, E. Izaurralde, and M. Sattler. 2005. The structure of the flock house virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition. EMBO Rep. 61149-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Moya, J. J., and J. A. García. 1999. Potyviruses (Potyviridae), p. 1369-1375. In R. G. Webster and A. Granoff (ed.), Encyclopedia of virology, 2nd ed. Academic Press Ltd., London, United Kingdom.
- 42.Lu, R., I. Malcuit, P. Moffett, M. T. Ruiz, J. Peart, A. J. Wu, J. P. Rathjen, A. Bendahmane, L. Day, and D. C. Baulcombe. 2003. High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 225690-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackay, J. P., and M. Crossley. 1998. Zinc fingers are sticking together. Trends Biochem. Sci. 231-4. [DOI] [PubMed] [Google Scholar]
- 44.Meng, C. Y., J. Chen, J. R. Peng, and S. M. Wong. 2006. Host-induced avirulence of hibiscus chlorotic ringspot virus mutants correlates with reduced gene-silencing suppression activity. J. Gen. Virol. 87451-459. [DOI] [PubMed] [Google Scholar]
- 45.Mérai, Z., Z. Kerényi, S. Kertész, M. Magna, L. Lakatos, and D. Silhavy. 2006. Double-stranded RNA binding may be a general plant RNA viral strategy to suppress RNA silencing. J. Virol. 805747-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mérai, Z., Z. Kerényi, A. Molnár, E. Barta, A. Válóczi, G. Bisztray, Z. Havelda, J. Burgyán, and D. Silhavy. 2005. Aureusvirus P14 is an efficient RNA silencing suppressor that binds double-stranded RNAs without size specificity. J. Virol. 797217-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moissiard, G., and O. Voinnet. 2006. RNA silencing of host transcripts by cauliflower mosaic virus requires coordinated action of the four Arabidopsis Dicer-like proteins. Proc. Natl. Acad. Sci. USA 10319593-19598. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Omarov, R., K. Sparks, L. Smith, J. Zindovic, and H. B. Scholthof. 2006. Biological relevance of a stable biochemical interaction between the tombusvirus-encoded p19 and short interfering RNAs. J. Virol. 803000-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Omarov, R. T., J. J. Ciomperlik, and H. B. Scholthof. 2007. RNAi-associated ssRNA-specific ribonucleases in Tombusvirus P19 mutant-infected plants and evidence for a discrete siRNA-containing effector complex. Proc. Natl. Acad. Sci. USA 1041714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pantaleo, V., G. Szittya, and J. Burgyan. 2007. Molecular bases of viral RNA targeting by viral small interfering RNA-programmed RISC. J. Virol. 813797-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Payre, F., P. Buono, N. Vanzo, and A. Vincent. 1997. Two types of zinc fingers are required for dimerization of the serendipity delta transcriptional activator. Mol. Cell. Biol. 173137-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pazhouhandeh, M., M. Dieterle, K. Marrocco, E. Lechner, B. Berry, V. Brault, O. Hemmer, T. Kretsch, K. E. Richards, P. Genschik, and V. Ziegler-Graff. 2006. F-box-like domain in the polerovirus protein P0 is required for silencing suppressor function. Proc. Natl. Acad. Sci. USA 1031994-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillai, R. S., S. N. Bhattacharyya, and W. Filipowicz. 2007. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 17118-126. [DOI] [PubMed] [Google Scholar]
- 54.Plisson, C., M. Drucker, S. Blanc, S. German-Retana, O. Le Gall, D. Thomas, and P. Bron. 2003. Structural characterization of HC-Pro, a plant virus multifunctional protein. J. Biol. Chem. 27823753-23761. [DOI] [PubMed] [Google Scholar]
- 55.Pruss, G., X. Ge, X. M. Shi, J. C. Carrington, and V. B. Vance. 1997. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qu, F., and T. J. Morris. 2005. Suppressors of RNA silencing encoded by plant viruses and their role in viral infections. FEBS Lett. 5795958-5964. [DOI] [PubMed] [Google Scholar]
- 57.Qu, F., T. Ren, and T. J. Morris. 2003. The coat protein of turnip crinkle virus suppresses posttranscriptional gene silencing at an early initiation step. J. Virol. 77511-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rakitina, D. V., N. E. Yelina, and N. O. Kalinina. 2006. Zinc ions stimulate the cooperative RNA binding of hordeiviral γb protein. FEBS Lett. 5805077-5083. [DOI] [PubMed] [Google Scholar]
- 59.Robaglia, C., M. Durand-Tardif, M. Tronchet, G. Boudazin, S. Astier-Manifacier, and F. Casse-Delbart. 1989. Nucleotide sequence of potato virus Y (N strain) genomic RNA. J. Gen. Virol. 70935-947. [DOI] [PubMed] [Google Scholar]
- 60.Rohila, J. S., M. Chen, R. Cerny, and M. E. Fromm. 2004. Improved tandem affinity purification tag and methods for isolation of protein heterocomplexes from plants. Plant J. 38172-181. [DOI] [PubMed] [Google Scholar]
- 61.Roth, B. M., G. J. Pruss, and V. B. Vance. 2004. Plant viral suppressors of RNA silencing. Virus Res. 10297-108. [DOI] [PubMed] [Google Scholar]
- 62.Ruiz-Ferrer, V., J. Boskovic, C. Alfonso, G. Rivas, O. Llorca, D. López-Abella, and J. J. López-Moya. 2005. Structural analysis of tobacco etch potyvirus HC-Pro oligomers involved in aphid transmission. J. Virol. 793758-3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwach, F., F. E. Vaistij, L. Jones, and D. C. Baulcombe. 2005. An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol. 1381842-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi, X. M., H. Miller, J. Verchot, J. C. Carrington, and V. B. Vance. 1997. Mutations in the region encoding the central domain of helper component-proteinase (HC-Pro) eliminate potato virus X/potyviral synergism. Virology 23135-42. [DOI] [PubMed] [Google Scholar]
- 65.Silhavy, D., and J. Burgyán. 2004. Effects and side-effects of viral RNA silencing suppressors on short RNAs. Trends Plant Sci. 976-83. [DOI] [PubMed] [Google Scholar]
- 66.Sontheimer, E. J. 2005. Assembly and function of RNA silencing complexes. Nat. Rev. Mol. Cell. Biol. 6127-128. [DOI] [PubMed] [Google Scholar]
- 67.Stenger, D. C., R. French, and F. E. Gildow. 2005. Complete deletion of Wheat streak mosaic virus HC-Pro: a null mutant is viable for systemic infection. J. Virol. 7912077-12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szittya, G., A. Molnar, D. Silhavy, C. Hornyik, and J. Burgyán. 2002. Short defective interfering RNAs of tombusviruses are not targeted but trigger post-transcriptional gene silencing against their helper virus. Plant Cell 14359-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas, C. L., V. Leh, C. Lederer, and A. J. Maule. 2003. Turnip crinkle virus coat protein mediates suppression of RNA silencing in Nicotiana benthamiana. Virology 30633-41. [DOI] [PubMed] [Google Scholar]
- 70.Thornbury, D. W., G. M. Hellmann, R. E. Rhoads, and T. P. Pirone. 1985. Purification and characterization of potyvirus helper component. Virology 144260-267. [DOI] [PubMed] [Google Scholar]
- 71.Trinks, D., R. Rajeswaran, P. V. Shivaprasad, R. Akbergenov, E. J. Oakeley, K. Veluthambi, T. Hohn, and M. M. Pooggin. 2005. Suppression of RNA silencing by a geminivirus nuclear protein, AC2, correlates with transactivation of host genes. J. Virol. 792517-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urcuqui-Inchima, S., I. G. Maia, P. Arruda, A. L. Haenni, and F. Bernardi. 2000. Deletion mapping of the potyviral helper component-proteinase reveals two regions involved in RNA binding. Virology 268104-111. [DOI] [PubMed] [Google Scholar]
- 73.Urcuqui-Inchima, S., I. G. Maya, G. Drugeon, A. L. Haenni, and F. Bernardi. 1999. Effect of mutations within the Cys-rich motif of potyvirus helper component-proteinase on self-interaction. J. Gen. Virol. 802809-2812. [DOI] [PubMed] [Google Scholar]
- 74.Urcuqui-Inchima, S., J. Walter, G. Drugeon, S. German-Retana, A. L. Haenni, T. Candresse, F. Bernardi, and O. Le Gall. 1999. Potyvirus helper component-proteinase self-interaction in the yeast two-hybrid system and delineation of the interaction domain involved. Virology 25895-99. [DOI] [PubMed] [Google Scholar]
- 75.Valli, A., J. J. López-Moya, and J. A. García. 2007. Recombination and gene duplication in the evolutionary diversification of P1 proteins in the family Potyviridae. J. Gen. Virol. 881016-1028. [DOI] [PubMed] [Google Scholar]
- 76.Valli, A., A. M. Martín-Hernández, J. J. López-Moya, and J. A. García. 2006. RNA silencing suppression by a second copy of the P1 serine protease of Cucumber vein yellowing ipomovirus (CVYV), a member of the family Potyviridae that lacks the cysteine protease HCPro. J. Virol. 8010055-10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Rij, R. P., M. C. Saleh, B. Berry, C. Foo, A. Houk, C. Antoniewski, and R. Andino. 2006. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 202985-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Wezel, R., X. L. Dong, H. T. Liu, P. Tien, J. Stanley, and Y. G. Hong. 2002. Mutation of three cysteine residues in Tomato yellow leaf curl virus-China C2 protein causes dysfunction in pathogenesis and posttranscriptional gene-silencing suppression. Mol. Plant-Microbe Interact. 15203-208. [DOI] [PubMed] [Google Scholar]
- 79.Vargason, J. M., G. Szittya, J. Burgyan, and T. M. Tanaka Hall. 2003. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115799-811. [DOI] [PubMed] [Google Scholar]
- 80.Varrelmann, M., E. Maiss, R. Pilot, and L. Palkovics. 2007. Use of pentapeptide-insertion scanning mutagenesis for functional mapping of the plum pox virus helper component proteinase suppressor of gene silencing. J. Gen. Virol. 881005-1015. [DOI] [PubMed] [Google Scholar]
- 81.Vazquez, F. 2006. Arabidopsis endogenous small RNAs: highways and byways. Trends Plant Sci. 11460-468. [DOI] [PubMed] [Google Scholar]
- 82.Voinnet, O. 2005. Induction and suppression of RNA silencing: insights from viral infections. Nat. Rev. Genet. 6206-220. [DOI] [PubMed] [Google Scholar]
- 83.Voinnet, O., Y. M. Pinto, and D. C. Baulcombe. 1999. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 9614147-14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Voinnet, O., S. Rivas, P. Mestre, and D. Baulcombe. 2003. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 33949-956. [DOI] [PubMed] [Google Scholar]
- 85.Wang, H., K. J. Buckley, X. J. Yang, R. C. Buchmann, and D. M. Bisaro. 2005. Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J. Virol. 797410-7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie, Q., and H. S. Guo. 2006. Systemic antiviral silencing in plants. Virus Res. 1181-6. [DOI] [PubMed] [Google Scholar]
- 87.Ye, K., L. Malinina, and D. J. Patel. 2003. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 426874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye, K. Q., and D. J. Patel. 2005. RNA silencing suppressor p21 of beet yellows virus forms an RNA binding octameric ring structure. Structure 131375-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang, X., Y.-R. Yuan, Y. Pei, S.-S. Lin, T. Tuschl, D. J. Patel, and N.-H. Chua. 2006. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 203255-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou, Z., M. Dell'orco, P. Saldarelli, C. Turturo, A. Minafra, and G. P. Martelli. 2006. Identification of an RNA-silencing suppressor in the genome of Grapevine virus A. J. Gen. Virol. 872387-2395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.