Abstract
Kaposi's sarcoma–associated herpesvirus (KSHV)/human herpesvirus 8, which is consistently present in tissues of patients with Kaposi's sarcoma and primary effusion lymphomas, contains a gene that encodes a G protein–coupled receptor (KSHV-GPCR). We recently showed that KSHV-GPCR exhibits constitutive signaling via activation of phosphoinositide-specific phospholipase C and stimulates cell proliferation and transformation. In this study, we determined whether normal cellular mechanisms could inhibit constitutive signaling by KSHV-GPCR and thereby KSHV-GPCR–stimulated proliferation. We show that coexpression of GPCR-specific kinases (GRKs) and activation of protein kinase C inhibit constitutive signaling by KSHV-GPCR in COS-1 monkey kidney cells and in mouse NIH 3T3 cells. Moreover, GRK-5 but not GRK-2 inhibits KSHV-GPCR–stimulated proliferation of rodent fibroblasts. These data provide evidence that cell regulatory pathways of receptor desensitization may be therapeutic targets in human diseases involving constitutively active receptors.
Kaposi's sarcoma–associated herpesvirus (KSHV)/human herpesvirus 8 is a gammaherpesvirus with homology to Herpesvirus saimiri and Epstein-Barr virus, which are viruses that cause transformation of lymphocytes (1, 2). Accumulating evidence suggests that KSHV may be involved in the pathogenesis of primary effusion (or body cavity-based) lymphomas (3) and Kaposi's sarcoma (KS) (4). KSHV contains a gene that encodes a G protein–coupled receptor (KSHV-GPCR; references 5, 6). We recently showed that KSHV-GPCR exhibits constitutive signaling, that is, signaling in the absence of agonist, via activation of phosphoinositide-specific phospholipase C (PLC) (7). Moreover, expression of KSHV-GPCR stimulates cell proliferation (7) and causes transformation of mouse fibroblasts (8). KSHV-GPCR is homologous to a GPCR encoded by H. saimiri (ECRF3) (9) and to human IL-8 receptors (human chemokine receptors CXCR1 and CXCR2; references 10, 11). However, ECRF3, CXCR1, and CXCR2 do not exhibit constitutive signaling although they appear to signal via PLC, like KSHV-GPCR. When activated by chemokines in a monkey kidney (COS) cell line, CXCR1 and CXCR2 stimulate the formation of inositol phosphate second messenger molecules (IPs) when G protein α subunit Gα16 is coexpressed (12). In Xenopus laevis oocytes, chemokine activation of ECRF3 leads to calcium efflux that is likely caused by PLC-mediated generation of inositol-1,4,5-trisphosphate (13).
GPCRs in general, and chemokine GPCRs specifically, may be desensitized by GPCR-specific kinases (GRKs) and by second messenger–activated protein kinases such as protein kinase C (PKC) (14). For phosphorylation and desensitization by GRKs, GPCRs must be in an active state. Desensitization occurs because GPCRs phosphorylated by GRKs bind arrestin proteins and this complexation inhibits GPCR coupling to G proteins. In general, GPCRs must be occupied by an agonist to be activated and, therefore, most unoccupied GPCRs are not phosphorylated by GRKs and are not desensitized. Constitutively active GPCRs, however, are GRK substrates and may be desensitized even in the absence of agonist (15, 16). GRKs themselves are activated by several mechanisms including binding to activated GPCRs, interacting with phospholipids, and binding to G protein βγ subunits (14). These processes allow translocation of the GRK to the cell surface membrane, but phosphorylation is limited by accessibility to activated GPCRs. In contrast to the mechanism of desensitization mediated by GRKs, second messenger–activated protein kinases must be activated and then may phosphorylate, and thereby desensitize, inactive or active GPCRs. The mechanism whereby second messenger–activated protein kinases inhibit coupling of receptors to G proteins has not been determined. Chemokine receptors are substrates for these kinases and may be desensitized by GRKs and PKC. For example, the monocyte chemoattractant protein-1 receptor (CCR2B) that is a GPCR for CC chemokines has been shown to be desensitized by GRK3 but not by GRK1 or 2 (17). CXCR1 and CXCR2 appear to undergo GRK-induced desensitization in neutrophils (18). In fact, CXCR1 appears to be desensitized by both GRKs and PKC (19). In this report, we show that increases in GRK and PKC activities lead to inhibition (desensitization) of constitutive signaling by KSHV-GPCR.
Materials and Methods
COS-1 cells were transfected by the DEAE-dextran method with plasmid encoding KSHV-GPCR (pcKSHV-GPCR) as described (7). In different experiments, plasmids encoding GRK2, 4, 5, or 6, CXCR2, or Gα16 were transfected. All transfection cocktails were made to contain equal amounts of plasmid DNA by the addition of appropriate amounts of vector. 24 h after transfection, cells were harvested and reseeded in 12-well plates in Dulbecco's modified Eagle's medium with 10% NuSerum and 1 μCi/ml myo- [3H]inositol (where indicated), and maintained in culture in 5% CO2 at 37°C for an additional 24 h. After removing the culture medium and washing with buffer, cells were incubated in Hank's balanced salt solution containing 25 mM Hepes, pH 7.4, and 10 mM LiCl. Cells were harvested after 90 min and IP accumulation was measured using ion exchange chromatography as described previously (20). IP accumulation was measured as the 3H-radioactivity in IPs as a fraction of the 3H-radioactivity in inositol lipids plus IPs (lipids + IPs).
Competition binding experiments were performed as described (7). In brief, COS-1 cells were transfected with plasmid encoding KSHV-GPCR (10 μg/ml) in the absence or presence of plasmid encoding GRK5 (3 μg/ml) and tested for IL-8 binding 48 h after transfection. Approximately 100,000 cells/well were washed with Hank's balanced salt solution containing 25 mM Hepes, pH 7.4. Between 0.3 and 0.5 nM 125I–IL-8 in the absence or presence of various concentrations of unlabeled IL-8 was added and binding was allowed to proceed at 4°C for 2 h. The binding buffer contained bovine serum albumin (1 mg/ml), bacitracin (1 mg/ml), and phenyl methyl sulfonyl fluoride (1 mM). Assays were terminated by aspirating the binding buffer, washing three times with chilled buffer, and solubilizing the cells with 0.4 N NaOH. An aliquot of this solution was counted in a gamma counter and the data were expressed as cpm bound. Binding curves were analyzed using Prism software (GraphPad, Inc., San Diego, CA).
For experiments with NIH 3T3 cells or NRK cells, the cDNA encoding KSHV-GPCR was subcloned into plasmid pCEFL (a gift from Dr. Silvio Gutkind, National Institute of Dental Research, National Institutes of Health). NIH 3T3 cells and NRK cells were transfected by the calcium phosphate method with plasmid encoding KSHV-GPCR (pCEFLKSHV-GPCR) or with pCEFL without or with plasmids encoding GRK2, 4, 5, or 6. On the day after transfection, the medium was removed and the cells were incubated in Dulbecco's modified Eagle's medium with 10% calf serum and 0.75 mg/ml Geneticin. Cell populations were maintained in medium containing Geneticin. Experiments were performed 5–9 d after transfection.
Materials.
Plasmids encoding bovine GRK2, human GRK4, bovine GRK5, and human GRK6 were provided by Dr. Robert J. Lefkowitz (Howard Hughes Medical Institute, Duke University Medical Center, Durham, NC). Plasmid encoding Gα16 was provided by Dr. Melvin I. Simon (California Institute of Technology, Pasadena, CA). Plasmid encoding CXCR2 was provided by Dr. Lijun Wu (LeukoSite, Inc., Cambridge, MA). COS-1 and NIH 3T3 cells were from the American Type Culture Collection (Rockville, MD). myo-[3H]inositol and 125I–IL-8 were purchased from DuPont New England Nuclear (Boston, MA). Dulbecco's modified Eagle's medium, Geneticin, calf serum, and Hank's balanced salt solution were from GIBCO BRL (Gaithersburg, MD). Nu-Serum was from Collaborative Biomedical Products (Bedford, MA). Recombinant human IL-8 was from R&D Sys. Inc. (Minneapolis, MN). All other chemicals were from Sigma Chemical Co. (St. Louis, MO).
Results and Discussion
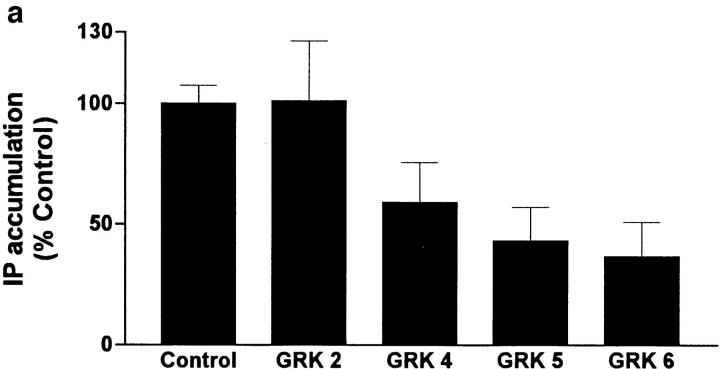
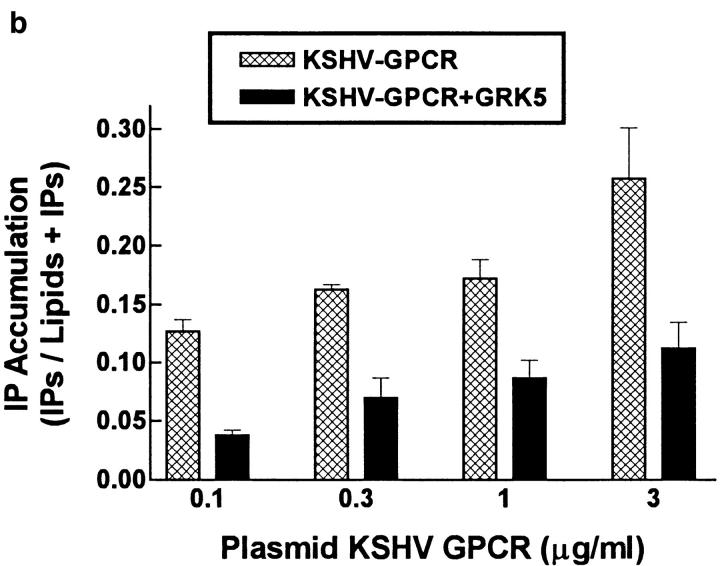
To determine whether constitutive signaling by KSHV-GPCRs could be inhibited by GRKs, we coexpressed KSHV-GPCR and GRK2, 4, 5, or 6 in COS-1 cells. We measured formation of IPs to assess signaling. Expression of GRK2, 4, 5, or 6 alone had no effect on IP formation (data not shown). Fig. 1 a illustrates that GRKs 4, 5, and 6, but not GRK2, inhibited KSHV-GPCR stimulation of IP formation by 41–63%. The lack of effect of GRK2 on KSHV-GPCR signaling was not due to lack of its expression because GRK2 inhibited agonist-stimulated signaling by the thyrotropin-releasing hormone receptor by 20 ± 9.4% in parallel incubations. Of note, GRKs 4, 5, and 6 appear to comprise a distinct subfamily of receptor-specific kinases (14). Specificity of desensitization by different GRKs has been observed (21, 22). Fig. 1 b confirms that GRK5 inhibits KSHV-GPCR signaling and shows that the extent of inhibition of IP formation by GRK5 was similar, ∼53%, at all levels of KSHV-GPCR expression. This level of inhibition is similar to that found for inhibition of agonist-stimulated IP second messenger formation when α1B-adrenergic receptors and GRKs were coexpressed in COS-7 cells (22).
Figure 1.
Effect of coexpression of KSHV-GPCR and GRKs on IP formation in COS-1 cells. (a) Comparison of the effects of GRK2, 4, 5, and 6. COS-1 cells were cotransfected with plasmids encoding KSHV-GPCR (3 μg/ml) and one of the GRKs (3 μg/ml), and IP accumulation was measured after 90 min. IP accumulation in cells expressing KSHV-GPCRs (Control) is from 4–10-fold higher than in untransfected or “mock-transfected” (cells transfected with pcDNA3.1(+) alone) COS-1 cells, which was 0.038 ± 0.0046 disintegrations per minute (dpm) in IPs/ (dpm in IPs + dpm in lipids) per 90 min. Data represent the mean ± SEM of four experiments. (b) Effect of expressing various levels of KSHV-GPCR on inhibition of IP accumulation by GRK5. COS-1 cells were cotransfected with various amounts of plasmid encoding KSHV-GPCR (from 0.1 to 3 μg/ml) in the presence or absence of plasmid encoding GRK5 (3 μg/ml). Data represent the mean ± SD of triplicate determinations in one of two experiments.
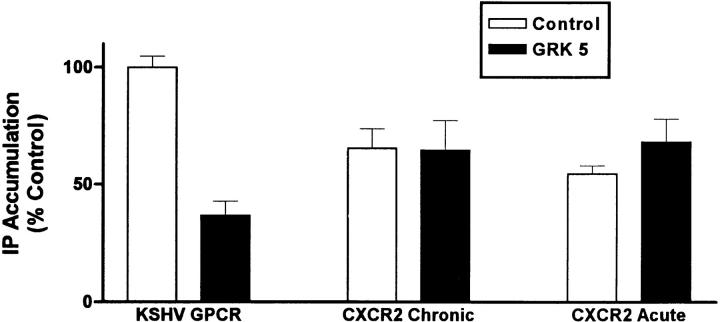
To determine whether the effect of GRK5 to inhibit IP formation was general or whether it may be specific for KSHV-GPCR, we coexpressed GRK5 and CXCR2. Because chemokine signaling via CXCR2 in COS cells requires Gα16 (or Gα14 or Gα15) (12), we coexpressed Gα16 also. Coexpression of Gα16 does not enhance KSHV-GPCR signaling in COS-1 cells (7). We reasoned that it may be necessary to activate CXCR2 before measuring the effect of the GRK on agonist-stimulated signaling so as to have activated CXCR2 as a substrate for GRK-mediated phosphorylation. Therefore, we measured the effects of GRK5 on IL-8 stimulation of IP formation via CXCR2 when IL-8 was added acutely or added for 1 or 48 h before IP measurement. As we showed previously (7), IL-8 stimulated IP formation via CXCR2 to a level only 55–65% of that stimulated constitutively by KSHV-GPCR under these conditions. In this series of experiments, KSHV-GPCR stimulation of IP formation was inhibited 63% by coexpression of GRK5 (Fig. 2). In contrast, GRK5 had no effect on IL-8 stimulation of IP formation via CXCR2 whether IL-8 was present only during the period of IP measurement or chronically. Thus, it appears that GRK5 does not desensitize all GPCRs under these conditions, but there is specificity to the effect of GRK5 on KSHV-GPCR signaling.
Figure 2.
Effect of expressing GRK5 on IP accumulation in COS-1 cells expressing human CXCR2. COS-1 cells were cotransfected with plasmid encoding KSHV-GPCR (3 μg/ml) or with plasmids encoding human CXCR2 (3 μg/ml), Gα16 (2 μg/ml), and a plasmid encoding GRK5 (3 μg/ml). CXCR2 expressing cells were treated with IL-8 (0.1 μM) chronically (for 1 or 48 h) or acutely (during measurement of IP accumulation). There was no difference between cells exposed to IL-8 for 1 or 48 h. Human recombinant IL-8 was added to a final concentration of 0.25 μg/ml. Data represent the mean ± SEM of three experiments.
It was recently proposed that GRKs (and arrestin) are involved in GPCR internalization (23). Because enhanced rates of internalization can lead to increased rates of receptor degradation (24), it was possible that the decreased signaling observed when KSHV-GPCR and GRK5 were coexpressed was secondary to decreased receptor levels due to increased receptor turnover. Decreased receptor expression might have occurred by other mechanisms also. We found that the levels (and affinities, equilibrium inhibitory constants of 35 ± 12 nM) of KSHV-GPCRs were not different in COS-1 cells expressing KSHV-GPCR alone or KSHV-GPCR and GRK5 (data not shown). Thus, GRK inhibition of KSHV-GPCR signaling occurred without a change in KSHV-GPCR expression.
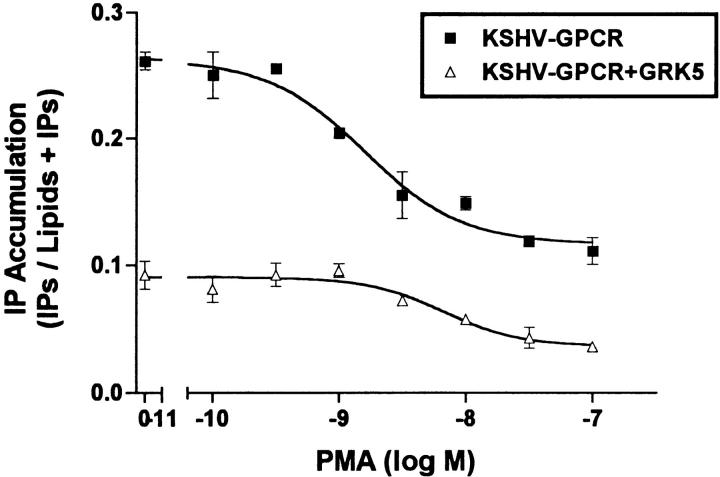
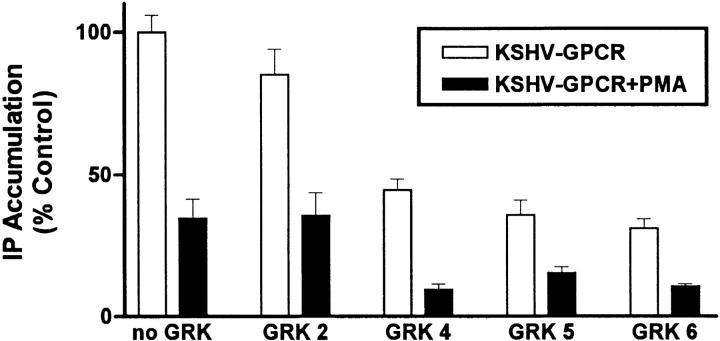
We considered the possibility that KSHV-GPCR signaling could be inhibited by PKC also. Fig. 3 illustrates that phorbol 12-myristate 13-acetate (PMA) causes a dose-dependent inhibition of IP formation by KSHV-GPCR. Only active phorbol esters, PMA (0.1 μM, to 36 ± 4.7% of control), and phorbol 12,13-didecanoate (0.1 μM, to 66 ± 5.4% of control), but not an inactive phorbol ester, 4α-phorbol 12,13-didecanoate (0.1 μM, to 96 ± 5.5% of control), inhibited KSHV-GPCR–stimulated IP formation. These effects occur exclusively via activation of PKC because they are prevented by a specific PKC inhibitor, calphostin C (25; data not shown). As was found for CXCR1 (19), we found that PMA inhibited IL-8 stimulation of IP formation via CXCR2 (data not shown). Thus, KSHV-GPCR signaling, like signaling by CXCR1 and CXCR2, is inhibited by PKC activation.
Figure 3.
Inhibition of KSHV-GPCR stimulation of IP formation by PMA. COS-1 cells were transfected with plasmid encoding KSHV-GPCR (3 μg/ml) in the absence or presence of plasmid encoding GRK5 (3 μg/ml) and IP accumulation was measured after 90 min. PMA was added 20 min before the experimental incubation at the final concentrations shown. Data represent the mean ± SD of triplicate determinations in one of two experiments.
Fig. 4 illustrates that the effects of expression of GRK4, 5, or 6 and PKC activation on constitutive IP formation stimulated by KSHV-GPCR are additive. GRK2 had no effect on KSHV-GPCR–stimulated IP formation and did not affect inhibiton by PMA. In this series of experiments, GRK4, 5, or 6 inhibited IP formation by 56–69%, PMA inhibited IP formation by 65%, and the combination of expression of GRK4, 5, or 6 and activation of PKC by PMA inhibited IP formation by 85–90%.
Figure 4.
Additive effects of GRK expression and PMA on IP formation stimulated by KSHV-GPCR. COS-1 cells were transfected with plasmid encoding KSHV-GPCR (3 μg/ml) in the absence or presence of plasmids encoding GRK2, 4, 5, or 6 (3 μg/ml). PMA was added 20 min before the experimental incubation and IP accumulation was measured for the subsequent 90 min. Data represent the mean ± SEM of two experiments.
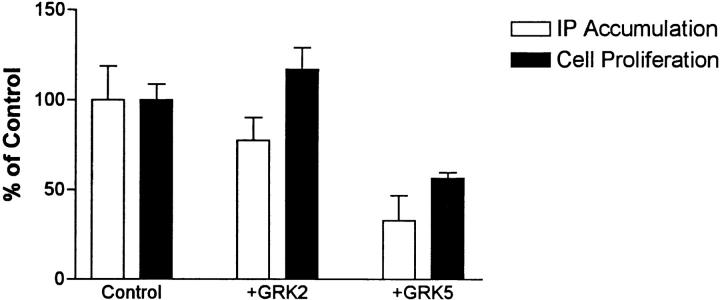
To show that GRK-mediated inhibition of KSHV-GPCR signaling was not peculiar to COS cells in which proteins may be markedly overexpressed, and that these protein kinases would inhibit an important cellular response to KSHV-GPCR, we constructed NIH 3T3 and NRK cell populations expressing KSHV-GPCRs, KSHV-GPCRs and GRK2, or KSHV-GPCRs and GRK5. Mouse NIH 3T3 cells and rat NRK cells are good models because proliferating fibroblasts are typically present in KS lesions (4) and KSHV-like viruses have recently been found in retroperitoneal fibromatosis tissues in monkeys (26). Fig. 5 shows that, as in COS-1 cells, expression of GRK5 inhibited IP accumulation in NIH 3T3 cells; GRK2 had no effect. More importantly, expression of GRK5, but not GRK2, inhibited proliferation of NIH 3T3 cells (Fig. 5). GRK4, 5, and 6, but not GRK2, inhibited KSHV-GPCR–stimulated proliferation of NRK cells by >75% (data not shown). Thus, inhibition of KSHV-GPCR signaling by expression of GRK5 was found in COS-1 and NIH 3T3 cells and of KSHV-GPCR stimulation of proliferation in NIH 3T3 and NRK cells. We recently showed that expression of KSHV-GPCR induces transformation of NIH 3T3 cells and that transformation is inhibited by coexpression of GRK5 but not of GRK2 (8).
Figure 5.
Effect of expressing GRK2 or GRK5 on KSHV-GPCR– stimulated IP accumulation and proliferation in NIH 3T3 cells. NIH 3T3 cells expressing KSHV-GPCRs (Control), KSHV-GPCRs and GRK2 (+GRK2), or KSHV-GPCRs and GRK5 (+GRK5) were used 8 or 9 d after transfection. For IP experiments, cells were labeled with [3H]myo-inositol for 48 h and IP accumulation was measured during a 90 min incubation. IP accumulation in cells expressing KSHV-GPCR alone was three- to fivefold greater than in mock-transfected cells (cells transfected with pCEFL). Data represent the mean ± SD of triplicate determinations in two experiments. For proliferation experiments, cells were harvested and seeded 5 d after transfection and cells were counted 4 d later. Data represent the mean ± SD of triplicate determinations in two experiments.
Constitutive signaling activity has been exhibited by some native GPCRs (27–29). More commonly, however, agonist-independent signaling has been observed with mutated GPCRs including a number that have been associated with human diseases (30), including nonmalignant tumors (31, 32). Moreover, GPCRs have been found to be oncogenic under some circumstances (33). Although Herpesvirus saimiri is an oncogenic virus (1), it is not known whether the GPCR encoded within the genome of this virus is involved in the transformation process. As KSHV is found in primary effusion lymphomas and KS tissues (3, 4, 34) and KSHV-GPCR stimulates proliferation of fibroblasts in tissue culture (7), it is possible that KSHV-GPCR is causally involved in tumorigenesis in these diseases. However, this has not yet been shown.
We (7) had previously suggested that if KSHV-GPCR were shown to be involved in the pathogenesis of primary effusion lymphoma or KS it would be a target for drug therapy using a negative antagonist (or inverse agonist). An inverse agonist is an antagonist that inactivates constitutively active receptors even in the absence of an agonist (35). Other investigators have suggested that the protein kinases involved in desensitization of GPCRs may be drug targets (36). In this report, we have shown that coexpression of GRK4, 5, or 6 as well as direct activation of PKC inhibit constitutive signaling by KSHV-GPCR. Moreover, inhibition of KSHV-GPCR signaling by GRKs causes inhibition of KSHV-GPCR–stimulated cell proliferation (Fig. 5) and transformation (8). Thus, drug-mediated activation of GRK4, 5, or 6 or of PKC might inhibit tumorigenesis. The GRKs would be the preferred targets as their activation would presumably lead to receptor-specific effects.
In conclusion, to our knowledge, this is the first demonstration of GRK and PKC inhibition of constitutive signaling by a GPCR associated with human disease. Although the molecular mechanism(s) of KSHV-GPCR desensitization was not proved, these data provide evidence that cell regulatory pathways of receptor desensitization may be therapeutic targets in diseases involving constitutively active receptors. Based on the findings reported here, we suggest that these protein kinases, along with the KSHV-GPCR itself, may be therapeutic targets for pleural effusion lymphomas and KS if KSHV-GPCR is shown to be involved in the pathogenesis of these diseases.
Footnotes
We thank Bianca Santomasso for excellent technical assistance.
This work was supported by National Institutes of Health grants DK-43036 (to M.C. Gershengorn), CA-73531 (to E. Cesarman) and AI-39192 (to E.A. Mesri).
Address correspondence to Marvin C. Gershengorn, Cornell University Medical College, 1300 York Ave., Rm A328, New York, NY 10021. Phone: 212-746-6275; Fax: 212-746-6289; E-mail: mcgersh@mail.med.cornell.edu
References
- 1.Rangan SR, Martin LN, Enright FM, Abee CR. Herpesvirus saimiri-induced lymphoproliferative disease in howler monkeys. J Natl Cancer Inst. 1977;59:165–171. doi: 10.1093/jnci/59.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Miller G. The oncogenicity of Epstein-Barr virus. J Infect Dis. 1974;130:187–205. doi: 10.1093/infdis/130.2.187. [DOI] [PubMed] [Google Scholar]
- 3.Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Said J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi's sarcoma–associated herpes virus. Blood. 1996;88:645–656. [PubMed] [Google Scholar]
- 4.Offermann MK. Kaposi's sarcoma and HHV-8. Trends Microbiol. 1996;4:419. doi: 10.1016/0966-842x(96)84953-5. [DOI] [PubMed] [Google Scholar]
- 5.Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, Moore PS, Chang Y, Knowles DM. Kaposi's sarcoma associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang YA, Moore PS. Nucleotide sequence of the Kaposi sarcoma–associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn MC, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 8.Bais C, Santomasso B, Coso O, Arvanitakis L, Geras-Raaka E, Gutkind JS, Asch AS, Cesarman E, Gershengorn MC, Mesri EA. G-protein–coupled receptor of Kaposi's sarcoma–associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 9.Nicholas J, Cameron KR, Honess RW. Herpesvirus saimiri encodes homologues of G protein–coupled receptors and cyclins. Nature. 1992;355:362–365. doi: 10.1038/355362a0. [DOI] [PubMed] [Google Scholar]
- 10.Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. [PubMed] [Google Scholar]
- 11.Holmes WE, Lee J, Kuang W-J, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1280. [PubMed] [Google Scholar]
- 12.Wu D, LaRosa GJ, Simon MI. G protein–coupled signal transduction pathways for interleukin-8. Science. 1993;261:101–103. doi: 10.1126/science.8316840. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja SK, Murphy PM. Molecular piracy of mammalian interleukin-8 receptor type B by herpesvirus saimiri. J Biol Chem. 1993;268:20691–20694. [PubMed] [Google Scholar]
- 14.Premont RT, Inglese J, Lefkowitz RJ. Protein kinases 3: protein kinases that phosphorylate activated G protein–coupled receptors. FASEB J. 1995;9:175–182. doi: 10.1096/fasebj.9.2.7781920. [DOI] [PubMed] [Google Scholar]
- 15.Ren Q, Kurose H, Lefkowitz RJ, Cotecchia S. Constitutively active mutants of the α2-adrenergic receptor. J Biol Chem. 1993;268:16483–1648. [PubMed] [Google Scholar]
- 16.Pei G, Samama P, Lohse M, Wang M, Codina J, Lefkowitz RJ. A constitutively active mutant β2-adrenergic receptor is constitutively desensitized and phosphorylated. Proc Natl Acad Sci USA. 1994;91:2699–2702. doi: 10.1073/pnas.91.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franci C, Gosling J, Tsou CL, Coughlin SR, Charo IF. Phosphorylation by a G protein–coupled kinase inhibits signaling and promotes internalization of the monocyte chemoattractant protein-1 receptor: critical role of carboxyl-tail serines/threonines in receptor function. J Immunol. 1996;157:5606–5612. [PubMed] [Google Scholar]
- 18.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 19.Richardson RM, DuBose RA, Ali H, Tomhave ED, Haribabu B, Snyderman R. Regulation of human interleukin-8 receptor A: identification of a phosphorylation site involved in modulating receptor functions. Biochemistry. 1995;34:14193–14201. doi: 10.1021/bi00043a025. [DOI] [PubMed] [Google Scholar]
- 20.Straub RE, Frech GC, Joho RH, Gershengorn MC. Expression cloning of a cDNA encoding the mouse pituitary thyrotropin-releasing hormone receptor. Proc Natl Acad Sci USA. 1990;87:9514–9518. doi: 10.1073/pnas.87.24.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiberi M, Nash SR, Bertrand L, Lefkowitz RJ, Caron MG. Differential regulation of dopamine D1A receptor responsiveness by various G protein–coupled receptor kinases. J Biol Chem. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- 22.Diviani D, Lattion AL, Larbi N, Kunapuli P, Pronin A, Benovic JL, Cotecchia S. Effect of different G protein–coupled receptor kinases on phosphorylation and desensitization of the α1B-adrenergic receptor. J Biol Chem. 1996;271:5049–5058. doi: 10.1074/jbc.271.9.5049. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson SSG, Zhang J, Barak LS, Caron MG. G-protein–coupled receptor kinases and arrestins: regulators of G-protein–coupled receptor sequestration. Biochem Soc Trans. 1996;24:953–959. doi: 10.1042/bst0240953. [DOI] [PubMed] [Google Scholar]
- 24.Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 26.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW, Jr, Thouless ME, Tsai C-C, Bosch ML. Identification of two homologs of the Kaposi's sarcoma–associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 28.Cohen DP, Thaw CN, Varma A, Gershengorn MC, Nussenzveig DR. Human calcitonin receptors exhibit agonist-independent (constitutive) signaling activity. Endocrinology. 1997;138:1400–1405. doi: 10.1210/endo.138.4.5046. [DOI] [PubMed] [Google Scholar]
- 29.Jinsi-Parimoo A, Gershengorn MC. Constitutive activity of native thyrotropin-releasing hormone receptors revealed using a protein kinase C–responsive reporter gene. Endocrinology. 1997;138:1471–1475. doi: 10.1210/endo.138.4.5059. [DOI] [PubMed] [Google Scholar]
- 30.Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- 31.Coughlin SR. Expanding horizons for receptors coupled to G proteins: diversity and disease. Curr Opin Cell Biol. 1994;6:191–197. doi: 10.1016/0955-0674(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 32.Van Sande J, Parma J, Tonacchera M, Swillens S, Dumont J, Vassart G. Somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metab. 1995;80:2577–2585. doi: 10.1210/jcem.80.9.7673398. [DOI] [PubMed] [Google Scholar]
- 33.Julius D, Livelli TJ, Jessell TM, Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989;244:1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- 34.Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. Establishment and characterization of a body cavity–based lymphoma cell line (BC-3) harboring Kaposi's sarcoma–associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;86:2708–2714. [PubMed] [Google Scholar]
- 35.Schutz W, Freissmuth M. Reverse intrinsic activity of antagonists on G protein-coupled receptors. Trends Pharmacol Sci. 1992;13:376–380. doi: 10.1016/0165-6147(92)90116-n. [DOI] [PubMed] [Google Scholar]
- 36.Chuang TT, Iacovelli L, Sallese M, De Blasi A. G protein–coupled receptors: heterologous regulation of homologous desensitization and its implications. Trends Pharmacol Sci. 1996;17:416–421. doi: 10.1016/s0165-6147(96)10048-1. [DOI] [PubMed] [Google Scholar]