Much is known about both microbial virulence and host defense mechanisms, and yet how they interact to produce different clinical outcomes is poorly appreciated. Here I review evidence that the clinical manifestations of gram-negative bacterial diseases can be influenced by how and where animal hosts sense the major gram-negative bacterial “signal” molecule, the cell wall lipopolysaccharide (LPS).
Clinicians know LPS best by the inaccurate name “endotoxin.” In fact, animals sense and react to LPS molecules that are on the bacterial surface, or released from it, and the ensuing inflammatory reaction is usually protective rather than harmful. Whereas the polysaccharide component of LPS differs between strains, the lipid A moiety retains certain features in almost all gram-negative bacteria, and it is this semiconserved structure that animals can sense to detect the presence of many gram-negative bacteria in their tissues (Fig. 1). Genes that encode the host sensory mechanism, a complex that includes both MD-2 (LPS binding) and Toll-like receptor 4 (TLR4; signal transduction), have been found in almost all studied vertebrate genomes (110). Intracellular signaling pathways downstream of TLR4 mediate both pro- and anti-inflammatory responses to infection.
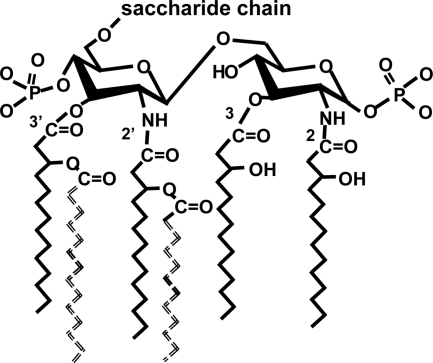
FIG. 1.
Structure of E. coli lipid A. The diglucosamine lipid A backbone has phosphates at positions 1 and 4′ and two molecules of 3-hydroxymyristate attached directly to each glucosamine. The hydroxyl groups of the hydroxymyristates at positions 2′ and 3′ are substituted by laurate (C12) and myristate (C14), respectively (shown as dashed lines in the drawing). Palmitate (C16) is sometimes found as a secondary acyl chain, and the primary (glucosamine-linked) acyl chains may have 12 carbons. This structure is sensed by the MD-2-TLR4 receptor. LPSs from different gram-negative bacteria may have more or fewer acyl chains, longer acyl chains, branched acyl chains, unsaturated acyl chains, only one phosphate, or other modifications. Each of these structures is less stimulatory to cells that express MD-2-TLR4 than is hexaacyl LPS. On the other hand, the two secondary (“piggyback”) chains can be attached to different 3-hydroxymyristate residues (for example, at 2 and 2′) without diminishing recognition by MD-2-TLR4.
A prominent role for host-LPS interactions in the pathogenesis of gram-negative bacterial diseases is plausible because LPS is the gram-negative bacterial molecule that vertebrates seem to detect most sensitively. As was first shown by Darveau and colleagues, intact Escherichia coli cells do not stimulate human endothelial cells when their LPS lacks one of the six fatty acyl chains that are attached to lipid A (124). Others have extended this finding to different gram-negative bacteria and host cells, and there is now much evidence that recognizing lipid A (LPS) is the most sensitive and specific mechanism by which animals detect gram-negative bacteria (10, 28, 30, 68, 72). On the other hand, gram-negative bacteria can also be sensed by other host receptors, and important innate defenses (such as complement activation) are not activated by lipid A. Moreover, not all gram-negative bacteria make LPS that can be recognized by MD-2-TLR4.
Many of the bacteria that can be sensed by MD-2-TLR4 are commensal or pathogenic aerobes that live on the mucosae of the upper respiratory or gastrointestinal (GI) tracts. In immunocompetent hosts, these bacteria usually elicit local inflammation without invading the bloodstream. One may thus hypothesize that the MD-2-TLR4 defense limits bacterial invasion to the submucosa, preventing systemic dissemination (91). In contrast, many gram-negative bacteria that cause systemic infections in humans produce LPS molecules that are poorly sensed by MD-2-TLR4, and so they escape recognition by this key defense mechanism. According to this schema, both the presence and the absence of TLR4-activating LPS can contribute to disease expression. The evidence reviewed below generally supports this notion, yet prominent exceptions highlight its weaknesses as a guiding framework and suggest areas for future research.
A discussion of how animals recognize microbes is given as a background and rationale for the description of clinical associations that follows. Here “disease” is used to denote infections that in some way damage the host; “commensal” refers to bacteria that can infect for long periods without inducing disease (normal microbiome); and “pathogens” are microbes that cause or elicit damage (99).
MD-2-TLR4 SENSES MUCOSAL COMMENSALS AND PATHOGENS
Since its formulation almost 20 years ago (61), a cornerstone of the innate immunity concept has been the idea that animals recognize microbes through “pattern recognition” receptors (PRRs) that sense “pathogen-associated molecular patterns” (PAMPs). Medzhitov and Janeway later suggested that “PRRs do not distinguish between microorganisms that colonize the host (pathogenic or commensal) and microorganisms that evolved to occupy habitats other than the host, because all of them produce PAMPs” (80). Other authors have endorsed the notion that animals can sense distinct molecular patterns that are found only in pathogens (113).
In another view, PRRs recognize conserved molecules (“patterns”) produced by microbes that influenced the evolution of the innate sensory mechanism. Members of the best-studied family of PRRs, the Toll-like receptors, have been found in all vertebrates studied, with minimal differences in molecular recognition between animals as phylogenetically distant as rodents and humans (110). Although the microorganisms that shaped the evolution of these ancient sensors are obviously not known, it is doubtful that they were the same pathogens that afflict contemporary humans. On the other hand, we can sense many of the microbes with which we normally coexist, our commensals. According to this notion, at one time these microbes or their ancestors were sufficiently pathogenic to have influenced the evolution of the innate sensory apparatus, or perhaps they benefited from interacting with vertebrate sensors (79). We sense pathogens today when they produce molecules that make them “look like” these microbes.
One way to test these ideas is to consider how innate immune sensors recognize specific kinds of microorganisms. Gram-negative bacteria are well suited for such a test, because lipid A has not been highly conserved. The structures of the lipid A moieties of the LPSs produced by over 40 gram-negative bacteria have been determined, and most of these LPSs have been tested for their ability to stimulate animal cells. Moreover, the structural requirements for LPS recognition by vertebrates have been defined using both natural and synthetic lipid A agonists (66). Knowing which LPSs can be recognized by MD-2-TLR4 should reveal a lot about how animals sense gram-negative bacteria.
If one matches the published lipid A structures with the ability of their LPSs to be recognized by MD-2-TLR4 (i.e., to activate inflammatory responses in immune cells), the results confirm conclusions that were reached in the 1980s by testing synthetic lipid A analogs (66, 91). Whereas remarkably diverse lipid A structures are found in the LPSs produced by different gram-negative bacteria, the requirements for maximal activation of animal cells are rather restricted. MD-2-TLR4 best senses a bis-phosphorylated diglucosamine to which are attached six saturated fatty acyl chains with lengths of 12 or 14 (occasionally 16) carbons (Fig. 1). For the following discussion, LPS molecules that have this structure are called “hexaacyl LPSs.”
It is evident from Table 1 that few of the gram-negative bacteria that have natural habitats in soil and water, or even in the anaerobic microbiome of the GI tract, produce LPS that has hexaacyl LPS (91). On the other hand, hexaacyl LPS is produced by most of the aerobic or facultatively anaerobic gram-negative bacteria that can inhabit the mucosal surfaces of the respiratory tract and gut. Importantly, the LPSs (lipid As) produced by commensals such as Klebsiella pneumoniae, Enterobacter cloacae, and E. coli are just as “visible” to MD2-TLR4 as are those of mucosal pathogens such as Shigella, Haemophilus, and Neisseria spp. These lipid As have closely similar structures, and they elicit the same inflammatory responses with similar potencies. Clearly, the MD-2-TLR4 receptor does not discriminate between mucosal commensals and pathogens (56, 91).
TABLE 1.
Gram-negative bacteria that produce LPS with predominantly hexaacyl (see text for definition) or nonhexaacyl lipid A structuresa
| Strain(s) |
|---|
| Hexaacyl lipid A structures |
| Aerobes or facultative anaerobes |
| Mucosal habitat |
| Escherichia coli |
| Klebsiella pneumoniae |
| Enterobacter cloacae |
| Serratia marcescens |
| Proteus mirabilis |
| Providencia rettgeri |
| Shigella sonnei, S. flexnerid |
| Haemophilus influenzae |
| Neisseria gonorrhoeae, N. meningitidis |
| Bordetella bronchiseptica |
| Vibrio cholerae O1 |
| Campylobacter sp. |
| Aeromonas salmonicida |
| Plesiomonas shigelloides |
| Actinobacillus actinomycetemcomitans |
| Soil, water habitat |
| Stenotrophomonas maltophilia |
| Burkholderia cenocepacia (some strains) |
| (Also E. coli, Aeromonas spp., Plesiomonas spp., others) |
| Strict anaerobes |
| None |
| Nonhexaacyl lipid A structures |
| Aerobes or facultative anaerobes |
| Mucosal habitat |
| Moraxella catarrhalis |
| Bordetella pertussis |
| Helicobacter pylori |
| Chlamydophila trachomatis |
| Vibrio cholerae O139 |
| Yersinia enterocolitica |
| Salmonella enterica serovar Typhimuriume |
| Vibrio vulnificusb |
| Animal habitat |
| Brucella abortus |
| Yersinia pestis |
| Francisella tularensis |
| Legionella pneumophila |
| Capnocytophaga canimorsusc |
| Bartonella henselae, B. quintana |
| Leptospira interrogans |
| Soil, water, or plant habitat |
| Leptospira interrogans |
| Pseudomonas aeruginosa |
| Burkholderia cenocepacia (some strains) |
| Burkholderia pseudomalleib |
| Acinetobacter radioresistens |
| Enterobacter agglomerans |
| Chromobacterium violaceum |
| Erwinia carotovora |
| Rhodobacter sphaeroides |
| Rhizobium etli |
| Sinorhizobium sp. |
| Xanthomonas campestris |
| Strict anaerobes |
| Bacteroides fragilis |
| Prevotella intermedia |
| Porphyromonas gingivalis |
Lipid A structure not published; LPS is at least 10-fold less potent than E. coli LPS in bioassays.
Data based on C. ochracea LPS results.
Both hexaacyl and pentaacyl structures have been reported (see text).
In the laboratory, Salmonella LPS may be hexaacyl; heptaacyl LPS was found in S. enterica serovar Typhimurium isolated from macrophages (44).
If our most robust system for sensing aerobic gram-negative bacteria mainly detects ones that can live on our mucosal surfaces, what adaptive role might this have served? One clue is the clinical observation that most mucosal diseases remain mucosal. In other words, mucosal inflammation can be vigorous, and yet systemic dissemination infrequently occurs. The MD-2-TLR4-based response may be disease inducing (in the form of inflammation-associated mucosal damage) and yet protective (preventing bacteremia). It is noteworthy that very little TLR4 has been found in the normal GI epithelium, and it is not expressed on lumenal surfaces except perhaps in colonic crypts (1, 5, 17, 96). Similarly, MD-2 is minimally expressed by human airway epithelial cells (62); TLR4 is produced by bladder epithelial cells but not by renal ones (5). The LPS receptor complex assembles mainly on the defense cells (macrophages, dendritic cells, mast cells, and neutrophils) that patrol the respiratory and gastrointestinal submucosae. This defense evidently does not sense gram-negative aerobes on epithelial surfaces, but it seems to detect and contain them once they transit the epithelium and reach submucosal spaces. In an experimental model of dextran sulfate sodium colitis, for example, mice that lacked TLR4 had diminished ability to prevent bacterial translocation to the mesenteric lymph nodes (40) and were more likely to succumb to their commensal microbes (106). There is also a backup system for preventing systemic dissemination: the hepatic and splenic clearance of gram-negative bacteria from the blood involves hexaacyl LPS recognition by, and activation of, Kupffer cells and splenic macrophages (22, 141). The phagocytes that express TLR4 thus seem to guard our most heavily populated mucosal barriers, stopping microbial invaders before they can get into the bloodstream or, when this fails, trapping and killing them in the major organs of hemofiltration. In addition, there are numerous mechanisms for preventing hexaacyl LPS sensing in the bloodstream, including binding LPS to circulating lipoproteins, LPS neutralization by circulating proteins (including bactericidal permeability-increasing protein and both natural and acquired antibodies), and efficient LPS clearance and inactivation by the liver and spleen (76, 86, 118). At least in theory, these mechanisms should minimize systemic inflammatory responses to transient bacteremia and endotoxemia (86).
Why might most mucosal gram-negative aerobes produce hexaacyl LPS? As has been noted by many others, having lipid A with six or more acyl chains can protect bacteria from the antibacterial molecules in mucosal secretions (e.g., bile salts and antimicrobial peptides) (19, 81, 115, 127, 155). These ancient host defenses may have shaped the composition of the normal aerobic mucosal microbiome. Bacteria that make nonhexaacyl LPS can also live on mucosal surfaces, but they often have special properties: Helicobacter pylori thrives in a special gastric niche, for example, and Chlamydophila LPS is packed in elementary bodies. It is also possible that host recognition is beneficial to mucosal aerobic gram-negative bacteria, favoring colonization by bacteria that can be sensed by MD-2-TLR4 (79). Conceivably the mucosal inflammatory response elicited by bacteria that transit the epithelium provides beneficial nutrients (iron?) or signals (catecholamines, cytokines?) that favor their persistence on the mucosa. Some degree of “beneficial mutualism” may be important for sustaining this host-microbe relationship, as it is for others (55). Indeed, evidence that certain host molecules (heme, hyaluronan, saturated fatty acids, others) (37, 134) can activate cells via TLR4 suggests that this receptor may have important host functions in addition to sensing gram-negative bacteria.
LPS recognition by MD-2-TLR4 may thus be viewed as an innate immune mechanism that protects vertebrates from the aerobic gram-negative bacteria that inhabit their mucosal surfaces throughout life. MD-2-TLR4 also recognizes some pathogens, but they are ones that, like most mucosal commensal gram-negative aerobes, produce hexaacyl LPS.
HOST RECOGNITION OF LPS AND THE PATHOGENESIS OF GRAM-NEGATIVE BACTERIAL DISEASES
Bacteria that produce hexaacyl LPS.
When mucosal pathogens that produce hexaacyl LPS cause damage to immunocompetent hosts, they generally induce either little or no inflammatory response (for example, secretory diarrhea) or local (sub)mucosal inflammation—the latter is clinically recognizable as pharyngitis, bronchitis, or cystitis (5) and as inflammatory diarrhea (enterocolitis). It is possible to view these local responses as representing innate immune success, since systemic dissemination rarely occurs. Here LPS is a virulence factor, since it elicits local inflammatory responses that may damage the host, and yet it also provides a signal that enables the host to prevent further invasion and systemic spread. It is interesting in this regard that Campylobacter jejuni produces hexaacyl LPS that has 16 carbon fatty acyl chains, is less stimulatory than E. coli LPS in various bioassays (84), and elicits less severe colonic inflammation than does Shigella flexneri or S. sonnei, species that produce hexaacyl LPS (6, 28). With colitis due to Campylobacter and most Shigella species (S. dysenteriae is an exception), bacteremia is unusual despite extensive mucosal injury. Nontypeable Haemophilus influenzae can cause bronchitis and can survive within adenoidal macrophages in asymptomatic children (38), and yet bacteremia is rarely noted. Salmonella enterica serovar Typhimurium elicits vigorous neutrophil recruitment to the gut mucosa; engineered mutants that produced pentaacyl LPS were less proinflammatory, but they were also found to be defective in elements of the type 3 secretion system that promotes pathogenicity (148). The latter example illustrates a well-known problem for students of microbial pathogenesis that is also a cautionary note for the present discussion: distinguishing the roles of individual bacterial molecules in disease processes is difficult when they are expressed or regulated in concert (137).
Successful innate immunity also prevents aerobic commensal mucosal bacteria such as Klebsiella spp. and (nonpathogenic) E. coli from causing disease in normal hosts. This may be a routine job for the MD-2-TLR4 recognition system—commensal bacteria probably traverse the mucosal epithelium frequently, via microabrasions, M cells, interdigitating dendritic cells, and other routes, and yet disease rarely ensues. By mobilizing inflammatory responses within the submucosa, cells that express MD-2-TLR4 would help contain and destroy these bacteria. Although other elements of innate and acquired immunity (antibodies, complement, antimicrobial peptides, NK cells, iron sequestration, etc.) also help prevent systemic dissemination by commensal microbes, even in concert these mechanisms may fail: in today's hospitals, the gram-negative bacteria isolated most often from patients with nosocomial severe sepsis and septic shock are E. coli and Klebsiella and Enterobacter species. Identifying virulence factors that favor the pathogenicity of these “extraintestinal” commensals-pathogens has been difficult (112, 129, 136), in part because the diseases they cause are often associated with epithelial barrier disruption or obstructed drainage conduits (146), because antimicrobial chemotherapy has selected resistant strains, or because their hosts are immunocompromised in some way (89). Bloodstream infection with these bacteria is typically low-grade and intermittent (154).
Stenotrophomonas maltophilia is a respiratory tract colonizer and pathogen in vulnerable hosts. It produces hexaacyl LPS and elicits airway inflammation, but it was poorly invasive in a mouse model (147). Although it can cause serious nosocomial pneumonia in immunocompromised patients, bacteremia occurs infrequently in nonneutropenic patients with S. maltophilia pneumonia (3). These observations raise the possibility that, even in some compromised hosts, S. maltophilia may be confined to the airways and lung by TLR4-based inflammation. The most abundant lipid A species in a nonpathogenic Acinetobacter strain was nonhexacylated (70), and yet LPS preparations obtained from clinical isolates of Acinetobacter baumannii were potently proinflammatory toward human cells (34). Structural information on A. baumannii LPS is awaited with interest; the clinical import of its host-LPS interaction may be similar to that suggested for Stenotrophomonas spp.
A prominent exception to this pattern is Neisseria meningitidis, a mucosal colonizer that can cross the nasopharyngeal epithelium and invade the bloodstream without eliciting clinically apparent local inflammation. It then can infect vascular endothelial cells and perivascular tissues (51), grow to high density in the blood, and release large amounts of LPS-containing membrane blebs. How encapsulated, hexaacyl LPS-producing bacteria like N. meningitidis, Haemophilus influenzae type b, and E. coli K1 (in neonates) manage to invade and grow to high density in the blood is not known with certainty (128), but these colonizers-pathogens are clearly exceptions to the general pattern described above, and the contribution of the host-LPS interaction to disease pathogenesis is uncertain (see below). Producing hexaacyl LPS is necessary for N. gonorrhoeae to survive within mucosal epithelial cells (101), yet certain gonococcal strains can invade the bloodstream without inducing local inflammation (or, at least, symptoms), suggesting that they also can avoid recognition by submucosal phagocytes that express MD-2-TLR4. Sialylation of gonococcal LPS may contribute to this apparent camouflage, just as the saccharide composition of the N. meningitidis lipooligosaccharide seems to influence interactions with MD-2-TLR4 and several stages in meningococcal pathogenesis (100, 160).
Bacteria that produce nonhexaacyl LPS. (i) Systemic diseases in immunocompetent hosts.
In contrast to the mucosal commensals, colonizers, and pathogens, most systemic gram-negative pathogens do not produce hexaacyl lipid A. Many of them have habitats in soil, water, free-living amoebae, other vertebrates, or insects, and they often breech the body's epithelial defenses via nonmucosal routes (cuts, bites, inhalation, transport across conjunctival membranes) (91). Examples include Yersinia pestis, Francisella tularensis, Burkholderia pseudomallei, Legionella pneumophila, Bartonella species, and Leptospira species. Although each of these bacterial species has distinctive features that allow it to elicit injurious host responses, they share the ability to avoid recognition by MD-2-TLR4. Some may proliferate to high densities within the systemic compartment, reaching >1,000 CFU per ml of blood in severe cases (15, 114, 145, 153). Perhaps the most striking example is Y. pestis, which produces hexaacyl LPS when grown at 25°C (flea) and tetraacyl LPS at 37°C (mammalian host), an adaptation that likely accounts for its ability to cause high-grade bacteremia in mammals (83).
Alternative host sensory mechanisms have been identified for some of these bacteria. Mice can sense F. tularensis via TLR2/TLR6 (67, 75), for example. The tetraacyl LPS produced by Leptospira interrogans is sensed by TLR4 in rodents (143), its natural reservoir, but in humans the predominant Toll-like receptor for Leptospira LPS is TLR2 (92, 149). This observation highlights an interesting and important difference, first noted many years ago (47), between human and murine LPS recognition. Whereas human MD-2-TLR4 senses only hexaacyl lipid A, murine MD-2-TLR4 can recognize pentaacyl and even tetraacyl lipid A. This difference has been exploited in elegant studies of the molecular specificity of LPS recognition by MD-2 (reviewed in reference 81). Another example is Pseudomonas aeruginosa, which produces a pentaacylated LPS that is sensed by murine respiratory epithelium via TLR4 and yet does not activate human respiratory epithelial cells (62, 122, 158). Coxiella burnettii is able to induce cytokine production and granuloma formation in mice in a TLR4-dependent manner (54), and yet its tetraacyl LPS is a very weak agonist for human cells (135) and its polysaccharide chain may mask other Coxiella TLR ligands (117). In contrast, producing tetraacyl or pentaacyl LPS renders many other gram-negative bacteria less proinflammatory even in mice (24, 68, 83). Unfortunately, these species-dependent differences prevent confident generalization from many murine models to their putative counterparts in human disease.
Some gram-negative bacteria that cause systemic disease are acquired by ingestion but they either possess (Brucella abortus, S. enterica serovar Typhimurium), or may adapt to produce (S. enterica serovar Typhimurium, Yersinia enterocolitica), nonhexaacyl LPS. Equipped with mechanisms that facilitate invasion and an intracellular lifestyle, they make their way across the GI mucosa into the systemic compartment, often translocating in the terminal ileum without provoking clinically apparent inflammation in the bowel wall. They then establish residence within host cells, particularly in monocytes/macrophages in the liver, spleen, and lymph nodes. Although they may also circulate in the blood for many days, often within phagocytes, these intracellular pathogens infrequently cause severe sepsis or shock (144). Brucella LPS is heptaacylated, with unusual fatty acyl chains, and F. tularensis (which also can be acquired by ingestion) makes a complex array of lipid A molecules (116) that are poorly sensed by Toll receptors (50). Y. enterocolitica produces hexaacyl LPS at low temperatures but adapts to growth at 37°C by making almost exclusively tetraacyl (108) or pentaacyl (8) LPS; its most common clinical presentation is mesenteric adenitis. The Vi capsule seems to prevent recognition of S. enterica serovar Typhi by shielding the LPS and flagella from TLR4 and TLR5, respectively; very little neutrophilic infiltration of the mucosa has been found in patients with typhoid fever (105). Moraxella catarrhalis (heptaacyl LPS) and Bordetella pertussis (pentaacyl) can establish residence within adenoidal macrophages with little or no accompanying inflammatory reaction (53). In all of these host-pathogen relationships, the absence of inflammation-inducing (hexaacyl) LPS could play a permissive role in disease pathogenesis. A contribution to Salmonella pathogenesis is less certain: hyperacylation of lipid A to seven acyl chains (regulated by the phoP-phoQ system) was reported to favor Salmonella persistence in monocytes and reduce recognition by MD-2-TLR4 (44, 49), but the absence of the enzyme that adds the seventh acyl chain (pagP) did not reduce S. enterica serovar Typhimurium virulence in mice (7). Other adaptations may also be important for preventing host recognition of salmonellae, including a drop in flagellin production that reduces sensing via TLR5 (43).
The mucosal gram-negative aerobes and facultative anaerobes are greatly outnumbered by the gram-negative anaerobes that also inhabit the upper respiratory and GI tracts. These bacteria (those studied include Bacteroides, Porphyromonas, and Prevotella spp.) produce nonhexaacyl LPS, and they infrequently cause disease in normal hosts. One possible exception is Fusobacterium necrophorum, which can invade from the oropharynx to cause septic thrombophlebitis of the jugular vein (Lemierre's syndrome); its lipid A structure has not been reported. Tissue invasion by strict anaerobes may be limited most effectively by high oxygen tension (redox potential), although some of these bacteria may be able to induce inflammatory defenses via TLR2 (25). It is noteworthy that, at least in vitro, Bacteroides and Porphyromonas LPS can block the ability of hexaacyl LPS to stimulate cells via MD-2-TLR4 (20, 29). Whether this competition between hexaacyl and nonhexacyl LPS can modulate TLR4-based sensing in the (sub)mucosae of the respiratory tract or intestine is not known.
(ii) Systemic diseases in abnormal hosts.
This category could include two uncommon but often lethal pathogens, Capnocytophaga canimorsus and Vibrio vulnificus. Although the structure of C. canimorsus LPS has not been solved, its cells have low inflammatory potency in vitro and a nonhexaacyl LPS can be anticipated (39). Whereas many patients who have developed overwhelming bacteremia with this canine oral commensal have had inadequate splenic function or advanced liver disease, others have evidently been immunocompetent. Vibrio vulnificus bacteremia occurs most often in individuals whose clearance mechanisms are compromised by hepatic cirrhosis; increased iron availability may also be important. Although its lipid A structure has also not been published, it was at least 10-fold less potent than E. coli LPS in some bioassays (102). Both V. vulnificus and C. canimorsus can grow to high density in vivo, particularly in splenectomized individuals; it is not unusual to find them on microscopical examination of peripheral blood smears from infected patients.
(iii) Local diseases in abnormal hosts.
As noted above, bacteria that have natural habitats in water, plants, or soil can cause disease in special hosts. Environmental isolates of Pseudomonas aeruginosa produce pentaacyl LPS, but this bacterium can adapt to the tracheobronchial tree of children with cystic fibrosis by making hexaacyl or even heptaacyl LPS (33); the extent to which this adaptation influences inflammatory responses in the airways is not known. Tissue P. aeruginosa isolates have also had pentaacyl LPS (32). Some local tissue infections caused by P. aeruginosa (for example, invasive external otitis) can be associated with minimal inflammatory reactions, as would be anticipated if the bacteria produced pentaacyl LPS and lacked other potent proinflammatory molecules. In some settings the inflammatory response to P. aeruginosa can be more vigorous; although the basis for this difference is not well understood, many strains express a type 3 secretion system that can deliver toxic proteins to host cells (58) and flagellated strains may be sensed by TLR2 and TLR5 (2). Burkholderia cepacia is a plant pathogen that can also colonize the airways of humans with cystic fibrosis. It has nine genomic species that differ in their ability to cause a life-threatening pneumonia (“cepacia syndrome”) and in the lipid A structures of their LPSs. Earlier investigations found that B. cepacia LPS was as stimulatory to human monocytes as E. coli LPS (159), and the LPS from one genomovar I strain was reported to have pentaacyl lipid A (120). More recently, De Soyza et al. found that the proinflammatory potency of strains from different genomovars was mimicked by that of their isolated LPSs; a representative ET-12 (pneumonia-associated) B. cenocepacia strain made hexaacyl LPS, whereas LPS from a less proinflammatory strain was pentaacyl (27).
Summary.
For most bacteria that produce hexaacyl LPS, LPS recognition via MD-2-TLR4 seems to contribute to disease within and beneath the mucosae of the GI, respiratory, and urinary tracts. One may infer that this defense also helps prevent dissemination into and within the systemic compartment (also see references 5 and 81). For bacteria that do not produce hexaacyl LPS, failure to activate host defenses via MD-2-TLR4 may allow or favor growth and dissemination in vivo.
Most of the experimental evidence for these generalizations has come from studies of bacterial mutants in animal models of disease. Since LPS-altering mutations can be associated with changes in other (possibly unrecognized) bacterial virulence determinants (124, 148, 156), reaching definitive conclusions regarding the role of LPS recognition per se may not be possible. Nonetheless, production of hexaacyl lipid A was associated with the ability of both Shigella flexneri and Salmonella enterica serovar Typhimurium to elicit gut-damaging inflammatory responses—in other words, to be pathogenic (28, 148). Moreover, S. enterica serovar Typhimurium bacteria mutated to produce pentaacyl LPS grew to high densities in vivo without causing death (68). In contrast, engineering a pathogenic strain of Yersinia pestis to produce hexaacyl instead of tetraacyl LPS rendered this otherwise lethal microbe avirulent—here the presence of hexaacyl LPS made Y. pestis recognizable by MD-2-TLR4 and thus very sensitive to killing by innate defenses, even when bacteria were injected subcutaneously or intravenously (83). This striking result is the most convincing evidence to date that the absence of hexaacyl LPS permits expression of other bacterial virulence determinants in vivo. Mutations in murine TLR4 also may not provide definitive tests of the LPS-MD-2-TLR4 interaction, but producing a functional TLR4 decreased susceptibility to bacteremia and/or systemic injury caused by several gram-negative bacteria that produce hexaacyl LPS (22, 23, 81, 93, 152). Conversely, the avirulent Y. pestis mutant that produced hexaacyl LPS was completely virulent in Tlr4−/− mice (83).
HOST-LPS RECOGNITION AND THE PATHOGENESIS OF SEVERE SEPSIS AND SHOCK
Different pathological pathways can lead to severe sepsis and septic shock, the often lethal states in which uninfected organs dysfunction and hypotension may supervene. For gram-negative commensals that have moved through a disrupted epithelium and multiplied within a submucosal tissue compartment, there is weak evidence that bacteremia or endotoxemia per se is the direct cause of distant organ dysfunction (88). In bacteremic patients, the occurrence of severe sepsis has been strongly related to the site of tissue infection rather than to the specific microbe cultured from the blood (14). Blood-borne commensal gram-negative bacteria may thus be a marker for uncontrolled tissue infection rather than the trigger for systemic inflammation. Harmful systemic responses are likely induced when tissue cells that express MD-2-TLR4 sense hexaacyl LPS and release inflammatory cytokines and other mediators, and yet how severe sepsis and shock develop is not understood (87). Since the dysfunctional organs typically remain anatomically normal and regain their function in survivors, neuroendocrine mechanisms may be dominant; important roles for blood-borne mediators, complement activation, and coagulopathy are also likely.
Y. enterocolitica produces hexaacyl LPS at low temperatures but not at 37°C (108). This bacterium has induced septic shock in humans, but the reported cases were those of individuals who had received infected blood that had been stored at 4°C; shock developed within 50 min of infusing the warmed blood (16). It is likely that the cold-enriched bacteria possessed hexaacyl LPS (108). The donors of the contaminated blood were asymptomatic, as would be expected if Y. enterocolitica LPS were weakly proinflammatory at 37°C and the bacteria were unable to grow to high density in the bloodstream.
A different route to severe sepsis is followed by many of the gram-negative bacteria that make nonhexaacyl LPS, including Y. pestis, B. pseudomallei, V. vulnificus, Leptospira sp., and C. canimorsus. Opposed by ineffective innate defenses, they grow within tissues and the bloodstream. Each of these bacteria likely has key virulence traits that enable it to injure the host in the absence of recognition by MD-2-TLR4. Shock may occur when there is massive bacterial growth within the blood and/or tissues (15, 119) or when inflammation is induced by non-TLR4-based recognition mechanisms (151). For example, a recent study using a mouse model found that host recognition via TLR2 contributed significantly to injury caused by B. pseudomallei (151). In contrast, other bacteria that make nonhexaacyl LPS may circulate for prolonged periods, often within phagocytes, without causing septic shock: examples include Bartonella quintana, S. enterica serovar Typhi and serovar Paratyphi, and Brucella sp. LPS recognition (or, here, the lack thereof) is obviously not the only determinant of pathogenicity, but it may influence the kind of host injury that occurs.
There is little doubt that the syndrome of fulminant meningococcemia is produced by the growth of N. meningitidis within the vasculature or that antibodies to the capsular polysaccharide can be protective, but the role played by the interaction of meningococcal LPS with MD-2-TLR4 is uncertain. In some individuals that lack protective antibodies, blood-borne meningococci can invade vascular endothelial cells, disrupt the endothelium, grow to high density in the blood, and release LPS-containing membrane blebs. The plasma concentrations of both endotoxin and meningococcal DNA copies have correlated strongly with mortality risk (13, 21, 97), raising the possibility that both LPS and non-LPS molecules trigger the host inflammatory response (60). Complement activation, a major feature of the syndrome, is evidently triggered by non-LPS components of meningococcal cells and blebs (126).
The meningococcus is thus an interesting exception to the general pattern noted above. Although N. meningitidis produces hexaacyl LPS, it both translocates from the nasopharynx into the bloodstream and multiplies within the systemic compartment. An effective TLR4-based defense should prevent each of these steps. It is possible that the host's ability to sense meningococcal LPS is prevented by the capsular polysaccharide and perhaps by sialylation of the LPS. It is also conceivable that a dominant anti-inflammatory (e.g., interleukin-10 [IL-10]) response to meningococcal LPS could prevent both symptoms and activation of host defenses while the bacteria multiply to high density within the blood (9, 150). Meningococci may also activate inflammatory responses via TLR2 and TLR9 (82), and yet these sensors also do not prevent massive bacterial growth in some patients. Preliminary evidence suggests that polymorphisms in several genes may predispose some individuals with meningococcemia to develop fulminant disease (31).
LIMITATIONS, POSSIBLE IMPLICATIONS
Limitations.
Although LPS sensing may play an important role in shaping many human diseases, many other innate mechanisms also contribute to defense against gram-negative bacteria. They include mobilizing inflammatory responses through recognition of other bacterial molecules, natural and acquired antibodies, complement activation, antimicrobial peptides, and many aspects of the acute phase response (90). Acquired immunity is also obviously critical for preventing recurrent disease.
The LPS analysis makes several assumptions not previously discussed. First, the structures produced by bacteria growing in culture are assumed to resemble those produced in vivo. Second, since many bacteria produce more than one lipid A structure, the most abundant one is assumed to dominate host recognition. Third, bioassays performed using isolated cells or experimental animals (often rodents) are assumed to reflect accurately how humans and other animals sense these structures. A fourth assumption, that the literature survey for LPS structures was complete and accurate, is compromised by the disparate nature of the sources and the author's unintended yet indisputable selection biases. Perhaps the most important of these limitations is that of the structural heterogeneity of the LPSs. Not only do many gram-negative bacteria produce more than one lipid A structure, but the relative abundances of the different structures can vary with environmental conditions that include the media and temperature used to grow the bacteria in culture. Such influences may account for reported differences in the acylation of S. flexneri lipid A, for example (18, 28). Improved methods are needed to analyze the lipid A structures that exist in vivo in different disease and nondisease states.
Another illustration emphasizes this heterogeneity. For many years, LPS has been thought to be transferred from bacterial membranes by LPS-binding protein to soluble or membrane-bound CD14, which in turn delivers it to MD-2-TLR4. In fact, there is strong evidence that LPS-binding protein and CD14 are most important for sensing LPS molecules that have long polysaccharide chains (smooth or S-form LPS) (41, 56, 131). Interestingly, CD14 can deliver S-form LPS to cells in a way that selectively activates the MyD88-independent intracellular pathway downstream of TLR4 (63). Early studies found that the lipid A moiety of S-form LPS is hypoacylated (64). If confirmed, this heterogeneity would further complicate the interpretation of the lipid A structures found in bacteria that produce both S-form and short chain (R-form) LPS molecules.
Finally, although many investigators have found that animal cells recognize hexaacyl LPS more sensitively than they detect other gram-negative bacterial components (24, 56, 83), few head-to-head comparisons of LPS with other bacterial components have been performed to ascertain their relative potencies as TLR ligands for different cell types (158).
Possible implications: innate immunity.
What does the LPS-TLR4 example suggest about innate recognition of other bacteria? Some of the other PRRs are also quite specific. NOD1 and NOD2 sense distinct substructures of peptidoglycan, TLR2/TLR6, and TLR2/TLR1 heterodimers can distinguish lipopeptides that have two and three acyl chains, respectively, and TLR9 recognizes definable oligonucleotide sequences (42). Since these microbial molecular “patterns” have been much more highly conserved than has the structure of lipid A, it is likely that these TLRs sense a much greater diversity of microbes than does MD-2-TLR4 (80). As might be expected, it is these sensors that seem to figure most prominently in diseases caused by gram-negative bacteria that make nonhexaacyl LPS.
Disease susceptibility.
Functional mutations in MD-2 or TLR4 could be expected to influence diseases caused by bacteria that make hexaacyl LPS. In keeping with this prediction, TLR4 polymorphisms were found more frequently in patients with meningococcal disease than in controls (123). On the other hand, the large number of studies with negative or marginally positive results published to date suggests that it may be difficult to find reproducible associations between TLR4 polymorphisms and disease susceptibility or severity. The association of the TLR4 Asp299Gly allele with protection from cerebral malaria suggests that nonbacterial diseases may have influenced the evolution of the gene in diverse human populations (35), although a confounding role for endotoxemia has not been excluded (139). It is also possible that the associations sought so far have not included mucosal infectious diseases, such as shigellosis, in which LPS recognition by MD-2-TLR4 seems to play an important role. Since LPS binds MD-2 (20, 45, 94), disease-linked polymorphisms in MD-2 also deserve more attention (48). Polymorphisms in other host sensors are more likely to be associated with increased susceptibility to microbes, such as Legionella pneumophila (52) and Y. pestis, that make nonhexaacyl LPS.
Pathogenesis.
Whereas LPS has long been regarded as necessary for gram-negative bacterial virulence, its contribution to the outcome of infectious diseases has seemed quite variable. Considering how and where animals sense LPS highlights the two faces of the host-parasite encounter: LPS recognition by an animal host can trigger inflammatory reactions that are both damaging and protective.
Many experts have regarded fulminant meningococcemia as a paradigm for serious systemic gram-negative bacterial diseases (46). As noted above, fulminant disease caused by N. meningitidis is actually a special case. It is not simply the most dramatic extreme of a continuous spectrum of sepsis severity, so information gained from patients with meningococcemia is not necessarily useful for understanding the pathophysiology of other kinds of sepsis (71).
It is also noteworthy that none of the major gram-negative bacterial biothreat agents produces hexaacyl LPS. Yersinia pestis and Francisella tularensis make tetraacyl LPS when grown at 37°C (104), and Burkholderia pseudomallei LPS (inferring from its weak potency [78] and the reported structures of other Burkholderia species) is probably pentaacyl. Producing an LPS that is poorly detected by MD-2-TLR4 may be essential for these microbes to cause disease (83).
Diagnosis.
The most widely employed test for the presence of endotoxin in fluids is based on LPS sensing by the clotting system of the horseshoe crab. This assay is not specific for hexaacyl LPS (132, 133). LPS recognition by the horseshoe crab likely evolved as a defense from aquatic bacteria; the assay's ability to recognize widely diverse lipid A structures enhances its ability to detect environmental contamination of biologicals, but it also may account for some of the problems observed when the test has been used to detect endotoxemia and gram-negative bacteremia in human patients (the correlations between disease severity, gram-negative bacteremia and endotoxemia have generally been poor) (57). A newer test for endotoxemia is based on an antibody that also does not discriminate hexaacyl from nonhexaacyl LPS (111).
Recent studies have found clear-cut differences in the mRNA profiles of blood leukocytes from children with gram-positive infections (by S. pneumoniae and S. aureus, TLR2 agonists) and gram-negative infections (by E. coli, a TLR4 agonist) (107). Future improvements may equip clinicians to distinguish inflammatory responses to gram-negative bacteria that produce hexaacyl LPS from responses elicited by bacteria that do not (30). It is noteworthy in this connection that TLR4 is the only TLR that activates both the MyD88 and TRIF (MyD88-independent) intracellular signaling pathways, although different hexaacyl LPSs may activate the two paths to different degrees (63, 161). Perhaps this property will assist molecular diagnosis. Future clinical trials may also benefit from categorizing gram-negative diseases according to the likely host-LPS interaction, since therapies that interfere with the LPS-TLR4 interaction should not be useful for diseases caused by bacteria that make nonhexaacyl LPS (12).
Therapy.
Two TLR4-targeting drugs are currently in clinical trials, and monoclonal antibodies that prevent LPS sensing by MD-2-TLR4 are in development (26). Eritoran is a tetraacyl synthetic lipid A analog that competes with LPS for binding MD-2 (69). It potently blocks human responses to intravenously injected LPS (74, 85). TAK-242 is a cyclohexene derivative that inhibits TLR4-mediated responses to LPS (59). Adjunctive therapy with these drugs should be most effective when the offending bacteria produce hexaacyl LPS and the drug can reach them in infected tissue spaces. Lipid A antagonists might hasten recovery from shigellosis or other mucosal gram-negative diseases, and yet it is also possible that, by diminishing the local inflammatory response, they could favor systemic dissemination. Also providing effective bactericidal therapy will thus be essential. Similarly, combining TLR4 inhibition with bactericidal chemotherapy might improve the outcome of tissue infections, such as pneumonia or pyelonephritis, due to bacteria that produce hexaacyl LPS (Stenotrophomonas spp., probably Acinetobacter spp., and any member of the Enterobacteriaceae family).
Experiments performed using mice have found that enzymatic deacylation of lipid A is required to prevent prolonged reactions to bacteria that produce hexaacyl LPS (73, 118). The deacylating enzyme, acyloxyacyl hydrolase, is made by phagocytes, including the Kupffer cells of the liver (118), and by renal cortical epithelial cells that secrete it into the urine (36). It is possible that increasing the amount or activity of this enzyme could hasten the recovery of patients with diseases caused by gram-negative bacteria that produce hexaacyl LPS.
Inhibiting the bacterial myristoyl or lauryl transferases, and thus preventing the addition of the secondary acyl chains to the lipid A backbone, should in theory prevent or diminish LPS recognition by MD-2-TLR4. This could reduce the local inflammatory response induced by mucosal pathogens such as H. influenzae or M. catarrhalis, but it also might enable invasive bacteria to evade TLR4-based host defenses. Inhibition of the early constitutive steps in lipid A biosynthesis (104) should be less likely to have unwanted consequences, since LPS hypoacylation may significantly weaken the barrier function of the bacterial cell wall.
Therapy-induced “endotoxic” shock was first noted in patients infected with B. melitensis or S. enterica serovar Typhi (109, 125). Since brucellae do not produce hexaacyl LPS (the S. enterica serovar Typhi lipid A structure has not been reported), the basis for shock is unlikely to have been treatment-induced endotoxin release. No clinical tests of this hypothesis have been reported from studies of patients with brucellosis or typhoid fever, but inflammatory reactions due to antibiotic-induced endotoxin release have been observed when treating local tissue infections due to bacteria that produce hexaacyl LPS (4, 103). In contrast, antibiotic treatment of patients with Burkholderia pseudomallei bacteremia increased endotoxin levels in plasma with no change in signs or symptoms (121).
Prevention.
Dutch workers found that the absence of one of its secondary acyl chains greatly reduced proinflammatory responses to meningococcal LPS while retaining its ability to act as an adjuvant in mice (140). Of the other lipid A-derived adjuvants, the monophosphoryl lipid A (MPLA) from Salmonella minnesota LPS has been studied most intensively (98, 138). Its most active congener, which has six acyl chains, potently induces human macrophages to produce chemokines and cytokines via a TLR4-dependent mechanism. Synthetic analogs based on the MPLA structure are also good adjuvants and immunomodulators, at least in mice (98). The basis for their lack of toxicity is not known, since they and LPS have induced very similar responses in some cell types (130). One recent report noted that MPLA failed to activate caspase-1, which catalyzes IL-1β precursor processing, so that IL-1 secretion was impaired (95). Another found that MPLA predominantly activates cell responses via a MyD88-independent pathway that is associated with less production of IL-1 and gamma interferon (77).
Despite producing a full complement of virulence factors, the Y. pestis strain engineered to make hexaacyl LPS at 37°C was so avirulent that it could be used as a vaccine in mice (83). This strategy might be used with other bacteria that make nonhexaacyl LPS, including other biothreat agents, provided that hexaacyl LPS-containing vaccines are not too proinflammatory for injection into humans.
Questions for future research.
In addition to the important unknowns mentioned in the paragraphs above, there are many unanswered questions. (i) How do cells that express MD-2-TLR4 sense native LPS? If the LPS acyl chains must fit into a pocket in MD-2, as recently reported for tetraacyl lipid A (69, 94), how does hexaacyl LPS bind to MD-2 and induce TLR4 activation (dimerization)? What extracts LPS from the bacterial membrane in a way that allows it to bind MD-2? Is this a job for LPS-binding protein (142), and, if so, does it occur both on the cell surface and within host cell phagosomes (11)? (ii) What is the basis for “mutualism” between aerobic, hexaacyl LPS-producing gram-negative bacteria and the mammalian upper respiratory and gastrointestinal mucosae? Do these bacteria derive some benefit from being sensed by host TLR4? Does producing a hexaacyl LPS contribute to this mutualism? (iii) How do gram-negative aerobic bacteria that lack hexaacyl lipid A survive in the gut? This question is especially interesting with regard to the pathogens that translocate across the gastrointestinal mucosa: might brucellae be protected within macrophages from an infected animal host or Vibrio vulnificus by oyster cells? If not, what are the cell wall molecules in these bacteria that prevent killing by antimicrobial peptides or bile salts? (iv) How do some bacteria that make hexaacyl LPS, most notably the pathogenic Neisseria spp., evade submucosal defenses and cross into the bloodstream without eliciting a (protective) local inflammatory response? In particular, what roles do capsule, LPS sialylation, and pili play in this process (65)? (v) What are the most important innate defenses toward bacteria that lack hexaacyl LPS? How can these defenses be enhanced without provoking harmful systemic inflammation? (vi) How does MD-2-TLR4 discriminate between lipid A and the host carbohydrate and protein ligands that have been reported to activate cells via TLR4 (134)? What are the downstream intracellular and systemic consequences of recognizing these different agonists? (vii) Can TLR4 stimulation or inhibition be used to improve outcome from diseases caused by bacteria that make hexaacyl LPS?
CONCLUSIONS
Bacterial virulence and innate immunity are extremely complicated phenomena. Each disease-inducing microbe brings an array of virulence factors to its interaction with an animal host, whose multifaceted defenses can be modified by both genetic and environmental influences. Disease-bacterium associations based on LPS structures may give clues to how innate immunity to gram-negative bacteria succeeds and fails in humans, but they are merely patterns—numerous variables preclude firm conclusions regarding causality. Although many clinical observations seem to be in line with the hypothesis that the MD-2-TLR4-based defense is principally directed toward mucosal gram-negative aerobes, the evidence for humans is largely circumstantial. Moreover, species differences in the specificity of the LPS-MD-2 interaction prevent generalization to humans from murine disease models, the most extensive source of evidence to date. Finally, some bacterium-host interactions do not fit the general pattern; at a minimum these exceptions limit the hypothesis and require further explanation. It is hoped that this summary will encourage further tests of the hypothesis using LPS structure-clinical correlations, experiments in more informative animal models, and clinical interventions directed at the LPS-MD-2-TLR4 interaction.
Acknowledgments
This research was supported by grant AI18188 from the National Institute of Allergy and Infectious Diseases and by the Jan and Henri Bromberg Chair in Internal Medicine, UT-Southwestern Medical Center, Dallas, TX.
Thanks to Arturo Casedevall, David Gilbert, Mark Swancutt, and Shaw Warren for very helpful criticism.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 17 December 2007.
REFERENCES
- 1.Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 1744453-4460. [DOI] [PubMed] [Google Scholar]
- 2.Adamo, R., S. Sokol, G. Soong, M. I. Gomez, and A. Prince. 2004. Pseudomonas aeruginosa flagella activate airway epithelial cells through asialoGM1 and Toll-like receptor 2 as well as Toll-like receptor 5. Am. J. Respir. Cell Mol. Biol. 30627-634. [DOI] [PubMed] [Google Scholar]
- 3.Aisenberg, G., K. V. Rolston, B. F. Dickey, D. P. Kontoyiannis, I. I. Raad, and A. Safdar. 2007. Stenotrophomonas maltophilia pneumonia in cancer patients without traditional risk factors for infection, 1997-2004. Eur. J. Clin. Microbiol. Infect. Dis. 2613-20. [DOI] [PubMed] [Google Scholar]
- 4.Arditi, M., L. Ables, and R. Yogev. 1989. Cerebrospinal fluid endotoxin levels in children with H. influenzae meningitis before and after administration of intravenous ceftriaxone. J. Infect. Dis. 1601005-1011. [DOI] [PubMed] [Google Scholar]
- 5.Backhed, F., and M. Hornef. 2003. Toll-like receptor 4-mediated signaling by epithelial surfaces: necessity or threat? Microb. Infect. 5951-959. [DOI] [PubMed] [Google Scholar]
- 6.Bath, U. R., T. Kontrohr, and H. Mayer. 1987. Structure of Shigella sonnei lipid A. FEMS Microbiol. Lett. 40189-192. [Google Scholar]
- 7.Belden, W. J., and S. I. Miller. 1994. Further characterization of the PhoP regulon: identification of new PhoP-activated virulence loci. Infect. Immun. 625095-5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bengoechea, J. A., K. Brandenburg, M. D. Arraiza, U. Seydel, M. Skurnik, and I. Moriyon. 2003. Pathogenic Yersinia enterocolitica strains increase the outer membrane permeability in response to environmental stimuli by modulating lipopolysaccharide fluidity and lipid A structure. Infect. Immun. 712014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjerre, A., B. Brusletto, E. A. Hoiby, P. Kierulf, and P. Brandtzaeg. 2004. Plasma interferon-gamma and interleukin-10 concentrations in systemic meningococcal disease compared with severe systemic Gram-positive septic shock. Crit. Care Med. 32433-438. [DOI] [PubMed] [Google Scholar]
- 10.Bjorkbacka, H., K. A. Fitzgerald, F. Huet, X. Li, J. A. Gregory, M. A. Lee, C. M. Ordija, N. E. Dowley, D. T. Golenbock, and M. W. Freeman. 2004. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades Physiol. Genomics 19319-330. [DOI] [PubMed] [Google Scholar]
- 11.Blander, J. M., and R. Medzhitov. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440808-812. [DOI] [PubMed] [Google Scholar]
- 12.Bochud, P. Y., and T. Calandra. 2003. Science, medicine, and the future—pathogenesis of sepsis: new concepts and implications for future treatment. Br. Med. J. 326262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandtzaeg, P., P. Kierulf, P. Gaustad, A. Skulberg, J. N. Bruun, S. Halvorsen, and E. Sorensen. 1989. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J. Infect. Dis. 159195-204. [DOI] [PubMed] [Google Scholar]
- 14.Brun-Buisson, C., F. Doyon, J. Carlet, B. Bedock, C. H. Annonay, E. Valente, O. Lescale, B. Misset, P. Charbonneau, M. Vergnaud, R. Cohen, M. Coloignier, J. L. Frances, A. Combes, O. Duval, P. Dellamonica, J. M. Descamps, Y. Domart, J. L. Galiacy, F. Gouin, G. Guivarc'h, C. Hennequin, A. Krajevitch, and P. Delmas. 1996. Bacteremia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. Am. J. Respir. Crit. Care Med. 154617-624. [DOI] [PubMed] [Google Scholar]
- 15.Butler, T., J. Levin, N. N. Linh, D. M. Chau, M. Adickman, and K. Arnold. 1976. Yersinia pestis infection in Vietnam. II. Quantitative blood cultures and detection of endotoxin in the cerebrospinal fluid of patients with meningitis. J. Infect. Dis. 133493-499. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 1991. Epidemiologic notes and reports update: Yersinia enterocolitica bacteremia and endotoxin shock associated with blood transfusions—United States, 1991. Morb. Mortal. Wkly. Rep. 40176-178. [PubMed] [Google Scholar]
- 17.Chabot, S., J. S. Wagner, S. Farrant, and M. R. Neutra. 2006. TLRs regulate the gatekeeping functions of the intestinal follicle-associated epithelium. J. Immunol. 1764275-4283. [DOI] [PubMed] [Google Scholar]
- 18.Chan, S., and V. N. Reinhold. 1994. Detailed structural characterization of lipid A: electrospray ionization coupled with tandem mass spectrometry. Anal. Biochem. 21863-73. [DOI] [PubMed] [Google Scholar]
- 19.Clements, A., D. Tull, A. W. Jenney, J. L. Farn, S. H. Kim, R. E. Bishop, J. B. McPhee, R. E. W. Hancock, E. L. Hartland, M. J. Pearse, O. L. C. Wijburg, D. C. Jackson, M. J. McConville, and R. A. Strugnell. 2007. Secondary acylation of Klebsiella pneumoniae lipopolysaccharide contributes to sensitivity to antibacterial peptides. J. Biol. Chem. 28215569-15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coats, S. R., C. T. Do, L. M. Karimi-Naser, P. H. Braham, and R. P. Darveau. 2007. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell. Microbiol. 91191-1202. [DOI] [PubMed] [Google Scholar]
- 21.Cohen, J. 2000. Meningococcal disease as a model to evaluate novel anti-sepsis strategies. Crit. Care Med. 28S64-S67. [DOI] [PubMed] [Google Scholar]
- 22.Cross, A., L. Asher, M. Seguin, L. Yuan, N. Kelly, C. Hammack, J. Sadoff, and P. Gemski, Jr. 1995. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J. Clin. Investig. 96676-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cross, A. S., J. C. Sadoff, N. Kelly, E. Bernton, and P. Gemski. 1989. Pretreatment with recombinant murine tumor necrosis factor α/cachectin and murine interleukin 1 α protects mice from lethal bacterial infection. J. Exp. Med. 1692021-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Darveau, R. P. 1998. Lipid A diversity and the innate host response to bacterial infection. Curr. Opin. Microbiol. 136-42. [DOI] [PubMed] [Google Scholar]
- 25.Darveau, R. P., T. T. Pham, K. Lemley, R. A. Reife, B. W. Bainbridge, S. R. Coats, W. N. Howald, S. S. Way, and A. M. Hajjar. 2004. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect. Immun. 725041-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daubeuf, B., J. Mathison, S. Spiller, S. Hugues, S. Herren, W. Ferlin, M. Kosco-Vilbois, H. Wagner, C. J. Kirschning, R. Ulevitch, and G. Elson. 2007. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J. Immunol. 1796107-6114. [DOI] [PubMed] [Google Scholar]
- 27.De Soyza, A., C. D. Ellis, C. M. A. Khan, P. A. Corris, and R. D. de Hormaeche. 2004. Burkholderia cenocepacia lipopolysaccharide, lipid A, and proinflammatory activity. Am. J. Respir. Crit. Care Med. 17070-77. [DOI] [PubMed] [Google Scholar]
- 28.D'Hauteville, H., S. Khan, D. J. Maskell, A. Kussak, A. Weintraub, J. Mathison, R. J. Ulevitch, N. Wuscher, C. Parsot, and P. J. Sansonetti. 2002. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J. Immunol. 1685240-5251. [DOI] [PubMed] [Google Scholar]
- 29.Dixon, D. R., and R. P. Darveau. 2005. Lipopolysaccharide heterogeneity: innate host responses to bacterial modification of lipid A structure. J. Dent. Res. 84584-595. [DOI] [PubMed] [Google Scholar]
- 30.Elson, G., I. Dunn-Siegrist, B. Daubeuf, and J. Pugin. 2007. Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 1091574-1583. [DOI] [PubMed] [Google Scholar]
- 31.Emonts, M., J. A. Hazelzet, R. De Groot, and P. W. Hermans. 2003. Host genetic determinants of Neisseria meningitidis infections. Lancet Infect. Dis. 3565-577. [DOI] [PubMed] [Google Scholar]
- 32.Ernst, R. K., S. M. Moskowitz, J. C. Emerson, G. M. Kraig, K. N. Adams, M. D. Harvey, B. Ramsey, D. P. Speert, J. L. Burns, and S. I. Miller. 2007. Unique lipid A modifications in Pseudomonas aeruginosa isolated from the airways of patients with cystic fibrosis. J. Infect. Dis. 1961088-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ernst, R. K., E. C. Yi, L. Guo, K. B. Lim, J. L. Burns, M. Hackett, and S. I. Miller. 1999. Specific lipopolysaccharide found in cystic fibrosis airway Pseudomonas aeruginosa. Science 2861561-1565. [DOI] [PubMed] [Google Scholar]
- 34.Erridge, C., O. L. Moncayo-Nieto, R. Morgan, M. Young, and I. R. Poxton. 2007. Acinetobacter baumannii lipopolysaccharides are potent stimulators of human monocyte activation via Toll-like receptor 4 signalling. J. Med. Microbiol. 56165-171. [DOI] [PubMed] [Google Scholar]
- 35.Ferwerda, B., M. B. B. McCall, S. Alonso, E. J. Giamarellos-Bourboulis, M. Mouktaroudi, N. Izagirre, D. Syafruddin, G. Kibiki, T. Cristea, A. Hijmans, L. Hamann, S. Israel, G. ElGhazali, M. Troye-Blomberg, O. Kumpf, B. Maiga, A. Dolo, O. Doumbo, C. C. Hermsen, A. F. H. Stalenhoef, R. van Crevel, H. G. Brunner, D. Y. Oh, R. R. Schumann, C. de la Rua, R. Sauerwein, B. J. Kullberg, A. J. A. M. van der Ven, J. W. M. van der Meer, and M. G. Netea. 2007. TLR4 polymorphisms, infectious diseases, and evolutionary pressure during migration of modern humans. Proc. Natl. Acad. Sci. USA 10416645-16650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feulner, J. A., M. Lu, J. M. Shelton, M. Zhang, J. A. Richardson, and R. S. Munford. 2004. Identification of acyloxyacyl hydrolase, a lipopolysaccharide-detoxifying enzyme, in the murine urinary tract. Infect. Immun. 723171-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Figueiredo, R. T., P. L. Fernandez, D. S. Mourao-Sa, B. N. Porto, F. F. Dutra, L. S. Alves, M. F. Oliveira, P. L. Oliveira, A. V. Graca-Souza, and M. T. Bozza. 2007. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 28220221-20229. [DOI] [PubMed] [Google Scholar]
- 38.Forsgren, J., A. Samuelson, A. Ahlin, J. Jonasson, B. Rynnel-Dagoo, and A. Lindberg. 1994. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect. Immun. 62673-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frieling, J. T., J. A. Mulder, T. Hendriks, J. H. Curfs, C. J. Van der Linden, and R. W. Sauerwein. 1997. Differential induction of pro- and anti-inflammatory cytokines in whole blood by bacteria: effects of antibiotic treatment. Antimicrob. Agents Chemother. 411439-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukata, M., K. S. Michelsen, R. Eri, L. S. Thomas, B. Hu, K. Lukasek, C. C. Nast, J. Lechago, R. Xu, Y. Naiki, A. Soliman, M. Arditi, and M. T. Abreu. 2005. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 288G1055-G1065. [DOI] [PubMed] [Google Scholar]
- 41.Gangloff, S. C., N. Hijiya, A. Haziot, and S. M. Goyert. 1999. Lipopolysaccharide structure influences the macrophage response via CD14-independent and CD14-dependent pathways. Clin. Infect. Dis. 28491-496. [DOI] [PubMed] [Google Scholar]
- 42.Gay, N. J., and M. Gangloff. 2007. Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 76141-165. [DOI] [PubMed] [Google Scholar]
- 43.Gerold, G., A. Zychlinsky, and J. L. de Diego. 2007. What is the role of Toll-like receptors in bacterial infections? Semin. Immunol. 1941-47. [DOI] [PubMed] [Google Scholar]
- 44.Gibbons, H. S., S. R. Kalb, R. J. Cotter, and C. R. Raetz. 2005. Role of Mg2+ and pH in the modification of Salmonella lipid A after endocytosis by macrophage tumour cells. Mol. Microbiol. 55425-440. [DOI] [PubMed] [Google Scholar]
- 45.Gioannini, T. L., A. Teghanemt, D. Zhang, E. N. Levis, and J. P. Weiss. 2005. Monomeric endotoxin:protein complexes are essential for TLR4-dependent cell activation. J. Endotoxin Res. 11117-123. [DOI] [PubMed] [Google Scholar]
- 46.Giroir, B. P. 2000. Meningococcemia as a model for testing the hypothesis of antisepsis therapies. Crit. Care Med. 28S57-S59. [DOI] [PubMed] [Google Scholar]
- 47.Golenbock, D. T., R. Y. Hampton, and C. R. H. Raetz. 1990. Lipopolysaccharide antagonism by lipid A precursor lipid IVA is species dependent. FASEB J. 4A2055. [Google Scholar]
- 48.Gu, W., Y. A. Shan, J. Zhou, D. P. Jiang, L. Zhang, D. Y. Du, Z. G. Wang, and J. X. Jiang. 2007. Functional significance of gene polymorphisms in the promoter of myeloid differentiation-2. Ann. Surg. 246151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276250-253. [DOI] [PubMed] [Google Scholar]
- 50.Hajjar, A. M., M. D. Harvey, S. A. Shaffer, D. R. Goodlett, A. Sjostedt, H. Edebro, M. Forsman, M. Bystrom, M. Pelletier, C. B. Wilson, S. I. Miller, S. J. Skerrett, and R. K. Ernst. 2006. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect. Immun. 746730-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison, O. B., B. D. Robertson, S. N. Faust, M. A. Jepson, R. D. Goldin, M. Levin, and R. S. Heyderman. 2002. Analysis of pathogen-host cell interactions in Purpura Fulminans: expression of capsule, type IV pili, and PorA by Neisseria meningitidis in vivo. Infect. Immun. 705193-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hawn, T. R., A. Verbon, M. Janer, L. P. Zhao, B. Beutler, and A. Aderem. 2005. Toll-like receptor 4 polymorphisms are associated with resistance to Legionnaires' disease. Proc. Natl. Acad. Sci. USA 1022487-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heiniger, N., V. Spaniol, R. Troller, M. Vischer, and C. Aebi. 2007. A reservoir of Moraxella catarrhalis in human pharyngeal lymphoid tissue. J. Infect. Dis. 1961080-1087. [DOI] [PubMed] [Google Scholar]
- 54.Honstettre, A., E. Ghigo, A. Moynault, C. Capo, R. Toman, S. Akira, O. Takeuchi, H. Lepidi, D. Raoult, and J. L. Mege. 2004. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through Toll-like receptor 4. J. Immunol. 1723695-3703. [DOI] [PubMed] [Google Scholar]
- 55.Hooper, L. V., and J. I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science 2921115-1118. [DOI] [PubMed] [Google Scholar]
- 56.Huber, M., C. Kalis, S. Keck, Z. Jiang, P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, B. Beutler, C. Galanos, and M. A. Freudenberg. 2006. R-form LPS, the master key to the activation ofTLR4/MD-2-positive cells. Eur. J. Immunol. 36701-711. [DOI] [PubMed] [Google Scholar]
- 57.Hurley, J. C. 2003. Endotoxemia and Gram-negative bacteremia as predictors of outcome in sepsis: a meta-analysis using ROC curves. J. Endotoxin Res. 9271-279. [DOI] [PubMed] [Google Scholar]
- 58.Ichikawa, J. K., S. B. English, M. C. Wolfgang, R. Jackson, A. J. Butte, and S. Lory. 2005. Genome-wide analysis of host responses to the Pseudomonas aeruginosa type III secretion system yields synergistic effects. Cell. Microbiol. 71635-1646. [DOI] [PubMed] [Google Scholar]
- 59.Ii, M., N. Matsunaga, K. Hazeki, K. Nakamura, K. Takashima, T. Seya, O. Hazeki, T. Kitazaki, and Y. Iizawa. 2006. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol. Pharmacol. 691288-1295. [DOI] [PubMed] [Google Scholar]
- 60.Ingalls, R. R., E. Lien, and D. T. Golenbock. 2001. Membrane-associated proteins of a lipopolysaccharide-deficient mutant of Neisseria meningitidis activate the inflammatory response through toll-like receptor 2. Infect. Immun. 692230-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janeway, C. A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 541-13. [DOI] [PubMed] [Google Scholar]
- 62.Jia, H. P., J. N. Kline, A. Penisten, M. A. Apicella, T. L. Gioannini, J. Weiss, and P. B. McCray, Jr. 2004. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am. J. Physiol. Lung Cell Mol. Physiol. 287L428-L437. [DOI] [PubMed] [Google Scholar]
- 63.Jiang, Z., P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, M. Huber, C. Kalis, S. Keck, C. Galanos, M. Freudenberg, and B. Beutler. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6565-570. [DOI] [PubMed] [Google Scholar]
- 64.Jiao, B., M. Freudenberg, and C. Galanos. 1989. Characterization of the lipid A component of genuine smooth-form lipopolysaccharide. Eur. J. Biochem. 180515-518. [DOI] [PubMed] [Google Scholar]
- 65.Johansson, L., A. Rytkönen, P. Bergman, B. Albiger, H. Källström, T. Hökfelt, B. Agerberth, R. Cattaneo, and A. B. Jonsson. 2003. CD46 in meningococcal disease. Science 301373-375. [DOI] [PubMed] [Google Scholar]
- 66.Kanegasaki, S., K. Tanamoto, T. Yasuda, J. Y. Homma, M. Matsuura, M. Nakatsuka, Y. Kumazawa, A. Yamamota, T. Shiba, S. Kusumoto, M. Imoto, H. Yoshimura, and T. Shimamoto. 1986. Structure-activity relationship of Lipid A: comparison of biological activities of national and synthetic Lipid A's with different fatty acid compositions. J. Biochem. 991203-1210. [DOI] [PubMed] [Google Scholar]
- 67.Katz, J., P. Zhang, M. Martin, S. N. Vogel, and S. M. Michalek. 2006. Toll-Like Receptor 2 Is Required for Inflammatory Responses to Francisella tularensis LVS. Infect. Immun. 742809-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan, S. A., P. Everest, S. Servos, N. Foxwell, U. Zahringer, H. Brade, E. T. Rietschel, G. Dougan, I. G. Charles, and D. J. Maskell. 1998. A lethal role for lipid A in Salmonella infections. Mol. Microbiol. 29571-579. [DOI] [PubMed] [Google Scholar]
- 69.Kim, H. M., B. S. Park, J. I. Kim, S. E. Kim, J. Lee, S. C. Oh, P. Enkhbayar, N. Matsushima, H. Lee, O. J. Yoo, and J. O. Lee. 2007. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 130906-917. [DOI] [PubMed] [Google Scholar]
- 70.Leone, S., L. Sturiale, E. Pessione, R. Mazzoli, C. Giunta, R. Lanzetta, D. Garozzo, A. Molinaro, and M. Parrilli. 2007. Detailed characterization of the lipid A fraction from the nonpathogen Acinetobacter radioresistens strain S13. J. Lipid Res. 481045-1051. [DOI] [PubMed] [Google Scholar]
- 71.Levin, M., P. A. Quint, B. Goldstein, P. Barton, J. S. Bradley, S. D. Shemie, T. Yeh, S. S. Kim, D. P. Cafaro, P. J. Scannon, and B. P. Giroir for the rBPI21 Meningococcal Sepsis Study Group. 2000. Recombinant bactericidal/permeability-increasing protein (rBPI21) as adjunctive treatment for children with severe meningococcal sepsis: a randomised trial. Lancet 356961-967. [DOI] [PubMed] [Google Scholar]
- 72.Low, K. B., M. Ittensohn, T. Le, J. Platt, S. Sodi, M. Amoss, O. Ash, E. Carmichael, A. Chakraborty, J. Fischer, S. L. Lin, X. Luo, S. I. Miller, L. Zheng, I. King, J. M. Pawelek, and D. Bermudes. 1999. Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor-targeting in vivo. Nat. Biotechnol. 1737-41. [DOI] [PubMed] [Google Scholar]
- 73.Lu, M., M. Zhang, A. Takashima, J. Weiss, M. A. Apicella, X. H. Li, D. Yuan, and R. S. Munford. 2005. Lipopolysaccharide deacylation by an endogenous lipase controls innate antibody responses to Gram-negative bacteria. Nat. Immunol. 6989-994. [DOI] [PubMed] [Google Scholar]
- 74.Lynn, M., D. P. Rossignol, J. L. Wheeler, R. J. Kao, C. A. Perdomo, R. Noveck, R. Vargas, T. D'Angelo, S. Gotzkowsky, and F. G. McMahon. 2003. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J. Infect. Dis. 187631-639. [DOI] [PubMed] [Google Scholar]
- 75.Malik, M., C. S. Bakshi, B. Sahay, A. Shah, S. A. Lotz, and T. J. Sellati. 2006. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect. Immun. 743657-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marshall, J. C. 2005. Lipopolysaccharide: an endotoxin or an exogenous hormone? Clin. Infect. Dis. 41(Suppl. 7)S470-S480. [DOI] [PubMed] [Google Scholar]
- 77.Mata-Haro, V., C. Cekic, M. Martin, P. M. Chilton, C. R. Casella, and T. C. Mitchell. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 3161628-1632. [DOI] [PubMed] [Google Scholar]
- 78.Matsuura, M., K. Kawahara, T. Ezaki, and M. Nakano. 1996. Biological activities of lipopolysaccharide of Burkholderia (Pseudomonas) pseudomallei. FEMS Microbiol. Lett. 13779-83. [DOI] [PubMed] [Google Scholar]
- 79.Matzinger, P. 1998. An innate sense of danger. Semin. Immunol. 10399-415. [DOI] [PubMed] [Google Scholar]
- 80.Medzhitov, R. and C. A. Janeway, Jr. 2002. Decoding the patterns of self and nonself by the innate immune system. Science 296298-300. [DOI] [PubMed] [Google Scholar]
- 81.Miller, S. I., R. K. Ernst, and M. W. Bader. 2005. LPS, TLR4 and infectious disease diversity. Nat. Rev. Micro. 336-46. [DOI] [PubMed] [Google Scholar]
- 82.Mogensen, T. H., S. R. Paludan, M. Kilian, and L. Ostergaard. 2006. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 80267-277. [DOI] [PubMed] [Google Scholar]
- 83.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 71066-1073. [DOI] [PubMed] [Google Scholar]
- 84.Moran, A. P. 1995. Biological and serological characterization of Campylobacter jejuni lipopolysaccharides with deviating core and lipid A structures. FEMS Immunol. Med. Microbiol. 11121-130. [DOI] [PubMed] [Google Scholar]
- 85.Mullarkey, M., J. R. Rose, J. Bristol, T. Kawata, A. Kimura, S. Kobayashi, M. Przetak, J. Chow, F. Gusovsky, W. J. Christ, and D. P. Rossignol. 2003. Inhibition of endotoxin response by E5564, a novel Toll-like receptor 4-directed endotoxin antagonist. J. Pharmacol. Exp. Ther. 3041093-1102. [DOI] [PubMed] [Google Scholar]
- 86.Munford, R. S. 2005. Detoxifying endotoxin: time, place, person. J. Endotoxin Res. 1169-84. [DOI] [PubMed] [Google Scholar]
- 87.Munford, R. S. 2005. Sepsis, severe sepsis and septic shock, p. 906-926. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases. Elsevier Churchill-Livingstone, London, United Kingdom.
- 88.Munford, R. S. 2006. Severe sepsis and septic shock: the role of Gram-negative bacteremia, p. 7-496. In A. K. Abbas, S. J. Galli, and P. M. Howley (ed.), Annual review of pathology: mechanisms of disease. Annual Reviews, Palo Alto, CA. [DOI] [PubMed]
- 89.Munford, R. S., and J. Pugin. 2001. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am. J. Respir. Crit. Care Med. 163316-321. [DOI] [PubMed] [Google Scholar]
- 90.Munford, R. S., and J. Pugin. 2001. The crucial role of systemic responses in the innate (non-adaptive) host defense. J. Endotoxin Res. 7327-332. [PubMed] [Google Scholar]
- 91.Munford, R. S., and A. W. Varley. 2006. Shield as signal: lipopolysaccharides and the evolution of immunity to Gram-negative bacteria. PLoS Pathog. 2e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nahori, M. A., E. Fournie-Amazouz, N. S. Que-Gewirth, V. Balloy, M. Chignard, C. R. H. Raetz, I. Saint Girons, and C. Werts. 2005. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J. Immunol. 1756022-6031. [DOI] [PubMed] [Google Scholar]
- 93.O'Brien, A. D., D. L. Rosenstreich, I. Scher, G. H. Campbell, R. P. MacDermott, and S. B. Formal. 1980. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J. Immunol. 12420-24. [PubMed] [Google Scholar]
- 94.Ohto, U., K. Fukase, K. Miyake, and Y. Satow. 2007. Crystal structures of human MD-2 and its complex with antiendotoxic Lipid IVa. Science 3161632-1634. [DOI] [PubMed] [Google Scholar]
- 95.Okemoto, K., K. Kawasaki, K. Hanada, M. Miura, and M. Nishijima. 2006. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1β or activation of caspase-1. J. Immunol. 1761203-1208. [DOI] [PubMed] [Google Scholar]
- 96.Ortega-Cava, C. F., S. Ishihara, M. A. Rumi, K. Kawashima, N. Ishimura, H. Kazumori, J. Udagawa, Y. Kadowaki, and Y. Kinoshita. 2003. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 1703977-3985. [DOI] [PubMed] [Google Scholar]
- 97.Øvstebø, R., P. Brandtzaeg, B. Brusletto, K. B. Haug, K. Lande, E. A. Hoiby, and P. Kierulf. 2004. Use of robotized DNA isolation and real-time PCR to quantify and identify close correlation between levels of Neisseria meningitidis DNA and lipopolysaccharides in plasma and cerebrospinal fluid from patients with systemic meningococcal disease. J. Clin. Microbiol. 422980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10S32-S37. [DOI] [PubMed] [Google Scholar]
- 99.Pirofski, L. A., and A. Casadevall. 2002. The meaning of microbial exposure, infection, colonisation, and disease in clinical practice. Lancet Infect. Dis. 2628-635. [DOI] [PubMed] [Google Scholar]
- 100.Plant, L., J. Sundqvist, S. Zughaier, L. Lovkvist, D. S. Stephens, and A. B. Jonsson. 2006. Lipooligosaccharide structure contributes to multiple steps in the virulence of Neisseria meningitidis. Infect. Immun. 741360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Post, D. M. B., N. J. Phillips, J. Q. Shao, D. D. Entz, B. W. Gibson, and M. A. Apicella. 2002. Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A. Infect. Immun. 70909-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Powell, J. L., A. C. Wright, S. S. Wasserman, D. M. Hone, and J. G. Morris, Jr. 1997. Release of tumor necrosis factor alpha in response to Vibrio vulnificus capsular polysaccharide in in vivo and in vitro models. Infect. Immun. 653713-3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prins, J. M., M. A. Van Agtmael, E. J. Kuijper, S. J. H. van Deventer, and P. Speelman. 1995. Antibiotic-induced endotoxin release in patients with gram-negative urosepsis: a double-blind study comparing imipenem and ceftazidime. J. Infect. Dis. 172886-891. [DOI] [PubMed] [Google Scholar]
- 104.Raetz, C. R. H., C. M. Reynolds, M. S. Trent, and R. E. Bishop. 2007. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76295-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raffatellu, M., D. Chessa, R. P. Wilson, R. Dusold, S. Rubino, and A. J. Baumler. 2005. The Vi capsular antigen of Salmonella enterica serotype Typhi reduces Toll-like receptor-dependent interleukin-8 expression in the intestinal mucosa. Infect. Immun. 733367-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rakoff-Nahoum, S., J. Paglino, F. Eslami-Varzaneh, S. Edberg, and R. Medzhitov. 2004. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118229-241. [DOI] [PubMed] [Google Scholar]
- 107.Ramilo, O., W. Allman, W. Chung, A. Mejias, M. Ardura, C. Glaser, K. M. Wittkowski, B. Piqueras, J. Banchereau, A. K. Palucka, and D. Chaussabel. 2007. Gene expression patterns in blood leukocytes discriminate patients with acute infections. Blood 1092066-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rebeil, R., R. K. Ernst, B. B. Gowen, S. I. Miller, and B. J. Hinnebusch. 2004. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 521363-1373. [DOI] [PubMed] [Google Scholar]
- 109.Reilly, J., A. Compagnon, P. Tournier, R. Bastin, and H. Du Buit. 1950. Les accidents du traitement des fievres typhoides par la chloromycetine. Ann. Med. 51597-629. [PubMed] [Google Scholar]
- 110.Roach, J. C., G. Glusman, L. Rowen, A. Kaur, M. K. Purcell, K. D. Smith, L. E. Hood, and A. Aderem. 2005. The evolution of vertebrate Toll-like receptors. Proc. Na. Acad. Sci. USA 1029577-9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romaschin, A. D., D. M. Harris, M. B. Ribeiro, J. Paice, D. M. Foster, P. M. Walker, and J. C. Marshall. 1998. A rapid assay of endotoxin in whole blood using autologous neutrophil dependent chemiluminescence. J. Immunol. Methods 212169-185. [DOI] [PubMed] [Google Scholar]
- 112.Sannes, M. R., M. A. Kuskowski, K. Owens, A. Gajewski, and J. R. Johnson. 2004. Virulence factor profiles and phylogenetic background of Escherichia coli isolates from veterans with bacteremia and uninfected control subjects. J. Infect. Dis. 1902121-2128. [DOI] [PubMed] [Google Scholar]
- 113.Sansonetti, P. J., and J. P. Di Santo. 2007. Debugging how bacteria manipulate the immune response. Immunity 26149-161. [DOI] [PubMed] [Google Scholar]
- 114.Segura, E. R., C. A. Ganoza, K. Campos, J. N. Ricaldi, S. Torres, H. Silva, M. J. Cespedes, M. A. Matthias, M. A. Swancutt, L. R. Lopez, E. Gotuzzo, H. Guerra, R. H. Gilman, and J. M. Vinetz. 2005. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin. Infect. Dis. 40343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Selsted, M. E., and A. J. Ouellette. 2005. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6551-557. [DOI] [PubMed] [Google Scholar]
- 116.Shaffer, S. A., M. D. Harvey, D. R. Goodlett, and R. K. Ernst. 2007. Structural heterogeneity and environmentally regulated remodeling of Francisella tularensis subspecies novicida lipid A characterized by tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 181080-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc. Natl. Acad. Sci. USA 1028722-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shao, B., M. Lu, S. C. Katz, A. W. Varley, J. Hardwick, T. E. Rogers, N. Ojogun, D. C. Rockey, R. P. DeMatteo, and R. S. Munford. 2007. A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen. J. Biol. Chem. 28213726-13735. [DOI] [PubMed] [Google Scholar]
- 119.Shenep, J. L., P. M. Flynn, F. F. Barrett, G. L. Stidham, and D. F. Westenkirchner. 1988. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J. Infect. Dis. 157565-568. [DOI] [PubMed] [Google Scholar]
- 120.Silipo, A., A. Molinaro, P. Cescutti, E. Bedini, R. Rizzo, M. Parrilli, and R. Lanzetta. 2005. Complete structural characterization of the lipid A fraction of a clinical strain of B. cepacia genomovar I lipopolysaccharide. Glycobiology 15561-570. [DOI] [PubMed] [Google Scholar]
- 121.Simpson, A. J. H., S. M. Opal, B. J. Angus, J. M. Prins, J. E. Palardy, N. A. Parejo, W. Chaowagul, and N. J. White. 2000. Differential antibiotic-induced endotoxin release in severe melioidosis. J. Infect. Dis. 1811014-1019. [DOI] [PubMed] [Google Scholar]
- 122.Skerrett, S. J., C. B. Wilson, H. D. Liggitt, and A. M. Hajjar. 2007. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am. J. Physiol. Lung Cell Mol. Physiol. 292L312-L322. [DOI] [PubMed] [Google Scholar]
- 123.Smirnova, I., N. Mann, A. Dols, H. H. Derkx, M. L. Hibberd, M. Levin, and B. Beutler. 2003. Assay of locus-specific genetic load implicates rare Toll-like receptor 4 mutations in meningococcal susceptibility. Proc. Natl. Acad. Sci. USA 1006075-6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Somerville, J. E., Jr., L. Cassiano, and R. P. Darveau. 1999. Escherichia coli msbB Gene as a virulence factor and a therapeutic target. Infect. Immun. 676583-6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spink, W. W., A. I. Braude, M. R. Castaneda, and R. S. Goytia. 1948. Aureomycin therapy in human brucellosis due to Brucella melitensis. JAMA 1381145-1148. [DOI] [PubMed] [Google Scholar]
- 126.Sprong, T., A. S. Moller, A. Bjerre, E. Wedege, P. Kierulf, J. W. M. van der Meer, P. Brandtzaeg, M. van Deuren, and T. E. Mollnes. 2004. Complement activation and complement-dependent inflammation by Neisseria meningitidis are independent of lipopolysaccharide. Infect. Immun. 723344-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Starner, T. D., W. E. Swords, M. A. Apicella, and P. B. McCray, Jr. 2002. Susceptibility of nontypeable Haemophilus influenzae to human β-defensins is influenced by lipooligosaccharide acylation. Infect. Immun. 705287-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stephens, D. S., B. Greenwood, and P. Brandtzaeg. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 3692196-2210. [DOI] [PubMed] [Google Scholar]
- 129.Stewart, L., A. L. Oesterle, J. M. Griffiss, G. A. Jarvis, B. Aagaard, and L. W. Way. 2002. Gram-negative bacteria killed by complement are associated with more severe biliary infections and produce more tumor necrosis factor-α in sera. Surgery 132408-414. [DOI] [PubMed] [Google Scholar]
- 130.Stover, A. G., J. Silva Correia, J. T. Evans, C. W. Cluff, M. W. Elliott, E. W. Jeffery, D. A. Johnson, M. J. Lacy, J. R. Baldridge, P. Probst, R. J. Ulevitch, D. H. Persing, and R. M. Hershberg. 2004. Structure-activity relationship of synthetic Toll-like receptor 4 agonists. J. Biol. Chem. 2794440-4449. [DOI] [PubMed] [Google Scholar]
- 131.Suda, Y., T. Kirikae, T. Shiyama, T. Yasukochi, F. Kirikae, M. Nakano, E. T. Rietschel, and S. Kusumoto. 1995. Macrophage activation in response to S-form lipopolysaccharides (LPS) separated by centrifugal partition chromatography from wild-type LPS: effects of the O-polysaccharide portion of LPS. Biochem. Biophys. Res. Commun. 210678-685. [DOI] [PubMed] [Google Scholar]
- 132.Takada, H., S. Kotani, S. Tanaka, T. Ogawa, I. Takahashi, M. Tsujimoto, T. Komuro, T. Shiba, S. Kusumoto, N. Kusunose, A. Hasegawa, and M. Kiso. 1988. Structural requirements of lipid A species in activation of clotting enzymes from the horseshoe crab, and the human complement cascade. Eur. J. Biochem. 175573-580. [DOI] [PubMed] [Google Scholar]
- 133.Takayama, K., N. Qureshi, C. R. H. Raetz, E. Ribi, J. Peterson, J. L. Cantrell, F. C. Pearson, J. Wiggins, and A. G. Johnson. 1984. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect. Immun. 45350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Taylor, K. R., K. Yamasaki, K. A. Radek, A. D. Nardo, H. Goodarzi, D. Golenbock, B. Beutler, and R. L. Gallo. 2007. Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Toll-like receptor 4, CD44, and MD-2. J. Biol. Chem. 28218265-18275. [DOI] [PubMed] [Google Scholar]
- 135.Toman, R., P. Garidel, J. Andra, K. Slaba, A. Hussein, M. Koch, and K. Brandenburg. 2004. Physicochemical characterization of the endotoxins from Coxiella burnetii strain Priscilla in relation to their bioactivities. BMC Biochem. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tseng, C. C., J. J. Wu, H. L. Liu, J. M. Sung, and J. J. Huang. 2002. Roles of host and bacterial virulence factors in the development of upper urinary tract infection caused by Escherichia coli. Am. J. Kidney Dis. 39744-752. [DOI] [PubMed] [Google Scholar]
- 137.Tükel, C., M. Raffatellu, D. Chessa, R. P. Wilson, M. Akcelik, and A. J. Baumler. 2006. Neutrophil influx during non-typhoidal salmonellosis: who is in the driver's seat? FEMS Immunol. Med. Microbiol. 46320-329. [DOI] [PubMed] [Google Scholar]
- 138.Ulrich, J. T., and K. R. Myers. 1995. Monophosphoryl lipid A as an adjuvant, p. 495-524. In M. F. Powell and M. J. Newman (ed.), Vaccine design: the subunit and adjuvant approach. Plenum Press, New York, NY.
- 139.Usawattanakul, W., S. Tharavanij, D. A. Warrell, S. Looareesuwan, N. J. White, S. Supavej, and S. Soikratoke. 1985. Factors contributing to the development of cerebral malaria. II. Endotoxin. Clin. Exp. Immunol. 61562-568. [PMC free article] [PubMed] [Google Scholar]
- 140.Van der Ley, P., L. Steeghs, H. J. Hamstra, J. ten Hove, B. Zomer, and L. Van Alphen. 2001. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect. Immun. 695981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vazquez-Torres, A., B. A. Vallance, M. A. Bergman, B. B. Finlay, B. T. Cookson, J. Jones-Carson, and F. C. Fang. 2004. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J. Immunol. 1726202-6208. [DOI] [PubMed] [Google Scholar]
- 142.Vesy, C. J., R. L. Kitchens, G. Wolfbauer, J. J. Albers, and R. S. Munford. 2000. LPS binding protein and phospholipid transfer protein release lipopolysaccharides from gram negative bacterial membranes. Infect. Immun. 682410-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Viriyakosol, S., M. A. Matthias, M. A. Swancutt, T. N. Kirkland, and J. M. Vinetz. 2006. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect. Immun. 74887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wain, J., V. B. Pham, V. Ha, N. M. Nguyen, S. D. To, A. L. Walsh, C. M. Parry, R. P. Hasserjian, V. A. HoHo, T. H. Tran, J. Farrar, N. J. White, and N. P. Day. 2001. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J. Clin. Microbiol. 391571-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Walsh, A. L., M. D. Smith, V. Wuthiekanun, Y. Suputtamongkol, W. Chaowagul, D. A. B. Dance, B. Angus, and N. J. White. 1995. Prognostic significance of quantitative bacteremia in septicemic melioidosis. Clin. Infect. Dis. 211498-1500. [DOI] [PubMed] [Google Scholar]
- 146.Wang, M. C., C. C. Tseng, C. Y. Chen, J. J. Wu, and J. J. Huang. 2002. The role of bacterial virulence and host factors in patients with Escherichia coli bacteremia who have acute cholangitis or upper urinary tract infection. Clin. Infect. Dis. 351161-1166. [DOI] [PubMed] [Google Scholar]
- 147.Waters, V. J., M. I. Gomez, G. Soong, S. Amin, R. K. Ernst, and A. Prince. 2007. Immunostimulatory properties of the emerging pathogen Stenotrophomonas maltophilia. Infect. Immun. 751698-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Watson, P. R., A. Benmore, S. A. Khan, P. W. Jones, D. J. Maskell, and T. S. Wallis. 2000. Mutation of waaN reduces Salmonella enterica serovar Typhimurium-induced enteritis and net secretion of type III secretion system 1-dependent proteins. Infect. Immun. 683768-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2346-352. [DOI] [PubMed] [Google Scholar]
- 150.Westendorp, R. G. J., J. A. M. Langermans, T. W. J. Huizinga, A. H. Elouali, C. L. Verweij, D. I. Boomsma, and J. P. Vandenbrouke. 1997. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 349170-173. [DOI] [PubMed] [Google Scholar]
- 151.Wiersinga, W. J., C. W. Wieland, M. C. Dessing, N. Chantratita, A. C. Cheng, D. Limmathurotsakul, W. Chierakul, M. Leendertse, S. Florquin, A. F. de Vos, N. White, A. M. Dondorp, N. P. Day, S. J. Peacock, and T. van der Poll. 2007. Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med. 4e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Woods, J. P., J. A. Frelinger, G. Warrack, and J. G. Cannon. 1988. Mouse genetic locus Lps influences susceptibility to Neisseria meningitidis infection. Infect. Immun. 561950-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wormser, G. P. 2007. Discovery of new infectious diseases—Bartonella species. N. Engl. J. Med. 3562346-2347. [DOI] [PubMed] [Google Scholar]
- 154.Yagupsky, P., and F. S. Nolte. 1990. Quantitative aspects of septicemia. Clin. Microbiol. Rev. 3269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yeaman, M. R., and N. Y. Yount. 2007. Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 5727-740. [DOI] [PubMed] [Google Scholar]
- 156.Yoon, J. W., J. Y. Lim, Y. H. Park, and C. J. Hovde. 2005. Involvement of the Escherichia coli O157:H7(pO157) ecf operon and lipid A myristoyl transferase activity in bacterial survival in the bovine gastrointestinal tract and bacterial persistence in farm water troughs. Infect. Immun. 732367-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zähringer, U., B. Lindner, and E. T. Rietschel. 1999. Chemical structure of lipid A: recent advances in structural analysis of biologically active molecules, p. 93-114. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Marcel Dekker, New York, NY.
- 158.Zhang, Z., J. P. Louboutin, D. J. Weiner, J. B. Goldberg, and J. M. Wilson. 2005. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect. Immun. 737151-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zughaier, S. M., H. C. Ryley, and S. K. Jackson. 1999. Lipopolysaccharide (LPS) from Burkholderia cepacia is more active than LPS from Pseudomonas aeruginosa and Stenotrophomonas maltophilia in stimulating tumor necrosis factor alpha from human monocytes. Infect. Immun. 671505-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zughaier, S. M., Y. L. Tzeng, S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2004. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zughaier, S. M., S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2005. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect. Immun. 732940-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]