Abstract
The endothelium is morphologically and functionally adapted to meet the unique demands of the underlying tissue. At the present time, little is known about the molecular basis of endothelial cell diversity. As one approach to this problem, we have chosen to study the mechanisms that govern differential expression of the endothelial cell–restricted von Willebrand factor (vWF) gene. Transgenic mice were generated with a fragment of the vWF gene containing 2,182 bp of 5′ flanking sequence, the first exon and first intron coupled to the LacZ reporter gene. In multiple independent lines of mice, β-galactosidase expression was detected within endothelial cells in the brain, heart, and skeletal muscle. In isogeneic transplantation models, LacZ expression in host-derived auricular blood vessels was specifically induced by the microenvironment of the heart. In in vitro coculture assays, expression of both the transgene and the endogenous vWF gene in cardiac microvascular endothelial cells (CMEC) was upregulated in the presence of cardiac myocytes. In contrast, endothelial cell levels of thrombomodulin protein and mRNA were unchanged by the addition of ventricular myocytes. Moreover, CMEC expression of vWF was not influenced by the addition of 3T3 fibroblasts or mouse hepatocytes. Taken together, the results suggest that the vWF gene is regulated by vascular bed–specific pathways in response to signals derived from the local microenvironment.
The endothelium exhibits a remarkable diversity of cellular properties that are uniquely adapted to the needs of the underlying tissue. Heterogeneity within the endothelium has been described at the level of cell structure, antigen composition, mRNA expression, and cell function (Gerritsen, 1987; Kumar et al., 1987; Turner et al., 1987; Tomlinson et al., 1991; Page et al., 1992; Gerritsen and Bloor, 1993). For example, the postcapillary, high venule endothelial cells in lymphoid organs support the binding and migration of lymphocytes via the specific interaction of adhesion molecules with lymphocyte homing receptors (Streeter et al., 1988; Berg et al., 1989; Girard and Springer, 1995). On the other hand, the endothelial cells that line the small blood vessels of the brain possess a unique expression pattern of cell surface receptors, transporters, and intracellular enzymes that serve to tightly regulate the exchange of solutes between blood and brain parenchyma (Bradbury, 1993; Schlosshauer, 1993). Distinct endothelial cell phenotypes have also been documented in other organs such as the liver, kidney, and lung (DeFouw, 1988; Fleming and Jones, 1989). In addition, the endothelium has been shown to vary in its response to pathophysiological stimuli. Escherichia coli–induced sepsis in baboons results in the selective activation of tissue factor in a subpopulation of endothelial cells within the marginal zone of splenic follicles (Drake et al., 1993). In mice, the systemic delivery of lipopolysaccharide results in a specific upregulation of the pentraxin gene family member, ptx-3, specifically within the vascular beds of the heart and skeletal muscle (Introna et al., 1996). These and other examples of the vascular bed–specific endothelial cell response underscore the potential role of phenotypic heterogeneity in mediating focal vasculopathic disease states.
Beyond a large descriptive catalogue of endothelial cell phenotypes, surprisingly little is known about the molecular basis of vascular diversity. An important question that continues to elude us is whether the phenotypic patterns are genetically inherited from distinct sublineages or rather governed by signals residing within the microenvironment. In vitro investigations using embryonic stem cell cultures suggest that endothelial cell differentiation and early vasculogenesis are genetically predetermined (Wang et al., 1992). Retroviral cell tagging studies in chicken embryos have shown different clonal origins for endocardial versus coronary artery endothelial cells (Mikawa and Fischman, 1992). On the other hand, in vivo transplant studies using avian species have pointed to the critical role of environmental cues in establishing blood vessel patterning during development (Poole and Coffin, 1989; Noden, 1990). Unfortunately, these experimental approaches are difficult to adapt to the mammalian system, owing to poor accessibility of embryos and the lack of appropriate cell markers. Nevertheless, there is evidence that regional specialization of the endothelium in mammals may be conditioned by exogenous factors. Perhaps the best examples are found in studies of the blood–brain barrier, in which both in vitro culture and in vivo transplant studies have documented the ability of astrocytes to induce the appropriate phenotype in endothelial cells (Stewart and Wiley, 1981; Beck et al., 1984; Janzer and Raff, 1987; Maxwell et al., 1987; Tao-Cheng et al., 1987; Lobrinus et al., 1992). Additional studies have demonstrated a direct influence of extracellular signals on gene expression in other endothelial cell types. For example, preproendothelin-1 mRNA in rat cardiac microvascular endothelial cells was found to be upregulated when these cells were grown in coculture with ventricular myocytes (Nishida et al., 1993). Shear stress has been shown to modulate the transcription of a number of endothelial cell genes through the induction of specific DNA–protein interactions (Resnick et al., 1993; Resnick and Gimbrone, 1995). At this time, it is not clear what role these and other examples of modulatable gene expression play in establishing and/or maintaining the phenotype of a given endothelial cell in the intact animal.
Regardless of the relative roles of clonality and environment in mediating phenotypic differences within the endothelium, the establishment and maintenance of vascular diversity is ultimately controlled at a transcriptional level. Regulation of endothelial cell gene expression has been shown to vary between blood vessel types and vascular beds (Bahnak et al., 1989; Hadley et al., 1994; Kaipainen et al., 1995; Lassalle et al., 1996; Smith et al., 1996). For example, the multimeric glycoprotein von Willebrand factor (vWF),1 a cofactor for platelet adhesion and a carrier for the antihemophiliac factor (for review see Sadler, 1991; Ruggeri and Ware, 1993), is heterogeneously distributed throughout the vascular tree and is associated with regional variations in mRNA levels (Rand et al., 1987; Wu et al., 1987; Bahnak et al., 1989; Coffin et al., 1991; Page et al., 1992; Smith et al., 1996; Aird, W., and R.D. Rosenberg, unpublished results). vWF is expressed at higher levels on the venous side of the circulation compared with arteries and arterioles. By contrast, consistently low levels of vWF are present within the sinusoidal endothelial cells of the liver and spleen. In en face preparations of the rat aorta, expression of vWF appears to vary from one endothelial cell to another (Senis et al., 1996). The gene product is present in clusters of endothelial cells oriented along the longitudinal axis of blood flow and is particularly concentrated in endothelial cells lining the ostia of the intercostal arteries. The administration of thrombin resulted in an increase of histochemically detected vWF expression, suggesting that previously nonexpressing endothelial cells may be recruited to produce vWF (Senis et al., 1996). Taken together, the available evidence suggests that the transcriptional control of vWF varies from one endothelial cell to another and that cell-to-cell variation may be programmed by the extracellular environment. Indeed, an understanding of how these transcriptional networks operate selectively in subsets of endothelial cells should provide an initial framework with which to unravel the molecular mechanisms of differential gene expression and endothelial cell heterogeneity.
Until recently, little was known about the transcriptional regulation of vWF. In transgenic mice, a segment of the human vWF gene containing 487 bp of 5′ flanking sequence, as well as the first exon (+1 – +246) was found to direct expression to a subpopulation of endothelial cells in the adult brain (Aird et al., 1995). These observations suggested that the transgene is under vascular bed–specific transcriptional control and implied that more widespread expression of the vWF gene might be dependent on promoter sequences either proximal to or distal to the 733-bp fragment. To test this hypothesis, a larger segment of the vWF gene containing 2,182 bp of 5′ flanking sequence, the first exon, and the first intron was coupled to the LacZ reporter gene, and the resulting construct (vWFlacZ-2) was used to generate additional lines of transgenic mice. As we report below, transgene expression in these mice was detected not only within blood vessels of the brain but also within the microvasculature of the heart and skeletal muscle. These findings indicate that vWF expression is indeed regulated by distinct organ-specific transcriptional pathways. We also show by transplantation and coculture techniques that cardiac vascular bed–specific control of the vWF transgene and the endogenous gene is modulated by interactions between microvascular endothelial cells and cardiomyocytes. The results support the existence of novel, tissue-specific pathways that regulate the function of endothelial cells in response to signals derived from their local microenvironment.
Materials and Methods
Generation and Analysis of Transgenic Mice
The vWF sequence in vWFlacZ-2 was cloned from a human genomic library (Stratagene, La Jolla, CA). Through sequential cloning steps, the sequence including 2,182 bp of 5′ flanking sequence, the first exon, the first intron, and the translational start site of the human vWF gene was coupled to the SDK sequence, LacZ cDNA and simian virus polyadenylation signal of pSDKlacZpA (generous gift from J. Rossant, Mount Sinai Hospital, Toronto, Canada). The generation and identification of transgenic mice as well as the analysis of tissue sections and whole mounts for LacZ activity and vWF immunohistochemistry were carried out as previously described (Aird et al., 1995). For reverse transcriptase (RT)-PCR, total RNA isolation was isolated from vWFlacZ-2 mouse organs using a guanidinium thiocyanate phenol–chloroform single-step extraction (Stratagene). Approximately 10 μg of total RNA from each organ was treated with DNase and then incubated with reverse transcriptase in the presence of [32P]dCTP. First strand cDNA was then used as template for PCR with primer sets specific for E. coli LacZ (5′-GCATCGAGCTGGGTAATAA GCGTTGGCAAT-3′, 5′-GACACCAGACCAACTGGTAATGGTAG-CGAC-3′), mouse vWF (5′-ATGATGGAGAGGTTACACATC-3′, 5′ GGCAGTTGCAGACCCTCCTTG-3′) and mouse thrombomodulin (5′ ACTGATCGGACGCTGCAGAAGTTCTGA 3′, 5′-GGCCCAGTATGTCTCAAGATAGCAATG-3′). The PCR parameters were 95°C for 3 min, 95°C for 45 s and 72°C for 3.5 min, for a total of 40 cycles, followed by 7 min of elongation at 72°C. PCR products were resolved on a 1.2% agarose gel and visualized with ethidium bromide.
Cardiac and Lung Transplantations
Cardiac transplantation experiments were performed as previously described (Rossi, 1992). Briefly, adult vWFlacZ-2 recipient mice were anesthetized with intraperitoneal avertin, one or both ears were cleaned with 70% ethanol and a subdermal incision 2–5 mm in length was made with a scalpel along the transverse axis of the ear. A pair of microdissection scissors was then used to dissect away intradermal tissue towards the apex of the ear, creating a subdermal ear pouch. The donor heart was removed from the wild-type neonates (12–24-h old) and inserted into the ear pouch. Gentle pressure with delicate curved forceps was then used to express free air from the pocket and to close the incision. Transplanted mice were returned to their cages and cared for according to standard protocols. Neonatal wild-type lung tissue was transplanted into the pinna of the ear of isogeneic adult recipients according to a similar protocol. In mock transplant experiments, a subdermal ear pouch was created and then closed as described above without insertion of donor tissue.
In Vitro Coculture Assays
Cardiac muscular endothelial cells (CMEC) were harvested from neonatal and adult mice according to modified protocols (Lodge et al., 1992; Nishida et al., 1993). Adult hearts were excised from anesthetized mice and retrogradely perfused with Hanks' balanced salt solution buffer through the ascending aorta to remove blood cells. The left ventricle was separated from remaining heart tissue, stripped of its epicardium and minced in HBSS containing 40 mg/ml collagenase. The resulting cell suspension was incubated at 37°C in a shaking water bath for 20 min, supplemented with trypsin (final concentration 25 mg/ml), incubated for an additional 15 min at 37°C and then centrifuged at 100 g for 5 min. The endothelial cell–rich supernatant was resuspended and plated in DME/ 20% FCS. To harvest CMEC from neonatal FVB mice, hearts were removed aseptically from a total of 8–15 mice between 2 and 5 d of age, placed in ADS buffer (116 mM NaCl, 20 mM Hepes, 1.0 mM NaH2PO4, 5 mM KCl, 0.8 mM Mg2SO4, 5.5 mM glucose), and minced with a straight-edge razor blade. The tissue was then digested for 45 min at 37°C in DME supplemented with 5% FCS, 0.2% (wt/vol) collagenase and 0.0005% (wt/ vol) DNase. The cell-rich supernatant was centrifuged at 200 g for 10 min and the resulting pellet was resuspended in 2 ml 40% percoll, overlaid with 2 ml 25% percoll, followed by 2 ml PBS. The gradient was centrifuged at 400 g for 15 min and cells in the 40–25% interface were collected, pelleted at 200 g for 2 min, and then resuspended in DME/10% FCS. Cells were initially plated at a density of 2 × 105 cells ml and after a 1-h incubation, the adherent fraction was fed fresh media and subsequently grown at 37°C. After reaching confluence at 7 d, the adult and neonatal CMEC cultures exhibited uniform uptake of Dil-Ac-low density lipoprotein (LDL), uniformly bound both FITC-conjugated Griffonia simplicifolia lectin and were positive for the endothelial cell marker platelet endothelial cell adhesion molecule–1 (PECAM) (data not shown). In fluorescence-activated cell sorting studies, >95% of the cells in the CMEC cultures were positive for diI-acLDL (data not shown). For endothelial cell–myocyte coculture assays, ventricular myocytes were harvested from embryonic hearts (Woodley et al., 1991; Okazaki et al., 1994) and overlaid on established cultures of CMEC at a ratio of 1:1. To assay for endothelial cell proliferation, CMEC were cultured alone or cocultured with ventricular myocytes as described above. After 3 d in culture, the cells were incubated with diI-acLDL. 12 h later, the diI-acLDL–positive endothelial cells were quantitated and the results were used to calculate the proliferative index. The proliferative index for CMEC and CMEC in coculture was 3.0 ± 0.3 and 2.1 ± 0.2, respectively (data not shown). For coculture assays with nonmyocyte cell types, the murine 3T3 fibroblast cell line (CRL 1658), and the murine BNL CL.2 embryonic hepatocyte cell line (TIB 73) were obtained from the American Type Culture Collection (Rockville, MD), cultured in DME/10% FCS and coplated with monolayers of CMEC at a ratio of 1:1.
O-Nitrophenyl-β-d-galactopyranoside Assays and vWF, TM ELISA
CMEC were cultured either alone or in the presence of ventricular myocytes, 3T3 fibroblasts, or BNL hepatocytes for a total of 4 d, and then assayed for β-galactosidase activity, vWF, and TM. β-galactosidase activity was measured using the O-Nitrophenyl-β-d-galactopyranoside (ONPG) assay. Cells were washed with PBS, and then incubated with TEN (40 mM TrisHCl, pH 7.5, 10 mM EDTA, 150 mM NaCl) at room temperature for 10 min. Cells were collected with a cell scraper and centrifuged in an Eppendorf tube at 200 g for 5 min. The pellet was resuspended in 50 μl lysis buffer (250 mM TrisHCl, pH 7.8, 10 mM EDTA), freeze-thawed three times, and then incubated for 1–12 h at 37°C with 150 μl of buffer Z (60 mM dibasic NaHPO4.2H2O, 60 mM monobasic NaHPO4.2H2O, 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol), and 50 μl ONPG 4 μg/ml in 100 mM NaPO3 buffer, pH 7.0. The A420, A550, and A600 of each sample was measured by spectrophotometry and β-galactosidase activity was calculated as previously described (Miller, 1972). For TM ELISA, cells were fixed with ice-cold acetone for 2 min, air-dried, washed with PBS, and then blocked for 1 h with 1% BSA and 0.05% saponin in PBS. The cells were then washed with PBS and incubated with either anti– mouse CD31 monoclonal antibody (PharMingen, San Diego, CA) or anti– human TM (a gift from S. Kennel, University of Tennessee, Oak Ridge, TN) at a dilution of 1:1,000 for 45 min at room temperature. After five washes in PBS, the cells were incubated with anti–rat-HRP antibody at a dilution of 1:500 for 1 h at room temperature. Cells were washed five times with PBS, and then incubated with 100 μl of solution containing 8 mg orthophenylenediamine (OPD) substrate (Dako Corp., Carpinteria, CA) in 12 ml 0.1 M citric acid-phosphate, pH 5.0, and 0.0125% H2O2 at room temperature for 3 min. The reaction was stopped with equal volume 1 M sulfuric acid and the A490 was measured by spectrophotometry. For vWF ELISA, the cells were fixed with 4% paraformaldeyhde in PBS for 15 min on ice, washed three times with PBS, and incubated with 1% BSA and 0.05% saponin in PBS at room temperature for 1 h. After a wash in PBS, the cells were incubated with vWF-HRP antibody (Dako Corp.) at a dilution of 1:200 for 1 h at room temperature. Cells were washed five times with PBS, and then incubated with 100 μl of solution containing 8 mg OPD substrate (Dako Corp.) in 12 ml 0.1 M citric acid phosphate, pH 5.0, and 0.0125% H2O2 at room temperature for 3 min. The reaction was stopped with equal volume 1 M sulfuric acid, and the A490 was measured by spectrophotometry.
Ribonuclease Protection Assays
Template DNA containing sequences from the mouse TM cDNA and from exon 28 of the mouse vWF gene were subcloned into the Bluescript vector (Stratagene). The constructs were linearized by restriction digestion with NotI, and then incubated with T7 in the presence of [32P]UTP to generate single stranded radiolabeled RNA probe. The probe was then hybridized with 20 μg of total RNA in hybridization buffer at 42°C overnight and the reaction mixture subsequently treated with RNase and RNase T1 for 30 min at 37°C. A [32P]UTP-labeled, 250-bp mouse β-actin probe was included in each reaction mixture to control for amounts of RNA. The protected fragments were separated on a 5% nondenaturing polyacrylamide gel. The gel was then dried and exposed to x-ray film overnight. The relative intensity of the bands were quantitated with Betascope 603 Blot Analyzer (Betagen, Waltham, MA).
Results
The vWFlacZ-2 Transgene Is Expressed in the Microvascular Bed of the Heart and Skeletal Muscle
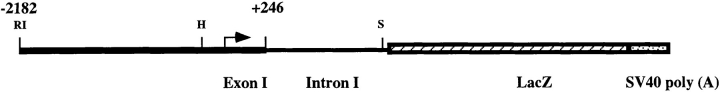
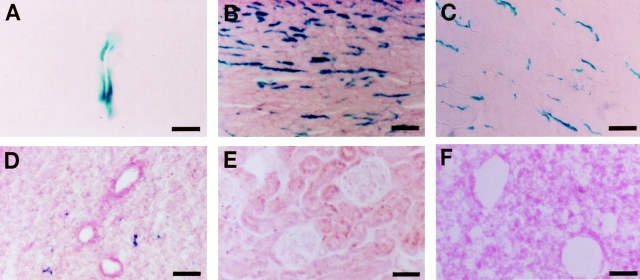
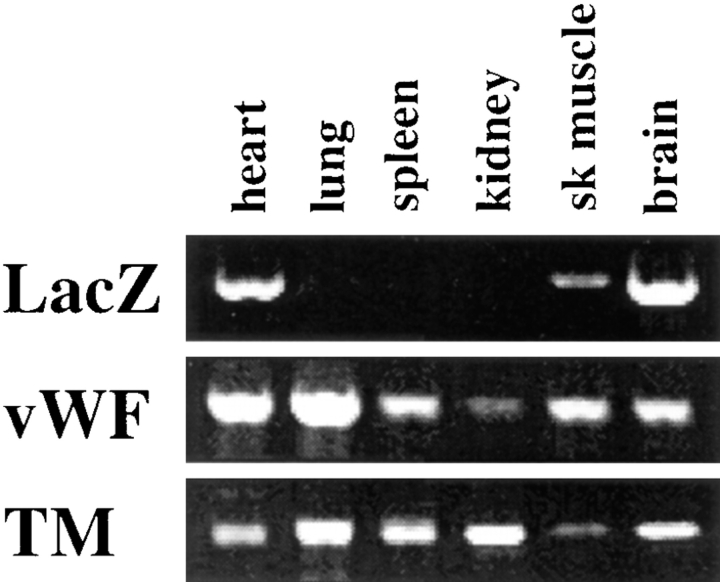
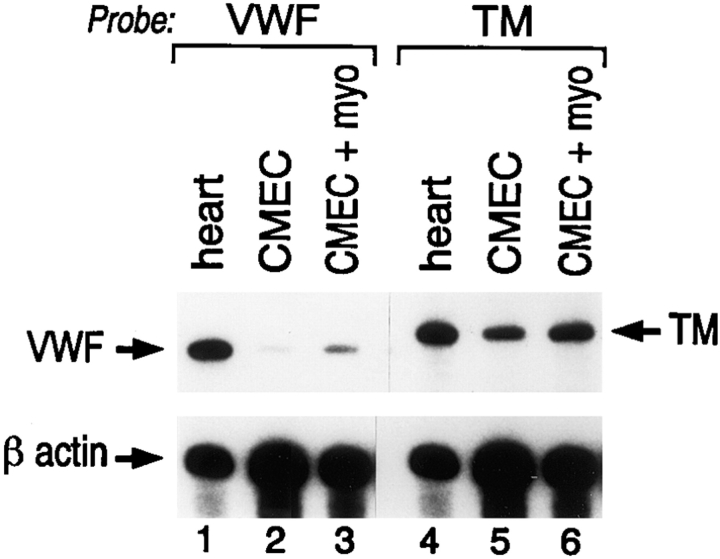
The limited pattern of transgene expression in mice harboring a 733-bp fragment of the human vWF gene suggested that DNA sequences outside this region contained information for more widespread endothelial cell expression (Aird et al., 1995). To confirm this speculation, we generated transgenic mice with a larger segment of the vWF gene containing 2,182 bp of 5′ flanking sequence, the first exon, and first intron was coupled to the LacZ reporter gene (vWFlacZ-2) (Fig. 1). In seven independent founder lines, the X-Gal reaction product was detected not only within blood vessels of the brain but also in a subset of microvessels within the heart and skeletal muscle (Figs. 2, A–C, and 3). In cardiac sections stained with X-Gal and then processed for immunoperoxidase detection of endogenous vWF, the transgene and endogenous gene products colocalized in the endothelial lining of capillary vessels (Fig. 3 B). In contrast, the endothelial cells of the coronary arteries, coronary veins, penetrating arteries, and endocardium of the heart exhibited no detectable β-galactosidase activity but possessed immunoreactive vWF (Fig. 3 A, arrowhead, absence of LacZ staining in epicardial coronary artery). Transgene expression was similarly absent in the vascular bed of other organs, including the liver, spleen, lung, and kidney, as well as in the aorta and megakaryocyte lineage (Fig. 2, D–F). In each of the seven lines of mice, ectopic reporter gene activity was detected within a subpopulation of neurons within the hypothalamus and cerebellum (data not shown). In RT-PCR analyses, LacZ mRNA was detected only in brain, heart, and skeletal muscle (Fig. 4). In contrast, mRNA from the endogenous vWF gene and from the endothelial cell- restricted TM gene was present in all tissues examined. vWF mRNA levels varied from one organ to another (Fig. 4) and correlated with transcript levels detected by ribonuclease protection assays (data not shown). Thus, the above vascular bed–specific expression pattern of vWFLacZ-2 provides further evidence that the vWF transgene is regulated through the interaction of regional transcriptional networks with distinct promoter elements.
Figure 1.
Schematic representation of the vWFlacZ-2 transgene. Arrow, transcriptional start site; SV40 poly(A), SV40 polyadenylation signal; RI, EcoRI; H, HindIII; S, SphI.
Figure 2.
The vWFlacZ-2 transgene directs vascular bed–specific expression in vivo. LacZ staining of 10-μm sections from vWFlacZ-2 mouse tissues showing reporter gene activity within the endothelial cell lining of a blood vessel in the white matter of the brain (A), and microvessels of the heart (B) and skeletal muscle (C). In contrast, β-galactosidase activity is not detectable in the lung (D), kidney (E), and spleen (F). The X-Gal reaction product was similarly absent in other organs, including the liver and aorta as well as the megakaryocyte/platelet lineage (data not shown). Bars: (A) 23 μm; (B–F) 63 μm.
Figure 3.
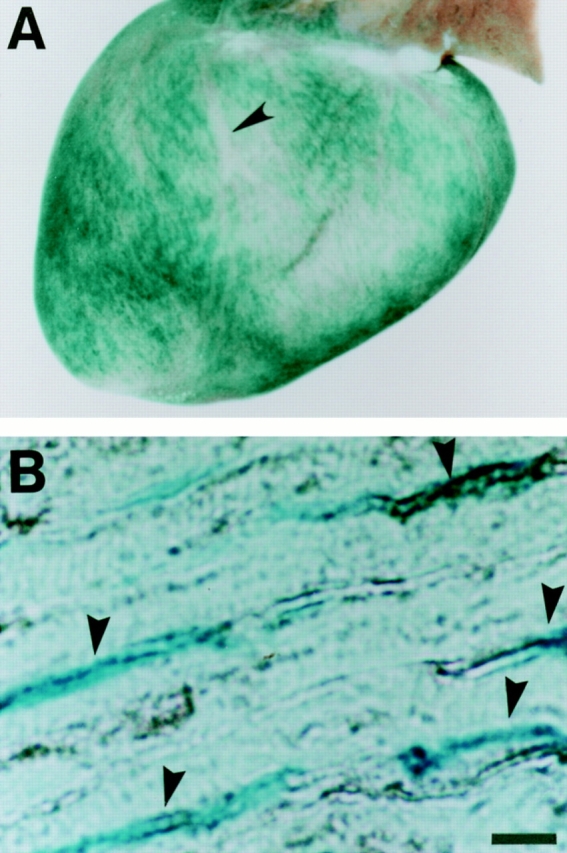
The vWFlacZ-2 transgene colocalizes with endogenous vWF within the microvessels of the heart. (A) Whole mounts of the vWFlacZ-2 adult heart incubated with the X-Gal substrate reveals diffuse LacZ staining in both ventricles and atria with distinct sparing of the epicardial coronary arteries (arrowhead). (B) 10-μm section through the left ventricular wall of the vWFlacZ-2 adult heart processed for β-galactosidase activity (blue) and immunoperoxidase detection of vWF (black) reveals co-localization (arrowheads) within the endothelial lining of the microvessels. Bar, 26 μm.
Figure 4.
β-galactosidase activity correlates with LacZ mRNA levels. RT-PCR analysis of LacZ, vWF, and TM in vWFlacZ-2 mouse tissues reveals the presence of detectable β-galactosidase transcripts exclusively within the brain, heart, and skeletal muscle of adult transgenic mice. This limited expression pattern contrasts with the more widespread, albeit heterogeneous, distribution of endogenous vWF and TM mRNA in adult mouse tissues. Each lane represents an RT-PCR analysis from identical cDNA template. Two independent experiments in two independent vWFlacZ-2 transgenic lines produced similar results.
Wild-type Heart Grafts Induce Transgene Expression in Endothelial Cells Derived from LacZ-negative Blood Vessels
We then wished to determine the relative importance of environmental cues and genetic factors in programming expression of the vWFlacZ-2 transgene in different vascular beds. To investigate this issue, we used a syngeneic cardiac transplantation model in which neonatal donor hearts are grafted into the ear of adult recipient mice (Rossi, 1992). In these experiments, wild-type neonatal were harvested within 24 h of birth, and immediately implanted under the subdermal layer of the ear pinna of adult vWFlacZ-2 transgenic mice. The blood vessels of the host ear rapidly vascularize the graft (Fig. 5 A), and endothelial cells from this nonexpressing bed are newly exposed to a myocardial environment. The grafts were analyzed between 3–6 wk after transplantation when the functional viability of the implanted organ was confirmed both by visible pulsations and electrocardiographic activity (Fig. 5 B). In six independent transplants, LacZ activity was detected in numerous blood vessels surrounding ventricular myocytes (Fig. 5, C and D). The X-Gal reaction product was not observed in the vascular bed of the ear proper, nor within the blood vessels of mock-transplanted transgenic littermates (data not shown). LacZ staining was also absent in neonatal lung tissue transplanted into the pinna of six vWFlacZ-2 transgenic mice (Fig. 5 F). As a control for neovascularization, wild-type lungs were also grafted into a transgenic mouse that contains β-galactosidase activity in endothelial cells of every vascular bed including the ear (Aird, W.C., and R.D. Rosenberg, unpublished observations). In these mice, an abundance of LacZ-positive blood vessels within the substance of the graft indicate that the transplanted lung is revascularized by host-derived endothelium (Fig. 5 E). Taken together, these results suggest that certain vascular beds outside the heart, skeletal muscle, and brain retain the competence to express the vWFlacZ-2 transgene in response to the microenvironment of the heart. In other words, the critical information required for cardiac microvascular–specific transcriptional activation of the vWF transgene is not contained within the endothelial cell, but rather within the surrounding myocytes or the extracellular milieu. These in vivo observations support the view that organ-specific endothelial cell gene expression is ultimately controlled by the interplay between local environmental factors and intracellular transcriptional networks.
Figure 5.
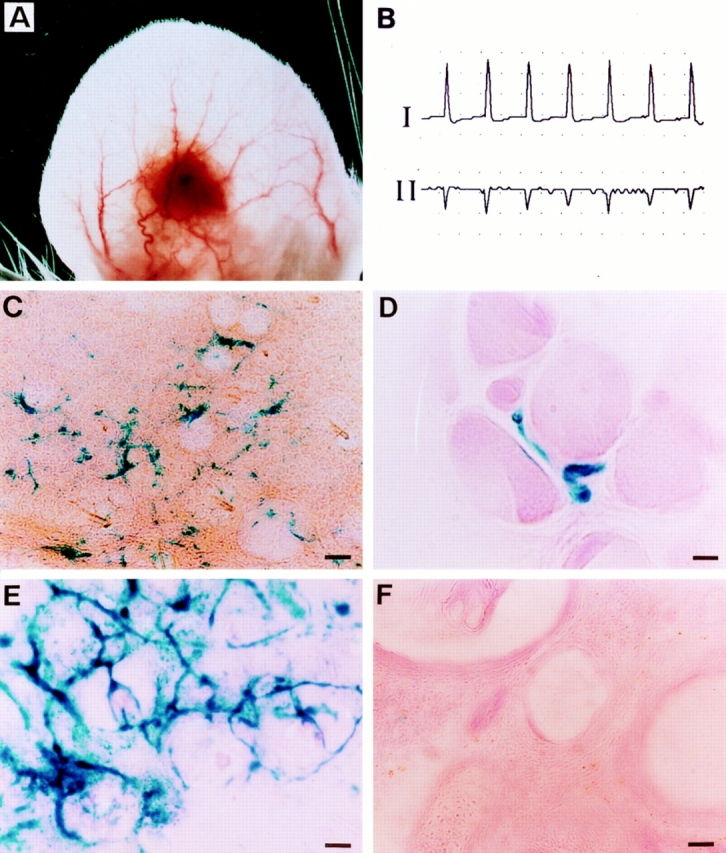
Environmental induction of transgene expression in cardiac transplantation model. (A) Whole mount photomicrograph of a 3-wk-old neonatal, wild-type cardiac graft in the ear of an adult vWFlacZ-2 mouse showing the complex network of anastomosing host auricular blood vessels. (B) Two-lead electrocardiogram of a transplanted heart revealing electrocardiographic activity. The heart rate of the graft was 150 beats per min, compared with the native heart rate of 320 beats per min under anesthesia (C) X-Gal staining of a thick 100-μm section from the cardiac graft reveals the presence of β-galactosidase activity in a linear pattern. (D) X-Gal staining of an 8-μm section from the cardiac graft reveals the presence of LacZ-containing endothelial cells next to wild-type ventricular myocytes. (E) X-Gal staining of a 12-μm section through a wild-type lung graft transplanted into the ear of a transgenic mouse that expresses LacZ in all vascular beds. The presence of LacZ-positive blood vessels indicates that the lung graft is revascularized by host-derived endothelium. (F) X-Gal staining of a 12-μm section through a wild-type lung graft in the ear of a vWFlacZ-2 mouse ear revealing absence of detectable LacZ activity. Bars: (C,E,F) 60 μm; (D) 12 μm.
Cardiac Myocytes Induce Expression of Both Transgene and Endogenous vWF under In Vitro Coculture Conditions
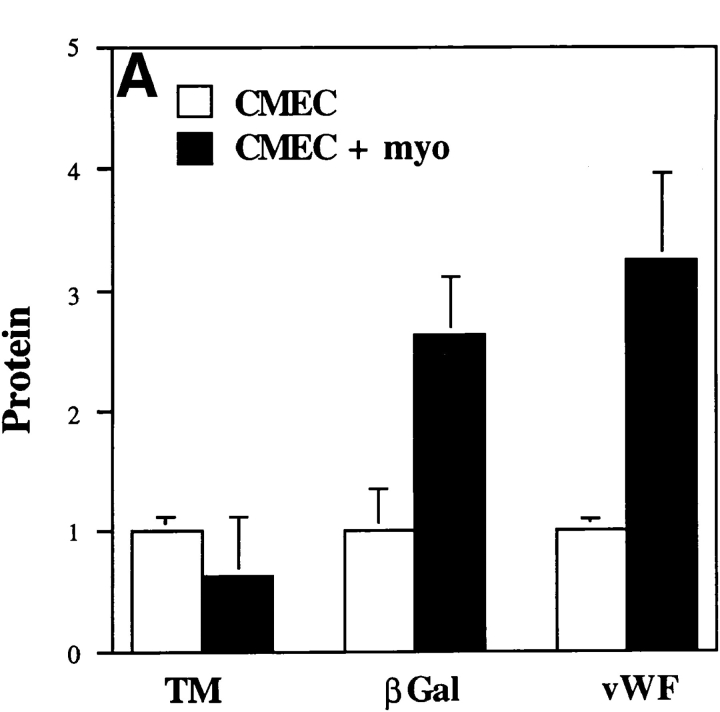
We next established a coculture system to delineate the interaction between CMEC and ventricular myocytes involved in transgene activation. To this end, neonatal or adult transgenic CMEC were harvested and grown under in vitro conditions. After 4 d of culture, pure populations of CMEC no longer exhibited LacZ activity (data not shown). However, when overlaid with wild-type ventricular myocytes, CMEC reacquired the X-Gal reaction product. Interestingly, most LacZ-positive endothelial cells occurred within clusters of spontaneously beating ventricular myocytes (Fig. 6 B). To quantitate the extent of transgene induction, LacZ activity was assayed in whole plate lysates with the ONPG substrate. On average, β-galactosidase activity in primary cultures of CMEC (120 U/105 cells) was 2.6-fold higher in cocultures of CMEC and myocytes as compared to CMEC alone (Fig. 6 A). Of importance, the total numbers of endothelial cells, as monitored by cell-specific markers, failed to increase upon the addition of ventricular myocytes which argues against a proliferative effect of the coculture conditions (data not shown). Moreover, the antigenic levels of the endothelial cell marker TM were not elevated, suggesting that the inductive process is specific for β-galactosidase (Fig. 6 A). Furthermore, cocultures of CMEC with BNL hepatocytes or 3T3 fibroblasts failed to induce LacZ expression (data not shown). Thus, the above data suggest that maintenance and reinduction of the vWFlacZ-2 transgene in CMEC are specifically mediated by cardiac myocytes.
Figure 6.
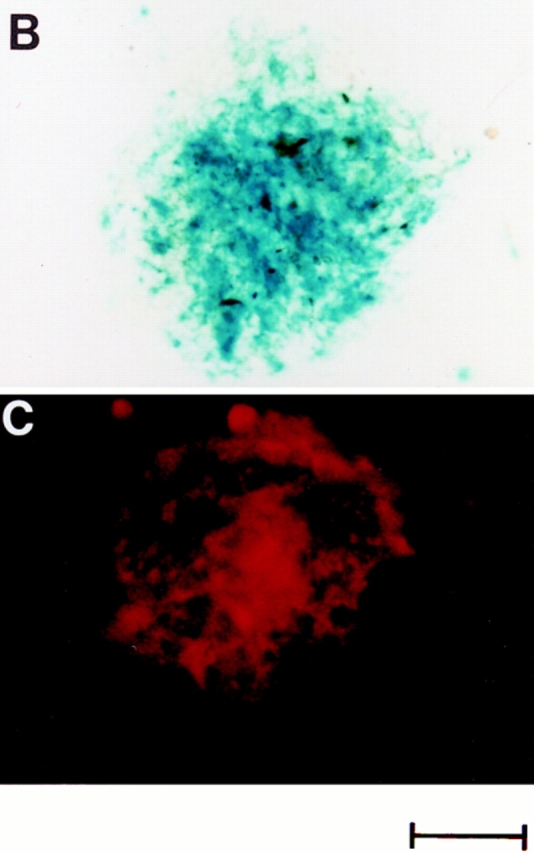
Protein expression in cardiac microvascular endothelial cell-ventricular myocyte coculture. (A) β-Galactosidase activity in CMEC from vWFlacZ-2 mice, as measured with the ONPG substrate, was 2.6-fold higher under coculture conditions (CMEC + myo) compared with either vWFlacZ-2 or wild-type CMEC alone. Antigenic levels of cellular vWF were stimulated 3.1-fold under similar conditions. In contrast, there was no change in the antigenic levels of the endothelial cell marker TM when CMEC was coplated with ventricular myocardial cells. The results are derived from at least three independent experiments, each performed in triplicate. Protein levels are calculated relative to values obtained from primary cultures of vWFlacZ-2 and wild-type–derived CMEC. (B) X-Gal staining of a coculture plate containing CMEC and cardiomyocytes reveals the presence of numerous LacZ-positive endothelial cells integrated within a cluster of myocytes. (C) vWF immunofluorescence under coculture conditions reveals a similar staining pattern with strongly positive endothelial cells interspersed within a colony of cardiomyocytes. Bar, 100 μm.
Finally, we asked whether endothelial cell expression of the endogenous vWF gene and the vWFlacZ-2 transgene is controlled in a similar fashion by cardiomyocytes. Primary cultures of CMEC between passages 1–4, exhibited barely visible vWF antigen as judged by immunohistochemical staining (data not shown). By comparison, strongly positive vWF-containing endothelial cells were readily discernible within clusters of ventricular myocytes under coculture conditions (Fig. 6 C). Total cellular vWF antigenic levels in CMEC (1 ng/105 cells) were increased by an average of 3.1-fold upon addition of cardiomyocytes (Fig. 6 A), but were not altered in the presence of mouse hepatocytes or 3T3 cells (data not shown). To exclude the possibility that a translational mechanism was responsible for elevating endogenous vWF concentrations, vWF mRNA levels were determined by ribonuclease protections assays under coculture conditions. Primary cultures of CMEC exhibited decreased concentrations of transcript as compared to whole heart or freshly harvested CMEC (Fig. 7). The addition of ventricular myocytes to CMEC resulted in a threefold induction of vWF expression (Fig. 7). In contrast, TM mRNA levels did not change significantly (Fig. 7).
Figure 7.
Changes in vWF antigen correlate with transcript levels. In ribonuclease protection assays, total RNA from freshly harvested heart (heart), CMEC and CMEC in coculture with ventricular myocytes (CMEC + myo) was hybridized to riboprobes specific for mouse vWF, TM, and β-actin mRNA.
Discussion
In the present study, a region of the human vWF gene encompassing 2,182 bp of 5′ flanking sequence, the first exon and first intron coupled to the coding region of LacZ was used to generate transgenic mice. In seven independent lines of mice, reporter gene activity and mRNA were limited to the endothelial lining of blood vessels in the brain, heart and skeletal muscle, indicating that this particular promoter fragment contains information sufficient for vascular bed–specific expression of vWF. The limited distribution of the transgene contrasts with the more widespread expression of the endogenous gene and suggests that alternative mechanisms of transcriptional activation are operative in LacZ-negative endothelial cells. These observations add vWF to a growing list of endothelial cell promoters that have been shown to direct limited, endothelial cell subtype-restricted expression in transgenic mice (Harats et al., 1995; Korhonen et al., 1995; Schlaeger et al., 1995). In one report, DNA promoter constructs containing either 1,200 or 600 bp of the murine Tie-2 promoter were shown to direct expression to distinct endothelial cell subpopulations within transgenic embryos (Schlaeger et al., 1995). In a similar study, a 735-bp region of the mouse Tie-1 gene was shown to confer endothelial cell subtype-specific expression during development (Korhonen et al., 1995). In contrast, activity of the Tie-1 and Tie-2 transgenes was downregulated in adult mice (Korhonen et al., 1995; Schlaeger et al., 1995). Finally, a 5.9-kb fragment of the murine preproendothelin-1 promoter directed differential expression within the endothelium and vascular smooth muscle cells of adult transgenic mice (Harats et al., 1995). Expression levels in these mice varied not only between arteries, veins, and capillaries, but also between vascular beds of different organs (Harats et al., 1995). Taken together, these studies provide strong support for the existence of regional differences in the mechanisms of endothelial cell gene regulation.
The existence of cell subtype-specific mechanisms of gene regulation is not limited to the endothelium. In transgenic mice, the α1(1) collagen gene was shown to possess different cis elements required for expression in fibroblasts of the skin as compared to fibroblasts within the fascia (Liska et al., 1994). In another investigation, expression of the CD4 gene in transgenic mice was shown to be governed by distinct regulatory elements in separate T cell subsets (Hanna et al., 1994). In a recent study of the muscle-specific SM22α gene, a 445-bp region of the promoter directed expression in the vascular smooth cells of arteries as well as cardiac and skeletal myocytes in a temporospatial pattern similar to that of the endogenous gene. However, in contrast to the endogenous gene, transgene expression was absent in venous and visceral smooth muscle cells (Li et al., 1996). The promoter region of yet another muscle-specific gene (MLC-3F) was found to contain distinct DNA regions capable of distinguishing between regulatory programs within the various chambers of the transgenic heart (Kelly et al., 1995). These reports provide additional evidence that transcriptional control mechanisms may differ between subpopulations of cells and reinforces the notion that not all cell types within a given lineage are alike.
The vWFlacZ-2 transgenic mouse provides a unique tool with which to study environmental modulation of endothelial gene expression. In cardiac transplantation experiments, endothelial cells derived from LacZ-negative blood vessels of the ear were shown to express the transgene in the presence of grafted heart tissue. These findings indicate that endothelial cells of vascular beds outside the heart, skeletal muscle, and brain retain the competence to express the vWFlacZ-2 transgene in response to appropriate environmental cues. In other words, the information for cardiac microvascular bed–specific transcriptional activation of the vWF transgene is contained not within the endothelial cell itself but rather within the surrounding extracellular milieu. The importance of the ventricular myocyte in mediating the inductive phenomenon was documented in coculture assays. In these experiments, maintenance and reinduction of both vWFlacZ and endogenous vWF gene expression in CMEC was found to be dependent on cardiomyocyte-derived signals. Taken together, these observations add strong support to the notion that organ-specific endothelial cell gene expression is ultimately controlled by interactions between local environmental factors and intracellular transcriptional networks. Indeed, the in vitro system should prove useful in identifying and characterizing signaling pathways involved.
In summary, the above data strongly suggest that cardiac microvascular endothelial cells possess a common transcriptional mechanism for expressing both endogenous vWF and the vWFlacZ-2 transgene. Based upon these results, we predict that transcriptional activation of vWF in LacZ-negative vascular beds is mediated by the interaction of local signaling pathways with cis-acting elements outside of the vWFlacZ-2 promoter region. According to this model of gene regulation, the expression pattern of vWF and perhaps other endothelial cell genes within the vascular tree reflect the combined activity of multiple signaling pathways that vary from one microenvironment to another. This array of local networks would provide an effective means of establishing functionally distinct endothelial cell populations responsive to the specific needs of the underlying tissues. As a logical extension of this model, pathophysiological alterations in one or more of these pathways might underlie the focal nature of vascular diseases.
Acknowledgments
We thank D. Beeler and E. Li for their technical assistance, and M. Krieger and R. Kelly for their critical reading of the manuscript. We are grateful to A. Hautzopolous for providing us with the mouse vWF riboprobe.
This work was supported in part by grant HL41484 from the National Institutes of Health.
Abbreviations used in this paper
- CMEC
cardiac microvascular endothelial cells
- LDL
low density lipoprotein
- ONPG
O-Nitrophenyl-β-d- galactopyranoside
- RT
reverse transcriptase
- TM
thrombomodulin
- vWF
von Willebrand factor
Footnotes
Please address all correspondence to W.C. Aird, Molecular Medicine, RW-663, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, Boston, MA 02215. Tel.: (617) 667-1031. Fax: (617) 667-2913. e-mail: waird@bidmc.harvard.edu
References
- Aird WC, Jahroudi N, Weiler-Guettler H, Rayburn HB, Rosenberg RD. Human von Willebrand factor gene sequences target expression to a subpopulation of endothelial cells in transgenic mice. Proc Natl Acad Sci USA. 1995;92:4567–4571. doi: 10.1073/pnas.92.10.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahnak BR, Wu Q, Coulombel L, Assouline Z, Kerbiriou-Nabias D, Pietu G, Drouet L, Caen JP, Meyer D. Expression of von Willebrand Factor in porcine vessels: heterogeneity at the level of von Willebrand Factor mRNA. J Cell Physiol. 1989;138:305–310. doi: 10.1002/jcp.1041380212. [DOI] [PubMed] [Google Scholar]
- Beck DW, Vinters HV, Hart MN, Cancilla PA. Glial cells influence polarity of the blood-brain barrier. J Neuropathol Exp Neurol. 1984;43:219–224. doi: 10.1097/00005072-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Berg EL, Goldstein LA, Jutila MA, Nakache M, Picker LJ, Streeter PR, Wu NW, Zhou D, Butcher EC. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocytes traffic. Immunol Rev. 1989;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB. The blood-brain barrier. Exp Physiol. 1993;78:453–472. doi: 10.1113/expphysiol.1993.sp003698. [DOI] [PubMed] [Google Scholar]
- Coffin JD, Harrison J, Schwartz S, Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev Biol. 1991;148:51–62. doi: 10.1016/0012-1606(91)90316-u. [DOI] [PubMed] [Google Scholar]
- DeFouw DO. Structural heterogeneity within the pulmonary microcirculation of the normal rat. Anat Rec. 1988;221:645–654. doi: 10.1002/ar.1092210210. [DOI] [PubMed] [Google Scholar]
- Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia colisepsis. Am J Pathol. 1993;142:1458–70. [PMC free article] [PubMed] [Google Scholar]
- Fleming S, Jones DB. Antigenic heterogeneity of renal endothelium. J Pathol. 1989;158:319–323. doi: 10.1002/path.1711580409. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME. Functional heterogeneity of vascular endothelial cells. Biochem Pharmacol. 1987;36:2701–2711. doi: 10.1016/0006-2952(87)90252-8. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Bloor CM. Endothelial cell gene expression in response to injury. FASEB (Fed Am Soc Exp Biol) J. 1993;7:523–532. doi: 10.1096/fasebj.7.6.8472891. [DOI] [PubMed] [Google Scholar]
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Hadley JT, Lu Z, Wasniowska K, Martin AW, Peiper SC, Hesselgesser J, Horuk R. Postcapillary venule endothelial cells in kidney express a multispecific chemokine receptor that is structurally and functionally identical to the erythroid isoform, which is the Duffy blood group antigen. J Clin Invest. 1994;94:985–991. doi: 10.1172/JCI117465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna Z, Simard C, Laperriere A, Jolicoeur P. Specific expression of the human CD4 gene in mature CD4+ CD8- and immature CD4+ CD8+ T cells and in macrophages of transgenic mice. Mol Cell Biol. 1994;14:1084–1094. doi: 10.1128/mcb.14.2.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harats D, Kurihara H, Belloni P, Oakley H, Ziober A, Ackley D, Cain G, Kurihara Y, Lawn R, Sigal E. Targeting gene expression to the vascular wall in transgenic mice using the murine preproendothelin-1 promoter. J Clin Invest. 1995;95:1335–1344. doi: 10.1172/JCI117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introna M, Alles VV, Castellano M, Picardi G, De Gioia L, Bottazzai B, Peri G, Breviario F, Salmona M, De Gregorio L, et al. Cloning of mouse ptx3, a new member of the pentraxin gene family expressed at extrahepatic sites. Blood. 1996;87:1862–72. [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature (Lond) 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VWM, Fang G, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen J, Lahtinen I, Halmekyto M, Alhonen L, Janne J, Dumont D, Alitalo K. Endothelial-specific gene expression directed by the tie gene promoter in vivo. . Blood. 1995;86:1826–1835. [PubMed] [Google Scholar]
- Kumar S, West DC, Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987;36:57–70. doi: 10.1111/j.1432-0436.1987.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Lassalle P, Molet S, Janin A, Van der Heyden J, Tavernier J, Fliers W, Devos R, Tonnel A. ESM-1 is a novel human endothelial cell-specific molecule expressed in the lung and regulated by cytokines. J Biol Chem. 1996;271:20458–20464. doi: 10.1074/jbc.271.34.20458. [DOI] [PubMed] [Google Scholar]
- Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska DJ, Reed MJ, Sage EH, Bornstein P. Cell-specific expression of alpha 1(I) collagen-hGH minigenes in transgenic mice. J Cell Biol. 1994;125:695–704. doi: 10.1083/jcb.125.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobrinus JA, Juillerat-Jeanneret L, Darekar P, Schlosshauer B, Janzer RC. Induction of the blood brain barrier specific HT7 and neurothelin epitopes in endothelial cells of the chick chorioallantoic vessels by a soluble factor derived from astrocytes. Brain Res Dev Brain Res. 1992;70:207–211. doi: 10.1016/0165-3806(92)90199-7. [DOI] [PubMed] [Google Scholar]
- Lodge PA, Haisch CE, Thomas FT. A simple method of vascular endothelial cell isolation. Transplant Proc. 1992;24:2816–2817. [PubMed] [Google Scholar]
- Maxwell K, Berliner JA, Cancilla PA. Induction of gamma-glutamyl transpeptidase in cultured cerebral endothelial cells by a product released by astrocytes. Brain Res. 1987;410:309–314. doi: 10.1016/0006-8993(87)90329-5. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.H. 1972. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. 446 pp.
- Nishida M, Springhorn JP, Kelly RA, Smith TW. Cell-cell signaling between adult rat ventricular myocytes and cardiac microvascular endothelial cells in heterotypic primary culture. J Clin Invest. 1993;91:1934–1941. doi: 10.1172/JCI116412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. Origins and assembly of avian embryonic blood vessels. Ann NY Acad Sci. 1990;588:236–249. doi: 10.1111/j.1749-6632.1990.tb13214.x. [DOI] [PubMed] [Google Scholar]
- Okazaki K, Ishikawa T, Inui M, Tada M, Goshima K, Okamoto T, Hidaka H. KN-62, a specific Ca++/calmodulin-dependent protein kinase inhibitor, reversibly depresses the rate of beating of cultured fetal mouse cardiac myocytes. J Pharmacol Exp Ther. 1994;270:1319–1324. [PubMed] [Google Scholar]
- Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- Poole TJ, Coffin JD. Vasculogenesis and angiogenesis: two distinct morphogenetic mechanisms establish embryonic vascular pattern. J Exp Zool. 1989;251:224–231. doi: 10.1002/jez.1402510210. [DOI] [PubMed] [Google Scholar]
- Rand JH, Badimon L, Gordon RE, Uson RR, Fuster V. Distribution of von Willebrand factor in porcine intima varies with blood vessel type and location. Arteriosclerosis. 1987;7:287–291. doi: 10.1161/01.atv.7.3.287. [DOI] [PubMed] [Google Scholar]
- Resnick N, Gimbrone MA. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB (Fed Am Soc Exp Biol) J. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- Resnick N, Collins T, Atkinson W, Bonthron DT, Dewey CF, Gimbrone MA. Platelet–derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci USA. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MA. Chronic hemodynamic unloading regulates the morphologic development of newborn mouse hearts transplanted into the ear of isogeneic adult mice. Am J Pathol. 1992;141:183–191. [PMC free article] [PubMed] [Google Scholar]
- Ruggeri ZM, Ware J. von Willebrand factor. FASEB (Fed Am Soc Exp Biol) J. 1993;7:308–316. doi: 10.1096/fasebj.7.2.8440408. [DOI] [PubMed] [Google Scholar]
- Sadler JE. von Willebrand factor. J Biol Chem. 1991;266:22777–22780. [PubMed] [Google Scholar]
- Schlaeger TM, Qin Y, Fujiwara Y, Magram J, Sato TN. Vascular endothelial cell lineage-specific promoter in transgenic mice. Development (Camb) 1995;121:1089–1098. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B. The blood brain barrier: morphology, molecules and neurothelin. Bioessays. 1993;15:341–346. doi: 10.1002/bies.950150508. [DOI] [PubMed] [Google Scholar]
- Senis YA, Richardson M, Tinlin S, Maurice DH, Giles AR. Changes in the pattern of distribution of von Willebrand factor in rat aortic endothelial cells following thrombin generation in vivo. . Br J Hematol. 1996;93:195–203. doi: 10.1046/j.1365-2141.1996.4661005.x. [DOI] [PubMed] [Google Scholar]
- Smith JM, Meinkoth JH, Hochstatter T, Meyers KM. Differential distribution of von Willebrand factor in canine vascular endothelium. Am J Vet Res. 1996;57:750–755. [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: A study using quail-chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Streeter PR, Berg EL, Rouse BTN, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature (Lond) 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, van Vlijmen H, Loesch A, Burnstock G. An immunohistochemical study of endothelial cell heterogeneity in the rat: observations in “en face” Hautchen preparations. Cell Tissue Res. 1991;263:173–181. doi: 10.1007/BF00318413. [DOI] [PubMed] [Google Scholar]
- Turner RR, Beckstead JH, Warnke RA, Wood GS. Endothelial cell phenotypic diversity. Am J Clin Pathol. 1987;87:569–575. doi: 10.1093/ajcp/87.5.569. [DOI] [PubMed] [Google Scholar]
- Wang R, Clark R, Bautch VL. Embryonic stem cell-derived cystic embryoid bodies form vascular channels: an in vitromodel of blood vessel development. Development (Camb) 1992;114:303–316. doi: 10.1242/dev.114.2.303. [DOI] [PubMed] [Google Scholar]
- Woodley SL, McMillan M, Shelby J, Lynch DH, Roberts LK, Ensley RD, Barry WH. Myocyte injury and contraction abnormalities produced by cytotoxic T lymphocytes. Circulation. 1991;83:1410–1418. doi: 10.1161/01.cir.83.4.1410. [DOI] [PubMed] [Google Scholar]
- Wu QY, Drouet L, Carrier JL, Rothschild C, Berard M, Rouault C, Caen JP, Meyer D. Differential distribution of von Willebrand factor in endothelial cells. Comparison between normal pigs and pigs with von Willebrand disease. Arteriosclerosis. 1987;7:47–54. doi: 10.1161/01.atv.7.1.47. [DOI] [PubMed] [Google Scholar]